Abstract
NB-003 and NB-003 gel formulations are oil-in-water nanoemulsions designed for use in bacterial infections. In vitro susceptibility of Propionibacterium acnes to NB-003 formulations and comparator drugs was evaluated. Both NB-003 formulations were bactericidal against all P. acnes isolates, including those that were erythromycin, clindamycin, and/or tetracycline resistant. In the absence of sebum, the MIC90s/minimum bactericidal concentrations (MBC90s) for NB-003, NB-003 gel, salicylic acid (SA), and benzoyl peroxide (BPO) were 0.5/2.0, 1.0/2.0, 1,000/2,000, and 50/200 μg/ml, respectively. In the presence of 50% sebum, the MIC90s/MBC90s of NB003 and BPOs increased to 128/1,024 and 400/1,600 μg/ml, respectively. The MIC90s/MBC90s of SA were not significantly impacted by the presence of sebum. A reduction in the MBC90s for NB-003 and BPO was observed when 2% SA or 0.5% BPO was integrated into the formulation, resulting in MIC90s/MBC90s of 128/256 μg/ml for NB003 and 214/428 μg/ml for BPO. The addition of EDTA enhanced the in vitro efficacy of 0.5% NB-003 in the presence or absence of 25% sebum. The addition of 5 mM EDTA to each well of the microtiter plate resulted in a >16- and >256-fold decrease in MIC90 and MBC90, yielding a more potent MIC90/MBC90 of ≤1/<1 μg/ml. The kinetics of bactericidal activity of NB-003 against P. acnes were compared to those of a commercially available product of BPO. Electron micrographs of P. acnes treated with NB-003 showed complete disruption of bacteria. Assessment of spontaneous resistance of P. acnes revealed no stably resistant mutant strains.
INTRODUCTION
Propionibacterium acnes is a Gram-positive, non-spore-forming, anaerobic bacillus and resides in normal facial and nasal microflora. P. acnes is one of the primary factors involved in the pathogenesis of acne vulgaris. It is a predominant microorganism of the pilosebaceous glands of human skin, with up to 107 viable organisms isolated from a single sebaceous unit (37, 38). Although aerotolerant, P. acnes typically grows in the anaerobic environment of the infrainfundibulum of the pilosebaceous unit, where it releases lipases and digests local accumulations of the skin and sebum (oily substance produced by sebaceous glands). Sebum is primarily composed of glycerides, triglycerides, wax esters, squalene, and free fatty acids (40, 61, 62).
Most cases of mild acne are treated with topical antibacterials containing benzoyl peroxide, erythromycin, clindamycin, or tetracycline with salicylic acid as a keratolytic agent (4).
If inflammatory acne lesions are present, the Global Alliance to Improve Outcomes in Acne recommends a combination therapy of an antibacterial with a topical retinoid that exhibits both direct anti-inflammatory and comedolytic activities as first-line therapy in patients with mild, moderate, and, in many cases, severe acne (12). Each year, 5 million prescriptions for oral antibiotics and 1.4 million prescriptions of oral isotretinoin are dispensed for treatment of moderate to severe acne (56). A combination of topical benzoyl peroxide (BPO) and a retinoid can cause erythema, scaling, burning, and stinging in some patients, particularly during the first 2 to 3 weeks of treatment. Therefore, there have been numerous attempts to minimize the irritation associated with BPO, and various drug delivery technologies have been developed (13, 14, 25, 64). Studies have confirmed that the combination of benzoyl peroxide and a topical antibiotic (e.g., clindamycin, erythromycin) is just slightly more effective than either agent alone, with lesser skin irritation observed than with benzoyl peroxide monotherapy (57). In more severe acne (grades III and IV) characterized by numerous papules and/or cysts, oral therapy consisting of an oral retinoid and/or oral antibiotic (erythromycin, tetracycline, minocycline, doxycycline) is prescribed. Oral retinoids (isotretinoin) shrink the sebaceous glands but have severe side effects (major birth defects, depression, hair loss, etc.) and are only used in very severe or cystic acne (52, 59).
Although antibiotic therapy has been used for more than 40 years to treat acne, resistance to antibiotics became noticeably prevalent in 1970 (36). Recent studies in six European countries identified that acne patients carried P. acnes isolates with at least one resistance determinate, with frequencies ranging from 51% in Hungary to 94% in Spain. Combined resistance to erythromycin and clindamycin was very common, with 91% of the isolates in Spain having this resistance phenotype (53, 54). Another study in the United Kingdom showed that the proportion of patients carrying strains resistant to one or more commonly used antiacne antibiotics rose steadily from 34.5% in 1991 to a peak of 64% in 1997. The prevalence dropped to 50.5% during 1999 and then rose again to 55.5% in 2000 (9). Resistance to erythromycin was the most common, and the majority of erythromycin-resistant strains were cross-resistant to clindamycin. Thus, any new agent seeking to treat acne by reducing the P. acnes burden should not be cross-resistant to erythromycin, clindamycin, or tetracycline, the most commonly used antibiotics for acne treatment. Quaternary ammonium compounds showed the in vitro MICs comparable to relevant antibiotics used in acne treatment, suggesting the possibility of replacing antibiotics in the antimicrobial therapy of acne by quaternary ammonium compounds (18).
Nanomaterials have unique structural properties, such as a large surface-area-to-mass ratio and high affinity of binding to surfaces. Nanoparticle-based therapeutics have been approved for clinical use, such as liposome and polymer-based technology (65). Antimicrobial nanoemulsions are highly stable oil-in-water emulsions composed of nanometer-sized, positively charged droplets that have broad-spectrum activity against enveloped viruses, fungi, and bacteria (2, 17, 19–21, 41, 47–50, 58). NB-003 (NanoStat, NanoBio Corp., Ann Arbor, MI) contains the cationic quaternary ammonium compound cetylpyridinium chloride (CPC) oriented at the oil-water interface, which also stabilizes the nanoemulsion droplets, contributes to the anti-infective activity, and serves as a marker for the pharmacokinetic, pharmacodynamic, and toxicological studies.
These studies assessed the in vitro antibacterial activity of NB-003 with and without other conventional antimicrobial agents against P. acnes, including isolates resistant to one or more common antibiotics used in the treatment of acne. Antibacterial synergy of NB-003 with other antiacne compounds was also evaluated, as was the effect of EDTA. Spontaneous resistance to NB-003 was also assessed.
