Abstract
There is no parenteral formulation of the neuraminidase inhibitor oseltamivir, the most widely used anti-influenza virus drug. Oseltamivir resistance is an increasing problem. Zanamivir is effective against the most prevalent oseltamivir-resistant influenza viruses. A parenteral formulation of zanamivir is in development for the treatment of severe influenza. It is not known if there is any pharmacokinetic interaction between the two drugs. Sixteen healthy Thai adult volunteers were studied in an open-label, four-period, randomized two-sequence crossover pharmacokinetic study in which zanamivir was given by constant-rate infusion or slow intravenous injection either alone or together with oral oseltamivir. Plasma concentration profiles of oseltamivir, the active metabolite oseltamivir carboxylate, and zanamivir were measured by liquid chromatography-mass spectrometry-mass spectrometry. Both drugs were well tolerated alone and in combination. The maximum plasma concentrations and the areas under the plasma concentration-time curves (AUC) of oseltamivir and oseltamivir carboxylate were not significantly different when oseltamivir was given separately or together with zanamivir. Maximum plasma concentrations of zanamivir were 10% (95% confidence interval, 7 to 12%) higher when zanamivir was infused concurrently with oral oseltamivir than with infusions before or after oral oseltamivir. The plasma zanamivir total AUC was positively correlated with the total oseltamivir carboxylate AUC (Pearson's correlation coefficient [rP] = 0.720, P = 0.002, n = 16) but not with the oseltamivir AUC (rp = 0.121, n = 16). There is no clinically significant pharmacokinetic interaction between oseltamivir and zanamivir.
INTRODUCTION
Pandemic influenza is considered by many experts to be the most significant potential global public health emergency caused by a naturally occurring pathogen. The human cases of highly pathogenic strains of avian influenza (H5N1) and the recent H1N1 2009 pandemic have heightened concerns about the potential consequences of a severe influenza pandemic. The H5N1 virus is endemic in birds in parts of Southeast Asia and has spread to other parts of Asia and Europe. Mortality in human cases consistently exceeds 50%. Antiviral drugs are a critical component of the clinical management of severe influenza in an influenza pandemic.
The prompt use of neuraminidase inhibitors has been recommended for the treatment of patients with suspected severe influenza such as avian influenza (2). There is increasing anecdotal experience with neuraminidase inhibitors, but they have not been evaluated rigorously in the treatment of severe influenza, and their optimal dose and duration of treatment are unknown. The currently available neuraminidase inhibitors are formulated for oral administration (oseltamivir [OS]) or inhalation (zanamivir), both of which are inappropriate formulations for critically ill patients. The high levels of oseltamivir resistance in prevalent influenza A viruses (all seasonal H1N1 viruses in the 2008-2009 season were oseltamivir resistant) and the ready selection of resistance in H5N1 infections further highlight the critical need for additional treatment options (6). As predicted by the chemical structure of zanamivir and its interaction with the neuraminidase active site, the oseltamivir-resistant H5N1 strains that have been recovered following the treatment of patients generally are not cross-resistant to zanamivir (6, 16, 11). The provision of an intravenous (i.v.) neuraminidase inhibitor for the treatment of influenza therefore could be of significant benefit to critically ill patients. Early in the development of zanamivir, an intravenous formulation was investigated in healthy volunteers. The pharmacokinetics of intravenously administered zanamivir were studied after single escalating doses of up to 600 mg. Repeated doses of 600 mg twice daily for 5 days also were studied. After a single intravenous dose of 16 mg, the elimination half-life was about 1.7 h, and about 90% of the dose was excreted unchanged in the urine within 10 h after dosing. No accumulation of zanamivir in serum was evident after repeated doses of 600 mg every 12 h (q12h) (4). The volume of distribution of zanamivir was 16 liters in the adult subjects studied, which approximated the volume of extracellular water. This is consistent with its physicochemical characteristics as a polar compound with low protein binding of <10%. Zanamivir is excreted in the urine primarily by the passive renal filtration of unchanged drug. Chromatographic profiling showed no evidence of biotransformation (5). These findings indicate that the drug does not undergo metabolism. Given its low protein binding and elimination primarily by the passive renal filtration of unchanged drug, it is unlikely that zanamivir would affect the elimination of other concurrently administered compounds or vice versa. Coadministration at clinically relevant concentrations of ibuprofen, cimetidine, pseudoephedrine, and cefuroxime did not affect the renal extraction ratio of zanamivir in the isolated perfused rat kidney model (5).
Oseltamivir is approved for the treatment of uncomplicated acute influenza infection. It is generally well tolerated. Oseltamivir is an ethyl ester prodrug requiring ester hydrolysis for conversion to the active form, oseltamivir carboxylate (OC), which then is exclusively excreted by glomerular filtration and active tubular secretion (via organic anion transporter 1 [hOAT1]) without further metabolism. Parent oseltamivir has a short half-life and does not accumulate with repeat dosing q12h (10, 15, 17). In contrast, the active metabolite oseltamivir carboxylate has a 5- to 11-h half-life and therefore does accumulate with repeated q12h dosing, and it achieves a steady state within 3 days (15, 17). Oseltamivir does not show dose-dependent kinetics. In human drug interaction studies, the anionic compound probenecid inhibits the renal secretion of oseltamivir carboxylate (10, 17) and also contracts the total apparent volume of distribution, thereby considerably increasing systemic exposure (17). Functional polymorphisms in human carboxylesterase 1 may affect oseltamivir bioactivation (17). As oseltamivir is widely available, it is very likely that during a pandemic intravenous zanamivir will be used in combination with oseltamivir. Although the potential for a drug interaction between the two compounds is very low, the likelihood of coadministration combined with the common route of renal clearance of both drugs and the current lack of information on the organic anion transporter (OAT) inhibition potential of zanamivir in vivo indicated a need to study the in vivo interaction between these agents.
