Abstract
We demonstrated direct assimilation of cellooligosaccharide using Escherichia coli displaying beta-glucosidase (BGL). BGL from Thermobifida fusca YX (Tfu0937) was displayed on the E. coli cell surface using a novel anchor protein named Blc. This strain was grown successfully on 0.2% cellobiose, and the optical density at 600 nm (OD600) was 1.05 after 20 h.
TEXT
The utilization of biomass as a source of renewable, environmentally friendly energy and/or chemicals has attracted much attention because of the depletion of fossil fuels and increasing environmental problems. Lignocellulosic biomass is regarded as a promising feedstock because it is abundant, inexpensive, and renewable and has favorable environmental properties (11). However, it requires costly and complex hydrolysis steps, such as pretreatment and/or lengthy cellulase treatment (11). Therefore, an efficient and cost-effective method for degradation and fermentation of lignocellulosic biomass into commodity products is required.
Escherichia coli is a promising host for the production of a variety of useful compounds and can metabolize all major sugar monomers existing in plant biomass (1). Metabolic engineering allows for the introduction of desirable pathways to produce target compounds, such as ethanol and other alcohols (2, 4, 10) and lactic acids and other organic acids (3, 17). Since E. coli cannot utilize cellobiose and cellooligosaccharides, most compounds are produced from monomeric sugars (i.e., glucose and/or xylose) as carbon sources. Although some E. coli isolates can assimilate cellobiose, their slow growth is a major limiting factor (8).
Efficient degradation of cellulose requires a synergistic reaction of the cellulolytic enzymes endoglucanase (EG), cellobiohydrolase (CBH), and beta-glucosidase (BGL). The cellulose is degraded by EG and CBH, resulting in cellobiose and some cellooligosaccharides, which can be converted to glucose by BGL. BGL catalyzes the final step in cellulose degradation as well as stimulates cellulose hydrolysis by relieving the cellobiose-mediated inhibition of EG and CBH (16). Many kinds of EGs have been expressed in E. coli for cellulose degradation. However, the enzymatic activity is insufficient for cellulose degradation because of the low secretion of proteins into the medium (15). In the case of BGL, there is only one study in which BGL was expressed using E. coli grown on cellobiose (5).
Here, we developed cellooligosaccharide-assimilating E. coli by expressing BGL on the cell surface using a novel anchor protein. Generally, the protein-secretive ability of E. coli is relatively low compared to those of other microorganisms (15). Alternatively, cell surface display allows for efficient transport of target proteins into the membrane guided by an anchor protein. Therefore, an appropriate anchor protein is an important feature of active protein display (6, 7, 12, 13, 14).
The bacterial strains, oligonucleotides, and plasmids used in this study are listed in Table 1. E. coli strain BW25113 (National Institute of Genetics, Japan) and E. coli strain JCM20137 (Japan Collection of Microorganisms, RIKEN BRC, which is participating in the National BioResource Project of the MEXT, Japan) were used as host strains. Minimal medium containing 0.2% cellobiose (Sigma-Aldrich Corp., St. Louis, MO) or cellooligosaccharide (Seikagaku Co., Tokyo, Japan) (0.6% Na2HPO4, 0.3% K2HPO4, 0.05% NaCl, 0.1% NH4Cl, 1 mM MgSO4, 0.001% thiamine, 0.1 mM CaCl2) was used for growth analysis. The plasmids for BGL expression on the cell surface using the PgsA anchor protein (9) were constructed as follows. The gene encoding BGL from Clostridium cellulovorans was amplified by PCR from genomic DNA of C. cellulovorans using the BglA_F and BglA_R primers. The amplified gene fragment was digested with BamHI/SpeI and ligated into plasmid pHLA (9). The resultant plasmid was named BglA-pHLA. Other BGL expression vectors were also constructed similarly (Table 1). The gene encoding BGL (Tfu0937) from Thermobifida fusca YX were amplified by PCR using the Tfu0937_F and Tfu0937_R primers. The genes encoding BGLs (Sde0245 and Sde2497) from Saccharophagus degradans 2-40 were amplified by PCR using the Sde0245_F and Sde0245_R or Sde2497_F and Sde2497_R primer pair. The genes encoding BGLs CHU2268 from Cytophaga hutchinsonii and Rumal_1801 from Ruminococcus albus were amplified by PCR using the CHU2268_F and CHU2268_R or Rumal_1801_F and Rumal_1801_R primer pair. Each of the amplified fragments was digested by XhoI/HindIII (Tfu0937), BamHI/XhoI (Sde0245, CHU2268, and Rumal_1801), or BamHI/SpeI (Sde2497) and ligated into plasmid pHLA. The resultant plasmids were named Tfu0937-pHLA, Sde0245-pHLA, Sde2497-pHLA, CHU2268-pHLA, and Rumal_1801-pHLA (Table 1). All plasmids were introduced into E. coli BW25113 or JCM20137 by electroporation using a Gene Pulser (Bio-Rad Laboratories, Hercules, CA), according to the manufacturer's procedure.
