Abstract
Staphylococcus aureus and other Gram-positive organisms, including methicillin-resistant S. aureus, continue to be the predominant pathogens associated with diabetic foot infections. Consequently, linezolid is often used to treat these infections. The purpose of the current study was to describe the pharmacokinetic profile and determine the level of penetration of linezolid into healthy thigh tissue and infected wound tissue of the same extremity in 9 diabetic patients with chronic lower limb infections by use of in vivo microdialysis. Hourly plasma and dialysate samples were obtained over a 12-h dosing interval following 3 to 4 doses of linezolid (600 mg intravenously every 12 h). Plasma protein binding was also assessed at 1, 6, and 12 h postdose. The means ± standard deviations (SD) for the maximum concentration in serum (Cmax), the volume of distribution at terminal phase (Vz), and the half-life (t1/2) for linezolid in plasma were 11.99 ± 3.67 μg/ml, 0.71 ± 0.25 liters/kg of body weight, and 4.71 ± 1.23 h, respectively. Mean protein binding was 14.78% (range, 3.85 to 32.03%). The mean areas under the concentration-time curves from 0 to 12 h for the free, unbound fraction of linezolid (fAUC0–12 values) ± SD for plasma, wound tissue, and thigh tissue were 51.24 ± 12.72, 82.76 ± 59.01, and 92.52 ± 60.44 μg · h/ml, respectively. Tissue penetration ratios (tissue fAUC to plasma fAUC) were similar for thigh (1.42; range, 1.08 to 2.23) and wound (1.27; range, 0.86 to 2.26) tissues (P = 0.648). With the currently approved dosing regimen, linezolid penetrated well into both healthy thigh tissue and infected wound tissue in these diabetic patients.
INTRODUCTION
Diabetic wound infections continue to be the most common cause of nontraumatic lower-extremity amputation both in the United States and abroad (21, 22, 29), with over 85% of these amputation cases starting with a foot ulcer that became severely infected (29). Approximately 15% of diabetic patients will develop a foot ulcer at some point in their lives, with an annual total cost of direct care in the United States of around $9 billion (29). It is estimated that the direct cost of medical care for a leg amputation in the United States is around $50,000, which adds up to a total cost of approximately $1.6 billion annually in the United States (29).
Gram-positive organisms, including both methicillin-susceptible and methicillin-resistant Staphylococcus aureus (MRSA), are the most common pathogens isolated from diabetic foot infections (12, 14). Linezolid is an oxazolidinone antibiotic that is FDA approved for the treatment of complicated skin and skin structure infections (cSSSI), including diabetic foot infections (23). Linezolid is a reasonable treatment option for diabetic foot infections, as it has activity against many of the causative Gram-positive organisms and is available in both intravenous and oral formulations, making it convenient for use in both inpatient and outpatient settings. Additionally, linezolid was at least as effective as vancomycin for treating cSSSI and was superior to vancomycin for treating MRSA in one randomized controlled trial (30). A pharmacoeconomic study also demonstrated lower treatment costs for patients receiving linezolid over those receiving vancomycin, likely due to shorter treatment duration with intravenous linezolid than with intravenous vancomycin, as well as a shorter average length of stay (16).
The pharmacokinetic profile of linezolid in tissue has been described for several different patient populations. One study conducted with 6 patients undergoing lower limb amputation found a mean linezolid concentration in infected soft tissue samples collected at the time of amputation that was 51% of the corresponding concentration in serum (27). A second study, conducted with 15 patients with diabetic wound infections, found a mean linezolid concentration in infected wound tissue samples that was 102% of that in plasma at 3 h (15). Both of these studies utilized tissue homogenate, which can lead to inaccuracies in tissue concentration as a result of vascular and intracellular contamination, among other factors (19, 25). Additionally, the variability in these data is possibly due to the fact that a comparison between blood and tissue concentrations was made at only a single point in time for each patient, whereas the degree of tissue penetration should vary over the course of the concentration-time profile. For instance, penetration just after the dose is administered may appear low as a result of high blood concentrations, whereas penetration around the trough may appear higher, as concentrations in blood decline more rapidly than those in the tissue. As a result, tissue penetration should be evaluated over the entire exposure period by comparing the areas under the concentration-time curves of the free, unbound fraction of linezolid (fAUCs) in tissue and in blood. Two studies sought to do this, using in vivo microdialysis in both healthy and critically ill subjects receiving linezolid, which resulted in approximately 90% penetration into tissue (3, 6). However, neither of these populations were diabetic; therefore, the tissue penetration of linezolid into the interstitial fluid of diabetic wounds is still not fully understood. Since these patients also often suffer from diabetic neuropathy and peripheral vascular disease, which can further impair penetration into the target tissue site, it is also important to determine if there is a difference in penetration between infected peri-ulcer tissue and healthy tissue within the same patient. The objective of the current study was to describe the pharmacokinetic profile of linezolid within the interstitial fluids of infected and uninfected soft tissues of diabetic patients with in vivo microdialysis (18, 19, 20, 25).
