Abstract
The 8-aminoquinoline analogue sitamaquine (SQ) is an oral antileishmanial drug currently undergoing phase 2b clinical trials for the treatment of visceral leishmaniasis. In the present study, we investigated the mechanism of action of this drug in Leishmania donovani promastigotes. SQ causes a dose-dependent inhibition of complex II (succinate dehydrogenase) of the respiratory chain in digitonin-permeabilized promastigotes, together with a drop in intracellular ATP levels and a decrease of the mitochondrial electrochemical potential. This is associated with increases of reactive oxygen species and intracellular Ca2+ levels, a higher percentage of the population with sub-G1 DNA content, and exposure of phosphatidylserine. Taken together, these results support a lethal mechanism for SQ that involves inhibition of the respiratory chain complex II, which in turn triggers oxidative stress and finally leads to an apoptosis-like death of Leishmania parasites.
INTRODUCTION
Leishmaniasis, a protozoal infectious disease caused by a set of 17 species of the genus Leishmania, shows a wide spectrum of clinical manifestations, including, in order of increasing severity, cutaneous (CL), mucocutaneous (MCL), and visceral (VL) leishmaniasis (3). Although chemotherapy is the only current treatment option for leishmaniasis, its efficacy is increasingly limited by growing resistance to first-line drugs, especially antimonials, by the frequent side effects associated with their use, and by the high cost of treatment (30). The paucity of new drugs in the pipeline, together with the poor definition of Leishmania targets for the drugs in current clinical use, represents an additional concern for current chemotherapy. Solutions to curb this pessimistic scenario rely on combination therapy (40) and the rescue of old drugs, such as paromomycin (17, 36) and sitamaquine (SQ) (39), that were previously discarded.
8-Aminoquinolines are an important class of antiparasitic agents (37) with broad application and excellent efficacy but with limitations due to their hematological toxicity (primarily metahemoglobinemia and hemolysis). SQ, formerly known as WR6026, is an 8-aminoquinoline that was initially developed by the Walter Reed Army Institute (46). The results of phase 2b clinical trials of this drug against VL in India (16) and Kenya (45) by GlaxoSmithKline were encouraging. These results, together with its oral administration, represent a substantial advantage in terms of its future widespread implementation.
The targets for SQ remain elusive. Entry of SQ into the parasite starts with an electrostatic interaction with anionic phospholipids of the plasma membrane (11). In a seminal work, Vercesi and Docampo (43) observed a loss of mitochondrial electrochemical potential in digitonin-permeabilized parasites after SQ addition, together with alkalinization of acidocalcisomes (44), which also underwent a privileged SQ accumulation, although no correlation was found with its toxicity (18).
Herein we provide further insight into the leishmanicidal mechanism of SQ, which induces an apoptosis-like death of the parasite, as confirmed by phosphatidylserine (PS) externalization and chromatin fragmentation (sub-G1 population) in association with enhanced reactive oxygen species (ROS) production, elevation of intracellular Ca2+ levels, and depolarization of the mitochondrial membrane potential. The site of action was mapped to complex II (succinate dehydrogenase [SDH]) by systematic analysis of the different complexes in the respiratory chain, with SQ inhibiting its activity in a dose-dependent manner.
MATERIALS AND METHODS
Chemical compounds.
SQ (N,N-diethyl-N′-[6-methoxy-4-methylquinolin-8-yl]hexane-1,6-diamine) dihydrochloride was kindly provided by GlaxoSmithKline (Greenford, United Kingdom). A 40 mM SQ stock solution was prepared in dimethyl sulfoxide (DMSO). DMNPE-luciferin {D-luciferin-1[-(4,5-dimethoxy-2-nitrophenyl) ethyl ester]}, Fluo4-AM, H2DCF-DA (2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester), pluronic F127, rhodamine 123 (Rh123), Sytox green, DiBAC4(3) [bis-(1,3-dibutylbarbituric acid)trimethine oxonol], and Alexa Fluor 488-conjugated annexin V were purchased from Invitrogen (Carlsbad, CA). Fatty acid-free bovine serum albumin (BSA), digitonin, ADP, carbonyl cyanide 4-trifluoromethoxyphenylhydrazone (FCCP), carbonyl cyanide m-chlorophenylhydrazone (CCCP), KCN, tetramethyl-p-phenylenediamine (TMPD), α-glycerophosphate, malonate, oligomycin, succinate, propidium iodide (PI), and Triton X-100 were purchased from Sigma-Aldrich (Madrid, Spain). All other chemicals were of the highest quality available.
Parasites.
Leishmania donovani promastigotes (MHOM/ET/67/L82) and promastigotes of the derived line L. donovani 3-Luc (22), which expresses cytoplasmic firefly luciferase mutated at its C-terminal tripeptide, were grown at 28°C in RPMI 1640 modified medium (Invitrogen) supplemented with 20% heat-inactivated fetal bovine serum (Invitrogen).
Bioluminescence assays.
The in vivo variation in intracellular ATP levels was monitored in promastigotes expressing a cytoplasmic form of firefly luciferase, as described previously (19). Briefly, parasites from the L. donovani 3-Luc strain (2 × 107 promastigotes/ml) were resuspended in HEPES-buffered saline (HBS; 21 mM HEPES, 0.7 mM Na2HPO4, 137 mM NaCl, 5 mM KCl, and 6 mM d-glucose, pH 7.1), and DMNPE-luciferin was added to a final concentration of 25 μM. Aliquots of this suspension (100 μl/well) were immediately added to a 96-well black polystyrene microplate, and different SQ concentrations were added once the luminescence had reached a plateau. Changes in luminescence were recorded with an Infinite F200 microplate reader (Tecan Austria GmbH, Austria). Inhibition of recombinant firefly luciferase activity by SQ in vitro was discarded by using an ATP determination kit (Invitrogen) in the presence of saturable ATP concentrations. The release of ATP from L. donovani promastigotes into the external medium was determined using the same kit.