Table 1.
Strains, DNA, plasmids, and primers used in this study
| Strain, genomic DNA, plasmid, or primer | Relevant phenotype, description, or sequence (5′–3′)a | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| NovaBlue | endA1 hsdR17(rK12− mK12+) supE44 thi-1 recA1 gyrA96 relA1 lac[F′ proAB+lacIqZΔM15::Tn10 (Tetr)]; host for DNA manipulation | Novagen |
| BW25113 | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ−rph-1 Δ(rhaD-rhaB)568 hsdR514 | National Institute of Genetics, Japan |
| JCM20137 | B strain | Japan Collection of Microorganisms, RIKEN BRC |
| Genomic DNA | ||
| Clostridium cellulovorans | Genomic DNA was prepared by standard DNA extraction method from Clostridium cellulovorans 743B (ATCC 35296) | ATCC |
| Thermobifida fusca | YX (ATCC BAA-629D-5) | ATCC |
| Saccharophagus degradans | 2-40 (ATCC 43961D-5) | ATCC |
| Cytophaga hutchinsonii | ATCC 33406D-5 | ATCC |
| Ruminococcus albus | ATCC 27210 | ATCC |
| Plasmids | ||
| pHLA | Vector under HCE promoter control; carrying PgsA anchor protein | 10 |
| BglA-pHLA | Vector for BGL (BglA) expression using PgsA anchor protein | This study |
| Tfu0937-pHLA | Vector for BGL (Tfu0937) expression using PgsA anchor protein | This study |
| Sde0245-pHLA | Vector for BGL (Sde0245) expression using PgsA anchor protein | This study |
| Sde2497-pHLA | Vector for BGL (Sde2497) expression using PgsA anchor protein | This study |
| CHU2268-pHLA | Vector for BGL (CHU2268) expression using PgsA anchor protein | This study |
| Rumal_1801-pHLA | Vector for BGL (Rumal_1801) expression using PgsA anchor protein | This study |
| Blc-Tfu0937-pHLA | Vector for Tfu0937 expression using Blc anchor protein; the C terminus of Blc was fused to the N terminus of Tfu0937 | This study |
| Slp-Tfu0937-pHLA | Vector for Tfu0937 expression using Slp anchor protein; the C terminus of Blc was fused to the N terminus of Tfu0937 | This study |
| HdeD-Tfu0937-pHLA | Vector for Tfu0937 expression using HdeD anchor protein; the C terminus of Blc was fused to the N terminus of Tfu0937 | This study |
| Tfu0937-Blc-pHLA | Vector for Tfu0937 expression using Blc anchor protein; the N terminus of Blc was fused to the C terminus of Tfu0937 | This study |
| Tfu0937-Slp-pHLA | Vector for Tfu0937 expression using Slp anchor protein; the N terminus of Blc was fused to the C terminus of Tfu0937 | This study |