MATERIALS AND METHODS
Study protocol.
This was an open-label pharmacokinetic study of 9 diabetic patients admitted to Hartford Hospital in Hartford, CT, with lower-extremity wound infections. The study protocol was reviewed and approved by the Institutional Review Board at Hartford Hospital. Written informed consent was obtained from all patients prior to participation in the study.
Patients.
Patients had to have a documented history of type 1 or type 2 diabetes and be actively receiving pharmacologic therapy (insulin and/or an oral antihyperglycemic agent). Lower-extremity wound infections were limited to chronic complicated skin and skin structure infections (cSSSI) requiring surgical debridement and defined as mild or moderate by the Infectious Diseases Society of America (12) or as grade 2 or 3 by the International Consensus on the Diabetic Foot (13).
Patients were excluded prior to enrollment if they were less than 18 years of age, had a hypersensitivity to the study medication (linezolid) or anesthetics (lidocaine or lidocaine derivatives), were pregnant or breastfeeding, were receiving monoamine oxidase inhibitors (MAOIs) or selective serotonin reuptake inhibitors (SSRIs), had no palpable pedal pulses, were likely to require multiple procedures during the study, or had participated in another study of an investigational drug or device within the preceding 30 days. Patients were also excluded if their creatinine clearance was less than 30 ml/min when calculated by the Cockcroft-Gault equation (5); their platelets, hematocrit, or white blood cell count was less than 75% of the lower limit of the normal range; or their aspartate aminotransferase, alanine aminotransferase, or alkaline phosphate was greater than twice the upper limit of the normal range.
Study medication.
Linezolid for intravenous (i.v.) injection (lot 09H20Z35; expiration date, 1 August 2012) was provided by Pfizer Inc. (New York, NY). Patients received 600 mg of linezolid in addition to other antimicrobial therapies prescribed for the treatment of their infection. Linezolid was administered intravenously through either a peripheral catheter placed in the antecubital vein or a peripherally inserted central catheter (PICC) over the course of 1 h every 12 h for 3 to 4 doses to achieve steady state.
Microdialysis procedure.
The microdialysis procedure was performed as previously described (4, 8). Briefly, two microdialysis probes (CMA 60 microdialysis catheters; CMA Microdialysis AB, Solna, Sweden) with membrane lengths of 30 mm and a molecular mass cutoff of 20 kDa were inserted under sterile conditions into the subcutaneous tissue after patients underwent surgical debridement of their lower-extremity wound. Following the local injection of a 0.5% lidocaine solution, one catheter was placed within 10 cm of the wound margin, while the second was placed in the uninfected thigh tissue of the same extremity. After the catheters were inserted, a microinfusion pump was connected (CMA 107 microdialysis pump; CMA Microdialysis AB, Solna, Sweden) and the catheters were flushed at a rate of 15 μl/min and then continuously perfused with Lactated Ringer's solution at a rate of 2 μl/min.
Sample collection.
Venous blood was collected from either a peripheral intravenous catheter or a PICC at the following time points after the 3rd or 4th dose of linezolid: 0 (the start of the infusion), 1 (the end of the infusion), 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 h. Blood samples were collected using a 10-ml BD Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ) containing sodium heparin. Blood samples were centrifuged (2,000 × g for 10 min), and the separated plasma was immediately frozen in amber polypropylene tubes and stored at −80°C until analysis.
Dialysate samples of approximately 120 μl were obtained simultaneously from both microdialysis catheters at each of the corresponding blood sample time points. Dialysate samples were collected in 200-μl microvials (CMA Microdialysis AB, Solna, Sweden), which were then stored in amber polypropylene tubes and immediately frozen at −80°C until analysis.