Determination of ΔΨp.
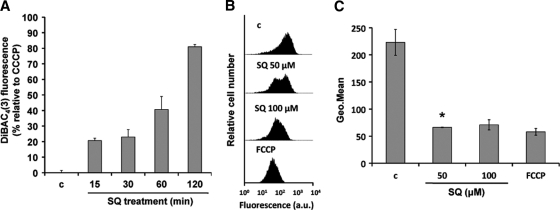
The membrane potential probe DiBAC4(3) was used to measure the plasma membrane potential (ΔΨp). Parasites (107 promastigotes/ml) were incubated with or without 100 μM SQ in HBS for 15, 30, 60, or 120 min at 28°C and then treated with 1 μM DiBAC4(3) for 10 min at 28°C. Parasites treated with a 10 μM concentration of the depolarizing agent CCCP for 15 min were used as a control. DiBAC4(3) fluorescence was analyzed by flow cytometry using a FACScan flow cytometer (Becton Dickinson, San Jose, CA) equipped with an argon laser operating at 488 nm. Fluorescence emission between 515 and 545 nm was quantified using Cell Quest software.
Plasma membrane permeabilization.
Sytox green dye was used to assess plasma membrane integrity as described previously (21), with some modifications. Briefly, parasites (4 × 106 promastigotes/ml) were treated with 100 μM SQ in HBS for 10, 30, and 60 min at 28°C, washed twice with HBS, and then incubated with 2 μM Sytox green (final concentration) for 15 min at 28°C. The parasites were subsequently transferred to a 96-well microplate (100 μl/well), and fluorescence due to binding of the dye to intracellular nucleic acids was recorded using an Infinite F200 microplate reader (Tecan Austria GmbH, Austria) equipped with 485- and 535-nm filters for excitation and emission wavelengths, respectively. The control for maximum fluorescence was obtained by addition of 0.05% Triton X-100.
Analysis of ΔΨm.
The variation of the mitochondrial membrane potential (ΔΨm) in promastigotes was monitored using Rh123 accumulation, as described previously (8). Parasites (107 promastigotes/ml) were incubated with 50 and 100 μM SQ in HBS for 15 min at 28°C, and then 0.8 μM Rh123 was added and incubated for 5 min. Afterwards, the parasites were washed twice, resuspended in phosphate-buffered saline (PBS), and analyzed by flow cytometry in a FACScan flow cytometer (Becton Dickinson, San Jose, CA) equipped with an argon laser operating at 488 nm. Fluorescence emission between 515 and 545 nm was quantified using Cell Quest software. Parasites either left untreated or fully depolarized by incubation with 10 μM FCCP for 10 min were used as controls.
Determination of oxygen consumption rates.
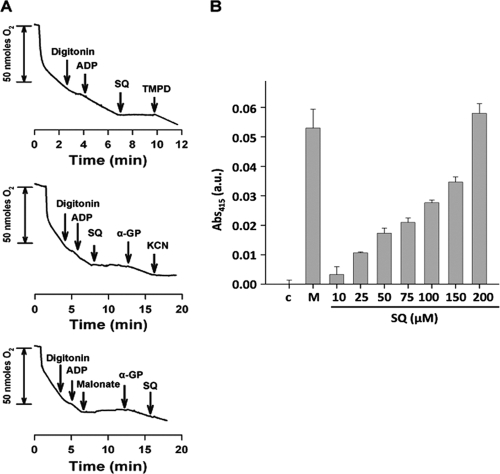
Oxygen consumption rates were measured using a Clark oxygen electrode (Hansatech, KingsLynn, United Kingdom) at 25°C, using 1 ml of promastigote suspension (108 cells/ml) in respiration buffer (10 mM Tris-HCl, 125 mM sucrose, 65 mM KCl, 1 mM MgCl2, 2.5 mM NaH2PO4, 0.3 mM EGTA, pH 7.2) supplemented with 5 mM succinate and 1 mg/ml fatty acid-free BSA, as described previously (1). Cells were permeabilized with 60 μM digitonin, which allows selective permeation of the plasma membrane but not of the inner mitochondrial membrane (42), and then 100 μM ADP was added to restore state 3 respiration, followed by 250 μM SQ once a steady rate had been reached. Although this concentration is much higher than the 50% effective concentration (EC50) for SQ, it mimics the intracellular concentration of SQ: a previous estimation has found a 10-fold greater enrichment of SQ in cytosol than on the membrane, in equilibrium with the external concentration (5). A set of substrates and inhibitors specific to the different complexes inside the respiratory chain was used to map the site of inhibition by SQ within the respiratory chain. Their final concentrations were 6.7 mM α-glycerophosphate, 1 mM KCN, 0.1 mM TMPD plus 1.7 mM ascorbate, and 2 mM malonate.
SDH activity.
A mitochondrion-enriched fraction was obtained as described by Chen et al. (4). Leishmania promastigotes were washed twice in PBS, resuspended in hypo-osmotic buffer (5 mM Tris-HCl, pH 7.4) for 10 min at 25°C, and then homogenized on ice using a Potter-Elvehjem homogenizer. Cell debris was removed by centrifugation (1,000 × g, 10 min, 4°C). The supernatant was then centrifuged at 13,000 × g (20 min, 4°C). The resulting pellet, containing the mitochondrial fraction, was resuspended in isotonic phosphate saline buffer (50 mM Na2HPO4, 90 mM NaCl, and 5 mM KCl, pH 7.2) at a protein concentration of 0.2 mg/ml. Aliquots (100 μl/well) of the mitochondrial fraction were added to a 96-well microplate and incubated for 1 h at 25°C with either SQ (10, 25, 50, 75, 100, 150, and 200 μM) or 10 mM malonate. The SDH activity was measured spectrophotometrically at 415 nm, using a model 680 Bio-Rad microplate enzyme-linked immunosorbent assay (ELISA) reader, in the presence of 10 mM succinate and 1 mM potassium ferricyanide (13).
