Abstract
The object of this study was to investigate the pharmacokinetics of darunavir-ritonavir and atazanavir-ritonavir once-daily dosing over 72 h (h) following drug intake cessation. Volunteers received darunavir-ritonavir at 800 and 100 mg, respectively, once daily for 10 days, followed by a 7-day washout period, and atazanavir-ritonavir at 300 and 100 mg, respectively, once daily for 10 days. Full pharmacokinetic profiles were assessed for each phase for the 72 h following day 10. Pharmacokinetic parameters were determined over 24 h and to the last measurable concentration by noncompartmental methods. Seventeen subjects completed the study. The geometric mean (GM) terminal elimination half-life to 72 h of darunavir was 6.48 h, which was lower than the 0- to 24-h half-life (10.70 h). The terminal elimination half-life of atazanavir was 6.74 h, which was lower than the 0- to 24-h half-life (13.72 h). All subjects but one had darunavir concentrations higher than the target of 550 ng/ml for protease-resistant HIV isolates (equivalent to 10 times the protein-binding-corrected 50% inhibitory concentration [IC50] for wild-type virus) at 24 h postdose, and 14 out of 17 had concentrations higher than the target at 30 h postdose (GM of 1,088 and 851 ng/ml). All subjects had atazanavir concentrations above the suggested minimum effective concentration of 150 ng/ml (equivalent to 10 times the protein-binding-corrected IC50 for wild-type virus) at 24 and 30 h postdose (GM of 693 and 392 ng/ml). Two of 17 and 5 of 17 subjects were above target at 48 h postdose while on darunavir-ritonavir and atazanavir-ritonavir. Ritonavir half-life to 72 h was 6.84 h with darunavir and 6.07 with atazanavir. This study investigated the pharmacokinetic forgiveness of two boosted protease inhibitors. Although the rates of decline of darunavir and atazanavir slightly increased as ritonavir concentrations declined, most individuals had concentrations 6 h after the end of the ideal dosing interval of 24 h which were above the cutoff used to define therapeutic concentrations.
INTRODUCTION
The combination of a ritonavir-boosted protease inhibitor and two nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs) has shown sustained suppression of plasma HIV replication and continued immunological recovery in naïve and experienced HIV-infected individuals (11).
The advantages associated to ritonavir boosting are a consequence of the increased drug exposure and prolonged half-life that allow reducing the pill burden and dosing frequency and lead to the achievement of a high genetic barrier to resistance (11, 16).
Drug persistence (the presence of drug at a detectable level high enough to exert an effect) in plasma mainly depends on the half-life (which itself is dependent on clearance and volume of distribution). Long-half-life antiretroviral agents may allow for forgotten doses, especially if they are able to delay the decline in drug concentration to a subtherapeutic level for a period of time that is long enough for the concentrations to be above target until the patient remembers to take the drug.
Depending on pharmacokinetic properties, boosted protease inhibitors differ in terms of dosing schedule (11). Some are characterized by longer half-lives and are approved for once-daily dosing. The latter is critical for optimal adherence to antiretroviral therapy and therefore for virological success (17).
Data on drug persistence in plasma are limited, and the effect of forgotten and delayed dosing on outcome is largely unknown.
A recent study found that doses might be missed in the case of regimens containing long-half-life drugs, such as the nonnucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (8). However, the strategy appeared much riskier for patients on protease inhibitors like lopinavir-ritonavir or saquinavir-ritonavir that achieved subtherapeutic drug concentrations at the end of the second day off therapy, suggesting that despite the presence of the ritonavir boosting effect, lopinavir and saquinavir plasma concentrations declined rapidly (7).
We previously presented data on the pharmacokinetic forgiveness of atazanavir-ritonavir once-daily and lopinavir-ritonavir once- and twice-daily dosing, showing that whereas the decline in lopinavir concentrations and the boosting effect of ritonavir was rapid, the rate of decline of atazanavir remained constant to 72 h, resulting in a delayed onset of subtherapeutic concentrations (1). This could be explained mainly by the effect that the two different protease inhibitors have on ritonavir clearance (13). Ritonavir plasma concentrations are decreased by lopinavir and increased in the presence of a moderate CYP3A4 inhibitor such as atazanavir (2, 13). Therefore, the greater plasma persistence of ritonavir when it is given with atazanavir may be a key reason that atazanavir has slightly longer persistence than lopinavir.