MATERIALS AND METHODS
Nanoemulsion manufacturing and potency.
NB-003 is an oil-in-water nanoemulsion manufactured from ingredients that are on the FDA list of inactive ingredients of approved drug products. The emulsion is made by high-speed emulsification of highly purified oil, ethanol, polysorbate 20, cetylpyridinium chloride (CPC), and water. NB-003 gel formulation is modified to include a thickening agent. Placebo (vehicle) nanoemulsions were made from the same ingredients without CPC. The mean nanoemulsion droplet size is 180 nm as measured by dynamic light scattering using a Malvern Zetasizer Nano ZS3600 (Malvern Instruments Ltd., Worcestershire, United Kingdom). The relative activity of NB-003 is expressed in terms of the concentration of cationic surfactant (μg CPC/ml).
Source of comparator compounds.
Erythromycin and tetracycline were purchased from Sigma Chemicals (St. Louis, MO), clindamycin was obtained from USP (Rockville, MD), and salicylic acid was purchased from J.T. Baker (Phillipsburg, NJ). Benzoyl peroxide (BPO) was obtained from commercial products: Persa-Gel 10 (Johnson & Johnson, Skillman, NJ), Clearasil (Reckitt Benckiser, Parsippany, NJ), and Proactiv (Proactiv Solution, Des Moines, IA).
Source of P. acnes isolates.
Three ATCC isolates were obtained from American Type Culture Collection (Manassas, VA). Thirteen clinical isolates of P. acnes were obtained from Basilea Pharmaceutica (Basel, Switzerland) and have been previously described by Heller et al. (23). Twelve of these isolates had defined resistance mechanisms to erythromycin, clindamycin, and/or tetracycline. The resistance mechanisms as shown in Table 2 were mutations in either the 16S or 23S rRNA conferring tetracycline resistance or erythromycin resistance with or without clindamycin resistance, respectively, or resistance was conferred by an erm(X) methylase that dimethylates residue A2058 in 23S rRNA (Escherichia coli numbering of 23S rRNA residue), conferring high-level erythromycin and clindamycin resistance (23). Resistance is defined as erythromycin, ≥0.5 μg/ml; clindamycin, ≥0.25 μg/ml; or tetracycline, ≥2 μg/ml (44, 45).
Table 2.
Resistance phenotype characterization and FIC/FBC index values of NB-003–BPO against P. acnes isolates
| Strain no. | Resistance phenotypea | Average synergy indicesb |
|
|---|---|---|---|
| FIC | FBC | ||
| PAC-001 | Erys Clis Tets (ATCC6919) | 0.4 | 0.2 |
| PAC-003 | Erys Clis Tets (ATCC11828) | 0.4 | 0.4 |
| PAC-004 | Erys Clis Tets | 0.6 | 0.5 |
| PAC-005 | Erys Clir Tets (unknown) | 0.4 | 0.7 |
| PAC-006 | Eryr Clir Tetr (G2057A + G1058C) | 0.5 | 0.3 |
| PAC-007 | Eryr Clir Tetr (A2058G + G1058C) | 0.4 | 0.3 |
| PAC-008 | Eryr Clir Tetr (A2058G + G1058C) | 0.4 | 0.4 |
| PAC-009 | Eryr Clir Tets (A2059G) | 0.5 | 0.6 |
| PAC-010 | Eryr Clir Tetr (A2059G + G1058C) | 0.5 | 0.4 |
| PAC-011 | Eryr Clir Tets (ermX) | 0.4 | 0.4 |
| PAC-002 | Erys Clis Tets (ATCC11827) | NA | NA |
| PAC-012 | Erys Clis Tetr (G1058C) | NA | NA |
| PAC-013 | Eryr Clir Tetr (unknown) | NA | NA |
| PAC-014 | Eryr Clir Tetr (ermX) | NA | NA |
| PAC-015 | Eryr Clir Tets (G2057A) | NA | NA |
| PAC-016 | Eryr Clir Tets (A2058G) | NA | NA |
Erys/r, erythromycin susceptible/resistant; Clis/r, clindamycin susceptible/resistant; Tets/r, tetracycline susceptible/resistant. The resistance breakpoints are erythromycin, ≥0.5 μg/ml; clindamycin, ≥0.25 μg/ml; tetracycline, ≥2 μg/ml. Unknown, the genetic basis of antibiotic resistance is unknown.
NA, not tested in synergy study.
MIC/minimum bactericidal concentration (MBC) determination.
MICs in the presence or absence of 50% artificial sebum (40) were determined using the broth microdilution method in Wilkins-Chalgren broth (Becton, Dickinson & Co., Sparks, MD) as described in the Clinical and Laboratory Standards Institute (CLSI) methodology for anaerobic bacteria (7, 55). Sixteen P. acnes isolates were tested once per isolate, and then the MIC50s and MIC90s were calculated accordingly. Premade drug plates were inoculated with a culture suspension of P. acnes to give ∼106 CFU/ml. Two quality control (QC) isolates, ATCC 11827 and ATCC 11828, were included on each day of testing. Inoculum concentration was verified by plating the serially diluted inoculum on Trypticase soy agar containing 5% sheep blood (Becton, Dickinson & Co., Sparks, MD). Microtiter plates and blood agar plates (BAP) were incubated at 35 to 37°C in a 7-liter anaerobic incubation jar (Mitsubishi Gas Chemical, New York, NY) fitted with an anaerobic gas-generating system (Mitsubishi Gas Chemical, New York, NY) and a dry anaerobic indicator strip (Becton, Dickinson & Co., Sparks, MD). MICs were read after 48 h using an illuminated microtiter plate reader fitted with a magnifying mirror (Biodesign, New York, NY). Verification of viable counts of inocula was made after 72 h of incubation.
MBC was determined by using the standard CLSI methodology (8). Briefly, 10 μl was removed from the well that defined the MIC and four or more concentrations above the MIC and was plated onto blood agar plates. In our study, the antibacterial effect of CPC was neutralized by the ingredients present in the Wilkins-Chalgren broth used in the 96-well plate and by the dilution effect of spreading the 10 μl on the blood agar plates. Additionally, to avoid antibacterial carryover, the aliquots were allowed to soak into the agar and then were spread for isolation, thus removing the cells from the drug source. MBC was defined as the lowest concentration of drug that reduced the initial inoculum by ≥3 logs. A compound is considered bactericidal if the MBC/MIC ratio is ≤4. Bacteriostatic compounds have an MBC/MIC ratio of >4 (8). The MBC50 and the MBC90 are the lowest concentrations of an antimicrobial agent that collectively kill (≥3 log reduction) 50% or 90% of the isolates tested, respectively. Tolerance was defined as an MBC/MIC ratio of ≥32 (8, 51).
