Abstract
Vibrio cholerae, a bacterium autochthonous to the aquatic environment, is the causative agent of cholera, a severe watery, life-threatening diarrheal disease occurring predominantly in developing countries. V. cholerae, including both serogroups O1 and O139, is found in association with crustacean zooplankton, mainly copepods, and notably in ponds, rivers, and estuarine systems globally. The incidence of cholera and occurrence of pathogenic V. cholerae strains with zooplankton were studied in two areas of Bangladesh: Bakerganj and Mathbaria. Chitinous zooplankton communities of several bodies of water were analyzed in order to understand the interaction of the zooplankton population composition with the population dynamics of pathogenic V. cholerae and incidence of cholera. Two dominant zooplankton groups were found to be consistently associated with detection of V. cholerae and/or occurrence of cholera cases, namely, rotifers and cladocerans, in addition to copepods. Local differences indicate there are subtle ecological factors that can influence interactions between V. cholerae, its plankton hosts, and the incidence of cholera.
INTRODUCTION
Vibrio cholerae, the causative agent of cholera, is native to the aquatic environment. Cholera, the disease caused by V. cholerae, is endemic in regions of the world where inadequate sanitary practices and consumption of contaminated water and food are common. More than 200 serotypes of V. cholerae have been identified, of which several can cause mild to serious gastroenteritis and even local outbreaks of diarrheal illnesses with cholera-like symptoms. Toxigenic strains of V. cholerae serogroups O1 and O139 have been identified with cholera epidemics and pandemics (4, 24).
V. cholerae O1 and O139 are commensal to crustacean zooplankton, notably copepods, which are present both in their gut and in biofilms on their chitinous surfaces (15, 28). Furthermore, V. cholerae is present throughout the year in and on its zooplankton host (13), and V. cholerae serogroup O1 has been shown to attach preferentially to zooplankton, but also to some species of phytoplankton in Bangladesh waters (33). Its commensal existence provides protection from grazing by heterotrophic nanoflagellates (23) and also from toxic chemicals, including those used to disinfect drinking water, such as alum and chlorine (6). V. cholerae, like all Vibrio species, produces chitinase(s) (12), with chitin serving as a nutrient source (25, 27). In a recent study, Kirschner et al. (21) demonstrated that association with zooplankton is important for V. cholerae non-O1/non-O139 serogroup isolates endemic in Neusiedler See, a large, shallow, moderately saline-alkaline lake in Central Europe. A significant correlation was observed between the seasonal pattern in frequency of occurrence of V. cholerae and increased zooplankton biomass (21).
Zooplankton comprise a broad assortment of ecologically important heterotrophic groups, including small crustaceans, i.e., copepods, water fleas of the genera Daphnia, Bosmina, and Diaptomus, fairy shrimp, such as Artemia, Ostracods, the medusa stage of cnidarians, rotifers, and large crustacean nauplii. The composition of the zooplankton community changes constantly during an annual cycle, with a dramatic difference in species composition of freshwater and marine zooplankton communities. Plankton species composition also plays a role in Vibrio seasonality, as has been shown to occur in waters off the Georgia coast in the United States, highlighting the complex relationship between seasonal shifts in plankton composition and the number of vibrios in the aquatic environment (34).
Colwell and Huq (7) first proposed that zooplankton play a significant role in cholera epidemics in developing countries. The importance of copepods in cholera transmission was further demonstrated in a study showing that the number of cholera cases in Bangladeshi villages was significantly reduced when a simple filtration method was employed to remove plankton and particulate matter from pond and river water used for household purposes, including as a source of drinking water (8, 16). A significant positive correlation between copepod abundance and cholera cases in three different surveillance sites in Bangladesh was recently documented (7, 14).
Cholera is endemic to Bangladesh and occurs in a characteristic bimodal seasonal pattern (10, 22, 30). Thus, remote villages in Bangladesh, where cholera occurs each year, offer unique sites for study of the ecology of V. cholerae and its interaction with zooplankton, given that most of the drinking water in these rural areas is taken from man-made freshwater ponds, many of which are regularly flooded with seawater carrying zooplankton from the Bay of Bengal during seasonal cyclones. In this study, we examined the association of cholera incidence with presence of toxigenic V. cholerae and specific zooplankton taxa in the environment.
MATERIALS AND METHODS
Description of study sites.
The two areas under study were reported previously by Alam et al. (1, 2). Briefly, Bakerganj is located in the southern district of Barisal, about 70 km north of the coast of the Bay of Bengal and approximately 300 km southwest of Dhaka, the capital city of Bangladesh (Fig. 1). In the present study, which was part of an epidemiological and ecological surveillance of cholera and V. cholerae in Bakerganj (1, 14, 30), samples were collected biweekly from patients attending the Thana (local government) Health Complex (THC) and monthly from eight environmental sites (one river, five ponds, and two lakes). The sampling pond sites, located in villages, are heavily used by those villagers, preferentially for drinking water, and the river and lakes are used for domestic purposes because of the high salinity of the ground water. Mathbaria, the second study site, is located adjacent to the Bay of Bengal, in close proximity to the Sunderban mangrove forest and approximately 400 km southwest of Dhaka. In this study, samples were collected biweekly from patients attending the THC of Mathbaria and from six man-made ponds (one of which is linked to a river) that are heavily used as drinking water source and for other domestic purposes.