In this study we have assessed the pharmacokinetics of once-daily darunavir-ritonavir (15) and once-daily atazanavir-ritonavir over 72 h following drug intake cessation in HIV-negative healthy volunteers.
(Part of this research was presented at the 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 27 February to 2 March 2011.)
MATERIALS AND METHODS
Subjects and ethics.
The study protocol was approved by the Outer West London Ethics Committee, Northwick Park Hospital, Harrow, London, United Kingdom, as well as by the Medicines and Healthcare products Regulatory Agency ([MHRA] EudraCT 2009-017170-21) in the United Kingdom and was conducted according to Good Clinical Practice and the Declaration of Helsinki.
Written informed consent was obtained from male and female healthy volunteers between 18 and 65 years of age. Before entering the study, the volunteers were established to be HIV negative and in good health (by medical history, physical examination, electrocardiogram, and standard hematological and blood chemistry tests); in addition, they had not received any other drugs, including over-the-counter medications and herbal preparations, within 2 weeks prior to the first dose of the study drug.
Study design.
This was an open-label, two-phase, pharmacokinetic study carried out at the Pharmacokinetic Unit of the St. Stephen's Centre, Chelsea, and Westminster Hospital, London, United Kingdom.
During the first study phase, volunteers were administered darunavir-ritonavir at 800 and 100 mg, respectively, once daily (two 400-mg darunavir tablets plus one 100-mg ritonavir heat-stable tablet) in the morning for 10 days. On study days 10 to 13, darunavir and ritonavir plasma concentrations were assessed predose and at 1, 2, 3, 4, 6, 8, 10, 12, 16, 20, 24, 30, 36, 48, 60, and 72 h postdose.
After a washout period of 7 days, all subjects were administered atazanavir-ritonavir at 300 and 100 mg, respectively, once daily (one 300-mg atazanavir capsule plus one 100-mg ritonavir heat-stable tablet) for 10 days, and drug concentrations were measured on study days 27 to 30, over 72 h following the last dose. On the pharmacokinetic days, the study medication was taken with a standardized breakfast (626 kcal) and 240 ml of water, and subjects were admitted to the unit for 36 h. Following this time point, subjects attended the unit every 12 h on four different occasions for both study phases.
Compliance with study drug administration was assessed by pill counting by the study staff.
Safety assessment.
The safety and tolerability of study medications were evaluated throughout the study on the basis of clinical adverse events (using the NIAID Division of AIDS grading scale to characterize abnormal findings), clinical laboratory tests (at screening, on days 1, 5, 10, 22, and 27, and at follow-up), vital signs, and physical examinations.
Analytical and pharmacokinetic methods.
Concentrations of darunavir, atazanavir, and ritonavir in plasma were measured using validated high-pressure liquid chromatography–tandem mass spectrometry methods (10). The lower limits of quantification (LLQC) were 16.0, 5.0, and 3.0 ng/ml for darunavir, atazanavir, and ritonavir, respectively. For concentrations below the assay limit of quantification, a value of one-half of the quantification limit (8.0, 2.5, and 1.5 ng/ml, respectively) was used. Assay precision, assessed by the calculation of inter- and intra-assay variability of low (LQC)-, medium (MQC)-, and high (HQC)-quality control samples and expressed in terms of percent coefficient of variation (CV), has been previously described (10). Inter- and intra-assay precision (% CV) did not exceed 10% for all analytes.
The calculated pharmacokinetic parameters for darunavir, atazanavir, and ritonavir were the plasma concentration measured 24 h after the observed dose (C24h), the maximum observed plasma concentration (Cmax), and the area under the plasma concentration-time curve from 0 to 24 h (AUC0–24) and from 0 to 72 h (AUC0–72). The half-life was determined from the elimination phase within the normal dosing interval of 0 to 24 and as a terminal elimination half-life to the last measurable concentration within 72 h. All pharmacokinetic parameters were calculated using actual blood sampling times and noncompartmental modeling techniques (WinNonlin Phoenix, version 6.1; Pharsight Corp., Mountain View, CA).