The effect of EDTA on the antibacterial activity of the nanoemulsion formulations was evaluated in MIC/MBC assays by adding 1, 5, 10, or 20 mM EDTA to each well.
Determination of spontaneous resistance frequencies.
Agar-based single-step mutation studies were performed as described in the literature (1, 11, 29, 30, 42, 46). Blood agar plates containing 1, 2, 4, or 8 times the respective agar-based MIC of NB-003, clindamycin, tetracycline, or BPO were inoculated with 109 to 1010 CFU of P. acnes and incubated for 3 to 4 days at 35°C. The spontaneous resistance frequency for an isolate-drug combination was calculated from the number of colonies that grew on plates containing drug versus the number of colonies that grew on drug-free agar. To ensure that drug-selective pressure was maintained, randomly selected colonies recovered from the plates were subcultured to blood agar plates containing the compound at the selecting concentration and used as inoculum for broth-based MIC determination.
Time-kill studies.
In vitro time-kill studies were performed in broth as well as on the surface of pig skin. Assessment of bacterial reduction in broth was performed in a test tube using 10 times the MBC. Nanoemulsion samples were prepared at 2 times the final test concentration in deionized water, and a culture suspension in 2× broth was added in a 1:1 ratio to achieve the final drug concentration and inoculum. At predetermined time points, samples were serially diluted and 100 μl of each sample was plated onto a BAP. Colony counts were assessed after 3 to 5 days under anaerobic conditions.
Time-kill studies were also performed after incubation of P. acnes on the surface of swine skin. Swine abdominal skin was obtained from Sinclair Research Center (Auxvasse, MO). The subcutaneous fat was removed manually such that the pilosebaceous units were left intact. Glass rings (2-cm diameter by 1-cm height) were glued to the skin surface with super glue and placed in 6-well tissue culture plates and hydrated with 500 μl of sterile water and stored at 2 to 8°C overnight prior to use. The next day, water was removed from wells containing skin samples, and 30 μl of a P. acnes culture was applied to the surface of the pig skin within the confines of the glass ring to give a concentration of 6 to 7 logs of CFU/cm2. Inoculated skin samples were incubated under anaerobic conditions for 1 h to allow absorption and adherence of bacteria to the skin surface. Following 1 h of equilibration, 50 μl of drug was applied to the skin samples (17.7 μl/cm2 of skin); this dose of drug provided an application similar to the topical application of BPO used in humans. Drug-treated skin samples were incubated under anaerobic conditions and at predetermined times samples were removed from the incubator. Each skin sample was rinsed with 1 ml cold saline twice to collect any viable bacteria still existing on the skin. The combined rinses were pooled and serially diluted, and 100 μl of all dilutions, including the initial pooled rinse, was plated onto BAP and incubated anaerobically at 35 to 37°C. Control experiments determined that samples containing NB-002 and BPO had to be diluted 1:10 to remove residual activity (data not shown).
Electron microscopy.
To observe the morphology of P. acnes after different times of nanoemulsion exposure, 450-μl samples from different time points of a time-kill study were mixed with 113 μl of fixative (10% aqueous solution of glutaraldehyde). Mixtures were vortexed and placed at 4°C for at least 18 h. Samples were further processed for scanning electron microscopy as described previously (48).
Determination of interactions between drugs.
Interactions between NB-003 and BPO in the presence of 50% artificial sebum were determined using checkerboard methodology (3, 63). The fractional inhibitory concentration (FIC) for each drug was calculated for each strain as follows: FIC for NB-003 = MIC of NB-003 in combination/MIC of NB-003 alone; FIC of BPO = MIC of BPO in combination/MIC of BPO alone.
The average FIC index for each isolate was calculated using FIC indices of all tested drug combinations: FIC index (Σ FIC) = FIC of NB-003 + FIC of BPO.
The fractional bactericidal concentrations (FBC) and FBC index were calculated using MBCs instead of MICs.
Interpretation.
Synergism is a Σ FIC of ≤0.5; indifference is a Σ FIC of >0.5 and ≤4; and antagonism is a Σ FIC of >4.
RESULTS
NB-003 is bactericidal for P. acnes.
The antimicrobial activities of NB-003 and other antiacne agents, such as erythromycin, clindamycin, tetracycline, BPO, salicylic acid, and chlorhexidine, were evaluated using clinical isolates of P. acnes. In the absence of sebum, the MICs of NB-003 or NB-003 gel formulations ranged from 0.25 to 1.0 μg/ml, and the MBCs ranged from 0.5 to 4 μg/ml (Table 1). The MIC90s and MBC90s were 0.5 μg/ml and 2.0 μg/ml for 0.3% NB-003 and 1 μg/ml and 2 μg/ml for 0.3% NB-003 gel, respectively. More than 50% of the isolates tested in this study were resistant to erythromycin and clindamycin; 44% of the isolates were resistant to tetracycline. The NB-003 formulations were bactericidal against all the isolates, including strains that were erythromycin, clindamycin, and/or tetracycline resistant. The placebo formulation did not exhibit any antibacterial activity.
Table 1.