Microdialysis probe recovery: in vivo retrodialysis.
Thigh and wound tissue catheters were calibrated for each patient using the in vivo retrodialysis technique after all samples were obtained. A solution of linezolid for calibration was freshly prepared by diluting linezolid with Lactated Ringer's solution to a concentration of 200 μg/ml (Cperfusate) and was then perfused through the tissue at 2 μl/min for 1 h, and the disappearance of the drug through the membrane was determined, representing the amount of recovery. The in vivo recovery was calculated using the following equation: percent recovery in vivo = 100 − (100 × Cdialysate/Cperfusate), where Cdialysate is defined as the concentration obtained in the dialysate collected during the 1-h calibration.
Protein binding studies.
Protein binding studies were conducted in triplicate for each patient at 1, 6, and 12 h after the administration of the last dose of linezolid. A blood sample was collected into a 10-ml BD Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ) containing sodium heparin at each of these three time points and centrifuged (2,000 × g for 10 min) to obtain separated plasma. Exactly 0.9 ml of plasma was transferred into each regenerated-cellulose, 30-kDa molecular-mass-cutoff ultrafiltration device (Centrifree centrifugal filters; Millipore Corporation, Billerica, MA) and centrifuged at 2,000 × g using a fixed-angle rotor for 30 min at 10°C to obtain ultrafiltrate (Cultrafiltrate). An aliquot of plasma was also retained at each corresponding time point for determination of total drug concentration in the plasma (Cplasma). The amount of protein binding was calculated using the following equation: percent protein binding = 100 − (100 × Cultrafiltrate/Cplasma).
The adsorption of linezolid to the membrane of the ultrafiltration device was also investigated using an aqueous standard solution of analytical-grade linezolid (Pfizer Inc., New York, NY) prepared to a concentration of 6 μg/ml in Lactated Ringer's solution. Three independent test samples were ultrafiltered and analyzed using the same methodology as described above for plasma samples. Since adsorption of linezolid to the ultrafiltration membrane was negligible (<1.5%), no further adjustments were made to the protein binding data for nonspecific binding.
Analytical procedures.
Linezolid concentrations in plasma and dialysate were determined using a validated high-performance liquid chromatography (HPLC) assay that was developed using a previously published assay (2) at the Center for Anti-Infective Research and Development in Hartford, CT. The plasma assay was linear over a range of 0.2 to 30 μg/ml (r2 = 0.997). The mean interday coefficients of variation (CV) for high (20 μg/ml) and low (0.5 μg/ml) check samples were 2.5% and 2.4%, respectively. The mean intraday coefficients of variation were 3.2% and 1.3%, respectively. The dialysate assay was linear over a range of 0.1 to 20 μg/ml (r2 = 0.997). The mean interday coefficients of variation for high (15 μg/ml) and low (0.2 μg/ml) check samples were 3.9% and 4.4%, respectively. The mean intraday coefficients of variation were 1.4% and 1.9%, respectively.
Pharmacokinetic analysis. (i) Plasma.
Total linezolid concentrations in plasma were analyzed for each subject by noncompartmental analysis using WinNonlin software (version 5.2.1; Pharsight Corporation, Mountain View, CA). The maximum concentration of linezolid (Cmax) and time to maximum concentration (Tmax) were determined by visual inspection of the concentration-time profile. The area under the concentration-time profile from 0 to 12 h (AUC0–12) was calculated using the linear/log trapezoidal method. The half-life (t1/2) was as calculated as ln(2)/λz, where λz is the terminal elimination rate constant. The terminal elimination rate constant was estimated by linear-regression analysis of the terminal portion of the concentration-time profile. Clearance was calculated as dose/AUC0–12. The volume of distribution was calculated as dose/(λz × AUC0–12). The mean of each individual's protein-binding percentages was applied to each subject's corresponding AUC to determine that person's free-drug AUC.
(ii) Tissue.