Statistical analysis.
Descriptive statistics, including geometric mean (GM) and 90% confidence intervals (CIs), were calculated for darunavir, atazanavir, and ritonavir pharmacokinetic parameters.
Within-subject changes in ritonavir pharmacokinetic parameters were evaluated by calculating geometric mean ratios (GMRs) and 90% CIs. The ritonavir concentrations measured in the presence of atazanavir were used as a reference. The CIs were determined using logarithms of the individual geometric mean values; the calculated values were then expressed as linear values. The changes in pharmacokinetic parameters were considered significant when the CI did not cross the value 1.
Interindividual variability in drug pharmacokinetic parameters was expressed as a coefficient of variation, calculated as follows: (standard deviation/mean) × 100.
RESULTS
Study population.
Eighteen volunteers were screened, one withdrew for personal reasons, and 17 completed all phases of the study. Of the 17 evaluable subjects, median (range) age was 35 (19 to 62) years, and the median body mass index (BMI) was 23.6 (20.9 to 34.6) kg/m2. Eight were female, eight were Caucasians, eight were black, and one was Asian. No serious breaches to the protocol were recorded during the study.
Pharmacokinetics.
Geometric mean values and 90% CIs for the steady-state pharmacokinetic parameters measured over 24 and 72 h for darunavir, atazanavir, and ritonavir are summarized in Table 1.
Table 1.
Darunavir, atazanavir, and ritonavir steady-state pharmacokinetic parameters measured over 24 and 72 h
| Parameter | Value for darunavir-ritonavir (GM [90% CI])a |
Value for atazanavir-ritonavir (GM [90% CI])a |
||||||
|---|---|---|---|---|---|---|---|---|
| Darunavir (800 mg) |
Ritonavir (100 mg) |
Atazanavir (300 mg) |
Ritonavir (100 mg) |
|||||
| 0–24 h |
0–72 h |
0–24 h |
0–72 h |
0–24 h |
0–72 h |
0–24 h |
0–72 h |
|
| Day 10 | Days 10–13 | Day 10 | Days 10–13 | Day 27 | Days 27–30 | Day 27 | Days 27–30 | |
| AUC (ng · h/ml) | 53,910 (47,450–67,886) | 70,686 (60,570–94,380) | 5,279 (4,681–6,556) | 5,689 (5,027–7,086) | 34,851 (29,059–48,196) | 43,394 (34,823–65,189) | 8,529 (7,267–10,887) | 8,907 (7,578–11,410) |
| AUC0–∞ (ng · h/ml) | 72,298 (62,466–95,462) | 71,128 (60,875–95,086) | 5,621 (4,969–7,005) | 5,667 (5,005–7,067) | 47,631 (37,292–75,757) | 43,507 (34,871–65,532) | 8,872 (7,534–11,428) | 8,868 (7,540–11,376) |
| Cmax (ng/ml) | 5,021 (4,492–6,060) | 5,021 (4,492–6,060) | 746 (646–1,045) | 746 (646–1,045) | 3,416 (2,953–4,300) | 3,416 (2,953–4,300) | 1,378 (1,199–1,744) | 1,378 (1,199–1,744) |
| C24/72 h (ng/ml) | 1,008 (841–1,513) | 12 (1–52)b | 39 (32–55) | ND | 693 (522–1,233) | 6 (3–31)b | 44 (35–74) | ND |
| Half-life (h) | 10.70 (9.34–13.53) | 6.81 (5.96–7.30) | 5.48 (4.98–6.28) | 6.30 (6.16–7.97) | 11.87 (8.63–25.69) | 6.86 (6.30–7.31) | 4.64 (4.20–5.29) | 5.03 (5.30–7.35) |
GM, geometric mean; ND, not done.
This value indicates the concentrations measured at 72 h postdose for 8 subjects as the drug was not detectable in nine subjects.
Geometric mean concentrations of darunavir and atazanavir measured at 30, 36, 48, and 60 h postdose are illustrated in Table 2.
Table 2.