MIC/MBCs of NB-003 and comparators against P. acnes
| Agent(s) | MIC/MBC (μg/ml) |
|||||
|---|---|---|---|---|---|---|
| Without sebum (n = 16 isolates) |
With 50% sebum (n = 16 isolates) |
|||||
| 90% | 50% | Range | 90% | 50% | Range | |
| NB-003 | 0.5/2 | 0.5/2 | 0.25–1/0.5–2 | 128/1,024 | 128/1,024 | 64–256/256–1,024 |
| NB-003 gel | 1/2 | 0.5/21 | 0.5–1/1–4 | 128/1,024 | 128/1,024 | 64–256/128–>1,024 |
| Erythromycin | 1,000/>1,000 | 4/>1,000 | ≤0.25–>1,000/62.5–>1,000 | 1,000/>1,000 | 4/1,000 | ≤0.25–>1,000/62.5–>1,000 |
| Clindamycin | 500/>1,000 | 4/1,000 | ≤0.125–500/125–>1,000 | 500/>1,000 | 1/100 | ≤0.125–500/62.5–>1,000 |
| Tetracycline | 31/1,000 | 0.5/250 | ≤0.063–31.3/31.3–1,000 | 31.3/1,000 | 0.5/250 | ≤0.063–31.3/31.3–1,000 |
| Benzoyl peroxide | 50/200 | 50/200 | ≤50–100/100–400 | 400/1,600 | 200/800 | ≤50–400/800–1,600 |
| Salicylic acid | 1,000/2,000 | 1,000/2,000 | 50–1,000/2,000–>2,000 | 1,000/2,000 | 1,000/2,000 | 500–1,000/2,000–>2,000 |
| 0.3% NB-003 gel + 0.5% BPO | 0.5/4 | 0.5/4 | 0.5–1/2–4 | 128/256 | 128/128 | 128/128–256 |
| 0.3% NB-003 gel + 2% salicylic acid | 0.5/2 | 0.5/2 | 0.25–1/0.5–2 | 128/256 | 128/128 | 128/128–256 |
Synergism between NB-003 and BPO.
Since NB-003 is a nanoemulsion and is preferentially taken up by the transfollicular route (5), incorporation of another antiacne drug into the nanodroplets may also be effectively codelivered to the site of infection. Thus, we investigated the antibacterial activity of NB-003 gel formulated with either benzoyl peroxide or salicylic acid and compared the MICs and MBCs of the combination nanoemulsions to those of benzoyl peroxide or salicylic acid alone. In the presence of 50% artificial sebum, a decrease in MBCs of NB-003, BPO, and salicylic acid to P. acnes was observed. The two combination nanoemulsions (NB-003–BPO or NB-003–salicylic acid) had 4-times-lower MBCs (256 μg/ml) than NB-003 (1,024 μg/ml).
Interactions of NB-003 and BPO in the presence of 50% artificial sebum were evaluated by checkerboard microdilution methodology. Ten isolates of P. acnes were tested for susceptibility to NB-003–BPO combinations. The FIC and FBC indices for the NB-003–BPO combination ranged from 0.4 to 0.6 and 0.2 to 0.7, respectively (Table 2). This combination showed synergy against 90% of isolates based on MICs and 80% of isolates based on MBCs, with none of the isolates showing antagonism.
EDTA enhances the activity of NB-003.
Broth-based MICs of NB-003 with different concentrations of EDTA were evaluated in the presence of 25% artificial sebum. The addition of EDTA reduced the MIC and MBC of NB-003 in the presence of 25% artificial sebum (Table 3). In the absence of EDTA, the MIC90/MBC90 was 0.5/2 μg/ml for NB-003 without sebum and increased to 32/512 μg/ml in the presence of 25% sebum. With the addition of 1 mM to 20 mM EDTA to each well of a microtiter plate containing 0.5% NB-003, MBC90s and MBC50s ranged from >16 to ≤1 μg/ml. The addition of 5 mM EDTA resulted in an MBC90 of 4 μg/ml, a more than 256-fold decrease in MBC90 compared to the MBC90 of NB-003 alone.
Table 3.
MIC/MBCs of NB-003 tested with different concentrations of EDTA
| Agent(s) | MIC/MBC (μg/ml)a |
|||||
|---|---|---|---|---|---|---|
| Without sebum (n = 10 isolates) |
With 25% sebum (n = 10 isolates) |
|||||
| 90% | 50% | Range | 90% | 50% | Range | |
| 0.5% NB003 | 0.5/2 | 0.5/2 | 0.25–0.5/1–2 | 32/512 | 16/256 | 16–32/128–>512 |
| 0.5% NB-003 + 1 mM EDTA/well | ≤0.063/2 | ≤0.063/>1 | ≤0.063/1–>1 | ≤1/>16 | ≤1/>16 | ≤1/16–>16 |
| 0.5% NB-003 + 5 mM EDTA/well | ≤0.063/0.5 | ≤0.063/0.125 | ≤0.063/≤0.063–0.5 | ≤1/4 | ≤1/<1 | ≤1/<1–4 |
| 0.5% NB-003 + 10 mM EDTA/well | ≤0.063/≤0.063 | 0.063/≤0.063 | 0.063/≤0.063 | ND/≤1 | ND/≤1 | ND/≤1 |
| 0.5% NB-003 + 20 mM EDTA/well | ≤0.063/≤0.063 | 0.063/≤0.063 | 0.063/≤0.063 | ND/≤1 | ND/≤1 | ND/≤1 |
ND, not done.
Time-kill and mechanism-of-action studies.
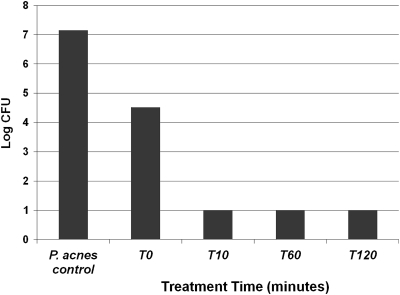
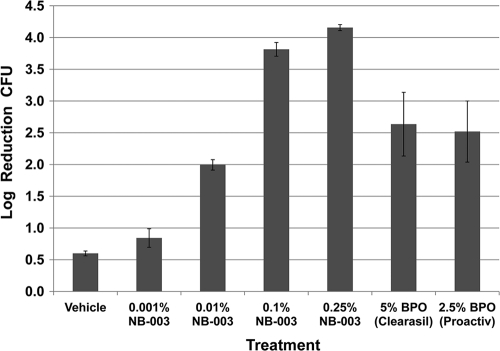
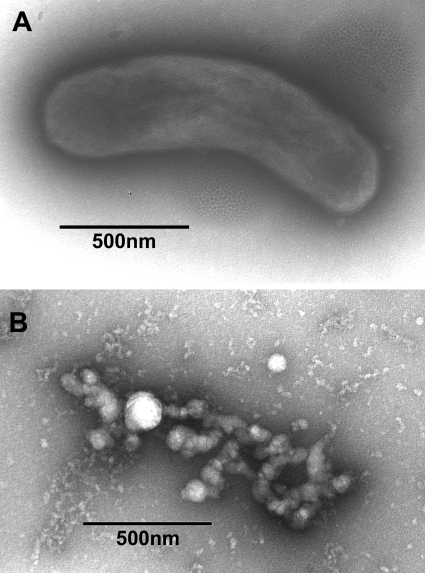
The kinetics of bactericidal activity of NB-003 and BPO were evaluated against two isolates of P. acnes, ATCC 6919 and multidrug-resistant clinical isolate PAC-008. Figure 1 shows that NB-003 at a concentration of 20 μg/ml is rapidly bactericidal in vitro (test tube), causing a more than 2-log reduction on contact (time zero) and a more than 6-log reduction after 10 min of exposure time. In a pig skin model, there was dose-dependent killing of P. acnes in the presence of 0.001 to 0.25% NB-003, and 0.1% and 0.25% NB-003 were shown to be more potent in killing P. acnes on pig skin than 2.50% BPO (Fig. 2). Both 0.1% and 0.25% NB-003 (3.8- and 4.2-log reduction, respectively) reduced the bacterial counts significantly more effectively than Clearasil (5% BPO) (2.6-log reduction) or Proactive (2.5% BPO) (2.5-log reduction). Figure 3 shows the morphology of P. acnes bacterium by using a scanning electron microscope before and after a 1-h treatment with 20 μg/ml (or 0.002%) NB-003. The integrity of the multidrug-resistant rod-shaped bacterium is rapidly destroyed, leaving no doubt that NB-003 is rapidly bactericidal.