MATERIALS AND METHODS
Sixteen healthy Thai adult volunteers were studied in an open-label, four-period, randomized two-sequence pharmacokinetic comparison conducted in the volunteer facility of the Hospital of Tropical Diseases, Faculty of Tropical Medicine, Mahidol University.
Participants were healthy nonpregnant HIV-1, hepatitis B- and C-uninfected individuals, 18 to 60 years of age, with body mass indices of 17 to 32 kg of body weight/m2 and body weight of ≥37 kg, with a normal electrocardiogram QTc of <450 ms. Participants understood the purpose of the study and provided written consent. Participants were screened for substance abuse and eligibility based on inclusion and exclusion criteria. Volunteers were required to agree to use the appropriate form of contraception and refrain from the ingestion of grapefruit-containing products for 72 h prior to the start of dosing.
Treatment.
Each adult volunteer received the following four regimens: A, 50 mg/h zanamivir (GlaxoSmithKline Laboratories) administered by continuous i.v. infusion in 0.9% normal saline administered for 16 h (total dose of 800 mg); B, 150 mg of oseltamivir (Tamiflu; Roche Laboratories) administered per os (p.o.) q12h for 3 days (for a total of five doses ending the morning of day 3); C, 150 mg of oseltamivir administered p.o. q12h for 3 days (for a total of five doses ending the morning of day 3) plus 50 mg/h zanamivir by continuous i.v. infusion administered for 3 days (72 h; total dose, 3.6 g) given concurrently; D, 150 mg of oseltamivir administered p.o. q12h for 3 days plus 600 mg zanamivir as an i.v. dose (30-min infusion in 0.9% normal saline) q12h for 3 days (for a total of five doses ending the morning of day 3). The zanamivir rapid infusion was given 4 h after the dose of oseltamivir to coincide with peak concentrations of oseltamivir carboxylate and to maximize the opportunity to characterize any interaction or adverse effects. Zanamivir is a polar compound (log P, −1.87) that is very soluble in water and saline (intrinsic solubility, 14.9 mg/ml). The zanamivir solutions for intravenous administration were prepared at the following concentrations: regimen A, 800 mg/500 ml; and regimen C, 1,200 mg/500 ml; and regimen D, 600 mg/100 ml saline. These mixtures resulted in final concentrations of 1.6, 2.4, and 6 mg/ml, respectively. The total volume given was 500, 1,500, and 500 ml in regimen A, B, and D, respectively.
The i.v. formulation of zanamivir was provided to the site as bulk open-labeled material and then was prepared for dosing individually. The oral study medication oseltamivir (Tamiflu; Roche Pharmaceuticals, Switzerland) was taken with water. To reduce the risk of incorrect dose administration, the dosing was observed by a second study nurse. Each subject attended a screening visit, four treatment periods, and a follow-up visit at 7 to 10 days after completing the last treatment assessments or withdrawing from the study. Subjects were randomized to receive either regimen A or B for period 1. Subjects who received regimen A first received regimen B in period 2 and vice versa. The subjects then continued with regimens C and D consecutively, with a more than 3-day washout period between each period. Volunteers were admitted to the volunteer facility at the Hospital of Tropical Diseases the evening before each study period began. At the screening visit, check in, and each morning, subjects were asked about their state of health, compliance with study restrictions, and use of any concomitant medication since screening or the previous study visit. Adverse events, hematology, clinical chemistry, urinalysis, electrocardiograms (ECGs), and vital signs were collected on a standard case record form throughout each treatment period.
Blood sample collection.
Blood samples (2 ml) were drawn via individual needle stick or an i.v. cannula. Predose samples were drawn within 30 min of dosing. Samples for plasma oseltamivir measurements were collected predose and at 4 h after the first dose on days 1 and 2 (regimens B and C) and predose, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, and 24 h after the last dose on day 3 (regimens B, C, and D). Samples for plasma zanamivir measurements were collected in regimen A predose and at 1, 2, 4, 6, 8, 10, 12, 14, 16 (end of i.v. infusion), 16.5, 16.75, 17, 18, 20, 22, 24, and 28 h after the start of the i.v. infusion; in regimen C at 48, 50, 52, 54, 56, 58, 60, 64, and 72 h after the start of i.v. infusion; and in regimen D predose and at 30 min (end of infusion) on days 1 and 2 and then again at predose, 0.25, 0.5 (end of infusion), 0.75, 1, 1.25, 1.5, 2, 3, 4, 6, 8, and 12 h after the start of the i.v. infusion on day 3. Blood samples were collected into 2-ml fluoride-oxalate blood collection tubes (12). Upon collection, blood samples were centrifuged for 7 min at 2,000 × g at 4°C, transferred via pipette to a prelabeled sample storage tube, and stored upright in a non-self-defrosting freezer at −70°C or lower until analyzed.
Drug assay.
Plasma zanamivir was quantified using liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) as previously described (14). Performance during analysis was evaluated through the analysis of three replicate quality control samples at three concentrations during each batch of samples. The total relative standard deviation (RSD) for the quality controls were 3.29, 1.46, and 0.82% at 150, 2,000 and 40,000 ng/ml, respectively. The assay uses 50 μl plasma and covers a low and a high range, with a total working range from 1.00 to 50,000 ng/ml.
Oseltamivir and oseltamivir carboxylate were measured by LC-MS/MS as described previously (13). Performance during analysis was evaluated through the analysis of three replicate quality control samples at three concentrations during each batch of samples. The total RSD for the quality controls were 3.03, 2.60, and 2.56% at 3, 20, and 150 ng/ml, respectively, for oseltamivir and 2.62, 1.84, and 1.85% at 30, 400, and 4,000 ng/ml, respectively, for oseltamivir carboxylate. The assay uses 50 μl plasma and covers a range from 1.00 to 300 ng/ml and 10.0 to 10,000 ng/ml for oseltamivir carboxylate. In both of these assays, interference and matrix effects by the other drug were investigated and excluded. Both assays used stable isotope-labeled internal standards. The lowest point in each calibration range was at a concentration equivalent to the lower limit of quantification.
Data analysis: sample size.