| Tfu0937-HdeD-pHLA | Vector for Tfu0937 expression using HdeD anchor protein; the N terminus of Blc was fused to the C terminus of Tfu0937 | This study |
| Oligonucleotide primers | ||
| BglA_F | CGCGGATCCATGGAAAAGCTAAGATTTCCCAAAGATTTTATTTTT | |
| BglA_R | GGACTAGTTTATAAATCTTCTTCACTAATTAATTTTTGTTCCTTATTAGATCTTTCTATAAGCTCCTTATACCAATAAG | |
| Tfu0937_F | CGACTCTAGACTCGAGGTGACCTCGCAATCGACGACTCCTCTGGGCAATCTGGAGGAGACTCCCAAACCGGATATCCGC | |
| Tfu0937_R | CAAAACAGCCAAGCTTCTATTCCTGTCCGAAGATTCCCCCGTTGCGCATCACCCGGGAGTACCACCAGCCGCTGTCC | |
| Sde0245_F | GCGGATCCATGCTCAAAAAGATAAACAAGAAAGGTCTTGCTTTAAGC | |
| Sde0245_R | CGAGCTCGAGTTAGTCACACTTAATAGCTGCGCTATCTGCACCGCCAGG | |
| Sde2497_F | GCGGATCCATGAAAAATACTTTATCCTTTAAAACATCCTTGCTTGCGGGC | |
| Sde2497_R | GCATACTAGTGTCGACCTATTCGCCCAGCATTTTTTTAAGGGTGGGTTCCATCGC | |
| CHU2268_F | GCGGATCCATGAAAAAAATAACCGTATTGATTTCCATCTGGCTCAGTGCAGCCGC | |
| CHU2268_R | ATAGCTCGAGTTACTCATTAAAATATATTTCTGTCTGGAGATTTCCG | |
| Rumal_1801_F | GCTGGATCCATGATAAAGCTTGATTGGAACGAATATCTCGAAAAGGCAGCAGAGGTAAACGC | |
| Rumal_1801_R | ATGCCTCGAGTTAATCGATAAGCACGGCGTCCTCGAAGCAGCTTTTTACTTTGAAAGTTCGC | |
| Tfu0937_F2 | AAAAGACCAGATCTGGCGGCCGCTACCTCGCAATCGACGACTCCTCTG | |
| Tfu0937_R2 | ACAGCCAAGCTTCTAAGCCTTATCGTCGTCATCCTTGTAATCGGATCCTTCCTGTCCGAAGATTCCCCCGTTGCG | |
| Tfu0937_F3 | TGGAAAAAGGAGATCTGATGGTGACCTCGCAATCGACGACTCCTCTGGGCAATCTGGAGGAGACTCCCAAACCGGATATC | |
| Tfu0937_R3 | CAAAACAGCCAAGCTTTTAAGCGGCCGCCTCGAGTTCCTGTCCGAAGATTCCCCCGTTGCGCATCACCCGGGAGTACC | |
| Blc_F1 | TGGAAAAAGGAGATCTGATGCGCCTGCTCCCTCTCGTTGCCGCAGCGACAGCTGCATTTCTGGTCGTTGCC | |
| Blc_R1 | TTGCGAGGTAGCGGCCGCACTACCAGGCTGCTGTACCCAAATAAATTTACTGACATCAAACCCTTCCCGGGTCGCG | |
| HdeD_F1 | TGGAAAAAGGAGATCTGATGTTATATATAGATAAGGCAACAATTTTGAAGTTTGATCTGGAGATGC | |
| HdeD_R1 | TTGCGAGGTAGCGGCCGCTTGCTGCTTAACGAACAAACTGGCGAAGCTGAACAGGCTGGCGGCGC | |
| Slp_F1 | TGGAAAAAGGAGATCTGATGAACATGACAAAAGGTGCACTCATCCTCAGCCTTTCATTTTTGC | |
| Slp_R1 | TTGCGAGGTAGCGGCCGCTTTGACCAGCTCAGGTGTTACCTGACTCACCGCATTGGTGTAGTAAGGCGC | |
| Blc_F2 | CGGACAGGAACTCGAGATGCGCCTGCTCCCTCTCGTTGCCGCAGCGACAGCTGCATTTCTGGTCGTTGCC | |
| Blc_R2 | AGCTTTTAAGCGGCCGCCTAACTACCAGGCTGCTGTACCCAAATAAATTTACTGACATCAAACCCTTCCCGGG | |
| Slp_F2 | CGGACAGGAACTCGAGATGAACATGACAAAAGGTGCACTCATCCTCAGCCTTTCATTTTTGCTTGCCGC | |
| Slp_R2 | AGCTTTTAAGCGGCCGCTTATTTGACCAGCTCAGGTGTTACCTGACTCACCGCATTGGTGTAGTAAGGCGC |
HCE, high-level constitutive expression.