Concentrations obtained from wound and thigh dialysate samples were corrected using the in vivo recovery calculated for each catheter prior to the performance of pharmacokinetic analyses with the following equation: Ctissue = 100 × (Cdialysate/percent recovery in vivo), where Ctissue is the drug concentration in the interstitium. Linezolid concentrations in wound and thigh tissue were also analyzed for each subject by noncompartmental analysis using WinNonlin. The Cmax and Tmax in the tissues were determined by visual inspection of the concentration-time profile. The AUC0–12 was calculated using the linear/log trapezoidal rule. The t1/2 was calculated as ln(2)/λz. Tissue penetration ratios were calculated using the AUC0–12 for wound or thigh tissues (AUCwound or AUCthigh) and the fAUC0–12 in plasma (fAUCplasma) as follows: the wound penetration ratio is equal to AUCwound/fAUCplasma, and the thigh penetration ratio is equal to AUCthigh/fAUCplasma.
Statistical analysis.
Pharmacokinetic parameters and penetration ratios were compared for wound and thigh tissue using a paired t test or Wilcoxon signed-rank test for nonnormally distributed data using SigmaStat (version 2.03; SPSS Inc., Chicago, IL). Plasma concentrations at 0 h and 12 h were also compared using a paired t test to confirm that steady state was achieved. A P value of <0.05 was considered statistically significant.
RESULTS
Patients.
A total of 9 male patients were enrolled in the study. Baseline characteristics for these patients are listed in Table 1. Seven of the 9 had microdialysis catheters placed in both healthy thigh and infected wound tissues, while the 2 remaining patients had only a wound catheter, due to logistical issues. Subsequently, only the data for the 7 patients who underwent sampling from both thigh and wound catheters were used for comparing pharmacokinetic parameters and levels of penetration for the two tissue sites. One error occurred during the administration of the study medication, with one patient receiving the final dose of linezolid over 2 h, and one error occurred in sampling for another subject, as the peak concentration drawn at 1 h was drawn from the same line in which the drug was infused, resulting in sample contamination. As such, data from these patients were not included in the mean data for Cmax and Tmax.
Table 1.
Characteristics of subjects
| Characteristic | Valuea |
|---|---|
| Total no. of subjects | 9 |
| Age (yr) | 55.1 ± 10.0 |
| % male | 100 |
| % of race: | |
| African American | 22.2 |
| Caucasian | 66.7 |
| Hispanic | 11.1 |
| Ht (in.) | 70.8 ± 3.1 |
| Wt (kg) | 104.7 ± 35.0 |
| Body mass index (kg/m2) | 31.8 ± 8.3 |
| % with diabetes of type: | |
| 1 | 33.3 |
| 2 | 66.7 |
| Glycosylated hemoglobin A1c (%) | 8.7 ± 1.8 |
| % with pedal pulse rate: | |
| +1 | 77.8 |
| +2 | 22.2 |
| % with PEDISb grade 3 | 100 |
Data are reported as means ± standard deviations unless otherwise noted.
The PEDIS (perfusion, extent/size, depth/tissue loss, infection, and sensation) grade was defined by the International Consensus on the Diabetic Foot (2).
Two adverse events occurred during the study. One patient experienced an increase in total bilirubin to 1.6 mg/dl while on therapy, and another experienced an increase in lactate dehydrogenase (LDH) to 321 units/liter while on therapy. Both levels returned to baseline the following day. All other laboratory values were either within normal limits or clinically insignificant over the course of the study.
Pharmacokinetic analysis. (i) Plasma.
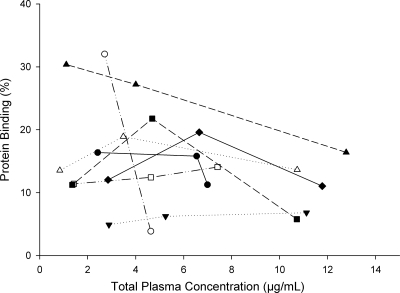
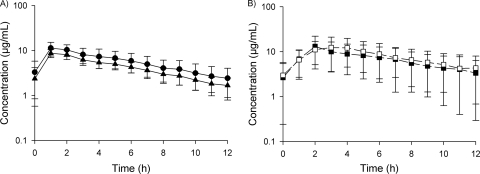
Protein binding was not evaluable at any of the three time points for one patient and at one of the three time points for a second patient. The mean protein binding for 8 patients was 15% (range, 4 to 32%) and did not appear to be concentration dependent, as depicted in Fig. 1. Both the total- and free-drug concentration-time profiles are depicted in Fig. 2A. The total drug pharmacokinetic parameters in plasma at steady state are described in Table 2. Plasma concentrations were not different between 0 h and 12 h for the dosing interval where sampling was conducted (P = 0.413), indicating that steady state had been achieved.