Detection of ROS production.
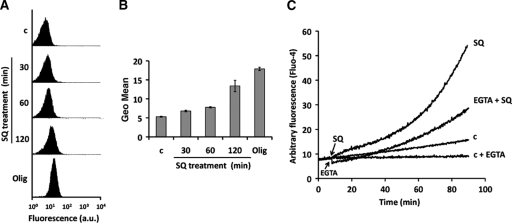
The generation of ROS was measured using the cell-permeating probe H2DCF-DA as described previously (35), with some modifications. The increase in fluorescence due to the oxidation of H2DCF (nonfluorescent) to the fluorogenic compound 2′,7′-dichlorofluorescein (DCF) is commonly used to detect the generation of reactive oxygen species. Parasites (1 × 107 promastigotes/ml) were incubated with 100 μM SQ in HBS for 30, 60, and 120 min at 28°C and then centrifuged and incubated with H2DCF-DA (2 μg/ml) in HBS for 15 min at 28°C in the dark. DCF fluorescence was measured by flow cytometry using a FACScan flow cytometer, and fluorescence emission was quantified using Cell Quest software. Parasites either left untreated or incubated with 10 μM oligomycin for 15 min to enhance ROS production were used as controls.
Measurement of free intracellular Ca2+.
Changes in the cytosolic Ca2+ level were monitored using the Ca2+-specific fluorescent probe Fluo4-AM, as described previously (9). Briefly, cells (1 × 107 promastigotes/ml) were incubated with 5 μM Fluo4-AM for 60 min at 28°C in RPMI 1640 medium devoid of phenol red and supplemented with 0.02% pluronic acid F127 to improve dispersion of the nonpolar acetyloxymethyl ester in aqueous media. After incubation, the cells were washed and incubated with 100 μM SQ in fresh medium supplemented or not with 8 mM EGTA. The fluorescence of Ca2+-bound Fluo4 was analyzed at 28°C by using an Aminco-Bowman series 2 fluorometer (excitation and emission wavelengths of 490 and 518 nm, respectively).
Analysis of PS externalization.
The exposure of PS at the outer leaflet of the plasma membrane is often a hallmark of apoptosis-like cell death. The externalization of PS was determined by measuring Alexa Fluor 488-conjugated annexin V binding to the cell, thereby exploiting its high Ca2+-dependent affinity for PS, according to the manufacturer's instructions, with slight modifications. Parasites (1 × 107 promastigotes/ml) were treated with or without 50 and 100 μM SQ in HBS for 1 h, washed twice with PBS, and resuspended in annexin binding buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM CaCl2, pH 7.4) at 5 × 106 parasites/100 μl. A 2.5-μl aliquot of Alexa Fluor-conjugated annexin V and PI at 0.4 μg/ml were added to 100 μl of parasite suspension, which was further incubated for 15 min at room temperature. Afterwards, 400 μl of annexin binding buffer was added, and the fluorescence of the stained parasites was analyzed by flow cytometry using a FACScan flow cytometer (Becton Dickinson, San Jose, CA) equipped with an argon laser operating at 488 nm. Fluorescence intensity was measured on the FL1-H channel for Alexa Fluor 488-conjugated annexin V and on the FL3-H channel for PI, using Cell Quest software.
DNA content analysis.
DNA content was analyzed by flow cytometry as described previously (12). Briefly, parasites (1 × 107 promastigotes/ml) were incubated with or without 50 and 100 μM SQ in culture medium for 24 h at 28°C, washed twice with PBS, fixed with ice-cold methanol by incubation for 3 min on ice, resuspended in 500 μl of PBS containing 1 μg/ml PI and 100 μg/ml RNase A, and incubated for 1 h in the dark at room temperature. Dye fluorescence was measured by flow cytometry using a FACScan flow cytometer (Becton Dickinson, San Jose, CA), and fluorescence emission was quantified using Cell Quest software.
Statistical analysis.
Statistical comparisons between groups were performed using Student's t test. Differences were considered significant at P values of <0.05.
RESULTS
SQ decreases intracellular ATP levels in L. donovani promastigotes.
We previously determined the EC50 of SQ for L. donovani promastigotes to be 19.8 ± 1.9 μM. The in vivo bioluminescence of Leishmania parasites of the 3-Luc strain was assayed after incubation with increasing concentrations of SQ (range, 0 to 200 μM). Above 30 μM SQ, the luminescence measured 10 min after drug addition decreased by 50% (Fig. 1). Since luminescence is directly related to the concentration of free intracellular ATP under these experimental conditions, SQ induces a fast and irreversible bioenergetic collapse in L. donovani promastigotes.
Fig. 1.