Fig. 1.
The impact of NB-003 on the viability of P. acnes PAC-001. Time course of P. acnes in broth treated with 20 μg/ml NB-003. The lower limit of detection was 10 CFU, because a 1:10 dilution was necessary to neutralize the antibacterial carryover.
Fig. 2.
The impact of NB-003 and BPO on the viability of P. acnes PAC-008, a clinical isolate resistant to tetracycline, erythromycin, and clindamycin. Log reduction in P. acnes on pig skin surface treated with 0.001% to 0.25% NB-003 and 5% to 2.5% BPO.
Fig. 3.
Scanning electron micrographs of P. acnes PAC-001 before (A) and after (B) 1 h of treatment with 20 μg/ml NB-003.
Spontaneous resistance frequency.
Sixteen isolates tested for agar-based MIC showed MIC ranges of 4 to 16, 781, 0.5 to >64, and 0.5 to >64 μg/ml for NB003, BPO, clindamycin, and tetracycline, respectively. Twelve to 16 isolates of P. acnes were evaluated for spontaneous resistance frequency on agar plates containing 1, 2, 4, or 8 times the respective MIC of NB-003, tetracycline, clindamycin, or BPO. At 2× the MIC of tested drugs, phenotypic mutants appeared at a frequency of 2.5 × 10−11, >3.8 × 10−7, and >2.2 × 10−7 for NB-003, tetracycline, and clindamycin, respectively. No mutants appeared on any plates containing BPO. The frequencies of mutants phenotypically resistant to NB-003 at 1×, 2×, 4×, and 8× MIC are given in Table 4. The ranges of spontaneous resistance frequency for NB-003, tetracycline, and clindamycin were 2.5 × 10−11 to <2.7 × 10−10, <2.5 × 10−11 to >3.8 × 10−7, and <3.4 × 10−11 to >2.2 × 10−7, respectively.
Table 4.
Frequency of spontaneous mutations for 16 strains of P. acnes
| Strain no. | Spontaneous mutation frequencya |
|||
|---|---|---|---|---|
| MIC | 2× MIC | 4× MIC | 8× MIC | |
| PAC-001 | 7.7 × 10−11 | <7.7 × 10−11 | <7.7 × 10−11 | <7.7 × 10−11 |
| PAC-002 | 3.4 × 10−10 | <6.9 × 10−11 | <6.9 × 10−11 | <6.9 × 10−11 |
| PAC-003 | <5.6 × 10−11 | <5.6 × 10−11 | <5.6 × 10−11 | <5.6 × 10−11 |
| PAC-004 | 2.0 × 10−10 | <4.0 × 10−11 | <4.0 × 10−11 | <4.0 × 10−11 |
| PAC-005 | 9.6 × 10−10 | <1.9 × 10−10 | <1.9 × 10−10 | <1.9 × 10−10 |
| PAC-006 | <9.1 × 10−11 | <9.1 × 10−11 | <9.1 × 10−11 | <9.1 × 10−11 |
| PAC-007 | <3.8 × 10−11 | <3.8 × 10−11 | <3.8 × 10−11 | <3.8 × 10−11 |
| PAC-008 | <2.7 × 10−11 | <2.7 × 10−11 | 5.5 × 10−11 | <2.7 × 10−11 |
| PAC-009 | 4.4 × 10−08 | <2.2 × 10−10 | <2.2 × 10−10 | <2.2 × 10−10 |
| PAC-010 | <1.3 × 10−10 | <1.3 × 10−10 | <1.3 × 10−10 | <1.3 × 10−10 |
| PAC-011 | <2.5 × 10−11 | 2.5 × 10−11 | <2.5 × 10−11 | <2.5 × 10−11 |
| PAC-012 | <2.7 × 10−10 | <2.7 × 10−10 | <2.7 × 10−10 | <2.7 × 10−10 |
| PAC-013 | <3.4 × 10−11 | <3.4 × 10−11 | <3.4 × 10−11 | <3.4 × 10−11 |
| PAC-014 | <1.0 × 10−07 | <1.0 × 10−07 | <1.0 × 10−07 | <1.0 × 10−07 |
| PAC-015 | <3.8 × 10−10 | <3.8 × 10−10 | <3.8 × 10−10 | <3.8 × 10−10 |
| PAC-016 | <1.0 × 10−07 | <1.0 × 10−07 | <1.0 × 10−07 | <1.0 × 10−07 |
Concentration shown as multiples of the MIC of NB-003 against the parent strain.
Despite the growth on agar plates containing higher concentrations of selecting compound, most of the phenotypically resistant isolates had MICs that were ±2-fold the initial MIC for all compounds when retested in broth. One mutant selected with tetracycline had a >16-fold increase in MIC for tetracycline compared to that of the parent isolate. MICs of recovered isolates tested by microtiter broth ranged from 0.25 to 0.5 μg/ml, ≤0.125 to 32 μg/ml, ≤0.125 to >64 μg/ml, and 24 to 98 μg/ml for NB-003, tetracycline, clindamycin, and BPO, respectively.