Fig. 1.
Protein binding of linezolid over the concentration range in 8 diabetic patients. Each line represents the protein binding profile over a range of total drug concentrations for an individual subject (n = 8).
Fig. 2.
Linezolid concentration-time profiles. (A) Plasma concentration-time profile of linezolid. Circles, total drug concentration; triangles, corrected free-drug concentration. (B) Free-drug tissue concentration-time profile of linezolid. Closed squares, wound tissue; open squares, healthy thigh tissue.
Table 2.
Steady-state pharmacokinetic parameters for linezolid concentrations in plasma and tissue samplesa
| Parameterb | Result for indicated sample typec |
P valued (n = 7) | ||
|---|---|---|---|---|
| Plasma (n = 9) | Wound tissue (n = 9) | Thigh tissue (n = 7) | ||
| Cmax (μg/ml) | 11.99 ± 3.67e | 13.45 ± 8.61 | 14.4 ± 7.49 | 0.714 |
| Tmax (h) | 1e | 2.11 ± 0.33 | 2.57 ± 0.79 | 0.289 |
| AUC0–12 (μg · h/ml) | 68.76 ± 29.31 | ND | ND | |
| ƒAUC0–12 (μg · h/ml) | 51.24 ± 12.72f | 82.76 ± 59.01 | 92.52 ± 60.44 | 0.942 |
| t1/2 (h) | 4.71 ± 1.23 | 5.14 ± 1.48 | 4.69 ± 1.78 | 0.275 |
| CL (liter/h/kg) | 0.11 ± 0.04 | ND | ND | |
| Vz (liter/kg) | 0.71 ± 0.25 | ND | ND | |
Steady state was achieved following 3 to 4 600-mg doses of linezolid administered as intravenous infusions for 1 h every 12 h.
Cmax, maximum concentration of linezolid; CL, clearance.
Values are reported as means ± standard deviations. ND, not determined.
The P value was obtained by comparing results for wound and thigh tissues.
Two subjects were excluded from this analysis (n = 7), as one subject received a 2-h infusion and another subject's concentration data at 1 h was not evaluable due to sample contamination.
Protein binding data were not available for one subject (n = 8).
(ii) Tissue.
The mean recovery rates ± the standard deviations (SD) for thigh and wound catheters were 39.3% ± 14.6% and 46.4% ± 17.3%, respectively. The steady-state concentration-time profiles for linezolid in wound and thigh interstitial fluid are displayed in Fig. 2B. Pharmacokinetic parameters were not statistically different between thigh and wound tissues (Table 2). Linezolid concentrations within the interstitia of both thigh and wound tissues were generally greater than 1 μg/ml throughout the dosing interval for all patients. Additionally, linezolid penetrated equally well into both healthy thigh tissue and infected wound tissue, as demonstrated by the tissue penetration ratios (AUCtissue/fAUCplasma) of 1.42 (range, 1.08 to 2.23; n = 8) in thigh tissue and 1.27 (range, 0.86 to 2.26; n = 7) in wound tissue. No significant difference was found between these ratios (P = 0.648) in the six patients who had both thigh and wound concentration data and evaluable free-drug exposures.
DISCUSSION
Wound infections are an ongoing problem among diabetic patients, often resulting in chronic infection, hospitalization, and amputation. Further complicating the treatment of these infections, diabetic patients often have reduced blood flow to their extremities as a result of peripheral vascular disease and diabetic neuropathy. Therefore, it is essential to determine the tissue penetration of linezolid, a commonly used antibiotic for these types of infections, into the interstitial fluid of both healthy and infected tissues of diabetic patients as described herein.