Concentrations of darunavir and atazanavir measured at 30, 36, 48, and 60 h postdose and number of subjects below target per time point
| Time point (h) | Darunavir (n = 17) |
Atazanavir (n = 17) |
||
|---|---|---|---|---|
| Concn (ng/ml)a | No. (%) of subjects below target (550 ng/ml) | Concn (ng/ml)a | No. (%) of subjects below target (150 ng/ml) | |
| 30 | 851 (125–2,692) | 3/17 (6) | 392 (182–1,845) | 0/17 (0) |
| 36 | 511 (34–2,141) | 8/17 (47) | 210 (69–1,391) | 8/17 (47) |
| 48 | 177 (8–1,456) | 15/17 (82) | 82 (20–681) | 12/17 (71) |
| 60 | 28 (8–662) | 16/17 (94) | 18 (3–290) | 15/17 (88) |
Values are geometric mean (range).
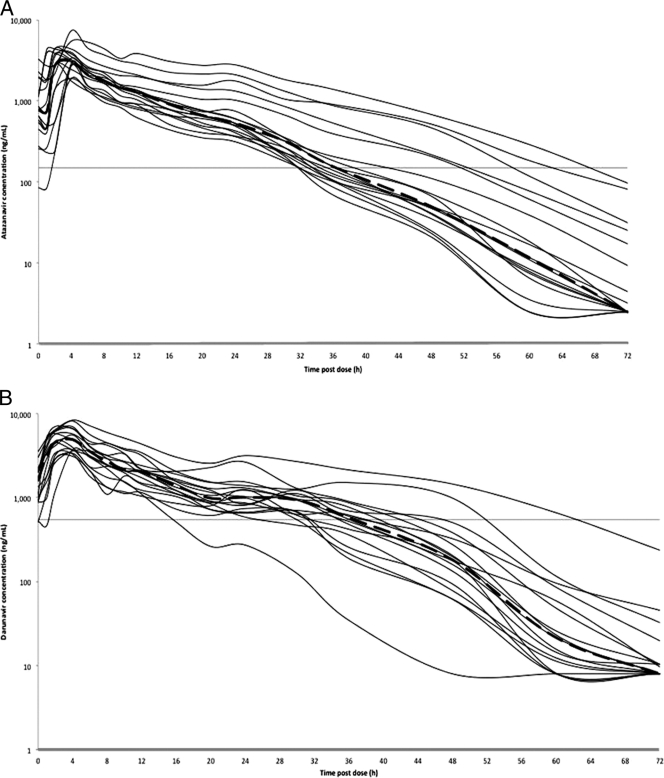
Plasma concentration-versus-time curves of darunavir and atazanavir are shown in Fig. 1.
Fig. 1.
Steady-state plasma concentrations from all studied subjects and geometric mean concentration-time curves (black thick lines) of darunavir (darunavir-ritonavir at 800 and 100 mg, respectively, once daily) (A) and atazanavir (atazanavir-ritonavir at 300 and 100 mg, respectively, once daily) (B). Horizontal lines indicate the targets used in this analysis: 550 ng/ml for darunavir and 150 ng/ml for atazanavir.
The geometric mean terminal elimination half-life to 72 h of darunavir was 6.48 h. This value was lower than the half-life measured over the dosing interval of 24 h (10.70 h).
Of the 17 subjects, 16 had darunavir plasma concentrations higher than the target of 550 ng/ml for protease-resistant HIV viral isolates (equivalent to 10 times the protein-binding-corrected 50% inhibitory concentration [IC50] for wild-type virus) (4, 19) at 24 h postdose, and 14 out of 17 had concentrations higher than the target at 30 h postdose (geometric mean, 1,088 and 851 ng/ml, respectively).
The geometric mean terminal half-life to 72 h of atazanavir was 6.74 h, and it was lower than the half-life measured over the 24-h dosing interval, which was 13.72 h.
No subject had atazanavir concentrations below the suggested minimum effective concentration (MEC) of 150 ng/ml (14) at 24 h (geometric mean C24h of 693 ng/ml) and at 30 h postdose (geometric mean of 392 ng/ml).
The interindividual variability in darunavir and atazanavir C24h values was 62% and 81%, respectively.