DISCUSSION
NB-003, an oil-in-water nanoemulsion with broad-spectrum antimicrobial activity, was optimized for topical treatment of acne vulgaris. NB-003 has a significant bactericidal activity against a collection of recent clinical isolates of P. acnes, including multidrug-resistant strains. Comparator drugs that have been used to treat acne, including erythromycin, clindamycin, tetracycline, benzoyl peroxide, and salicylic acid, were less effective against this strain panel. Resistance and cross-resistance of P. acnes to commonly used acne drugs are common and prevalent problems (15, 34, 53, 54). Consistent with its physical mechanism of action of interacting with the P. acne surface, NB-003 had no cross-resistance with known antiacne agents. Further, stably resistant isolates from cultures of P. acnes cultured on plates containing 2, 4, or 8 times the MIC of NB-003 were difficult to maintain.
Use of combination therapy to treat acne has been previously studied (10, 15, 32–35, 39). Benzoyl peroxide-clindamycin demonstrated improved efficacy and similar tolerability as benzoyl peroxide used alone. The efficacy of this combination therapy was similar to that of a benzoyl peroxide-erythromycin combination (10, 32, 35, 39). Erythromycin or clindamycin plus BPO have been reported to reduce drug resistance development compared to use of single antimicrobials.
Other combination strategies of BPO combined with a retinoid cause irritation and are often not well tolerated (35), resulting in discontinuation by patients. Studies on less irritating formulations have been reported; glycerin and dimethicone have been reported to reduce the formation of erythema and dryness caused by BPO and retinoids (14, 64) as well as micronized particles of BPO. In this study, NB-003 showed synergy with BPO for 90% of the isolates tested. Since individual MICs and MBCs of NB-003 and BPO decreased in the presence of sebum compared to those of NB-003 alone (Table 1), the synergistic interaction of the two drugs presents a potential opportunity for the nanoemulsion combination to be used for acne treatment. Under the best scenario that would likely cause less skin irritation and drying, 0.0025% BPO can be formulated with 0.3% NB-003. Combinations of the nanoemulsion NB-003 with BPO or salicylic acid were as effective as NB-003 alone, but synergy at the bactericidal level (MBCs) were seen when the combinations were used in the presence of sebum. Thus, the power of codelivering synergistic combinations by the transfollicular route conferred with nanoemulsion technology (5) could yield more effective treatment. Due to its kill-on-contact mechanism, NB-003 poses a very low risk for the development of resistance and cross-resistance with other drugs, and if resistance does emerge, it is not stable.
EDTA, a preservative in the food, drug, and cosmetic industries (16, 22, 28, 31, 43, 60), has been shown to potentiate the antibiotic activity of nanoemulsions containing CPC against Gram-positive and Gram-negative bacteria. Farca et al. have shown the synergistic effect of EDTA with five antimicrobial agents, ampicillin, cephalexin, oxytetracycline, streptomycin, and sulfadimethoxine, on three clinically isolated Gram-positive bacteria (Staphylococcus aureus, Staphylococcus hominis, and Enterococcus faecium) even in the absence of nanoemulsion delivery (16). We have shown that 20 mM EDTA enhances the activity of NB-003 up to 256-fold against P. acnes in the presence of 25% sebum. EDTA may enhance the activity of NB-003 by acting as a permeabilizer and chelating agent and enhancing the transmembrane diffusion of nanoemulsion.
NB-003 is a broad-spectrum topical that is being developed for treatment of diseases in the skin, hair, and nails. Previous work in human cadaver skin samples demonstrated that the antiviral NB-001 nanoemulsion uses a transfollicular route to enter the epidermal and dermal layers and that lateral diffusion occurred along tissue planes to sites distal from the application site (5, 6). Clinical studies for the topical treatment of herpes labialis and onychomycosis confirm that there is no systemic exposure in patients after multiple nanoemulsion applications per day, eliminating worry about drug-drug interactions and undesired systemic side effects (24, 26, 27). Thus, NB-003 offers a topical, empirical alternative to the oral or topical antibiotics used to treat acne, and clinical trials are ongoing to prove its value for the treatment of acne.
ACKNOWLEDGMENT
We acknowledge the laboratory of S. Shapiro for providing us the multidrug-resistant isolates of P. acnes.
Footnotes
Published ahead of print on 11 July 2011.