Fig. 1.
Map of Bangladesh showing the locations of Bakerganj and Mathbaria.
Collection and analysis of clinical specimens.
Biweekly clinical surveillance was carried out in Bakerganj and Mathbaria between January 2004 and September 2007 for a period of 45 months as part of an ongoing clinical surveillance, as previously reported (1). Rectal swabs were collected and analyzed for pathogenic V. cholerae, employing standard methods (19).
Collection of environmental samples and processing.
Water and plankton samples were collected monthly from seven ponds and one river site in Bakerganj and biweekly from six ponds in Mathbaria between March 2004 and September 2007 for a period of 43 months and processed as previously reported (1). Briefly, 100 liters of water was filtered successively through 64-μm and 20-μm mesh nylon nets (Millipore Corp., Bedford, MA), and 50 ml of retentate from the 64-μm net was collected as a crude measure of zooplankton. Also, during the process of filtration, 500 ml of filtrate from the 20-μm net was collected and analyzed for planktonic (unattached, free-living) bacteria. Both water and plankton samples were transported at ambient temperature to the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR, B), Dhaka, and processed within 12 to 16 h of collection (2).
Aliquots (10 ml) of the zooplankton samples were fixed in buffered formalin. The plankton samples were enumerated by predominant type, using a Sedgewick Rafter counting chamber, and taxonomic analyses were undertaken at Dhaka University, Dhaka, Bangladesh. From a 10-ml subsample, separately 3-ml subsamples (1 ml + 1 ml + 1 ml) were analyzed for a zooplankton count. Fresh plankton samples (40 ml) were further concentrated to 5 ml and homogenized. Subsamples (200 ml) of water that had been passed through the plankton nets were filtered using 0.2-μm-pore polycarbonate membrane filters. These concentrated samples were inoculated into alkaline peptone water (APW) and incubated at 37°C for 6 to 12 h. Pellicle growth from the surface of the APW was subcultured onto selective agar media, including thiosulfate citrate-bile salts-sucrose agar (TCBS) and tellurite-taurocholate-gelatin agar (TTGA), and incubated at 37°C for 24 to 48 h.
Determination of zooplankton population dynamics.
Zooplankton composition and density were measured by analyzing subsamples with a compound photomicroscope (Kruff MBL2100; Germany). Zooplankton taxa were identified to the lowest taxon, based on microscopic examination. When possible, organisms were identified to the species. For each sample, organisms were classified into four groups of abundance, based on enumeration of each subsamples, from most abundant (i.e., group 1) to least numerically abundant (i.e., group 4).
Direct detection of Vibrio cholerae in environmental samples.
Total DNA was extracted from all three fractions of the sample (i.e., >64 μm, >20 μm to <64 μm, and filtrate from the 20-μm filtration, following the method of Rivera et al. (29), using cetyltrimethylammonium bromide (CTAB) and phenol-chloroform-isopropyl alcohol. DNA was precipitated in 0.6 volume of isopropyl alcohol and centrifuged to form a pellet that was washed with 70% ethanol, resuspended in Tris-EDTA (TE) buffer (pH 8.0), and quantified by UV spectrophotometry. Molecular detection of toxigenic V. cholerae serogroups O1 and O139 and the cholera toxin gene ctxA was carried out by multiplex PCR, using primers and procedures described by Hoshino et al. (11). Unfiltered water samples inoculated directly into APW were similarly treated for DNA extraction (11, 29).
Statistical analysis.
A Monte Carlo approach was used to detect significant association between occurrence of cholera and presence of specific zooplankton, either genus or species, or the presence of molecular markers of V. cholerae serogroup O1 or O139 and ctxA in one of the three fractions of the sample (i.e., >64 μm, >20 to <64 μm, and filtrate from the 20-μm filtration. The number of simultaneous occurrences of positive observations in the two variables was divided by the number of observations. The bootstrap procedure, employing 100,000 series generated randomly and preserving the same first-order autocorrelation structure, as observed in the data, was established to compute a stable distribution of the statistic that can be expected to occur randomly. The P value of the test corresponds to 1 − the rank of the observed statistic over the 100,000 statistics obtained randomly divided by 100,001. If the P value was equal to or less than a significance level of 5% corrected by the Bonferonni method for multiple comparisons, we considered the association to be significantly different from an occurrence by chance. To avoid other potential biases in the results, we investigated a significant association only when the frequency of occurrence of a zooplankton genus (or group) or species was greater than or equal to 10. In most cases, analyses were performed using both genus- and group-level determinations of zooplankton, as well as species-level determination. There is a mechanistic basis for expecting a lag of up to 4 weeks from occurrence of increased abundance of zooplankton and subsequent presence of pathogenic V. cholerae in the environment as drivers of cholera. A time lag was considered between occurrence of positive observations in the two variables, equal to 4 weeks for Bakerganj and 2 and 4 weeks for Mathbaria. We investigated the association between occurrence of cholera and an earlier presence of both specific zooplankton and molecular markers of V. cholerae O1 or O139 and ctxA in the water. In addition, we also analyzed the association between occurrence of cholera and a later occurrence of molecular markers of V. cholerae O1 or O139 and ctxA.