The target sample size was chosen to address the primary pharmacokinetic study objective, which was to demonstrate that zanamivir does not have a significant effect on oseltamivir carboxylate pharmacokinetics as measured by maximum plasma concentrations (Cmax) and area under the plasma concentration-time curve between 0 and 12 h after dosing (AUC0-12). The absence of a significant effect was considered to have been demonstrated if the 90% confidence interval (CI) for the ratio of test to reference (C:B or D:B) lay within the range (0.80 to 1.25) for the Cmax and AUC0-12 (as per FDA guidance for bioequivalence). Assuming the within-subject coefficient of variation (CV) was not larger than 15.6% (based on our data previously published [17] and assuming a correlation between observations taken on the same subject of 0.6), it was estimated that enrolling 16 subjects, to ensure a sample size of 13 subjects with all studies completed, would provide at least 90% power to demonstrate no effect of zanamivir administration on the oseltamivir carboxylate Cmax or AUC0-12.
Pharmacokinetic analysis.
Noncompartmental pharmacokinetic analysis (NCA) was conducted for oseltamivir (OS; regimens B, C, and D), oseltamivir carboxylate (OC; regimens B, C, and D), and zanamivir (regimens A and D) using STATA (release 10.0; Stata Corporation, TX). The elimination half-life was estimated from the last five plasma concentration measurements, and the total area under the concentration-time curve was estimated using log-linear extrapolation. In compartmental modeling (using WinNonlin; Pharsight Co., Mountain View, CA), individual subject concentration-time profiles for OC (regimens B, C, and D) and zanamivir (regimens A and D) were modeled assuming either one- or two-compartment kinetics. Kinetic models were compared for goodness of fit with respect to the Akaike information criterion and residuals.
Following loge transformation, the effects of zanamivir on the total AUC, AUC0-12, and Cmax of oseltamivir and oseltamivir carboxylate were analyzed separately using a mixed-effect model, fitting a fixed-effect term for the regimen and treating subjects as a random effect. A maximum-likelihood random-effect estimator was used (18). Point and interval estimates for differences in log-transformed parameters from the model were exponentially back-transformed to construct point and 90% confidence interval estimates for the ratios of interest (C:B and D:B) on the original scale. Bioequivalence between oseltamivir administered alone and administered together with i.v. zanamivir was assessed using 90% confidence intervals, constructed in the same way but using standard errors based on residual variance (7, 8). Bioequivalence was determined when this confidence interval was in the 0.8 to 1.25 or 80 to 125% range. For zanamivir, formal bioequivalence testing was not performed because of different sampling or zanamivir dosing schedules between regimens, but measured steady-state concentrations for zanamivir administered alone (regimen A) were compared to measured steady-state concentrations in regimen C, and total body clearance rates estimated by noncompartmental analysis were compared between regimens A and D.
Ethics committee review.
This study was approved by the Ethics Review Committee of the Faculty of Tropical Medicine, Mahidol University.
RESULTS
Subjects.
In total, 31 volunteers were screened; 17 were enrolled, but one presented with increased aspartate transaminase (AST) and alanine aminotransferase (ALT) levels before first drug administration and was withdrawn from the study. Therefore, 16 volunteers completed all four pharmacokinetic series. Of the 16 participants, 14 were males and 2 were females, with a median age of 26 years and range of 20 to 45 years and median (range) body mass index of 22 kg/m2 (19 to 23.5 kg/m2). The median (range) oseltamivir doses administered were 2.4 mg/kg (2.1 to 3 mg/kg). The median (range) zanamivir doses administered were 12.8 mg/kg (11.3 to 16 mg/kg) in regimen A, 57.9 mg/kg (51.1 to 72 mg/kg) in regimen C, and 9.7 mg/kg (8.5 to 12 mg/kg) in each of the five infusions in regimen D. Three volunteers had short interruptions in the 72-h administration of zanamivir (regimen C) lasting 5 min for two of them and 10 min for the third volunteer.
Adverse events.
No serious adverse events were observed during the study. In total, 15 adverse events were recorded in 10 volunteers, with intensity graded as mild in 13, moderate in 1, and severe in 1 subject.
Only two adverse events were classified as possibly or probably related to a study drug: abnormal liver function and increased ALT, both with mild severity. They were observed after the administration of oral oseltamivir alone (regimen B). One had an oseltamivir carboxylate AUC above the 75th percentile, but the levels of oseltamivir and oseltamivir carboxylate were otherwise unexceptional. The other adverse events, classified as unrelated or unlikely to be related to the study drug, were increased AST (one subject), increased plasma creatinine (one subject), hypotension (one subject), nausea and vomiting (two subjects), vertigo (one subject), increased white blood count (two subjects), swollen injection site (one subject), increased plasma sodium (three subjects), and food poisoning (one subject). They were recorded either during the administration of oral oseltamivir and zanamivir continuous i.v. infusion for 72 h (regimen C; n = 8) or during the follow-up visit, 7 days after the completion of the last dosing (n = 5).
Plasma drug concentration profiles.
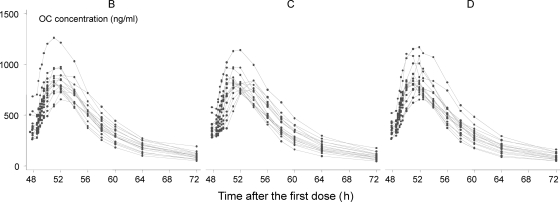
Oseltamivir (OS) was converted rapidly to the active metabolite oseltamivir carboxylate (OC) (Fig. 1 and 2) in all volunteers. No subjects showed markedly reduced conversion from OS to OC. The ratio of OS AUC to OC AUC was 4.0% (2.6 to 6.8%) and was not different between regimens (P = 0.420 by likelihood ratio test).
Fig. 1.
Plasma concentrations of oseltamivir (OS) after three oral regimens: B, oseltamivir administered alone; C, oseltamivir administered with zanamivir as an i.v. infusion during 72 h; D, oseltamivir administered with a 30-min rapid zanamivir i.v. infusion.