The pharmacokinetics of linezolid in plasma have been described previously for 6 hospitalized diabetic patients receiving 600 mg of linezolid i.v. infused over 1 h in a study by Stein et al. (27). The mean value for Cmax in the current study (11.99 ± 3.67 μg/ml) was comparable to that of the previous study (14.7 ± 6.1 μg/ml). The mean value for t1/2 (4.71 ± 1.23 h) was also similar to that of the previous study (5.5 ± 2.8 h). However, the mean AUC0–12 was 68.76 ± 29.31 μg · h/ml and the volume of distribution at steady state (Vss) was 0.73 ± 0.28 liters/kg of body weight for the current study, which is different from the previously reported values (AUC0–12, 114 ± 50 μg · h/ml, and Vss, 0.44 ± 0.11 liters/kg). This is potentially explained by the slightly larger mean total body weight observed in the current study (104 kg) than in the previous study (98 kg). Disparities in severity may also account for the observed differences in pharmacokinetics between these two studies; however, the severity of illness, the PEDIS (perfusion, extent/size, depth/tissue loss, infection, and sensation) score, and the level of peripheral vascular disease are not well characterized within the study population described by Stein et al. Despite achieving a slightly lower AUC0–12 with the same dosing regimen of linezolid, the coefficients of variation around these values in both of the studies were similar (∼43%). Notably, our values were similar to those observed in a critically ill population, where a mean AUC0–12 of 65.3 μg · h/ml (%CV, 57.9) and a Vss of 62.9 liters (%CV, 19.2) were reported for 12 patients with a median weight of 81 kg (3). Additionally, the mean protein binding observed in the current study was 15% (range, 4 to 32%), was not concentration dependent, and was quite variable between and within patients. Both of these observations are consistent with what has been reported previously (26, 28).
Linezolid penetrated well from plasma into both healthy and infected subcutaneous tissue, as demonstrated by mean penetration ratios of 1.42 (range, 1.08 to 2.23) and 1.27 (range, 0.86 to 2.26), respectively. Additionally, while between-patient variability for tissue penetration was observed, the level of penetration in healthy tissue was consistent with the level of penetration in infected tissue for each individual patient. While previous studies have also reported good tissue penetration for linezolid, the level of penetration observed in the current study is greater than those reported by other investigators for diabetic patients (15, 27). However, it may not be reasonable to compare levels of penetration from this study with levels in those two diabetic studies due to differences in methodologies. Levels of penetration reported herein are more reliable, as we used plasma and tissue ƒAUCs over an entire dosing interval at steady state to determine tissue penetration. This is especially important, as tissue penetration may actually change over the course of the dosing interval.
Linezolid tissue penetration into subcutaneous tissue has been investigated using microdialysis techniques in both healthy volunteers and critically ill patients but not in diabetic patients, where blood flow to the site of infection could potentially be diminished. In those studies with healthy volunteers and critically ill patients, tissue penetration ratios for linezolid were found to be approximately 90% (SD, 20%) and 89.6% (range, 20.2 to 118%), respectively (3, 6). These data were also lower than what was observed in the current study. This could be a result of differences in penetration among different patient populations, which also demonstrates the importance of determining tissue penetration in a diabetic population. Despite the assumption that blood flow to the site of infection was diminished in patients included in the current study due to microvascular and macrovascular complications of diabetes, the level of tissue penetration observed was in fact higher than what was previously described for noninfected, healthy volunteers and critically ill patients with similar plasma pharmacokinetics (3, 6). One potential explanation for increased drug penetration is that decreased blood flow might lead to the breakdown of cellular barriers and to enhanced penetration into the interstitial fluid. An alternative, yet undescribed, theory is that the an upregulation of pump- or carrier-mediated transportation across cellular barriers occurs in response to peripheral vascular disease in diabetic patients. The high level of linezolid penetration is likely not macrophage or leukocyte mediated, as penetration ratios were similar between healthy and infected tissues for all patients and linezolid does not exhibit a high level of intracellular accumulation within these cells (10, 11).
Two small case series reports in which linezolid penetration was studied in diabetic patients with microdialysis have recently been published. The first study evaluated linezolid tissue penetration in two diabetic patients with lower-extremity ulcers both prior to and after hyperbaric oxygen therapy (HBOT) following single-dose oral linezolid therapy. The linezolid tissue penetration ratios observed in the first and second subjects were 47.4% and 47.9%, respectively, before HBOT, and they increased to 95.0% and 75.7%, respectively, after HBOT (9). Values observed both before and after HBOT for both subjects were also lower than those observed in the current study. However, tissue penetration was measured following single-dose oral therapy, when distribution between plasma and tissue had not yet reached equilibrium. Therefore, it is not surprising that the level of penetration observed in the current study is higher, as it was measured at steady state following the administration of 3 to 4 doses. The second study, as in the current study, sought to determine tissue penetration at steady state in both healthy and infected tissues of diabetic patients; however, it was conducted with only three patients. The penetration ratios into both healthy (132%) and infected (112%) tissue (28) were similar to the levels of tissue penetration that were observed in the 9 patients in the current study.