RESULTS AND DISCUSSION
Zooplankton diversity.
The geographical region where Bakerganj and Mathbaria are located is in the southern part of Bangladesh, an area of low ground elevation where man-made ponds and open ground water reservoirs contain freshwater, but are inundated periodically by coastal flooding. Over the entire sampling period, six groups, Cladocera, Copepoda, crustacean nauplii, Ostracoda, rotifers, and protozoa, were identified at the two sampling sites. These assemblages of zooplankton are common in the freshwater environment of pelagic and littoral regions of ponds, lakes, and large rivers. Rotifers are found almost exclusively in fresh water (35). Copepods and cladocerans are groups within the large subphylum, Crustacea. Copepods constitute a class that is widespread in both freshwater and marine environments, while cladocerans comprise four orders occurring primarily in freshwater environments.
In total, 4,951 taxonomic identifications were made, with the three dominant zooplankton groups identified being rotifers (33.4%, n = 1,656), nauplii (29.5%, n = 1,459), and copepods (22.6%, n = 1,121). In total, 55 genera or subgroups were identified, with 44 genera found either in Bakerganj or Mathbaria, but not in both. The most commonly observed genera or subgroups were nauplii and metanauplius (15.6%, n = 773), rotifers of the Brachionus spp. (15.5%, n = 765), nauplii (13.9%, n = 686), copepods of the Cyclops spp. (11.3%, n = 560) and Diaptomus spp. (10.0%, n = 496), and rotifers of the Polyarthra spp. (7.5%, n = 374). As nauplius and metanauplius are early larval stages of crustaceans, these were treated as subgroups. For organisms for which identification to species was achieved, a total of 38 species were identified, and of these, 33 were present in Bakerganj and 27 in Mathbaria. Together, 11 genera or subgroups and four species were present in all sampling sites in the two geographical areas (Table 1), mainly freshwater genera and species. Taxa exclusive to one area comprised 18 genera and 11 species in Bakerganj and 14 genera and 5 species in Mathbaria (Table 2).
Table 1.
Ubiquitous genera and species of zooplankton observed at all sites of both areas of the study
| Group | Genus or species |
|---|---|
| Cladocera | Diaphanosoma |
| Moina | |
| Copepods | Cyclops |
| Diaptomus | |
| Nauplii | Metanauplius |
| Nauplius | |
| Protozoa | Difflugia |
| Rotifers | Asplanchna |
| Brachionus | |
| Filinia | |
| Polyarthra | |
| Brachionus angularis | |
| Brachionus diversicornis | |
| Brachionus falcatus | |
| Brachionus forficula |
Table 2.
Genera and species of zooplankton identified in Bakerganj and Mathbaria, Bangladesh, and exclusive to those areas
| Group | Genus or species |
|---|---|
| Bakerganj | |
| Cladocera | Bosmina coregoni |
| Bosmina longirostris | |
| Daphnia lumholtzi | |
| Daphnia | |
| Moinodaphnia | |
| Copepods | Heliodiaptomus latifi |
| Mesocyclops dybowskii | |
| Ostracoda | Herpetocypris/Heterocypris |
| Protozoa | Amphizonella |
| Endosphaera | |
| Pyxidicula | |
| Rotifers | Ascomorpha |
| Asplanchnopus | |
| Brachionus bidentata | |
| Brachionus calyciflorus | |
| Harringia | |
| Hexarthra intermedia | |
| Notommata | |
| Platyias patulus | |
| Platyias | |
| Rotaria neptunia | |
| Trichocerca longiseta | |
| Mathbaria | |
| Cladocera | Alonella |
| Scapholeberis kingi | |
| Scapholeberis | |
| Protozoa | Acanthocystis |
| Awerintzewia | |
| Frontonia | |
| Paraquadrula | |
| Vasicola ciliata | |
| Vasicola | |
| Rotifers | Brachionus donneri |
| Cephalodella | |
| Filinia camascela | |
| Lecane ohioensis | |
| Lepadella | |
| Monommata | |
| Notholca |
Zooplankton population heterogeneity was analyzed by pond or lake sampling site. Of the total identifications for each sampling site, maximum species richness at the genus level in Bakerganj was observed at sites 5 and 6, with 26 genera identified at each site, both of which are directly or indirectly connected to a nearby river. Site 5 is a lake connected to a river that flows into the Bay of Bengal, whereas site 6 is a large man-made pond, popularly named “Dighi,” channeled to a river by a controlled valve to maintain the level of this drinking water source. High plankton diversity observed at sites 5 and 6 is considered to be a consequence of the mixing of local and adjacent plankton communities or an intermediate disturbance. Minimum species richness in Bakerganj was observed at sites 3 and 8, with 21 genera enumerated at each site, both of which are shallow ponds surrounded by trees. Shade produced by the trees likely reduces phytoplankton abundance by limiting daily light availability and hence limits the food supply for the zooplankton, with the effect of reducing the zooplankton diversity. Twenty-five genera were identified at the four other sites.