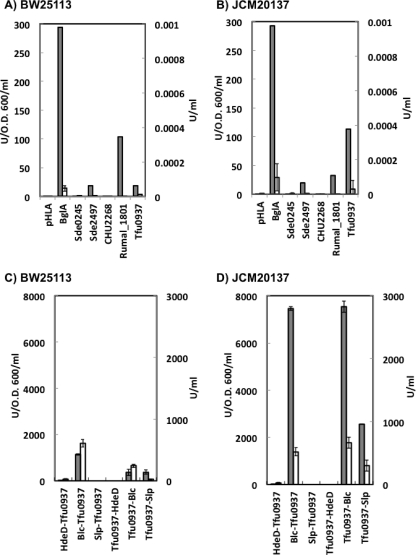
These BGL activities on the cell surface or in culture medium were quantitatively evaluated using p-nitrophenyl-β-d-glucopyranoside (pNPG; Nacalai Tesque, Inc., Kyoto, Japan) as a substrate. One unit of beta-glucosidase activity was defined as the amount of enzyme producing 1 μmol/min p-nitrophenol at 37°C and pH 5.0. As shown in Fig. 1 A, PgsA-BglA showed the highest BGL activity (294 U/optical density at 600 nm [OD600]/ml) in the case of E. coli BW25113, followed by PgsA-Rumal_1801 (103 U/OD600/ml) and PgsA-Tfu0937 (18 U/OD600/ml). In the case of E. coli JCM20137 (Fig. 1B), PgsA-BglA also showed the highest BGL activity (293 U/OD600/ml). In all strains, BGL activity on the cell surface was higher than that in the culture supernatant (under 0.0001 U/ml), showing that BGL was successfully expressed on the cell surface and retained its enzymatic function through the PgsA anchor protein.
Fig. 1.
BGL activity on the E. coli cell surface or in culture supernatant after 24 h of cultivation in LB medium. Gray bars show BGL activity on the cell surface (U/OD600/ml; left axis), and white bars show BGL activity in the culture supernatant (U/ml; right axis). (A and B) Each BGL was displayed on the E. coli cell surface using the PgsA anchor protein. (A) E. coli BW25113; (B) E. coli JCM20137. (C and D) Tfu0937 was displayed using various anchor proteins. In the case of HdeD-Tfu0937, Blc-Tfu0937, and Slp-Tfu0937, the C terminus of each anchor protein was fused to the N terminus of Tfu0937. In the case of Tfu0937-HdeD, Tfu0937-Blc, and Tfu0937-Slp, the N terminus of each anchor protein was fused to the C terminus of Tfu0937. (C) E. coli BW25113; (D) E. coli JCM20137. All data are averages from three independent experiments, and error bars represent standard deviations.
Using these strains, cell growth on 0.2% cellobiose as the sole carbon source was evaluated (data not shown). Interestingly, E. coli displaying Tfu0937 showed the highest growth compared to the other strains, even though the BGL activity of Tfu0937 was not the highest among them (Fig. 1A and B). The final OD600 values after 48 h of cultivation were approximately 0.41 for E. coli BW25113 and 0.85 for E. coli JCM20137. In the case of strains displaying BglA or Sde2497, the OD600 values after 48 h of cultivation were 0.24 (BglA) and 0.31 (Sde2497) for E. coli BW25113 and 0.38 (BglA) and 0.41 (Sde2497) for E. coli JCM20137. The strain carrying the pHLA control plasmid only did not have an increased OD600 (0.18 for E. coli BW25113 and 0.16 for E. coli JCM20137). These results clearly demonstrate that Tfu0937 was an appropriate BGL for cellobiose assimilation in E. coli.