Fig. 2.
Plasma concentrations of oseltamivir carboxylate (OC) after three oral regimens: B, oseltamivir administered alone; C, oseltamivir administered with zanamivir as an i.v. infusion during 72 h; D, oseltamivir administered with a 30-min rapid zanamivir i.v. infusion.
The OS maximum concentration (Cmax) was around 0.5 h after the last dose (Table 1). In all regimens a small second OS peak was observed in 15 volunteers on 34 occasions, at a median (range) of 2 h (1 to 4 h) after the last dose and with a median (range) increase of 40% (0.3 to 500%) from the lowest concentration value after the first peak. Seven out of these 34 peaks corresponded to less than a 10% increase in the concentration. There was no significant association between the number of volunteers with the second peak and the treatment regimen (P = 0.67 by chi-square test on 2 degrees of freedom).
Table 1.
Noncompartmental analysis of oseltamivir (OS) and oseltamivir carboxylate (OC) pharmacokineticsa
| Regimen | Oseltamivir |
Oseltamivir carboxylate |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t½ (h) | kel (h−1) | Cmax (ng/ml) | Tmax (h) | AUC0-∞ (ng/ml h) | AUC0-12 (ng/ml h) | AUCobs (ng/ml h) | t½ (h) | kel (h−1) | Cmax (ng/ml) | Tmax (h) | AUC0-∞ (ng/ml h) | AUC0-12 (ng/ml h) | AUCobs (ng/ml h) | |
| B | 2.321 | 0.3 | 206 | 0.5 | 387 | 373 | 377 | 6.291 | 0.11 | 825 | 4 | 10,215 | 7,041 | 9,023 |
| 1.428 | 0.076 | 115 | 0.5 | 270 | 259 | 264 | 5.286 | 0.07 | 653 | 3 | 7,459 | 5,684 | 6,976 | |
| 9.08 | 0.485 | 345 | 1.25 | 527 | 498 | 498 | 9.865 | 0.131 | 1,260 | 4 | 14,720 | 10,625 | 13,609 | |
| C | 3.664 | 0.19 | 216 | 0.5 | 409 | 379 | 396 | 6.456 | 0.107 | 825 | 3.5 | 9,904 | 6,794 | 8,793 |
| 1.499 | 0.063 | 106 | 0.5 | 270 | 257 | 262 | 5.25 | 0.082 | 726 | 2 | 7,464 | 5,456 | 6,850 | |
| 10.944 | 0.462 | 433 | 3 | 560 | 480 | 525 | 8.413 | 0.132 | 1,140 | 6 | 14,686 | 10,046 | 13,296 | |
| D | 2.565 | 0.275 | 235 | 0.5 | 413 | 395 | 400 | 6.175 | 0.112 | 831 | 3.9 | 10,080 | 6,857 | 9,002 |
| 1.05 | 0.041 | 103 | 0.5 | 277 | 267 | 267 | 5.014 | 0.073 | 653 | 2 | 7,382 | 5,787 | 6,863 | |
| 16.842 | 0.66 | 452 | 3 | 734 | 513 | 513 | 9.491 | 0.138 | 1,170 | 4.5 | 14,886 | 10,372 | 13,612 | |
Median values are given in the top line of each row, and underneath are the range values. Regimen B, 150 mg of oseltamivir administered p.o. q12h for 3 days; regimen C, the same as regimen B, with the addition of 50 mg/h zanamivir i.v. infusion for 72 h; regimen D, the same as regimen B, with the addition of zanamivir 600 mg i.v. q12h for 3 days. t½, elimination half life; kel, elimination rate constant; Cmax, maximum concentration; Tmax, time to maximum concentration; AUC0-∞, area under the plasma concentration-time curve extrapolated to infinity; AUC0-12, area under the plasma concentration-time curve between 0 and 12h; AUCobs, area under the plasma concentration-time curve between time zero and the time of the last measured concentration.
Zanamivir plasma concentrations after the three regimens are presented in Fig. 3. For steady-state concentrations, the between-subject coefficient of variation was 12% for regimen A and 13% for regimen C, and an adjustment for body weight did not decrease the variability.
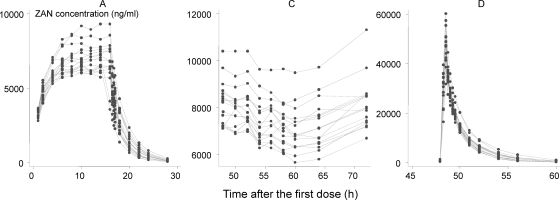
Fig. 3.
Plasma zanamivir concentrations after three dosage regimens: A, zanamivir administered alone as a slow (800 mg during 16 h) i.v. infusion; C, i.v. infusion of 3,600 mg during 72 h administered concurrently with oral oseltamivir; D, 30-min rapid i.v. infusion of zanamivir (600 mg) administered with oral oseltamivir.
The median (range) average steady-state plasma zanamivir concentration for each subject in regimen A, achieved between 10 and 14 h, was 6,878 ng/ml (5,927 to 9,140 ng/ml), with a median (range) coefficient of variation of 2.3% (0.2 to 4.3%). For regimen C, the median (range) average steady-state concentration for each subject was 7,517 ng/ml (6,598 to 10,063 ng/ml) with a median (range) coefficient of variation of 6.1% (4.3 to 11.6%) during the sampling period from 48 to 72 h.
In regimen C a relative decrease in concentrations of 14% (95% CI, 12 to 16%) was observed between 52 and 60 h, with the concentration reaching the initial levels again at approximately 72 h. This was not related to the interruption in zanamivir infusion observed in three subjects mentioned previously.