In Mathbaria, the largest number of genera was identified at site 4 (n = 31), a man-made pond located near a market bazaar containing more than 50 shops, which was maintained to meet daily drinking water needs. Interestingly, this pond was abandoned after the first V. cholerae O139 outbreak occurred in this area in 1993 (3, 31) because V. cholerae O139 was readily detected in the pond at that time. The smallest number of genera was observed at site 1 (n = 19), a pond used for fish culture. The numbers of genera identified at the four remaining sites were 28, 27, 25, and 24 for sites 2, 6, 3, and 5, respectively. At all sites, rotifers comprised the dominant group, with the numbers of genera identified ranging from 6 to 13, depending on the site. At least two and up to seven genera of copepods, cladocerans, and protozoa were identified. Two genera of ostracods, Cypris sp. and Herpetocypris/Heterocypris sp., were found in Bakerganj, and only a single genus of Ostracoda, Cypris sp., was found at three sites in Mathbaria.
By examining the relative abundance over the entire period of the study, rotifers were found to be the most abundant group, followed by nauplii, copepods, and cladocerans. Brachionus spp. were the most abundant genus or subgroup, followed by metanauplii, nauplii, Polyarthra spp., Cyclops spp., Diaptomus spp., and Keratella spp. (Table 3).
Table 3.
Relative abundance of zooplankton groups and genera
| Group | Genus | No. of times organism identifieda |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bakerganj |
Mathbaria |
Total | ||||||||||
| GR1 | GR2 | GR3 | GR4 | Subtotal | GR1 | GR2 | GR3 | GR4 | Subtotal | |||
| Nauplii | Metanauplius | 111 | 177 | 25 | 2 | 315 | 132 | 286 | 35 | 5 | 1,461 | 458 |
| Rotifers | Brachionus | 200 | 61 | 23 | 28 | 312 | 348 | 75 | 21 | 9 | 1,668 | 453 |
| Nauplii | Nauplius | 108 | 154 | 18 | 2 | 282 | 124 | 241 | 32 | 7 | 1,290 | 404 |
| Copepods | Cyclops | 11 | 60 | 156 | 46 | 273 | 9 | 47 | 189 | 42 | 597 | 287 |
| Copepods | Diaptomus | 8 | 51 | 116 | 38 | 213 | 8 | 66 | 178 | 31 | 617 | 283 |
| Rotifers | Polyarthra | 99 | 31 | 9 | 5 | 144 | 170 | 41 | 14 | 5 | 836 | 230 |
| Cladocera | Diaphanosoma | 8 | 11 | 46 | 66 | 131 | 4 | 15 | 47 | 64 | 219 | 130 |
| Cladocera | Moina | 6 | 8 | 28 | 56 | 98 | 2 | 6 | 20 | 53 | 119 | 81 |
| Rotifers | Keratella | 25 | 12 | 12 | 4 | 53 | 62 | 20 | 4 | 0 | 316 | 86 |
| Protozoa | Difflugia | 3 | 7 | 15 | 30 | 55 | 4 | 10 | 27 | 44 | 144 | 85 |
| Rotifers | Filinia | 32 | 14 | 7 | 5 | 58 | 53 | 12 | 4 | 1 | 257 | 70 |
| Rotifers | Trichocerca | 23 | 6 | 1 | 2 | 32 | 57 | 11 | 7 | 1 | 276 | 76 |
| Rotifers | Asplanchna | 23 | 5 | 1 | 4 | 33 | 8 | 9 | 3 | 0 | 65 | 20 |
| Rotifers | Horaella | 4 | 4 | 2 | 2 | 12 | 9 | 6 | 2 | 0 | 58 | 17 |
| Copepods | Mesocyclops | 2 | 5 | 12 | 6 | 25 | 0 | 5 | 10 | 3 | 38 | 18 |
| Protozoa | Glaucoma | 0 | 4 | 13 | 6 | 23 | 0 | 2 | 7 | 1 | 21 | 10 |
The GR no. corresponds to the categorical group of abundance from GR1, the most abundant, through GR4, the least abundant. Values in the GR columns correspond to the number of times the organism was identified as that categorical group of abundance. Only the top 16 groups are shown.
Seasonal patterns of the major zooplankton groups.