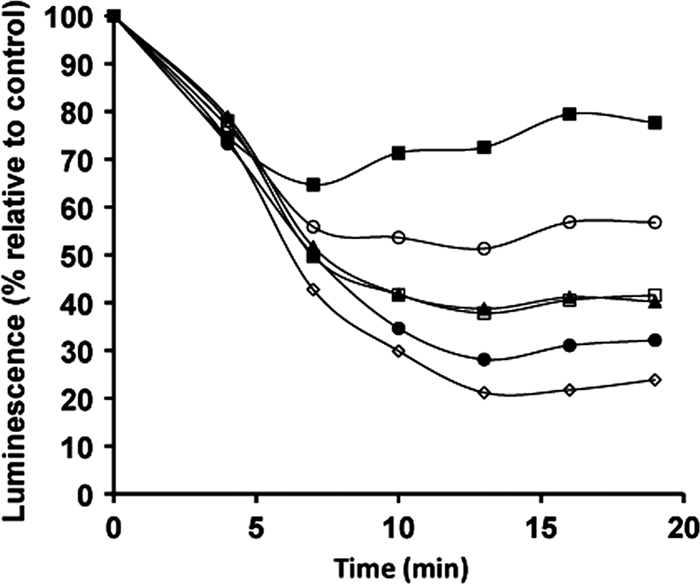
SQ reduces intracellular ATP levels. Changes in intracellular ATP levels were determined on the basis of changes in the luminescence of L. donovani 3-Luc promastigotes treated with the following SQ concentrations: 5 (■), 10 (○), 30 (□), 50 (▴), 100 (●), and 200 (♢) μM. Promastigotes were preloaded with 25 μM DMNPE-luciferin, SQ was added (time zero) once luminescence had reached a plateau, and the luminescence was monitored as described in Materials and Methods. Luminescence changes were normalized with respect to the control untreated parasites. Similar results were obtained in three independent experiments.
Effect of SQ on plasma membrane integrity.
Because SQ inserts into the Leishmania membrane, it may cause permeabilization by induction of faulty phospholipid packing (11). Depolarization of the plasma membrane and entry of the vital dye Sytox green (molecular weight = 600) were therefore measured after treatment with SQ to assess membrane permeabilization. SQ was found to induce plasma membrane depolarization, as monitored using DiBAC4(3) as a fluorescent probe (Fig. 2A); however, this cellular event occurred just after the decrease of intracellular ATP levels. Furthermore, the assays with the vital dye Sytox green revealed that incubation with 100 μM SQ for 60 min produced only 10% of the fluorescence increase obtained with 0.05% Triton X-100, which induces full permeabilization (data not shown). Leakage of ATP into the supernatant was also measured, and SQ did not induce ATP release into the medium compared with that for the control parasites (<50 pmol ATP × 10−6 promastigotes).
Fig. 2.
Effects of SQ on ΔΨp and ΔΨm. (A) Promastigotes were incubated without (c) and with 100 μM SQ in HBS for 15, 30, 60, and 120 min at 28°C and then treated with a 1 μM concentration of the specific plasma membrane potential probe DiBAC4(3) for 10 min at 28°C. DiBAC4(3) fluorescence is represented relative to that of parasites treated with 10 μM CCCP, used as 100% depolarization of the plasma membrane potential. Results are means ± standard deviations (SD) for three independent experiments. (B and C) SQ-induced ΔΨm depolarization. L. donovani promastigotes were treated without (c) and with 50 and 100 μM SQ for 15 min, stained with 0.8 μM Rh123, and analyzed for fluorescence by flow cytometry. Parasites treated with 10 μM FCCP for 10 min were used as a depolarization control. (B) Histograms from a representative experiment of three independent experiments. (C) Geometric mean (Geo.Mean) channel fluorescence values ± SD for three experiments. The experimental values were significantly different from control values by Student's t test (P < 0.02). *, geometric mean for the 47% of parasites that showed an Rh123 accumulation decrease compared to the control.
SQ induces depolarization of ΔΨm.
Mitochondrial oxidative phosphorylation is the main source of ATP in Leishmania parasites (41). To determine whether intracellular ATP decay was associated with an effect of SQ on the mitochondria, the variation of the mitochondrial electrochemical potential was monitored in parasites incubated with SQ. Parasites incubated for 15 min with 100 μM SQ or with 10 μM FCCP as a positive depolarization control showed significant decreases in Rh123 accumulation (3.1- and 3.8-fold, respectively) compared with untreated parasites (n = 3; P < 0.02), while only 47% of the parasites incubated with 50 μM SQ showed a 3.4-fold decrease of Rh123 accumulation with respect to control parasites (n = 3; P < 0.01) (Fig. 2B and C).
Oxygen consumption and SDH activity are inhibited by SQ.
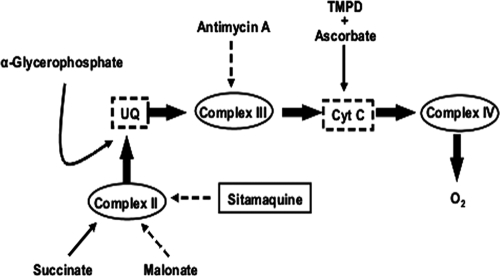
In the next step, we set out to determine the molecular target of SQ responsible for intracellular ATP decay. As noted above, oxidative phosphorylation is the main source of ATP for Leishmania. To afford unrestricted access of substrates and inhibitors to the respiratory chain, the plasma membrane was permeabilized with digitonin. Preservation of the mitochondrial inner membrane's integrity was confirmed by the increase of respiration after ADP addition (state 3), as it is no longer the limiting substrate for oxidative phosphorylation. The oxygen consumption rate in this state was 8.6 nmol/min × 108 cells (Fig. 3A). The addition of SQ (250 μM final concentration) to digitonin-treated parasites fully inhibited the succinate-dependent respiration (Fig. 3A). Furthermore, the oxygen consumption rate was restored after addition of TMPD-ascorbate, an electron donor for cytochrome c (Fig. 3A), thereby ruling out cytochrome c oxidase (complex IV) and ATP synthase (complex V), located downstream, as SQ targets and restricting the search to targets upstream of cytochrome c (Fig. 4). Addition of 6.7 mM α-glycerophosphate (Fig. 3A), which feeds the electron transport chain through the mitochondrial flavin adenine dinucleotide (FAD)-dependent glycerol-3-phosphate dehydrogenase by producing reduced ubiquinone (Fig. 4), partially reverted (60.3% of the original rate) SQ inhibition of respiration (14). This observation confirmed the functionality of complex III in the presence of SQ and pinpointed complex II as the drug target. To verify this hypothesis, succinate-dependent respiration was fully inhibited with malonate at the level of complex II, and α-glycerophosphate was added. Under these conditions, oxygen consumption proceeds only through complex III, and this rate was not inhibited by SQ (Fig. 3A), thereby ruling out complex III and confirming complex II as the sole SQ target within oxidative phosphorylation.