We developed a novel anchor protein appropriate for highly active BGL display to improve the cellobiose-assimilating ability. Unexpectedly, previously reported anchor proteins, such as LamB, OmpT, and OmpL (6, 13), did not work for active BGL display (data not shown). Hence, novel anchor proteins Blc, Slp, and HdeD derived from E. coli were selected. The plasmids for Tfu0937 expression on the cell surface using various anchor proteins were constructed as follows. The gene fragment encoding Tfu0937 was amplified by PCR from T. fusca YX genomic DNA using the Tfu0937_F2 and Tfu0937_R2 primers. The amplified fragment was digested with BglII/HindIII and ligated into plasmid pHLA. The resultant plasmid was named ΔPgsA-c-Tfu0937-pHLA. The genes encoding Blc, Slp, and HdeD were amplified using the following primer pairs Blc_F1 and Blc_R1, Slp_F1 and Slp_R1, and HdeD_F1 and HdeD_R1, respectively. Each amplified fragment was digested with BglII/NotI and ligated into plasmid ΔPgsA-c-Tfu0937-pHLA. The resultant plasmids were named Blc-Tfu0937-pHLA, Slp-Tfu0937-pHLA, and HdeD-Tfu0937-pHLA. In these constructions, the C terminus of the anchor protein was fused to the N terminus of Tfu0937 (Table 1).
As shown in Fig. 1C and D, Blc-Tfu0937 showed the highest BGL activity, 1,140 U/OD600/ml for E. coli BW25113 and 7,446 U/OD600/ml for E. coli JCM20137. The BGL activity of Blc-Tfu0937 was approximately 65-fold higher than that of PgsA-Tfu0937 in both E. coli strains. These results suggest that the specific activity of the Blc-Tfu0937 fusion protein might be improved rather than increasing the amount of protein expressed, because the amounts of fusion proteins (i.e., PgsA-Tfu0937 and Blc-Tfu0937) evaluated by Western blotting were at almost the same levels (data not shown). As expected, the BGL activity on the cell surface was higher than that in the culture supernatant (605 U/ml in E. coli BW25113 and 520 U/ml in E. coli JCM20137), showing that BGL was expressed successfully on the cell surface and retained its enzymatic function through the novel anchor protein.
The site of the BGL/anchor protein fusion (i.e., N or C terminus) is an important determinant of the displayed BGL activity. We prepared Tfu0937-Blc, Tfu0937-Slp, and Tfu0937-HdeD, in which the C terminus of Tfu0937 was fused to the N terminus of the anchor protein Blc, Slp, or HdeD. The gene fragment encoding Tfu0937 was amplified by PCR from T. fusca YX genomic DNA using the Tfu0937_F3 and Tfu0937_R3 primers. The amplified fragment was digested with BglII/HindIII and ligated into plasmid pHLA. The resultant plasmid was named ΔPgsA-n-Tfu0937-pHLA. The genes encoding Blc, Slp, and HdeD were amplified using the following primer pairs Blc_F2 and BlcR2, Slp_F2 and Slp_R2, and HdeD_F2 and HdeD_R2, respectively. Each amplified fragment was digested with XhoI/HindIII and ligated into plasmid ΔPgsA-n-Tfu0937-pHLA. The resultant plasmids were named Tfu0937-Blc-pHLA, Tfu0937-Slp-pHLA, and Tfu0937-HdeD-pHLA. In these constructions, the N terminus of the anchor protein was fused to the C terminus of Tfu0937. Their pNPG degradation activities were evaluated (Fig. 1C and D). In the case of JCM20137 (Fig. 1D), Tfu0937-Blc showed high activity (7,535 U/OD600/ml), the same as that of Blc-Tfu0937. These results show that Blc enables the display of target proteins regardless of whether a fusion protein is achieved at the N or the C terminus. In the case of E. coli BW25113 (Fig. 1C), low activity was found for both Tfu0937-Blc (353 U/OD600/ml) and Tfu0937-Slp (377 U/OD600/ml). HdeD showed no BGL activity when it was fused to the C terminus of Tfu0937 as an anchor protein.