Previous work with both mice and infected patients has determined AUC/MIC to be the pharmacodynamic parameter that drives efficacy (1, 24). A mean extrapolated total drug plasma AUC0–24 of 137.52 μg · h/ml was observed in this diabetic patient population, reaching the total drug AUC/MIC target of ≥80 for isolates with a MIC of ≤1 μg/ml. While an AUC/MIC target range of 80 to 120 has been established using plasma data, a pharmacodynamic target for tissue has not yet been determined (1, 24). The high level of linezolid penetration into both healthy and infected tissues of diabetic patients in this study resulted in extrapolated fAUC0–24 values of 185.0 μg · h/ml and 165.5 μg · h/ml for healthy thigh and infected wound tissues, respectively. Using a MIC90 of 2 μg/ml for both MRSA and methicillin-susceptible S. aureus isolates would yield free-drug AUC/MIC values of 92.5 and 82.8 μg · h/ml in healthy and infected tissues, respectively (7). If the pharmacodynamic target values are, in fact, similar for plasma and tissue, the target would be achieved against isolates with a MIC of ≤2 μg/ml. Furthermore, these values were calculated using free-drug exposures in tissue, whereas the target is based on total drug exposures in plasma; therefore, efficacy could potentially be achieved against isolates with MICs of >2 μg/ml.
In summary, 600 mg of linezolid every 12 h penetrated well into both healthy and infected tissues in diabetic patients but with substantial variability between patients. Based on current pharmacodynamic targets, exposures obtained at the site of infection should be sufficient to effectively treat a variety of susceptible and resistant Gram-positive pathogens often implicated in diabetic foot infections.
ACKNOWLEDGMENTS
We thank Christina Sutherland for conducting all HPLC analyses. We are grateful for the assistance with the sample collection provided by Rebecca Keel, Seth Housman, Catharine Bulik, and Lee Steere.
This study was supported by an investigator-initiated research grant from Pfizer Inc. (New York, NY).
D.P.N. has received research grants from and is a member of the advisory board and speakers' bureau for Pfizer Inc. J.L.K. has received research grants from Pfizer Inc.
Footnotes
Published ahead of print on 27 June 2011.
REFERENCES
- 1. Andes D., van Ogtrop M. L., Peng J., Craig W. A. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buerger C., Joukhadar C., Muller M., Kloft C. 2003. Development of a liquid chromatography method for the determination of linezolid and its application to in vitro and human microdialysis samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 796:155–164 [DOI] [PubMed] [Google Scholar]
- 3. Buerger C., Plock N., Dehghanyar P., Joukhadar C., Kloft C. 2006. Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis. Antimicrob. Agents Chemother. 50:2455–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bulik C. C., et al. 2010. Tissue penetration and pharmacokinetics of tigecycline in diabetic patients with chronic wound infections described by using in vivo microdialysis. Antimicrob. Agents Chemother. 54:5209–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cockcroft D. W., Gault M. H. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41 [DOI] [PubMed] [Google Scholar]
- 6. Dehghanyar P., et al. 2005. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob. Agents Chemother. 49:2367–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farrell D. J., Mendes R. E., Ross J. E., Jones R. N. 2009. Linezolid surveillance program results for 2008 (LEADER Program for 2008). Diagn. Microbiol. Infect. Dis. 65:392–403 [DOI] [PubMed] [Google Scholar]
- 8. Kim A., et al. 2008. In vivo microdialysis study of the penetration of daptomycin into soft tissues in diabetic versus healthy volunteers. Antimicrob. Agents Chemother. 52:3941–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koomanachai P., et al. 2011. Linezolid penetration into wound tissue of two diabetic patients before and after hyperbaric oxygen therapy. Undersea Hyperb. Med. 38:11–16 [PubMed] [Google Scholar]