Fig. 3.
Identification of the inhibition site of SQ in the respiratory chain of Leishmania. (A) Traces represent the oxygen consumption rates of L. donovani promastigotes in the presence of 5 mM succinate. Arrows indicate the addition of appropriate substrates and inhibitors at their respective final concentrations, namely, 60 μM digitonin, 100 μM ADP, 0.1 mM TMPD plus 1.7 mM ascorbate, 1 mM KCN, 2 mM malonate, 6.7 mM α-glycerophosphate (α-GP), and 250 μM SQ. (B) SDH activity in the presence of increasing concentrations of SQ. Activity was measured by the decrease of absorbance at 415 nm due to the reduction of ferricyanide by the SDH activity of the mitochondrial fraction in the presence of succinate, as described in Materials and Methods. Error bars indicate the SD. Samples not treated with SQ (c) and samples in the presence of malonate (M), an SDH inhibitor, were used as positive and negative controls, respectively. Activity was measured at 37°C.
Fig. 4.
Scheme of the respiratory chain in Leishmania, shown with the specific substrates and inhibitors used in this study. Sites of electron feeding and inhibition are indicated by solid and dotted lines, respectively. UQ, ubiquinone; Cyt C, cytochrome c. The role of complex I was based on the concepts compiled in reference 29.
To verify this finding, inhibition of SDH by increasing SQ concentrations was assayed in mitochondrial fractions. Figure 3B shows the dose-dependent inhibition of SDH activity by SQ. Total inhibition of this enzyme was achieved with 10 mM malonate as a control.
SQ increases ROS production in Leishmania.
Since mitochondrial depolarization is strongly associated with ROS production, which is induced by a variety of leishmanicidal drugs (2, 7, 23, 31, 33, 34), we determined the potential of SQ to induce ROS production. For this purpose, we used the ROS-sensitive probe H2DCF in L. donovani promastigotes treated with 100 μM SQ for different incubation times (30, 60, and 120 min) or treated with oligomycin (10 μM; 15 min) as a positive control for ROS formation. Flow cytometry analysis revealed that SQ induced significant (n = 3; P < 0.01) ROS production in a time-dependent manner (Fig. 5A and B).
Fig. 5.
SQ induces ROS generation and increases cytosolic Ca2+ levels. ROS levels were measured using the specific fluorescent dye H2DCF-DA. L. donovani promastigotes were incubated without (c) and with 100 μM SQ for 30, 60, and 120 min and then loaded with 2 μg/ml H2DCF-DA for 15 min. Oligomycin (Olig) (10 μM; 15 min) was used as a control for ROS generation. The fluorescence intensity was determined by flow cytometry analysis as described in Materials and Methods. (A) Histograms for a representative experiment of three independent ones. (B) Geometric mean (Geo.Mean) channel fluorescence values ± SD for three experiments. The experimental values were significantly different from control values by Student's t test (P < 0.01). (C) Fluo4-preloaded parasites were treated without (c) and with 100 μM SQ and then analyzed for increasing fluorescence over 90 min at 28°C, using an Aminco-Bowman series 2 spectrometer. The experiments were assessed with and without the presence of the Ca2+ chelator EGTA. Arrows indicate the addition of SQ and EGTA. Similar results were obtained in three independent experiments.
SQ increases free cytosolic Ca2+ levels.
ROS generation and changes of ΔΨm are associated with altered intracellular Ca2+ homeostasis (25). The fluorescent probe Fluo4 was used to monitor cytosolic Ca2+ variation. Promastigotes treated with 100 μM SQ showed an increased cytosolic Ca2+ level compared with untreated control parasites (Fig. 5C). To ascertain the source of the Ca2+ responsible for this effect, the experiment was repeated in the presence of EGTA to rule out the entry of external Ca2+. Under these conditions, the fluorescence increase was reduced by 56%, thereby evidencing a dual source for the observed Ca2+ increase, namely, release from intracellular stores and entry of external Ca2+, with their contributions being approximately equal.
SQ induces externalization of PS.
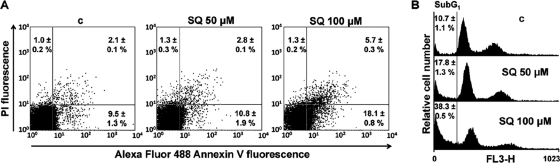
All of the previous experimental evidence pointed toward an apoptosis-like process of Leishmania death. To provide further experimental support for this process, we assayed the externalization of PS, a hallmark of apoptosis. Translocation of PS from the cytoplasmic space into the external leaflet of the plasma membrane was evidenced by the binding of fluorescent annexin V to the parasites. To rule out access of the reagent to the PS pool facing the cytoplasm due to membrane permeabilization, annexin V was incubated together with PI as a control for necrosis. Parasites treated with 100 μM SQ for 1 h showed a significant, 1.9-fold increase in the percentage of apoptotic promastigotes (annexin V positive and PI negative) by flow cytometry compared with untreated control parasites (n = 3; P < 0.02), while parasites treated with 50 μM SQ showed no significant difference (Fig. 6A).
Fig. 6.