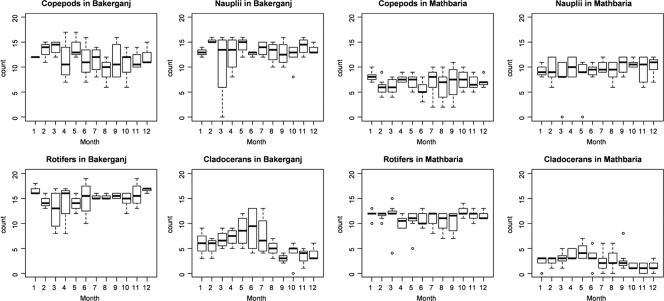
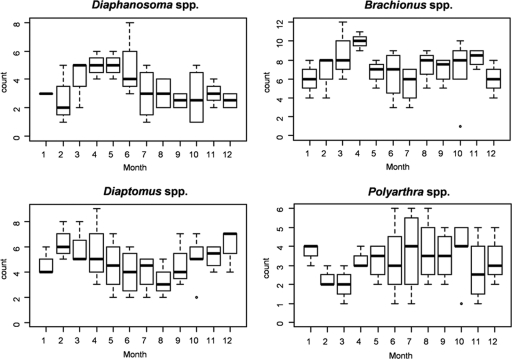
Figure 2 shows the monthly distribution of the four dominant groups of zooplankton over the 45-month sampling at Bakerganj and Mathbaria. Each group was present throughout the year, but two seasonal patterns were observed (with the understanding that variability within certain sampling periods was too large to conclude the patterns to be statistically significant), namely, that copepods and cladocerans occurred seasonally in Bakerganj. For example, the abundance of copepods was highest during the months of February through May and lowest in August and September. Cladocerans gradually increased in number from February until June and were less frequent from August until December. The same pattern for cladocerans occurred, although less pronounced, in Mathbaria. Of the genera most frequently identified in Bakerganj (Table 3), only four showed a seasonal pattern, i.e., Diaphanosoma spp. (cladocerans), Brachionus spp. (rotifers), Diaptomus spp. (copepods), and Polyarthra spp. (rotifers) (Fig. 3). In Mathbaria, the same organisms also varied in a seasonal pattern, but the pattern was less pronounced (results not shown).
Fig. 2.
Monthly distribution of the accumulated number of times dominant zooplankton groups were determined over each area during 45 months of sampling from March 2004 to September 2007 at Bakerganj and Mathbaria, Bangladesh (see Materials and Methods for details).
Fig. 3.
Monthly distribution of genera according to the number of times they were dominant (see Materials and Methods for details) during the 45-month sampling period from March 2004 to September 2007 in Bakerganj, Bangladesh.
Clinical surveillance.
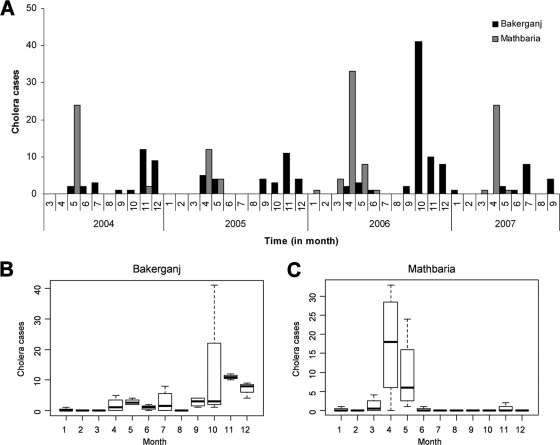
Of 616 rectal swabs collected in Bakerganj between January 2004 and September 2007, 144 (23.4%) were positive for V. cholerae O1 by culture (Fig. 4A). None of the samples yielded positive V. cholerae O139 cultures. The absence of V. cholerae O139 contrasts with the results of a study conducted at the same location with 70 cases of V. cholerae O139 recorded between June 1997 and December 2001 (30), indicating this serogroup had decreased in clinical incidence in this region. The seasonal pattern of cholera caused by V. cholerae O1 in Bakerganj, observed from cumulative cholera cases by month, showed a spring peak in April and May and a significantly larger number of cases from October to December (Fig. 4B).
Fig. 4.
(A) Temporal pattern of cholera caused by Vibrio cholerae O1 from March 2004 to April 2007. Seasonal patterns of cholera in Bakerganj and Mathbaria, Bangladesh, are shown in panels B and C, respectively.
Of 690 rectal swabs collected in Mathbaria between January 2004 and September 2007, 125 (18.1%) were confirmed positive for V. cholerae, with 115 (92%) of these being V. cholerae serogroup O1 (Fig. 4C). The remaining 10 (8%) were positive for V. cholerae serogroup O139. The V. cholerae O139 cases occurred between 19 March and 5 April 2005. In contrast to Bakerganj, the seasonal pattern of cholera in Mathbaria showed a significantly large outbreak of cholera in the spring months, between April and May, with only a few cases occurring in November 2004 (n = 2) and January 2006 (n = 1). This pattern is characteristic of this region, which is located very close to the coast of the Bay of Bengal.
Relationship of Vibrio cholerae O1 and O139, ctxA, and zooplankton.