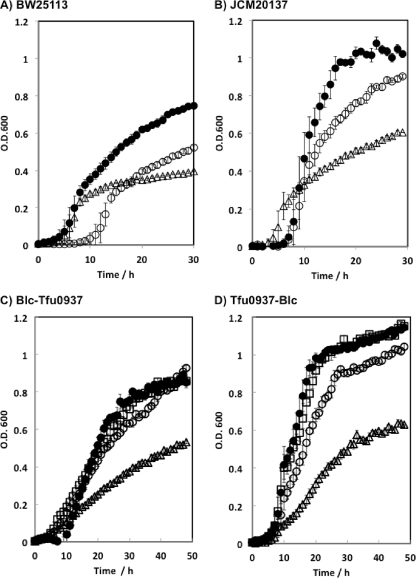
The growth on 0.2% cellobiose as the carbon source was evaluated. The initial OD600 was adjusted to 0.01. The time course analysis of OD600 during cultivation at 37°C is shown in Fig. 2 A and B. In the case of E. coli BW25113 (Fig. 2A), Blc-Tfu0937 showed high growth on cellobiose, followed by Tfu0937-Blc and Tfu0937-Slp. The values of OD600 after 30 h of cultivation were 0.75 (Blc-Tfu0937), 0.50 (Tfu0937-Blc), and 0.38 (Tfu0937-Slp). In the case of E. coli JCM20137 (Fig. 2B), the OD600 of Blc-Tfu0937 was up to 1.05 after 20 h of cultivation, which was a significantly improved growth rate and the same as its growth on 0.2% glucose after 16 h of cultivation (data not shown). The OD600 of Tfu0937-Blc also reached 0.9 after 30 h of cultivation, followed by Tfu0937-Slp. Two types of E. coli strains were used in this study to show the versatility of BGL display. The cell growth of E. coli BW25113 was lower than that of E. coli JCM20137, which might be due to the specific character of each E. coli strain, because the growth of JCM20137 on glucose was slightly superior to that of BW25113 (data not shown).
Fig. 2.
Growth analysis of Tfu0937-displaying E. coli using cellobiose (A and B) or cellooligosaccharide (C and D) as the sole carbon source. (A) E. coli BW25113; (B) E. coli JCM20137. Symbols shown in panels A and B are as follows: Blc-Tfu0937 (closed circles), Tfu0937-Blc (open circles), and Tfu0937-Slp (triangles). Symbols shown in panels C and D are as follows: cellotriose (closed circles), cellotetraose (squares), cellopentaose (open circles), and cellohexaose (triangles). Data are averages from three independent experiments, and standard deviations were within 10%.
Encouraged by these findings, cell growth on 0.2% cellooligosaccharide (cellotriose, cellotetraose, cellopentaose, or cellohexaose) as the sole carbon source was evaluated (Fig. 2C and D). Tfu0937-Blc showed high growth on each cellooligosaccharide, followed by Blc-Tfu0937. In the case of Tfu0937-Blc, the values of OD600 after 30 h of cultivation were 1.03 (cellotriose), 1.04 (cellotetraose), 0.90 (cellopentaose), and 0.49 (cellohexaose). These results clearly demonstrate that Tfu0937-Blc is appropriate for cellooligosaccharide assimilation in E. coli JCM20137.
In conclusion, we developed BGL-displaying E. coli using the novel anchor protein Blc and successfully demonstrated direct growth on cellobiose and cellooligosaccharide. Optimization of BGL, the anchor protein, and the E. coli strain can massively improve both BGL activity and cellobiose-assimilating ability. To date, engineered E. coli has produced many kinds of useful compounds from glucose or xylose as a carbon source (2, 3, 4, 10, 17). Our results suggest the possibility to produce useful compounds from cellooligosaccharide as well as glucose. In previous reports, we also have developed a Saccharomyces cerevisiae (yeast) cell surface display system and have demonstrated ethanol production from cellulose directly (7, 12). The codisplay of more than one enzyme, such as BGL, EG, and CBH, is required for direct assimilation of cellulose. Our approach will be also useful for other kinds of cellulases, such as EG and/or CBH, and we are currently undergoing further study along these lines.
Acknowledgments
This work was supported mainly by the Development of Preparatory Basic Bioenergy Technologies of the New Energy and Industrial Technology Development Organization (NEDO), Tokyo, and partially supported by The Noguchi Institute and the Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe), MEXT, Japan.
Footnotes
Published ahead of print on 8 July 2011.