SQ induces PS externalization and DNA fragmentation. (A) Parasites were treated without (c) and with 50 and 100 μM SQ in HBS for 1 h, costained with Alexa Fluor 488-conjugated annexin V and PI, and analyzed by flow cytometry (FL1 versus FL3) as described in Materials and Methods. Representative dot plots are divided into four quadrants. The percentage of cells in each quadrant is expressed as the mean ± SD for three independent experiments. Viable cells that did not bind annexin V or incorporate PI are represented in the lower left quadrant of each dot plot. The bottom right quadrants indicate apoptotic cells. The percentages of cells in these quadrants, expressed as means ± SD for three independent experiments, were significantly different from control values by t test (P < 0.02) only for parasites treated with 100 μM SQ. (B) SQ causes an increase of parasites in the sub-G1 phase. DNA fragmentation was quantified by measuring the percentage of cells in the sub-G1 DNA region. The DNA content degradation profiles of parasites were determined by flow cytometry and PI staining. Parasites were incubated without (c) or with 50 and 100 μM SQ for 24 h and then loaded with PI as described in Materials and Methods. The distribution of DNA content was analyzed by flow cytometry. The percentages of cells in the sub-G1 phase, expressed as means ± SD for three independent experiments, were significantly different from control values by t test (P < 0.02).
SQ increases the sub-G1 DNA parasite population.
SQ-induced chromatin degradation was measured by determining the hypodiploid DNA content in parasites, as monitored by PI fluorescence using flow cytometry. The fluorescence values for cells with DNA degradation were lower than those for G1 cells (sub-G1 peak in the DNA histograms) (28). After 24 h of incubation with 50 and 100 μM SQ in culture medium, 17.8 and 38.3% of the parasites, respectively, had DNA in the sub-G1 region, compared with only 10.7% of untreated control parasites (n = 3; P < 0.02) (Fig. 6B).
DISCUSSION
Definition of a drug target is an important step in establishing a robust rationale for optimization and design of new surrogate generations from an initial lead. Examples of drug targets in Leishmania are rather scarce, with ergosterol, a membrane ligand targeted by amphotericin B, and redox metabolism, a target for antimonials, perhaps being the best-defined targets (6).
SQ was rescued from temporary oblivion after its initial successful assay in the 1970s by the Walter Reed Army Institute. Although mitochondria and acidocalcisomes were initially proposed as SQ targets (43, 44), further studies of parasites with acidocalcisomes defective for SQ accumulation showed identical SQ susceptibilities, thus providing clear evidence that the antileishmanial action of SQ is unrelated to its accumulation in acidocalcisomes (18).
In this work, we aimed to pinpoint the target for SQ in L. donovani promastigotes. To attain this goal, we carried out a systematic analysis, starting with the general effects of the drug on the parasite and finishing by defining the molecular entity inhibited by this drug. SQ induces a fast and significant decrease of intracellular free ATP levels, thus leading to a bioenergetic collapse of the parasite. This may be due either to a direct inhibition of ATP synthesis or to a plasma membrane permeabilization phenomenon where ATP is leaked through membrane lesions or hydrolyzed in a futile manner by ionic pumps in an attempt to recover initial ionic gradients across the plasma membrane. In support of the last hypothesis, it has been reported that SQ interacts with the plasma membranes of parasites in a two-step mode involving electrostatic binding to anionic phospholipids and further insertion into the hydrophobic matrix to minimize aqueous exposure of its rather hydrophobic quinoline nucleus (5, 11). Hence, a feasible perturbation of phospholipid packing (5, 11) that leads to membrane permeability may ensue. This assumption was not supported experimentally, however, as plasma membrane permeabilization barely reached 10% of full permeabilization after 1 h of incubation with 100 μM SQ. Thus, inhibition of ATP synthesis was the only remaining option to account for collapse of the ATP levels. In contrast to the case for other trypanosomatids, such as the bloodstream trypomastigotes of African trypanosomes, the contribution of glycolysis to the overall ATP pool in Leishmania is considerably lower than that from oxidative phosphorylation (38). Hence, inhibition of ATP synthesis necessarily implies inhibition of oxidative phosphorylation, as confirmed by the SQ-induced inhibition of the oxygen consumption rate of these parasites. Polarographic analysis of the oxygen consumption rate of digitonin-treated parasites showed that complex II (SDH) was the target for SQ, after other targets within the oxidative phosphorylation pathway were systematically discarded by the use of selective substrates and inhibitors. Thus, reversion of SQ inhibition by TMPD plus ascorbate and α-glycerophosphate excluded complex IV and III as SQ targets, respectively, as well as the ATP synthase downstream of complex IV. Although complex I is present in trypanosomatids, it is defective in four subunits supposedly involved in proton extrusion, thereby excluding this complex from energy generation and relegating its activity to maintenance of the mitochondrial NADH/NAD ratio (29). SDH activity in the mitochondrion-enriched fractions was inhibited in a dose-dependent manner by SQ.
Although complexes I and III are the main producers of ROS in mammalian cells (26), complex II may also act as a source of ROS (15). Indeed, ROS production by inhibition of complex II is increasingly considered an objective for anticancer drugs that target mitochondria (10), thereby triggering apoptosis in these cells. Complex II has also been demonstrated to be a good target in Leishmania for phenyl-phenalenones (19), benzophenone-derived biphosphonium surrogates (20), and thenoyltrifluoroacetone (24).
ROS production triggers the increase of intracellular Ca2+ and subsequent steps of the apoptotic process in Leishmania. Interestingly, the Ca2+ pools involved may vary according to the stimulus. Thus, whereas oxidative stress induced by H2O2 involves mobilization from intra- and extracellular Ca2+ pools (25), only the intracellular pool is implicated after complex II poisoning with thenoyltrifluoroacetone plus pentamidine (24). Although both external and internal sources account for this process for SQ, the question of whether the entry of external Ca2+ is due to unspecific and transitory membrane permeabilization by SQ or to effects on Ca2+ channels remains unanswered.