In Bakerganj, 14 genera or groups and 9 species were analyzed over 44 (no lag period) and 43 (lag period of 4 weeks) samplings. The corrected risk α with the Bonferonni method for multiple comparison procedure was equal to 0.05/14 = 0.0036 and 0.05/9 = 0.0055 for genus- or group-level comparisons and for species-level comparisons, respectively. None of the associations was significant between detection of Vibrio cholerae O1 and O139, ctxA, and the occurrence of zooplankton genera or groups and species, either simultaneously or with a lag (earlier) of 4 weeks in Bakerganj.
In Mathbaria, 14 genera or groups and 11 species were investigated over the 88 (no time lag), 87 (lag of 2 weeks), and 86 (lag of 4 weeks) samplings. The corrected risk α with the Bonferroni method for multiple comparison procedure was equal to 0.05/33 = 0.0036 and 0.05/11 = 0.0045 for the genus- or group-level comparisons and for species-level comparisons, respectively. At the genus or group level, none of the comparisons were significant. At the species level, the presence of the rotifer Brachionus angularis was significantly associated with presence of V. cholerae O139 and with ctxA at a lag of 2 weeks, with P values equal to 0.0016 and 0.0013, respectively. At a lag of 4 weeks, Brachionus angularis was significantly associated with V. cholerae O1 and ctxA molecular detections, with P values equal to 0.00006 and 0.00004, respectively. B. angularis is a common freshwater rotifer species considered to be a detritivore. Tamplin et al. (33) found that Vibrio cholerae serogroup O1 attached preferentially to exuviae of zooplankton, including the rotifer Brachionus sp., in samples collected from the rivers and ponds of Matlab, Bangladesh.
Other associations, which might be of ecological interest for the purpose of screening and which were observed in this study, were close to the significance level, employing the corrected risk α by the Bonferonni method, which is known to be conservative in nature. These included cladocerans of the Moina spp. and the presence of ctxA at lag of 4 weeks in Bakerganj and in Mathbaria, with P values equal to 0.006 and 0.0065, respectively. Cladocerans of Moina spp. also were detected with the presence of V. cholerae O139 at a lag of 2 weeks in Mathbaria (P = 0.0088). Moina spp. of cladocerans are primitive freshwater crustaceans found in eutrophic ponds and temporary waters, both tropical and temperate, with fish as their predators.
These results indicate specific zooplankton not previously known to be associated with toxigenic V. cholerae O1 and V. cholerae O139 and should be targeted for further investigation with respect to their ecology and population dynamics in the water bodies of Bangladesh, where they may serve as potential reservoirs and amplifiers of V. cholerae. Indeed, the ability of strains and serogroups of V. cholerae to colonize zooplankton preferentially should prove a productive path for further investigation. As shown by Rawlings et al. (28), colonization of zooplankton by pathogenic strains of V. cholerae can play a significant role in the abundance of V. cholerae, especially in the environment of rural Bangladesh, where drinking water is taken directly from ponds and rivers without treatment. Since cholera is a dose-dependent disease (5), enhanced numbers of V. cholerae O1 and O139 in drinking water will influence cholera outbreak dynamics. Detection of zooplankton species and determination of selected association of V. cholerae with the individual taxa using molecular genetics and genomic methods may allow early warning of the presence of pathogenic strains of V. cholerae and the presence of pathogenic strains of V. cholerae and possible prevention of the disease in the human population by simple methods of filtration (8, 16).
Association between occurrence of cholera and presence of specific zooplankton.
In Bakerganj, the presence of the rotifer Brachionus angularis at a lag of 4 weeks was found to be significantly associated with occurrence of cholera caused by V. cholerae O1 (P = 0.0017), and in Mathbaria, the rotifer Brachionus forficula at lags of 0 weeks and 4 weeks was significantly associated with occurrence of cholera caused by V. cholerae O1, with P values equal to 0.0023 and 0.0025, respectively.
As before, there were a number of other associations that were close to the significance level, with the correction of the risk α by the Bonferonni method. In Bakerganj, cladocerans of Moina spp. and Diphanosoma spp. at a lag of 4 weeks were associated with cholera caused by V. cholerae O1 (P = 0.005 and 0.0082, respectively). At the species level, the rotifer Brachionus angularis at a lag of 0 weeks and Brachionus forficula at a lag of 4 weeks were associated with cholera caused by V. cholerae O1, with P values equal to 0.0067 and 0.0080, respectively. In Mathbaria, Diphanosoma spp. of cladocerans, at a lag of 0 weeks, and Moina spp., at a lag of 4 weeks, were associated with cholera caused by V. cholerae O1 (P = 0.0099 and 0.0066, respectively). At the species level, the rotifer Brachionus angularis at lags of 2 and 4 weeks and Brachionus diversicornis at a lag of 0 weeks were associated with occurrence of cholera caused by V. cholerae O1, with P values equal to 0.0049, 0.0051, and 0.0098, respectively. These specific associations observed between zooplankton in water samples collected from the village water source and the occurrence of cholera with those village populations merit further investigation employing molecular genetics and genomic methods for detection of the presence of V. cholerae on and/or in individual organism of these taxa because these organisms likely play a significant role in the ecology and transmission of epidemic serogroups of V. cholerae that cause cholera.