Between-subject variation was similar after each dose in regimen D compared between samples taken at 0.5 h after the dose, and the coefficient of variation was estimated as 16% after each dose. There was no evidence of dose accumulation, and the median (range) plasma concentrations measured half an hour after the dose were 49,600 ng/ml (34,400 to 58,800 ng/ml) after the first dose, 49,150 ng/ml (34,500 to 61,000 ng/ml) after the third dose, and 48,800 ng/ml (31,600 to 60,200 ng/ml) after the fifth dose. The variation in concentrations increased with time, reaching a coefficient of variation of 24, 38, 54, and 65% at 4, 6, 8, and 12 h, respectively, after the last dose.
Pharmacokinetics. (i) Oseltamivir.
The median and range of maximum plasma concentrations in the three studies were very similar; Cmax ranged from 206 to 235 ng/ml at a median of 0.5 h in each study. The median elimination half-life in the three studies ranged from 2.3 to 3.7 h.
(ii) Oseltamivir carboxylate.
OC kinetics were best described by a one-compartment model. Estimates of the apparent volume of distribution (Vd/F; median, 2.35 to 2.55 liters/kg) and oral clearance (CL/F; 0.33 to 0.34 liters/kg/h) were very similar in the three series. The elimination half-life was estimated to be approximately 5 to 6 h, with maximum concentrations around 4 h (Table 1). Estimates of the pharmacokinetic parameters for three different regimens were similar (Tables 1 and 2) and showed bioequivalence between OC concentrations when oral oseltamivir was administered alone or with parenteral zanamivir (Table 3). Between-subject variation explained 83% of the total variation in OC total AUC, 75% of the variation in OC Cmax, and 89% of the variation in OC AUC0-12.
Table 2.
Oseltamivir carboxylate pharmacokinetic parameters estimated from a one-compartment modela
| Regimenb | AUC0-∞ (ng/ml h) | Cmax (ng/ml) | Ka (h−1) | tlag (h) | kel (h−1) | t½ (h) | Tmax (h) | CL (liter/kg/h) | V (liter/kg) |
|---|---|---|---|---|---|---|---|---|---|
| B | 7,072 | 587 | 0.606 | 0.434 | 0.129 | 5.384 | 3.737 | 0.329 | 2.495 |
| 5,828 | 454 | 0.316 | 0.241 | 0.085 | 3.914 | 2.761 | 0.222 | 2.001 | |
| 10,816 | 856 | 1.198 | 1.607 | 0.177 | 8.138 | 5.581 | 0.472 | 3.956 | |
| C | 6,871 | 603 | 0.65 | 0.535 | 0.129 | 5.356 | 4.066 | 0.335 | 2.549 |
| 5,880 | 495 | 0.15 | 0.292 | 0.11 | 1.634 | 2.359 | 0.237 | 0.721 | |
| 10,140 | 767 | 2.514 | 2.496 | 0.424 | 6.322 | 5.572 | 0.468 | 3.816 | |
| D | 6,901 | 629 | 0.582 | 0.41 | 0.148 | 4.695 | 3.59 | 0.341 | 2.35 |
| 5,846 | 378 | 0.283 | 0.283 | 0.091 | 3.441 | 2.911 | 0.231 | 1.582 | |
| 10,416 | 871 | 0.865 | 1.335 | 0.201 | 7.654 | 5.496 | 0.471 | 4.52 |
Median values are given in the top line of each row, and underneath are the range values.
Regimen B, 150 mg of oseltamivir administered p.o. q12h for 3 days; regimen C, the same as regimen B, with the addition of 50 mg/h zanamivir i.v. infusion for 72 h; regimen D, the same as regimen B, with the addition of zanamivir 600 mg i.v. q12h for 3 days. AUC0-∞, total area under the plasma concentration time curve; Cmax, maximum plasma concentration; Tmax, time of maximum plasma concentration; Ka, absorption rate constant; tlag, lag time; kel, elimination rate constant; t½, elimination half life; CL, clearance; V, apparent volume of distribution.
Table 3.
Comparison of oseltamivir (OS) and oseltamivir carboxylate (OC) concentrations when oseltamivir was administered alone (regimen B) or together with zanamivir (regimens C and D)a
| Drug and pharmacokinetic parameter | C:B |
D:B |
||
|---|---|---|---|---|
| Ratio (95% CI) | Bioequivalence CIb | Ratio (95% CI) | Bioequivalence CIb | |
| OC | ||||
| AUC0-12 | 0.969 (0.940−0.999) | 94.4–99.5 | 0.989 (0.960−1.020) | 96.4–101.5 |
| AUC0-∞ | 0.982 (0.932−1.035) | 93.8–102.8 | 0.977 (0.927−1.030) | 93.3–102.2 |
| Cmax | 1.005 (0.958−1.055) | 96.4–104.8 | 1.025 (0.977−1.075) | 98.3–106.8 |
| OS | ||||
| AUC0-12 | 0.996 (0.932−1.064) | 94.0–105.5 | 1.008 (0.943−1.078) | 95.2–106.8 |
| AUC0-∞ | 1.019 (0.952−1.090) | 96.0–108.0 | 1.022 (0.955−1.094) | 96.4–108.4 |
| Cmax | 0.938 (0.743−1.185) | 76.6–114.8 | 1.073 (0.850−1.355) | 87.7–131.3 |
AUC0-∞, area under the concentration-time curve extrapolated to infinity; AUC0-12, area under the concentration-time curve between 0 and 12 h; Cmax, maximum concentration. Pharmacokinetic parameters were estimated by noncompartmental analysis.
Bioequivalence CIs were calculated per FDA recommendations (7, 8): exp(Δ ± s√(2/n)t0.05,30, where Δ is the estimated difference in log-transformed values, s is the square root of the mean square error from the mixed-effect model, and t0.05,30 is the critical value of t distribution at α = 0.05 and degrees of freedom = 30. Equivalence is concluded when this CI lies within the 80 to 125% range.
The median (range) ratio of OC clearance to creatinine clearance was 3.26 (2.40 to 8.49) and 3.10 (2.04 to 4.92) on days 2 and 3 of regimen C and 3.96 (2.37 to 5.68) and 3.18 (1.83 to 5.35) on days 2 and 3 of regimen D.
(iii) Zanamivir.