In any case, we propose the following sequence of events triggered by SQ, as described for other leishmanicidal drugs, including antimonials (24, 25, 35): ROS production → oxidative stress → increase of intracellular Ca2+ → final steps of apoptosis (PS externalization plus chromatin degradation).
In summary, we have demonstrated that SQ acts against the respiratory chain complex II, an essential target for Leishmania. This result, together with related findings for tafenoquine, which acts on the next downstream complex in the respiratory chain (2), demonstrates the versatility of the 8-aminoquinoline scaffold for the development of leishmanicidal drugs. The recently described improvement of 8-aminoquinolines (27) therefore appears to be an attractive strategy for development of analogs with promising antimalarial, antimicrobial, and antileishmanial activities, together with lower hematological toxicity. Furthermore, SQ was recently suggested as a candidate for drug combinations (32), thus supporting its use for reduced drug dosage, toxicity, and therapeutic failure.
ACKNOWLEDGMENTS
This work was supported by Spanish grant SAF2009-07440 (to F.G.), by ISCIII-Red de Investigación Cooperativa en Enfermedades Tropicales (RICET)-FEDER grants RD06/0021/0002 (F.G.) and RD 06/0021/0006 (L.R.), by European Union grants HEALTH-2007-223414 and FIS PS09-01928 (L.R.), by the Plan Andaluz de Investigación (BIO130), and by FEDER funds from the European Union to F.G.
We acknowledge the support of GlaxoSmithKline (Greenford, United Kingdom), who provided the sitamaquine used throughout this research work.
Footnotes
Published ahead of print on 13 June 2011.
REFERENCES
- 1. Alvarez-Fortes E., Ruiz-Perez L. M., Bouillaud F., Rial E., Rivas L. 1998. Expression and regulation of mitochondrial uncoupling protein 1 from brown adipose tissue in Leishmania major promastigotes. Mol. Biochem. Parasitol. 93:191–202 [DOI] [PubMed] [Google Scholar]
- 2. Carvalho L., et al. 2010. Tafenoquine, an antiplasmodial 8-aminoquinoline, targets Leishmania respiratory complex III and induces apoptosis. Antimicrob. Agents Chemother. 54:5344–5351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chappuis F., et al. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 5:873–882 [DOI] [PubMed] [Google Scholar]
- 4. Chen M., Zhai L., Christensen S. B., Theander T. G., Kharazmi A. 2001. Inhibition of fumarate reductase in Leishmania major and L. donovani by chalcones. Antimicrob. Agents Chemother. 45:2023–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coimbra E. S., et al. 2010. Mechanism of interaction of sitamaquine with Leishmania donovani. J. Antimicrob. Chemother. 65:2548–2555 [DOI] [PubMed] [Google Scholar]
- 6. Croft S. L., Sundar S., Fairlamb A. H. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Das R., Roy A., Dutta N., Majumder H. K. 2008. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis 13:867–882 [DOI] [PubMed] [Google Scholar]
- 8. Diaz-Achirica P., Ubach J., Guinea A., Andreu D., Rivas L. 1998. The plasma membrane of Leishmania donovani promastigotes is the main target for CA(1-8)M(1-18), a synthetic cecropin A-melittin hybrid peptide. Biochem. J. 330:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dolai S., Yadav R. K., Pal S., Adak S. 2009. Overexpression of mitochondrial Leishmania major ascorbate peroxidase enhances tolerance to oxidative stress-induced programmed cell death and protein damage. Eukaryot. Cell 8:1721–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dong L. F., et al. 2011. Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II. J. Biol. Chem. 286:3717–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duenas-Romero A. M., Loiseau P. M., Saint-Pierre-Chazalet M. 2007. Interaction of sitamaquine with membrane lipids of Leishmania donovani promastigotes. Biochim. Biophys. Acta 1768:246–252 [DOI] [PubMed] [Google Scholar]
- 12. Dutta A., et al. 2007. Racemoside A, an anti-leishmanial, water-soluble, natural steroidal saponin, induces programmed cell death in Leishmania donovani. J. Med. Microbiol. 56:1196–1204 [DOI] [PubMed] [Google Scholar]
- 13. Elingold I., et al. 2008. Mitochondrial toxicity and antioxidant activity of a prenylated flavonoid isolated from Dalea elegans. Chem. Biol. Interact. 171:294–305 [DOI] [PubMed] [Google Scholar]
- 14. Guerra D. G., Decottignies A., Bakker B. M., Michels P. A. 2006. The mitochondrial FAD-dependent glycerol-3-phosphate dehydrogenase of Trypanosomatidae and the glycosomal redox balance of insect stages of Trypanosoma brucei and Leishmania spp. Mol. Biochem. Parasitol. 149:155–169 [DOI] [PubMed] [Google Scholar]
- 15. Ishii N., Ishii T., Hartman P. S. 2007. The role of the electron transport SDHC gene on lifespan and cancer. Mitochondrion 7:24–28 [DOI] [PubMed] [Google Scholar]
- 16. Jha T. K., et al. 2005. A phase II dose-ranging study of sitamaquine for the treatment of visceral leishmaniasis in India. Am. J. Trop. Med. Hyg. 73:1005–1011 [PubMed] [Google Scholar]
- 17. Jhingran A., Chawla B., Saxena S., Barrett M. P., Madhubala R. 2009. Paromomycin: uptake and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 164:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lopez-Martin C., Perez-Victoria J. M., Carvalho L., Castanys S., Gamarro F. 2008. Sitamaquine sensitivity in Leishmania species is not mediated by drug accumulation in acidocalcisomes. Antimicrob. Agents Chemother. 52:4030–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luque-Ortega J. R., et al. 2004. Fungus-elicited metabolites from plants as an enriched source for new leishmanicidal agents: antifungal phenyl-phenalenone phytoalexins from the banana plant (Musa acuminata) target mitochondria of Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 48:1534–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luque-Ortega J. R., Reuther P., Rivas L., Dardonville C. 2010. New benzophenone-derived bisphosphonium salts as leishmanicidal leads targeting mitochondria through inhibition of respiratory complex II. J. Med. Chem. 53:1788–1798 [DOI] [PubMed] [Google Scholar]