Interestingly, when we compared the ritonavir pharmacokinetic parameters measured with darunavir to those measured with atazanavir (Table 3), we observed that the ritonavir AUC0–24 and AUC0–72 and the Cmax and C24h were significantly lower (38%, 36%, 46%, and 12%, respectively), while ritonavir half-life values for 0 to 24 h and 0 to 72 h were higher (18% and 13%, respectively).
Table 3.
Comparison of ritonavir pharmacokinetic parameters during coadministration with darunavir and atazanavir once daily
| Parameter | Ritonavir-darunavir vs ritonavir-atazanavir (GMR [90% CI])a |
|---|---|
| AUC0–24 (ng · h/ml) | 0.62 (0.38–0.45) |
| AUC0–72 (ng · h/ml) | 0.64 (0.40–0.47) |
| Cmax (ng/ml) | 0.54 (0.30–0.40) |
| C24h (ng/ml) | 0.88 (0.62–0.93) |
| Half-life, 0–24 h | 2.31 (1.91–2.79) |
| Half-life, 0–72 h | 1.25 (1.16–1.36) |
Ritonavir was coadministered once daily at 100 mg with darunavir (800 mg) or atazanavir (300 mg).
Safety and tolerability.
Treatment was generally well tolerated, and no serious adverse events occurred during the study. The most common adverse events observed throughout the study were headache and hyperbilirubinemia (during the atazanavir-ritonavir phase), recorded in 4 and 16 volunteers, respectively. No clinically relevant changes in laboratory parameters were reported.
DISCUSSION
We report here the steady-state plasma pharmacokinetics of once-daily darunavir-ritonavir at 800 and 100 mg, respectively, and of once-daily atazanavir-ritonavir at 300 and 100 mg, respectively, over 72 h following drug intake cessation in 17 male and female healthy volunteers as we investigated the pharmacokinetic forgiveness of two commonly administered boosted protease inhibitors. The rates of decline of both darunavir and atazanavir slightly increased as ritonavir concentrations declined. However, most individuals showed therapeutic concentrations up to 6 h after the end of the ideal dosing interval of 24 h. Furthermore, both darunavir and atazanavir showed a terminal half-life (0 to 72 h) longer than 6 h (6.48 and 6.74 h, respectively) and a 0- to 24-h half-life longer than 10 h (10.70 and 13.72 h, respectively).
Interestingly, in our previous study investigating the pharmacokinetic forgiveness of atazanavir-ritonavir and lopinavir-ritonavir and using the same methodologies, atazanavir half-lives (to 24 and 72 h) were slightly different (9.91 and 8.35 h). However, the number of subjects with concentrations below the MEC at the different time points studied were similar (1).
Darunavir-ritonavir and atazanavir-ritonavir were well tolerated, with adverse events limited to a small number of study subjects who reported headache; as expected, hyperbilirubinemia was commonly observed during the atazanavir-ritonavir study phase.
Poor adherence to medications is common among patients with chronic diseases. Nevertheless, when adherence is reduced, antiretroviral agent concentrations are not sufficient to suppress HIV replication and to control plasma viral load. In addition, poor adherence to antiretrovirals accelerates the development of drug-resistant HIV (12). While it is clear that omitting drug doses leads to these consequences, it is unclear whether delayed dosing can affect drug efficacy long-term. Therefore, identifying antiretroviral agent persistence may be important to increase knowledge and ensure prolonged viral load suppression.
Importantly, antiretrovirals are prescribed in combinations of three to four drugs that may be characterized by different half-lives, and therefore persistence times will vary in plasma following drug intake delay. Newer protease inhibitors in combination with low-dose ritonavir are today administered once daily as they are characterized by longer half-lives; however, when these drugs are combined with other NRTIs or NNRTIs, they may still decline more rapidly.
One of the limitations of this study is the use of cutoff values to define drug therapeutic concentrations. These have been proposed for some antiretroviral agents following a careful review of the literature and by considering studies evaluating pharmacokinetic/pharmacodynamic relationships retrospectively in small numbers of patients (14). These cutoffs are used to interpret therapeutic drug monitoring (TDM) results in clinical practice, where this test is available. The atazanavir MEC, for example, has been proposed to be 150 ng/ml (14). This differs by a factor of 10-fold from the in vitro protein-binding-corrected IC50 of 14 ng/ml for wild-type virus which was calculated during drug development (5).