Among zooplankton significantly associated with occurrence of cholera or presence of toxigenic strains of V. cholerae, the rotifer Brachionus angularis is a species of particular interest for future studies of the ecology of V. cholerae. Brachionus angularis is typically a freshwater species indicator of high trophic levels in water bodies; however, this rotifer is also found in marine water bodies. Two other rotifer species, Brachionus forficula and B. diversicornis, were associated with occurrence of V. cholerae O1 cases of cholera in Mathbaria. Cladocerans of the Moina and Diphanosoma spp. were associated with occurrence of V. cholerae O1 cholera or the ctxA gene in water samples in Bakerganj, and V. cholerae O1 cholera, ctxA, and V. cholerae O139 in water samples collected in Mathbaria. Cladocerans merit further study since they have been shown to be a vector of paralytic shellfish toxins active against fish larvae (17). Future studies should investigate the presence of pathogenic strains of V. cholerae in or on those organisms and their potential capability to serve as hosts for pandemic strains of V. cholerae.
Association between occurrence of cholera and Vibrio cholerae O1 and O139, and ctxA.
For Bakerganj, significant associations were found between occurrence of cholera caused by V. cholerae O1 and the presence of V. cholerae O1 in the environment at lags of 0 and 4 weeks (P = 0.008 and 0.002, respectively). These results suggest that detection of V. cholerae O1 in water samples provides at least a 4-week early warning of a cholera outbreak in the population of Bakerganj. Significant association was not observed for the presence of V. cholerae O1 or the detection of ctxA alone or together with V. cholerae O1 in the environment 4 weeks after the occurrence of cholera caused by V. cholerae O1, indicating that once a cholera outbreak occurs, other factors become dominant.
In Mathbaria, significant association was observed between occurrence of cholera caused by V. cholerae O1 and the presence of ctxA alone (P = 0.0018) or combined with V. cholerae O1 (P = 0.0027) in environmental samples at a lag of 0 weeks. None of the associations were significant 2 and 4 weeks earlier. In contrast to Bakerganj, a highly significant association was found between occurrence of cholera caused by V. cholerae O1 2 weeks earlier and detection of ctxA or the combination of V. cholerae O1 and ctxA (P = 0.0006 and 0.0011, respectively). Bakerganj is more densely populated than Mathbaria, with a cholera dynamic of two peaks per year—April to May and September to December. Mathbaria, less populated, is located in a very-low-lying coastal area, with a single cholera outbreak per year during March to May and with sporadic cases in November. The contrast between the two locations highlights local specificity, even though both are geographically close, indicating the need for fine-scale data analysis to understand underlying processes in cholera epidemiology. The argument has been made that cholera outbreaks are local in their source (32), and DNA-based methods are being applied to confirm this hypothesis, results of which will be published elsewhere.
In conclusion, significant associations between certain members of the zooplankton community and occurrence of cholera in both Bakerganj and Mathbaria, Bangladesh, have been found, along with significant differences in zooplankton diversity, incidence of cholera, and their association in the two locations Bakerganj and Mathbaria. Local differences, as observed in this study, indicate there are more subtle ecological factors that can influence interaction between V. cholerae and its plankton host and that these factors are host related.
ACKNOWLEDGMENTS
Support for the research was provided by National Institutes of Health grant no. 1 R01 A139129-01, National Oceanic and Atmospheric Administration (NOAA) grant no. S0660009, and National Science Foundation grant no. 0813066. C.J.G. was supported by an IC Postdoctoral Research Fellowship (NGA grant no. HM15820612010). The International Centre for Diarrheal Disease Research, Bangladesh is supported by donor countries and agencies that provide unrestricted support to the center for its operation and research.
Footnotes
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Alam M., et al. 2006. Seasonal cholera caused by Vibrio cholerae serogroups O1 and O139 in the coastal aquatic environment of Bangladesh. Appl. Environ. Microbiol. 72:4096–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alam M., et al. 2006. Effect of transport at ambient temperature on detection and isolation of Vibrio cholerae from environmental samples. Appl. Environ. Microbiol. 72:2185–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albert M. J., et al. 1993. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet 341:704. [DOI] [PubMed] [Google Scholar]
- 4. Bagchi K., et al. 1993. Epidemic of diarrhea caused by Vibrio cholerae non-O1 that produced heat-stable toxin among Khmers in a camp in Thailand. J. Clin. Microbiol. 31:1315–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cash R. A., et al. 1974. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J. Infect. Dis. 129:45–52 [DOI] [PubMed] [Google Scholar]
- 6. Chowdhury M. A., Huq A., Xu B., Madeira F. J., Colwell R. R. 1997. Effect of alum on free-living and copepod-associated Vibrio cholerae O1 and O139. Appl. Environ. Microbiol. 63:3323–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colwell R. R., Huq A. 1994. Environmental reservoir of Vibrio cholerae. The causative agent of cholera. Ann. N. Y. Acad. Sci. 740:44–54 [DOI] [PubMed] [Google Scholar]
- 8. Colwell R. R., et al. 2003. Reduction of cholera in Bangladeshi villages by simple filtration. Proc. Natl. Acad. Sci. U. S. A. 100:1051–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reference deleted.