- 21. Luque-Ortega J. R., Rivas L. 2007. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 51:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luque-Ortega J. R., Saugar J. M., Chiva C., Andreu D., Rivas L. 2003. Identification of new leishmanicidal peptide lead structures by automated real-time monitoring of changes in intracellular ATP. Biochem. J. 375:221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mandal G., et al. 2007. Increased levels of thiols protect antimony unresponsive Leishmania donovani field isolates against reactive oxygen species generated by trivalent antimony. Parasitology 134:1679–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehta A., Shaha C. 2004. Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition: complex II inhibition results in increased pentamidine cytotoxicity. J. Biol. Chem. 279:11798–11813 [DOI] [PubMed] [Google Scholar]
- 25. Mukherjee S. B., Das M., Sudhandiran G., Shaha C. 2002. Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J. Biol. Chem. 277:24717–24727 [DOI] [PubMed] [Google Scholar]
- 26. Murphy M. P. 2009. How mitochondria produce reactive oxygen species. Biochem. J. 417:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nanayakkara N. P., et al. 2008. Antiparasitic activities and toxicities of individual enantiomers of the 8-aminoquinoline 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline succinate. Antimicrob. Agents Chemother. 52:2130–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271–279 [DOI] [PubMed] [Google Scholar]
- 29. Opperdoes F. R., Michels P. A. 2008. Complex I of Trypanosomatidae: does it exist? Trends Parasitol. 24:310–317 [DOI] [PubMed] [Google Scholar]
- 30. Richard J. V., Werbovetz K. A. 2010. New antileishmanial candidates and lead compounds. Curr. Opin. Chem. Biol. 14:447–455 [DOI] [PubMed] [Google Scholar]
- 31. Roy A., et al. 2008. Mitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3,3′-diindolylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol. Pharmacol. 74:1292–1307 [DOI] [PubMed] [Google Scholar]
- 32. Seifert K., Munday J., Syeda T., Croft S. L. 2011. In vitro interactions between sitamaquine and amphotericin B, sodium stibogluconate, miltefosine, paromomycin and pentamidine against Leishmania donovani. J. Antimicrob. Chemother. 66:850–854 [DOI] [PubMed] [Google Scholar]
- 33. Sen N., et al. 2007. Apoptosis is induced in leishmanial cells by a novel protein kinase inhibitor withaferin A and is facilitated by apoptotic topoisomerase I-DNA complex. Cell Death Differ. 14:358–367 [DOI] [PubMed] [Google Scholar]
- 34. Sen N., et al. 2004. Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ. 11:924–936 [DOI] [PubMed] [Google Scholar]
- 35. Sudhandiran G., Shaha C. 2003. Antimonial-induced increase in intracellular Ca2+ through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes. J. Biol. Chem. 278:25120–25132 [DOI] [PubMed] [Google Scholar]
- 36. Sundar S., Chakravarty J. 2008. Paromomycin in the treatment of leishmaniasis. Expert Opin. Invest. Drugs 17:787–794 [DOI] [PubMed] [Google Scholar]
- 37. Tekwani B. L., Walker L. A. 2006. 8-Aminoquinolines: future role as antiprotozoal drugs. Curr. Opin. Infect. Dis. 19:623–631 [DOI] [PubMed] [Google Scholar]
- 38. Tielens A. G., van Hellemond J. J. 2009. Surprising variety in energy metabolism within Trypanosomatidae. Trends Parasitol. 25:482–490 [DOI] [PubMed] [Google Scholar]
- 39. Vale N., Moreira R., Gomes P. 2009. Primaquine revisited six decades after its discovery. Eur. J. Med. Chem. 44:937–953 [DOI] [PubMed] [Google Scholar]
- 40. van Griensven J., et al. 2010. Combination therapy for visceral leishmaniasis. Lancet Infect. Dis. 10:184–194 [DOI] [PubMed] [Google Scholar]
- 41. van Hellemond J. J., Tielens A. G. 1997. Inhibition of the respiratory chain results in a reversible metabolic arrest in Leishmania promastigotes. Mol. Biochem. Parasitol. 85:135–138 [DOI] [PubMed] [Google Scholar]
- 42. Vercesi A. E., Bernardes C. F., Hoffmann M. E., Gadelha F. R., Docampo R. 1991. Digitonin permeabilization does not affect mitochondrial function and allows the determination of the mitochondrial membrane potential of Trypanosoma cruzi in situ. J. Biol. Chem. 266:14431–14434 [PubMed] [Google Scholar]
- 43. Vercesi A. E., Docampo R. 1992. Ca2+ transport by digitonin-permeabilized Leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. Biochem. J. 284:463–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vercesi A. E., Rodrigues C. O., Catisti R., Docampo R. 2000. Presence of a Na(+)/H(+) exchanger in acidocalcisomes of Leishmania donovani and their alkalization by anti-leishmanial drugs. FEBS Lett. 473:203–206 [DOI] [PubMed] [Google Scholar]
- 45. Wasunna M. K., et al. 2005. A phase II dose-increasing study of sitamaquine for the treatment of visceral leishmaniasis in Kenya. Am. J. Trop. Med. Hyg. 73:871–876 [PubMed] [Google Scholar]
- 46. Yeates C. 2002. Sitamaquine (GlaxoSmithKline/Walter Reed Army Institute). Curr. Opin. Invest. Drugs 3:1446–1452 [PubMed] [Google Scholar]