For darunavir-ritonavir, an in vivo cutoff value of 550 ng/ml is suggested for treatment-experienced patients. This corresponds to the protein-binding-corrected 50% effective concentration (EC50) for protease inhibitor-resistant strains, and it is used as a reference by TDM services. For treatment-naïve patients with wild-type virus, the darunavir target is 55 ng/ml (EC50 for wild-type virus) (4, 19).
A further limitation was that all subjects received darunavir-ritonavir followed by atazanavir-ritonavir, and the impact of the sequencing of the drug regimen cannot be investigated. However, as atazanavir-ritonavir pharmacokinetics was studied for 17 days (7 days of washout and 10 days of atazanavir-ritonavir intake) following darunavir-ritonavir intake cessation, an effect of the latter on the former was not expected.
We measured geometric mean concentrations of 1,088, 693, and 851 ng/ml for darunavir and of 392, 511, and 210 ng/ml for atazanavir at 24, 30, and 36 h postdose, respectively; these values were higher than the cutoff values used in our study.
As our study involved withholding antiretroviral drug intake, it had to be performed in HIV-negative healthy volunteers. This can be a further limitation as differences in boosted protease inhibitor exposure have been seen between HIV-positive and -negative individuals (9), suggesting that patients with HIV infection may have lower plasma concentrations than those measured in our study. However, when our findings are compared to the concentrations of both darunavir and atazanavir measured in previously published studies (historical controls), the concentrations are similar to those observed in HIV-positive subjects (3, 5, 18).
Interestingly, we measured higher ritonavir AUC, Cmax, and C24h during coadministration with atazanavir than with darunavir. Ritonavir is not only an inhibitor but also a substrate of CYP3A4. Therefore, its plasma concentrations are increased in the presence of a moderate CYP3A4 inhibitor such as atazanavir (6). However, the ritonavir terminal half-life and 0- to 24-h half-life were slightly higher (13% and 18%, respectively) during coadministration with darunavir, which in part explains why the concentrations of darunavir are maintained above target for longer than the dosing interval of 24 h. Furthermore, the ritonavir Cmax was approximately 50% higher with atazanavir than with darunavir, suggesting an effect of the former on ritonavir absorption.
The clinical significance of the slow decay in darunavir and atazanavir concentrations is not fully clear as data on how many antiretroviral doses can be delayed in order to prevent virological failure and development of resistance are lacking. Moreover, the wide variability in maintaining therapeutic concentrations over time following missed doses further complicates the translation of our data into clinical practice.
Nonetheless, in the context of life-long treatments, it is reassuring to have data describing maintenance of therapeutic concentrations of protease inhibitors following delayed doses.
In conclusion, our study has explored the pharmacokinetic forgiveness of two boosted protease inhibitors and showed a relatively constant decline of both darunavir and atazanavir over 72 h following drug intake cessation, resulting in a delayed onset of subtherapeutic concentrations.
ACKNOWLEDGMENTS
We thank Bristol-Myers Squibb UK and the St. Stephen's AIDS Trust for funding the study.
M.B., A.J., D.B., S.K., B.G., and G.M. have received travel and research grants from and have been advisers for Tibotec, Roche, Pfizer, GlaxoSmithKline, Viiv, Bristol-Myers Squibb, Merck, Abbott, and Boehringer-Ingelheim.
Footnotes
Published ahead of print on 27 June 2011.