REFERENCES
- 1. Bogdanovich T., Ednie L. M., Shapiro S., Appelbaum P. C. 2005. Antistaphylococcal activity of ceftobiprole, a new broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 49:4210–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boivin G., Goyette N., Sutcliffe J. 2008. A novel nanoemulsion active against acyclovir (ACV)- and foscarnet (FOS)-resistant herpes simplex virus (HSV) 1 and 2. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. V-3538 [Google Scholar]
- 3. Bonapace C. R., Bosso J. A., Friedrich L. V., White R. L. 2002. Comparison of methods of interpretation of checkerboard synergy testing. Diagn. Microbiol. Infect. Dis. 44:363–366 [DOI] [PubMed] [Google Scholar]
- 4. Bowe W. P., Shalita A. R. 2008. Effective over-the-counter acne treatments. Semin. Cutan. Med. Surg. 27:170–176 [DOI] [PubMed] [Google Scholar]
- 5. Ciotti S., Eisma R., Ma L., Baker J. R., Jr 2008. Novel nanoemulsion NB-001 permeates skin by the follicular route. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1898 [Google Scholar]
- 6. Ciotti S., Eisma R., Vengroff L., Ma L., Baker J. R., Jr 2008. Mechanism of skin penetration and distribution of a novel antimicrobial nanoemulsion. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-2135 [Google Scholar]
- 7. CLSI 2007. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 7th ed Approved standard M11-A7. Clinical and Laboratory Standards Institute, Wayne, PA: [PubMed] [Google Scholar]
- 8. CLSI 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline M26-A. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Coates P., et al. 2002. Prevalence of antibiotic-resistant propionibacteria on the skin of acne patients: 10-year surveillance data and snapshot distribution study. Br. J. Dermatol. 146:840–848 [DOI] [PubMed] [Google Scholar]
- 10. Cunliffe W. J., Holland K. T., Bojar R., Levy S. F. 2002. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin. Ther. 24:1117–1133 [DOI] [PubMed] [Google Scholar]
- 11. Davies T. A., Pankuch G. A., Dewasse B. E., Jacobs M. R., Appelbaum P. C. 1999. In vitro development of resistance to five quinolones and amoxicillin-clavulanate in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:1177–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Del Rosso J. Q. 2007. Highlights from the latest acne treatment guidelines. Skin Aging 15:84–88 [Google Scholar]
- 13. Dhawan S. S. 2009. Comparison of 2 clindamycin 1%-benzoyl peroxide 5% topical gels used once daily in the management of acne vulgaris. Cutis 83:265–272 [PubMed] [Google Scholar]
- 14. Draelos Z. D., Callender V., Young C., Dhawan S. S. 2008. The effect of vehicle formulation on acne medication tolerability. Cutis 82:281–284 [PubMed] [Google Scholar]
- 15. Eady E. A., et al. 1996. The effects of acne treatment with a combination of benzoyl peroxide and erythromycin on skin carriage of erythromycin-resistant propionibacteria. Br. J. Dermatol. 134:107–113 [PubMed] [Google Scholar]
- 16. Farca A. M., Nebbia P., Re G. 1994. Potentiation of antibiotic activity by EDTA-tromethamine against three clinically isolated Gram-positive resistant bacteria. An in vitro investigation. Vet. Res. Commun. 18:1–6 [DOI] [PubMed] [Google Scholar]
- 17. Fritsche T., Biedenhach R., Jones R., Sutcliffe J. 2008. Antimicrobial activity of nanoemulsions tested against seven Gram-negative species. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-3945 [Google Scholar]
- 18. Gloor M., Schorch B., Hoeffler U. 1979. The feasibility of replacing antibiotics by quaternary ammonium compounds in topical antimicrobial acne therapy. Arch. Dermatol. Res. 265:207–212 [DOI] [PubMed] [Google Scholar]
- 19. Hamouda T., Baker J. R., Jr 2000. Antimicrobial mechanism of action of surfactant lipid preparations in enteric Gram-negative bacilli. J. Appl. Microbiol. 89:397–403 [DOI] [PubMed] [Google Scholar]
- 20. Hamouda T., et al. 1999. A novel surfactant nanoemulsion with broad-spectrum sporicidal activity against Bacillus species. J. Infect. Dis. 180:1939–1949 [DOI] [PubMed] [Google Scholar]
- 21. Hamouda T., et al. 2001. A novel surfactant nanoemulsion with a unique non-irritant topical antimicrobial activity against bacteria, enveloped viruses and fungi. Microbiol. Res. 156:1–7 [DOI] [PubMed] [Google Scholar]
- 22. Hart J. R. 1984. Chelating agents as preservative potentiators, p. 54–58 In Kabara J. J. (ed.), Cosmetic and drug preservation: principles and practices. Marcell Dekker, New York, NY [Google Scholar]
- 23. Heller S., Kellenberger L., Shapiro S. 2007. Antipropionibacterial activity of BAL19403, a novel macrolide antibiotic. Antimicrob. Agents Chemother. 51:1956–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jarrat M., Ijzerman M. M., Flack M. R., Baker J. J. R. 2008. Safety, tolerability and pharmacokinetics of NB-001 in a phase 2 dose ranging trial in subjects with recurrent herpes labialis. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. V-3539 [Google Scholar]
- 25. Jelvehgari M., Siahi-Shadbad M. R., Azarmi S., Martin G. P., Nokhodchi A. 2006. The microsponge delivery system of benzoyl peroxide: preparation, characterization and release studies. Int. J. Pharm. 308:124–132 [DOI] [PubMed] [Google Scholar]
- 26. Jones T., Flack M., Ijzerman M. M., Baker J. R., Jr 2008. Safety, tolerance, and pharmacokinetics of topical nanoemulsion (NB-002) for the treatment of onychomycosis. J. Am. Acad. Dermatol. 58:AB83 [Google Scholar]
- 27. Jones T., Flack M., Stanberry L., Baker J. R., Jr 2008. Safety, tolerance, pharmacokinetics, and efficacy of topical nanoemulsion (NB-001) for the treatment of herpes labialis. J. Am. Acad. Dermatol. 58:AB93 [Google Scholar]
- 28. Kalchayanand N., Hanlin M. B., Ray B. 1992. Sublethal injury makes Gram-negative and resistant Gram-positive bacteria sensitive to the bacteriocins, pediocin AcH and nisin. Lett. Appl. Microbiol. 15:239–243 [Google Scholar]
- 29. Kosowska K., et al. 2005. Antipneumococcal activity of ceftobiprole, a novel broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 49:1932–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kosowska-Shick K., et al. 2006. Single- and multistep resistance selection studies on the activity of retapamulin compared to other agents against Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 50:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lambert R. J. W., Hanlon G. W., Denye S. P. 2004. The synergistic effect of EDTA/antimicrobial combinations on Pseudomonas aeruginosa. J. Appl. Microbiol. 96:244–253 [DOI] [PubMed] [Google Scholar]