- 10. Glass R. I., et al. 1982. Endemic cholera in rural Bangladesh, 1966-1980. Am. J. Epidemiol. 116:959–970 [DOI] [PubMed] [Google Scholar]
- 11. Hoshino K., et al. 1998. Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139. FEMS Immunol. Med. Microbiol. 20:201–207 [DOI] [PubMed] [Google Scholar]
- 12. Hunt D. E., Gevers D., Vahora N. M., Polz M. F. 2008. Conservation of the chitin utilization pathway in the Vibrionaceae. Appl. Environ. Microbiol. 74:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huq A., et al. 1990. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl. Environ. Microbiol. 56:2370–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huq A., et al. 2005. Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl. Environ. Microbiol. 71:4645–4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huq A., et al. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 45:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huq A., et al. 1996. A simple filtration method to remove plankton-associated Vibrio cholerae in raw water supplies in developing countries. Appl. Environ. Microbiol. 62:2508–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang T.-J., Wang D.-Z., Niu T., Xu Y.-X. 2007. Trophic transfer of paralytic shellfish toxins from the cladoceran (Moina mongolica) to larvae of the fish (Sciaenops ocellatus). Toxicon 50:639–645 [DOI] [PubMed] [Google Scholar]
- 18. Reference deleted.
- 19. Kay B. A., Bopp C. A., Wells J. G. 1994. Isolation and identification of Vibrio cholerae O1 from fecal specimens, p. 3–26In Wachsmuth I. K., Blake P. A., Olsvik O. (ed.), Vibrio cholerae and cholera. ASM Press, Washington, DC [Google Scholar]
- 20. Reference deleted.
- 21. Kirschner A. K., et al. 2008. Rapid growth of planktonic Vibrio cholerae non-O1/non-O139 strains in a large alkaline lake in Austria: dependence on temperature and dissolved organic carbon quality. Appl. Environ. Microbiol. 74:2004–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Longini I. M., Jr., et al. 2002. Epidemic and endemic cholera trends over a 33-year period in Bangladesh. J. Infect. Dis. 186:246–251 [DOI] [PubMed] [Google Scholar]
- 23. Matz C., Kjelleberg S. 2005. Off the hook—how bacteria survive protozoan grazing. Trends Microbiol. 13:302–307 [DOI] [PubMed] [Google Scholar]
- 24. Morris J. G., Jr 1994. Non-O group A Vibrio cholerae strains not associated with epidemic disease, p. 103–116In Wachsmuth I. K., Blake P. A., Olsvik O.(ed.), Vibrio cholerae and cholera. ASM Press, Washington, DC [Google Scholar]
- 25. Nalin D. R., Daya V., Reid A., Levine M. M., Cisneros L. 1979. Adsorption and growth of Vibrio cholerae on chitin. Infect. Immun. 25:768–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reference deleted.
- 27. Pruzzo C., Vezzulli L., Colwell R. R. 2008. Global impact of Vibrio cholerae interactions with chitin. Environ. Microbiol. 10:1400–1410 [DOI] [PubMed] [Google Scholar]
- 28. Rawlings T. K., Ruiz G. M., Colwell R. R. 2007. Association of Vibrio cholerae O1 El Tor and O139 Bengal with the copepods Acartia tonsa and Eurytemora affinis. Appl. Environ. Microbiol. 73:7926–7933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rivera I. N. G., et al. 2003. Method of DNA extraction and application of multiplex PCR to detect toxigenic Vibrio cholerae O1 and O139 from aquatic ecosystems. Environ. Microbiol. 5:599–606 [DOI] [PubMed] [Google Scholar]
- 30. Sack R. B., et al. 2003. A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. J. Infect. Dis. 187:96–101 [DOI] [PubMed] [Google Scholar]
- 31. Siddique A. K., et al. 1994. Emergence of a new epidemic strain of Vibrio cholerae in Bangladesh. An epidemiological study. Trop. Geogr. Med. 46:147–150 [PubMed] [Google Scholar]
- 32. Stine O. C., et al. 2008. Seasonal cholera from multiple small outbreaks, rural Bangladesh. Emerg. Infect. Dis. 14:831–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamplin M. L., Gauzens A. L., Huq A., Sack D. A., Colwell R. R. 1990. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl. Environ. Microbiol. 56:1977–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Turner J. W., Good B., Cole D., Lipp E. K. 2009. Plankton composition and environmental factors contribute to Vibrio seasonality. ISME J. 3:1082–1092 [DOI] [PubMed] [Google Scholar]
- 35. Wallace R. L., Snell T. W. 1991. Rotifera, p. 187–248In Thorp J. H., Covich A. P. (ed.), Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego, CA [Google Scholar]