- 10. Lemaire S., Tulkens P. M., Van Bambeke F. 2010. Cellular pharmacokinetics of the novel biaryloxazolidinone radezolid in phagocytic cells: studies with macrophages and polymorphonuclear neutrophils. Antimicrob. Agents Chemother. 54:2540–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lemaire S., Van Bambeke F., Appelbaum P. C., Tulkens P. M. 2009. Cellular pharmacokinetics and intracellular activity of torexolid (TR-700): studies with human macrophage (THP-1) and endothelial (HUVEC) cell lines. J. Antimicrob. Chemother. 64:1035–1043 [DOI] [PubMed] [Google Scholar]
- 12. Lipsky B. A., et al. 2004. Diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 39:885–910 [DOI] [PubMed] [Google Scholar]
- 13. Lipsky B. A., Berendt A. R., Embil J. M., De Lalla D. 2004. Diagnosing and treating diabetic foot infections. Diabetes Metab. Res. Rev. 20:S56–S64 [DOI] [PubMed] [Google Scholar]
- 14. Lipsky B. A., et al. 2010. Skin and soft tissue infections in hospitalized patients with diabetes: culture isolates and risk factors associated with mortality, length of stay and cost. Diabetologia 53:914–923 [DOI] [PubMed] [Google Scholar]
- 15. Majcher-Peszynska J., et al. 2008. Pharmacokinetics and penetration of linezolid into inflamed soft tissue in diabetic foot infections. Eur. J. Clin. Pharmacol. 64:1093–1100 [DOI] [PubMed] [Google Scholar]
- 16. McKinnon P. S., Sorensen S. V., Liu L. Z., Itani K. M. F. 2006. Impact of linezolid on economic outcomes and determinants of cost in a clinical trial evaluating patients with MRSA complicated skin and soft-tissue infections. Ann. Pharmacother. 40:1017–1023 [DOI] [PubMed] [Google Scholar]
- 17. Reference deleted.
- 18. Müller M. 2002. Science, medicine, and the future: microdialysis. BMJ 324:588–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Müller M., dela Pena A., Derendorf H. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob. Agents Chemother. 48:1441–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Müller M., et al. 1996. Characterizations of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob. Agents Chemother. 40:2703–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicolau D. P., Stein G. E. 2010. Therapeutic options for diabetic foot infections. J. Am. Pod. Med. Assoc. 100:52–63 [DOI] [PubMed] [Google Scholar]
- 22. Pecoraro R. E., Reiber G. E., Burgess E. M. 1990. Pathways to diabetic limb amputation. Diabetes Care 13:513–521 [DOI] [PubMed] [Google Scholar]
- 23. Pfizer Inc 2007. Zyvox® (linezolid for injection, tablets, oral suspension). Prescribing information. Pfizer Inc., New York, NY [Google Scholar]
- 24. Rayner C. R., Forrest A., Meagher A. K., Birmingham M. C., Schentag J. J. 2003. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin. Pharmacokinet. 15:1411–1423 [DOI] [PubMed] [Google Scholar]
- 25. Schmidt S., Banks R., Kumar V., Rand K. H., Derendorf H. 2008. Clinical microdialysis in skin and soft tissues: an update. J. Clin. Pharmacol. 48:351–364 [DOI] [PubMed] [Google Scholar]
- 26. Slatter J. G., et al. 2001. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [14C]linezolid to healthy human subjects. Drug Metab. Dispos. 29:1136–1145 [PubMed] [Google Scholar]
- 27. Stein G. E., Schooley S., Peloquin C. A., Missavage A., Havlichek D. H. 2007. Linezolid tissue penetration and serum activity against strains of methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility in diabetic patients with foot infections. J. Antimicrob. Chemother. 60:819–823 [DOI] [PubMed] [Google Scholar]
- 28. Traunmuller F., et al. 2010. Linezolid concentrations in infected soft tissue and bone following repetitive doses in diabetic patients with bacterial foot infections. Int. J. Antimicrob. Agents 36:84–86 [DOI] [PubMed] [Google Scholar]
- 29. Ulbrecht J. S., Cavanagh P. R., Caputo G. M. 2004. Foot problems in diabetes: an overview. Clin. Infect. Dis. 39:S73–S82 [DOI] [PubMed] [Google Scholar]
- 30. Weigelt J., et al. 2005. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob. Agents Chemother. 49:2260–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]