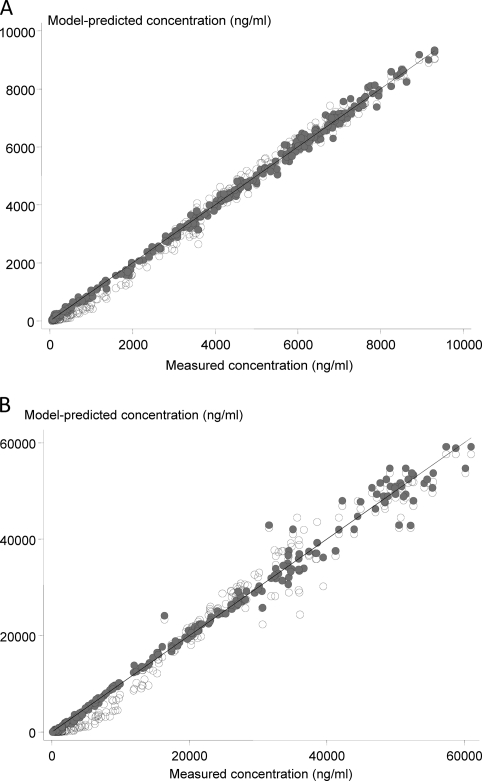
Zanamivir kinetics were best described by a two-compartment model for all except one subject in regimen D (Tables 4, 5, and 6). A one-compartment model consistently showed worse fits at low concentrations in all profiles (Fig. 4A and B). This model misspecification had little impact on most estimates of high concentrations or the areas under the plasma concentration-time curve.
Table 4.
Zanamivir pharmacokinetics by noncompartmental analysisa
| Regimen | t½ (h) | kel (h−1) | Cmax (ng/ml) | Tmax (h) | AUC0-∞ (ng/ml h) | AUC0-obs (ng/ml h) | CL (liter/kg/h) |
|---|---|---|---|---|---|---|---|
| A | 2.117 | 0.327 | 7025 | 14 | 111,056 | 110,818 | 0.111 |
| 1.632 | 0.279 | 6040 | 8 | 98,002 | 97,825 | 0.085 | |
| 2.484 | 0.425 | 9310 | 16 | 150,653 | 149,718 | 0.157 | |
| D | 1.937 | 0.358 | 48800 | 0.5 | 83,655 | 82,113 | 0.111 |
| 1.498 | 0.274 | 32600 | 0.5 | 72,566 | 71,805 | 0.078 | |
| 2.531 | 0.463 | 60200 | 0.75 | 122,952 | 119,254 | 0.155 |
Median values are given in the top line of each row, and underneath are the range values. Regimen A, 800 mg zanamivir infused during 16 h. t½, elimination half life; kel, elimination rate constant; Cmax, maximum concentration; Tmax, time to maximum concentration; AUC 0-∞, area under concentration-time curve extrapolated to infinity; AUCobs, area under concentration-time curve between time 0 h and the time of the last measured concentration; CL, total body clearance.
Table 5.
Zanamivir pharmacokinetics estimated from one- and two-compartment models using regimen Aa
| Model | kel (h−1) | t1/2 (h) | V1 (liter/kg) | CL (liter/kg/h) | V2 (liter/kg) | CL2 (liter/kg/h) | AUC (ng/ml h) | Cmax (ng/ml) | tα1/2 (h) | tβ1/2 (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Compartment | 0.537 | 1.291 | 0.222 | 0.114 | 108,584 | 6,833 | ||||
| 0.388 | 0.985 | 0.178 | 0.088 | 94,314 | 5,895 | |||||
| 0.704 | 1.787 | 0.252 | 0.16 | 145,017 | 9,048 | |||||
| 2 Compartment | 0.813 | 0.853 | 0.138 | 0.111 | 0.109 | 0.07 | 110,924 | 7,014 | 0.386 | 2.044 |
| 0.602 | 0.568 | 0.078 | 0.085 | 0.069 | 0.025 | 97,191 | 6,061 | 0.139 | 1.607 | |
| 1.221 | 1.152 | 0.206 | 0.157 | 0.164 | 0.237 | 150,502 | 9,337 | 0.838 | 4.039 |
Median values are given in the top line of each row, and underneath are the range values. Regimen A, 800 mg zanamivir infused during 16 h. kel, elimination rate constant; t1/2, elimination half life; V1, volume of the central (or only) compartment; CL, clearance from the central (or only) compartment; V2, volume of the second compartment; CL2, intercompartmental clearance; AUC, total area under the concentration-time curve; Cmax, maximum concentration; tα1/2, half life corresponding to the initial elimination phase (in a two-compartment model); tβ1/2, terminal elimination half life.
Table 6.
Zanamivir pharmacokinetics estimated from one- and two-compartment modelsa
| Model | kel (h−1) | t1/2 (h) | V1 (liter/kg) | CL (liter/kg/h) | V2 (liter/kg) | CL2 (liter/kg/h) | AUC (ng/ml h) | Cmax (ng/ml) | tα1/2 (h) | tβ1/2 (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Compartment | 0.832 | 0.834 | 0.17 | 0.149 | 63,705 | 48,317 | ||||
| 0.669 | 0.588 | 0.127 | 0.11 | 56,589 | 35,854 | |||||
| 1.179 | 1.037 | 0.2 | 0.198 | 92,939 | 57,596 | |||||
| 2 Compartmentb | 0.939 | 0.127 | 0.119 | 0.09 | 0.174 | 79,489 | 49,271 | 0.165 | 1.428 | |
| 0.613 | 0.06 | 0.085 | 0.052 | 0.053 | 67,307 | 36,624 | 0.04 | 0.957 | ||
| 1.802 | 0.178 | 0.165 | 0.128 | 0.875 | 114,477 | 58,871 | 0.434 | 2.141 |
Median values are given in the top line of each row, and underneath are the range values. Regimen D, 600 mg zanamivir infused during 30 min. kel, elimination rate constant; t1/2, elimination half life (in a one-compartment model); V1, volume of the central (or only) compartment; CL, clearance from the central (or only) compartment; V2, volume of the second compartment; CL2, intercompartmental clearance; AUC, total area under the concentration-time curve; Cmax, maximum concentration; tα1/2, half life corresponding to the initial elimination phase (in a two-compartment model); tβ1/2, terminal elimination half life.