- 32. Leyden J. J. 2002. Effect of topical benzoyl peroxide/clindamycin versus topical clindamycin and vehicle in the reduction of Propionibacterium acnes. Cutis 69:475–480 [PubMed] [Google Scholar]
- 33. Leyden J. J., et al. 2001. Comparison of the efficacy and safety of a combination topical gel formulation of benzoyl peroxide and clindamycin with benzoyl peroxide, clindamycin and vehicle gel in the treatments of acne vulgaris. Am. J. Clin. Dermatol. 2:33–39 [DOI] [PubMed] [Google Scholar]
- 34. Leyden J. J., Del Rosso J. Q., Webster G. F. 2009. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: a status report. Dermatol. Clin. 27:1–15 [DOI] [PubMed] [Google Scholar]
- 35. Leyden J. J., Hickman J. G., Jarratt M. T., Stewart D. M., Levy S. F. 2001. The efficacy and safety of a combination benzoyl peroxide/clindamycin topical gel compared with benzoyl peroxide alone and a benzoyl peroxide/erythromycin combination product. J. Cutan. Med. Surg. 5:37–42 [DOI] [PubMed] [Google Scholar]
- 36. Leyden J. J., Marples R. R., Mills O. H., Jr., Kligman A. M. 1973. Gram-negative folliculitis—a complication of antibiotic therapy in acne vulgaris. Br. J. Dermatol. 88:533–538 [DOI] [PubMed] [Google Scholar]
- 37. Leyden J. J., McGinley K. J., Mills O. H., Kligman A. M. 1975. Age-related changes in the resident bacterial flora of the human face. J. Investig. Dermatol. 65:379–381 [DOI] [PubMed] [Google Scholar]
- 38. Leyden J. J., McGinley K. J., Mills O. H., Kligman A. M. 1975. Propionibacterium levels in patients with and without acne vulgaris. J. Investig. Dermatol. 65:382–384 [DOI] [PubMed] [Google Scholar]
- 39. Leyden J. J., Wortzman M., Baldwin E. K. 2008. Antibiotic-resistant Propionibacterium acnes suppressed by a benzoyl peroxide cleanser 6%. Cutis 82:417–421 [PubMed] [Google Scholar]
- 40. Lu G. W., et al. 2009. Comparison of artificial sebum with human and hamster sebum samples. Int. J. Pharm. 367:37–43 [DOI] [PubMed] [Google Scholar]
- 41. Myc A., Vanhecke T., Landers J. J., Hamouda T., Baker J. R., Jr 2002. The fungicidal activity of novel nanoemulsion (X8W60PC) against clinically important yeast and filamentous fungi. Mycopathologia 155:195–201 [DOI] [PubMed] [Google Scholar]
- 42. Nagai K., et al. 2000. In vitro selection of resistance to clinafloxacin, ciprofloxacin, and trovafloxacin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:2740–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagai T., Oita S. 2004. Anti-Helicobacter pylori activity of EDTA. J. Gen. Appl. Microbiol. 50:115–118 [DOI] [PubMed] [Google Scholar]
- 44. Oprica C., et al. 2004. Antibiotic-resistant Propionibacterium acnes on the skin of patients with moderate to severe acne in Stockholm. Anaerobe 10:155–164 [DOI] [PubMed] [Google Scholar]
- 45. Oprica C., Nord C. E. 2005. European surveillance study on the antibiotic susceptibility of Propionibacterium acnes. Clin. Microbiol. Infect. 11:204–213 [DOI] [PubMed] [Google Scholar]
- 46. Pankuch G. A., Jueneman S. A., Davies T. A., Jacobs M. R., Appelbaum P. C. 1998. In vitro selection of resistance to four beta-lactams and azithromycin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2914–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pannu J., Ciotti S., Eisma R., Ma L., Sutcliffe J. 2009. In vitro susceptibility of Propionibacterium acnes and skin permeation of NB00X formulations. Abstr. 69th Annu. Meet. Soc. Investigat. Dermatol., abstr. 616 [Google Scholar]
- 48. Pannu J., et al. 2009. NB-002, a novel nanoemulsion with broad antifungal activity against dermatophytes, other filamentous fungi, and Candida albicans. Antimicrob. Agents Chemother. 53:3273–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pannu J., et al. 2008. A novel nanoemulsion with anti-dermatophyte activity. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-2134 [Google Scholar]
- 50. Pannu J., McCarthy A., Martin A., Sutcliffe J. 2009. Susceptibility of Propionibacterium acnes in the presence of sebum to NB-003 formulations, abstr. P106. Abstr. 2009 Summer Acad. Meet. Am. Acad. Dermatol [Google Scholar]
- 51. Pfaller M. A., Sheehan D. J., Rex J. H. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17:268–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roche Laboratories 2010. Accutane (isotretinoin) drug description facts. Roche Laboratories, Nutley, NJ [Google Scholar]
- 53. Ross J. I., Eady E. A., Carnegie E., Cove J. H. 2002. Detection of transposon Tn5432-mediated macrolide-lincosamide-streptogramin B (MLSB) resistance in cutaneous propionibacteria from six European cities. J. Antimicrob. Chemother. 49:165–168 [DOI] [PubMed] [Google Scholar]
- 54. Ross J. I., et al. 2003. Antibiotic-resistant acne: lessons from Europe. Br. J. Dermatol. 148:467–478 [DOI] [PubMed] [Google Scholar]
- 55. Smith M. A., Alperstein P., France K., Vellozzi E. M., Isenberg H. D. 1996. Susceptibility testing of Propionibacterium acnes comparing agar dilution with E test. J. Clin. Microbiol. 34:1024–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stern R. S. 2000. Medication and medical service utilization for acne 1995-1998. J. Am. Acad. Dermatol. 43:1042–1048 [DOI] [PubMed] [Google Scholar]
- 57. Strauss J. S., et al. 2007. Guidelines of care for acne vulgaris management. J. Am. Acad. Dermatol. 56:651–663 [DOI] [PubMed] [Google Scholar]
- 58. Sutcliffe J., Biedenbach D., Jones R., Fritsche T. 2008. Novel nanoemulsion antimicrobials tested against nine Gram-positive species. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-3944 [Google Scholar]
- 59. Tzellos T., Zampeli V., Makrantonaki E., Zouboulis C. C. 2011. Treating acne with antibiotic-resistant bacterial colonization. Expert Opin. Pharmacother. 12:1233–1247 [DOI] [PubMed] [Google Scholar]
- 60. Vaara M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Valiveti S., Lu G. W. 2007. Diffusion properties of model compounds in artificial sebum. Int. J. Pharm. 345:88–94 [DOI] [PubMed] [Google Scholar]
- 62. Valiveti S., Wesley J., Lu G. W. 2008. Investigation of drug partition property in artificial sebum. Int. J. Pharm. 346:10–16 [DOI] [PubMed] [Google Scholar]
- 63. Verma P. 2007. Methods for determining bactericidal activity and antimicrobial interactions: synergy testing, time-kill curves, and population analysis, p. 275–298 In Schwalbe R., Steele-Moore L., Goodwin A. C. (ed.), Antimicrobial susceptibility testing protocols. CRC Press, Boca Raton, FL [Google Scholar]
- 64. Weinberg J. M. 2006. The utility of benzoyl peroxide in hydrophase base (Brevoxyl) in the treatment of acne vulgaris. J. Drugs Dermatol. 5:344–349 [PubMed] [Google Scholar]
- 65. Zhang L., et al. 2008. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 83:761–769 [DOI] [PubMed] [Google Scholar]