REFERENCES
- 1. Alterthum F., Ingram L. O. 1989. Efficient ethanol production from glucose, lactose, and xylose by recombinant Escherichia coli. Appl. Environ. Microbiol. 55:1943–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atsumi S., Hanai T., Liao J. C. 2008. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89 [DOI] [PubMed] [Google Scholar]
- 3. Chang D. E., Jung H. C., Rhee J. S., Pan J. G. 1999. Homofermentative production of D- or L-lactate in metabolically engineered Escherichia coli RR1. Appl. Environ. Microbiol. 65:1384–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J., Zhang W., Tan L., Wang Y., He G. 2009. Optimization of metabolic pathways for bioconversion of lignocellulose to ethanol through genetic engineering. Biotechnol. Adv. 27:593–598 [DOI] [PubMed] [Google Scholar]
- 5. Kosugi A., Arai T., Doi R. H. 2006. Degradation of cellulosome-produced cello-oligosaccharides by an extracellular non-cellulosomal beta-glucan glucohydrolase, BglA, from Clostridium cellulovorans. Biochem. Biophys. Res. Commun. 349:20–23 [DOI] [PubMed] [Google Scholar]
- 6. Lee S. Y., Choi J. H., Xu Z. 2003. Microbial cell-surface display. Trends Biotechnol. 21:45–52 [DOI] [PubMed] [Google Scholar]
- 7. Matsumoto T., Fukuda H., Ueda M., Tanaka A., Kondo A. 2002. Construction of yeast strains with high cell surface lipase activity by using novel display systems based on the Flo1p flocculation functional domain. Appl. Environ. Microbiol. 68:4517–4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moniruzzaman M., Lai X., York S. W., Ingram L. O. 1997. Isolation and molecular characterization of high-performance cellobiose-fermenting spontaneous mutants of ethanologenic Escherichia coli KO11 containing the Klebsiella oxytoca casAB operon. Appl. Environ. Microbiol. 63:4633–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Narita J., et al. 2006. Display of alpha-amylase on the surface of Lactobacillus casei cells by use of the PgsA anchor protein, and production of lactic acid from starch. Appl. Environ. Microbiol. 72:269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohta K., Beall D. S., Mejia J. P., Shanmugam K. T., Ingram L. O. 1991. Genetic improvement of Escherichia coli for ethanol production: chromosomal integration of Zymomonas mobilis genes encoding pyruvate decarboxylase and alcohol dehydrogenase. Appl. Environ. Microbiol. 57:893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sánchez O. J., Cardona C. A. 2008. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 99:5270–5295 [DOI] [PubMed] [Google Scholar]
- 12. Shigechi H., et al. 2004. Direct production of ethanol from raw corn starch via fermentation by use of a novel surface-engineered yeast strain codisplaying glucoamylase and α-amylase. Appl. Environ. Microbiol. 70:5037–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Bloois E., Winter R. T., Kolmar H., Fraaije M. W. 2011. Decorating microbes: surface display of proteins on Escherichia coli. Trends Biotechnol. 29:79–86 [DOI] [PubMed] [Google Scholar]
- 14. Washida M., Takahashi S., Ueda M., Tanaka A. 2001. Spacer-mediated display of active lipase on the yeast cell surface. Appl. Microbiol. Biotechnol. 56:681–686 [DOI] [PubMed] [Google Scholar]
- 15. Wood B. E., Beall D. S., Ingram L. O. 1997. Production of recombinant bacterial endoglucanase as a co-product with ethanol during fermentation using derivatives of Escherichia coli KO11. Biotech. Bioeng. 55:547–555 [DOI] [PubMed] [Google Scholar]
- 16. Yan T. R., Lin Y. H., Lin C. L. 1998. Purification and characterization of an extracellular beta-glucosidase II with high hydrolysis and transglucosylation activities from Aspergillus niger. J. Agric. Food Chem. 16:431–437 [DOI] [PubMed] [Google Scholar]
- 17. Zhou S., Causey T. B., Hasona A., Shanmugam K. T., Ingram L. O. 2003. Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl. Environ. Microbiol. 69:339–407 [DOI] [PMC free article] [PubMed] [Google Scholar]