One subject was excluded, since the fit of the two-compartment model was poor.
Fig. 4.
Comparison of predicted plasma zanamivir concentrations from one-compartment (light gray circles) and two-compartment (dark gray circles) models. (A) Results for regimen A, i.e., 16-h i.v. zanamivir infusion (800 mg) administered alone; (B) results for regimen D, i.e., 0.5-h i.v. infusion (600 mg) administered with oral oseltamivir.
For regimen A, the median (range) Cmax was 2.6% (1.6 to 3.4%), and the AUC was 2.9% (1.9 to 4.6%) higher when estimated by the two-compartment model than by the one-compartment model. Similarly, for regimen D the corresponding relative difference for Cmax was 1.8% (1.0 to 2.9%), but the difference for the AUC was much higher, at 23.1% (12.9 to 34.9%).
Predictions of the zanamivir concentrations at steady state after regimen A were very similar based on both models, with estimates from the two-compartment model being 3.2% (95% CI, 2.6 to 3.8) higher than estimates coming from the one-compartment model. The median (range) elimination half lives in the noncompartmental analysis of series A and D were very similar: 2.1 h (1.6 to 2.5 h) and 1.9 h (1.5 to 2.5 h), respectively (Table 4).
The median (range) ratio of zanamivir clearance to creatinine clearance was 1.35 (0.90 −1.82) and 1.17 (0.61 to 1.62) on days 2 and 3 of regimen D, respectively.
Interaction between oseltamivir and zanamivir.
There was no evidence that zanamivir affected the pharmacokinetic properties of oseltamivir. The peak concentrations and areas under the plasma concentration-time curves for the active metabolite oseltamivir carboxylate were similar with or without zanamivir coadministration (Table 3). Bioequivalence could be concluded for all parameters except for OS Cmax.
Measured steady-state concentrations of zanamivir in regimen C (concurrent administration) were slightly higher than concentrations in regimen A (P < 0.001). There was no significant sequence effect. The ratio was estimated as 110% (95% CI, 107 to 112%). Of the total variation in steady-state concentrations, 73% was explained by the between-subject variation. There was no significant difference between noncompartmental total body clearance in regimen A and that in regimen D (P = 0.412), and the ratio was estimated as 98% (95% CI, 94 to 103%).
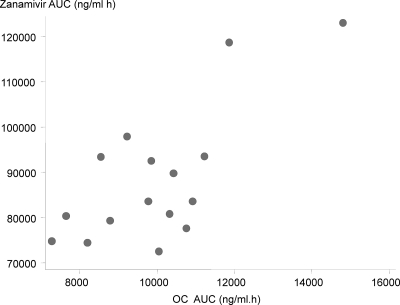
In regimen D, in which AUCs for both oseltamivir and zanamivir could be calculated, the zanamivir total AUC was significantly and positively correlated with the OC total AUC (Pearson correlation coefficient [r] = 0.720; P = 0.002; n = 16) (Fig. 5) but not with the OS total AUC (r = 0.121; P = 0.655; n = 16). Significant correlation between AUCs was due to two subjects with high AUCs. These subjects had the second and third lowest creatinine levels in this regimen series.
Fig. 5.
Correlation between plasma AUC values for zanamivir and oseltamivir carboxylate in regimen D, i.e., 0.5-h i.v. zanamivir (600 mg) infusion administered concurrently with oral oseltamivir.
DISCUSSION
The pharmacokinetic properties of intravenous zanamivir generally were similar to those observed in the early development of the drug (4). Zanamivir had a small apparent volume of distribution, compatible with distribution confined to extracellular water, and it was cleared rapidly with a terminal elimination half life of approximately 2 h with relatively little interindividual variability. Although a single-compartment model described plasma concentration profiles adequately, a two-compartment fit was slightly better, with a central compartment approximately 30% larger than the peripheral compartment.
The pharmacokinetic properties of oseltamivir in this investigation were generally similar to those we reported earlier for Thai subjects, although the elimination of the prodrug oseltamivir phosphate was slightly slower than that in the earlier series (17). The active metabolite kinetics were generally similar to those of previous reports, confirming the rapid absorption of the prodrug and rapid conversion to the active carboxylate metabolite. None of the subjects showed reduced biotransformation, indicating all had normal functions of human carboxylesterase 1 (HCE-1).
The anti-influenza neuraminidase inhibitors oseltamivir and zanamivir have different drug resistance profiles (9, 16). The most likely explanation for this is that the neuraminidase catalytic site has to change shape to accommodate oseltamivir, but this is not necessary for zanamivir binding. The rotation of the glutamate 276 residue in the neuraminidase site occurs on oseltamivir binding. This creates a pocket to accommodate the lipophilic side chain of the drug. Mutations resulting in resistance to oseltamivir are clustered around this E276 residue, notably the H274Y mutation, which has appeared readily in seasonal, pandemic, and highly pathogenic avian influenza viruses. Fortunately, because of the structural differences, most oseltamivir-resistant mutations remain sensitive to zanamivir. As a result, zanamivir is effective against all clinically relevant strains of influenza, including H5N1, the oseltamivir-resistant H274Y mutation of H1N1, and H1N1 2009. Fifty percent inhibitory concentrations against influenza A (H5N1) viral neuraminidase range from 0.3 to 0.7 ng/ml for various strains. Zanamivir also is effective in animal models of influenza A (H5N1) infection when given before or soon after infection. Zanamivir-resistant strains of influenza virus can be generated in vitro, but to date these strains have not been shown to be clinically relevant, although the use of the drug has been much less extensive than that of oseltamivir. One case of drug resistance to zanamivir has been reported in an immunocompromised patient (9). However, mutations conferring resistance to both drugs can and do occur (e.g., mutations E119 and R292) and would be under selection pressure if there is widespread use of zanamivir. Antivirals acting against a different target would be preferable, emphasizing the need for further pharmaceutical development. Nevertheless, the availability of a parenteral formulation of zanamivir and these currently favorable resistance properties make parenteral zanamivir an attractive empirical treatment for suspected severe influenza. Parenteral anti-infective drug administration is required for the optimum treatment of severe infections. This interaction study supports the preliminary studies of the manufacturer describing the excellent tolerability of intravenous zanamivir. Furthermore, this drug exhibits predictable pharmacokinetic properties in healthy subjects. It seems unlikely that there would be significant differences between populations. This parenteral formulation can be given either by rapid or continuous intravenous infusion.