REFERENCES
- 1. Boffito M., et al. 2008. Pharmacokinetics of atazanavir/ritonavir once daily and lopinavir/ritonavir twice and once daily over 72 h following drug cessation. Antivir. Ther. 13:901–907 [PubMed] [Google Scholar]
- 2. Boffito M., Hill A. 2007. Effect of protease inhibitors on ritonavir pharmacokinetics, abstr. 50. 8th Int. Workshop Clin. Pharmacol. HIV Ther., Budapest, Hungary, 16 to 18 April [Google Scholar]
- 3. Boffito M., et al. 2004. Atazanavir enhances saquinavir hard-gel concentrations in a ritonavir-boosted once-daily regimen. AIDS 18:1291–1297 [DOI] [PubMed] [Google Scholar]
- 4. Boffito M., Miralles D., Hill A. 2008. Pharmacokinetics, efficacy, and safety of darunavir/ritonavir 800/100 mg once-daily in treatment-naive and -experienced patients. HIV Clin. Trials 9:418–427 [DOI] [PubMed] [Google Scholar]
- 5. Bristol-Myers Squibb 2007. Reyataz (R) (atazanavir). Summary of product characteristics. Bristol-Myers Squibb, Uxbridge, United Kingdom [Google Scholar]
- 6. Busti A. J., Hall R. G., Margolis D. M. 2004. Atazanavir for the treatment of human immunodeficiency virus infection. Pharmacotherapy 24:1732–1747 [DOI] [PubMed] [Google Scholar]
- 7. Cohen C., et al. 2004. The FOTO study: a pilot study of short-cycle treatment interruption, taking antiretroviral medications for five days on, two days off (FOTO) for those with viral load suppression, abstr. TUPEB4575. XV Int. AIDS Conf., Bangkok, Thailand, 11 to 16 July 2004 [Google Scholar]
- 8. Cohen C. J., Colson A. E., Sheble-Hall A. G., McLaughlin K. A., Morse G. D. 2007. Pilot study of a novel short-cycle antiretroviral treatment interruption strategy: 48-week results of the five-days-on, two-days-off (FOTO) study. HIV Clin. Trials 8:19–23 [DOI] [PubMed] [Google Scholar]
- 9. Dickinson L., Khoo S., Back D. 2008. Differences in the pharmacokinetics of protease inhibitors between healthy volunteers and HIV-infected persons. Curr. Opin. HIV AIDS 3:296–305 [DOI] [PubMed] [Google Scholar]
- 10. Else L., et al. 2010. Validation of a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J. Chromatogr B Analyt. Technol. Biomed. Life Sci. 878:1455–1465 [DOI] [PubMed] [Google Scholar]
- 11. Gazzard B., et al. 2006. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy (2006). HIV Med. 7:487–503 [DOI] [PubMed] [Google Scholar]
- 12. Glass T. R., et al. 2010. Longitudinal analysis of patterns and predictors of changes in self-reported adherence to antiretroviral therapy: Swiss HIV Cohort Study. J. Acquir. Immune Defic. Syndr. 54:197–203 [DOI] [PubMed] [Google Scholar]
- 13. Hill A., van der Lugt J., Sawyer W., Boffito M. 2009. How much ritonavir is needed to boost protease inhibitors? Systematic review of 17 dose-ranging pharmacokinetic trials. AIDS 23:2237–2245 [DOI] [PubMed] [Google Scholar]
- 14. la Porte C., et al. 2006. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev. Antiviral Ther. 3:4–14 [Google Scholar]
- 15. McKeage K., Perry C. M., Keam S. J. 2009. Darunavir: a review of its use in the management of HIV infection in adults. Drugs 69:477–503 [DOI] [PubMed] [Google Scholar]
- 16. Moyle G. J., Back D. 2001. Principles and practice of HIV-protease inhibitor pharmacoenhancement. HIV Med. 2:105–113 [DOI] [PubMed] [Google Scholar]
- 17. Raboud J., et al. 28 September 2010. Once Daily Dosing Improves Adherence to Antiretroviral Therapy. AIDS Behav. doi: 10.1007/s10461-010-9818-5 [DOI] [PubMed] [Google Scholar]
- 18. Sekar V., et al. 2008. Pharmacokinetic-pharmacodynamic analyses of once-daily darunavir in the ARTEMIS study, abstr. L-103. Abstr. 15th Conf. Retrovir. Oppor. Infect., Boston, MA, 3 to 6 February 2008 [Google Scholar]
- 19. Sekar V., et al. 2006. Absence of TMC114 exposure-efficacy and exposure-safety relationships in POWER 3, abstr. TUPE0078. XVI Int. AIDS Conf., Toronto, Canada, 13 to 18 August 2006 [Google Scholar]