For severe influenza, parenteral treatment is ideal. It is hoped that at least two parenterally administered agents will become widely available in the near future, zanamivir and peramivir. Peramivir has a resistance profile similar to that of oseltamivir. It is more slowly eliminated than zanamivir, which may allow once-daily dosing (1). It is very likely that some patients receiving intravenous treatment will have taken oseltamivir before being admitted to the hospital. It also is likely that oral oseltamivir will be given to some patients as they recover and can swallow reliably. Zamanivir and oseltamivir therefore will encounter each other frequently in clinical practice. It is reassuring that there is no evidence for a clinically significant pharmacokinetic interaction between the two main anti-influenza drugs, and these data suggest that they can be given safely together. Studies to establish efficacy and characterize better pharmacokinetic-pharmacodynamic relationships for the two drugs alone and in combination both in experimental models and in patients are needed (3). Investigations of sick patients also are required to determine whether disease severity significantly affects drug disposition.
ACKNOWLEDGMENTS
We are very grateful to the nurses and staff of the Hospital for Tropical Diseases, Bangkok, Thailand. We are grateful to GSK Pharmaceuticals for providing the parenteral zanamivir.
This study was supported by the South East Asia Influenza Clinical Research Network. Funding was provided by the U.S. National Institute of Allergy and Infectious Diseases (NIH N01-AO-50042), and the study was part of the Wellcome Trust Mahidol University-Oxford Tropical Medicine Research Programme (077166/Z/05/Z) funded by the Wellcome Trust of Great Britain. The funders had no role in the design, conduct, analysis or interpretation of this study.
Footnotes
Published ahead of print on 20 June 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Arya V., Carter W. W., Robertson S. M. 2010. The role of clinical pharmacology in supporting the emergency use authorization of an unapproved anti-influenza drug, peramivir. Clin. Pharmacol. Ther. 88:587–589 [DOI] [PubMed] [Google Scholar]
- 2. Beigel J. H., et al. 2005. Current concepts–avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353:1374–1385 [DOI] [PubMed] [Google Scholar]
- 3. Brown A. N., et al. 2011. Effect of half-life on the pharmacodynamic index of zanamivir against influenza virus delineated by a mathematical model. Antimicrob. Agents Chemother. 55:1747–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cass L. M. R., Efthymiopoulos C., Bye A. 1999. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin. Pharmacokinet. 36(Suppl. 1):1–11 [DOI] [PubMed] [Google Scholar]
- 5. Daniel M. J., Barnett J. M., Pearson B. A. 1999. The low potential for drug interactions with zanamivir. Clin. Pharmacokinet. 36(Suppl. 1):41–50 [DOI] [PubMed] [Google Scholar]
- 6. de Jong M. D., et al. 2005. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 353:2667–2672 [DOI] [PubMed] [Google Scholar]
- 7. Food and Drug Administration 2001. Guidance for industry: statistical approaches to establishing bioequivalence. FDA, Washington, DC [Google Scholar]
- 8. Food and Drug Administration 2003. Guidance for industry: bioavailability and bioequivalence, studies for orally administered drug products–general consideration. FDA, Washington, DC [Google Scholar]
- 9. Gubareva L. V., Matrosovich M. N., Brenner M. K., Bethell R. C., Webster R. G. 1998. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J. Infect. Dis. 178:1257–1262 [DOI] [PubMed] [Google Scholar]
- 10. Hill G., et al. 2002. The anti-influenza drug oseltamivir exhibits low potential to induce pharmacokinetic drug interactions via renal secretion-correlation of in vivo and in vitro studies. Drug Metab. Dispos. 30:13–19 [DOI] [PubMed] [Google Scholar]
- 11. Kiso M., et al. 2004. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 364:759–765 [DOI] [PubMed] [Google Scholar]
- 12. Lindegårdh N., et al. 2007. Importance of collection tube during clinical studies of oseltamivir. Antimicrob. Agents Chemother. 51:1835–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lindegårdh N., et al. 2007. Development and validation of a liquid chromatographic-tandem mass spectrometric method for determination of oseltamivir and its metabolite oseltamivir carboxylate in plasma, saliva and urine. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 859:74–83 [DOI] [PubMed] [Google Scholar]
- 14. Lindegardh N., et al. 2011. Quantification of the anti-influenza drug zanamivir in plasma using high-throughput HILIC-MS/MS. Bioanalysis 3:157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Massarella J. W., et al. 2000. The pharmacokinetics and tolerability of the oral neuraminidase inhibitor oseltamivir (Ro 64–0796/GS4104) in healthy adult and elderly volunteers. J. Clin. Pharmacol. 40:836–843 [DOI] [PubMed] [Google Scholar]
- 16. Moscona A. 2005. Oseltamivir resistance–disabling our influenza defences. N. Engl. J. Med. 353:2633–2637 [DOI] [PubMed] [Google Scholar]
- 17. Wattanagoon Y., et al. 2009. Pharmacokinetics of high-dose oseltamivir in healthy volunteers. Antimicrob. Agents Chemother. 53:945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wooldridge J. M. 2002. Econometric analysis of cross section and panel data. Chapter 10 MIT Press, Cambridge, MA [Google Scholar]