Abstract
Feruloyl esterases (Faes) constitute a subclass of carboxyl esterases that specifically hydrolyze the ester linkages between ferulate and polysaccharides in plant cell walls. Until now, the described microbial Faes were mainly from fungi. In this study, we report that Cellulosilyticum ruminicola H1, a previously described fibrolytic rumen bacterium, possesses three different active feruloyl esterases, FaeI, FaeII, and FaeIII. Phylogenetic analysis classified the described bacterial Faes into two types, FaeI and FaeII in type I and FaeIII in type II. Substrate specificity assays indicated that FaeI is more active against the ester bonds in natural hemicelluloses and FaeIII preferentially attacks the ferulate esters with a small moiety, such as methyl groups, while FaeII is active on both types of substrates. Among the three feruloyl esterase genes, faeI was the only one induced significantly by xylose and xylan, while pectin appeared to moderately induce the three genes during the late log phase to stationary phase. Western blot analysis determined that FaeI and FaeIII were secreted and cytoplasmic proteins, respectively, whereas FaeII seemed to be cell associated. The addition of FaeI and FaeII but not FaeIII enhanced the activity of a xylanase on maize cob, suggesting a synergy of the former two with xylanase. Hence, we propose that the three feruloyl esterases work in concert to hydrolyze ferulate esters in natural hemicelluloses.
INTRODUCTION
Feruloyl esterases (Faes) represent a subclass of carboxyl esterases that can release phenolic acids, such as ferulic acids or other cinnamic acids, from esterified polysaccharides, especially xylan and pectin (42). Therefore, Faes are regarded as the key enzymes to loosen the internal cross-linking of plant cell walls by acting as important accessory enzymes in synergy with (hemi)cellulases in plant cell wall hydrolysis (43). Ferulic acids are the main phenolic acids to covalently link to the polysaccharides through ester bonds in the plant cell wall. In xylan, ferulic acids are linked to arabinose and are then attached to a xylan backbone, while in pectin, ferulic acids are ester linked to pectin polysaccharides mainly through arabinose or galactose (37). These cross-links formed via ferulic acids dramatically reduce the biodegradability of plant cell walls by microorganisms (7, 23).
Since the 1990s, microbial Faes have been studied due to their potential application in biotechnological processes. Faes are not only relevant in biofuel production but also used in the medical industry for synthesis of bioactive food components (24). Dozens of microorganisms have been reported to secrete Faes, but the Faes described to date are mainly from fungi (4), though several bacterial Faes have been studied (15, 20, 25, 28, 31, 32). On the basis of their primary sequences and substrate specificity against four model substrates, methyl ferulate (MFA), methyl caffeate (MCA), methyl p-coumarate (MpCA), and methyl sinapate (MSA), Faes are classified as types A, B, C, and D (8, 36). Despite having various primary amino acid sequences, Faes share similar three-dimensional structures, with an α/β-hydrolase having a serine, histidine, and aspartic acid catalytic triad (22, 26, 38).
Although many fungi and bacteria have been found to produce Faes, only a limited number of Fae-encoding genes have been characterized thus far. Bacteria that are reported to possess Fae genes are restricted to a few species, including Butyrivibrio fibrisolvens (9, 10), Prevotella ruminicola (15), Clostridium thermocellum (5), and Dickeya dadantii (25). Bioinformatics-based gene screening has indicated that each of these Fae-producing bacteria generally possesses one or two such genes, encoding proteins with distinct enzymatic characteristics. A few studies indicate that the production of Faes is induced by certain compounds, such as saccharides, phenolic acids, or phenolic acid sugar esters; for example, feruloyl esterase in Aspergillus niger is regulated through the xylanolytic transcriptional activator, XlnR (13), as well as through a second system that responds to aromatic compounds (14), while in a plant soft rot disease-causing bacterium, Dickery dadantii, the expression of faeD is regulated by ferulic acid via a LysR family regulator (25). However, cinB, a gene encoding cinnamoyl esterase in a ruminal bacterium, Butyrivibrio fibrisolvens, appears to be regulated by CinR in response to ferulated oligosaccharides isolated from plant cell walls but not in response to xylobiose or ferulic acid (9).
Recently, we have identified a comprehensive set of enzymes essential for decomposing plant cell walls, including feruloyl esterase activity, in a newly described yak ruminal bacterium, Cellulosilyticum ruminicola H1 (6). Strain H1 grew robustly on natural plant fibers, such as corn cob, alfalfa, and ryegrass, as the sole carbon and energy sources, as well as on a variety of polysaccharides, including cellulose, xylan, mannan, and pectin. The draft of the genome sequences displayed the gene repertoire targeting the plant cell wall as well. An Avicel-bound protein shows Fae activity, and its corresponding gene has been identified as encoding an active feruloyl esterase (FaeI). In further investigations of this bacterium for action on ferulated compounds in this study, we identified another two feruloyl esterases and analyzed the possible synergetic actions of the three enzymes on the basis of their enzymatic characterization and cellular localization and the patterns of induction of their gene expression.
MATERIALS AND METHODS
Strains and culture media.
C. ruminicola (CGMCC 1.5065T) was preserved in our laboratory and routinely cultured with 0.2% (wt/vol) cellobiose at 38°C under 1.01 × 105 Pa of gas-phase CO2 in van Rijn and Cohen (RC) medium, as described previously (6). For analysis of feruloyl esterase expression on different substrates, the following carbon sources were used instead of cellobiose: 0.2% (wt/vol) xylose, 0.5% (wt/vol) xylan (oat spelt), 0.3% (wt/vol) pectin (citrus fruits), 0.03% (wt/vol) ferulic acid, and 0.03% (wt/vol) p-coumaric acid.
Bioinformatic analysis of the putative feruloyl esterases.
Using BLAST software (version 2.2.24) available at the NCBI website (http://www.ncbi.nlm.nih.gov), we first built a local BLAST database with the translated sequences of putative genes in the C. ruminicola genome. The deduced protein sequence encoded by CGSCsYakCAS ORF18248 was then used as a probe to screen for homologies in the database using the BLASTP program. The signal peptides of the deduced amino acid sequences were predicted using SignalP (http://www.cbs.dtu.dk/services/SignalP). Domain structures were constructed based on Pfam (http://pfam.sanger.ac.uk) analysis. Phylogenetic analysis for bacterial Faes was performed using MEGA 4.0 software (40) with manual editing of sequences.
Cloning and expression of the feruloyl esterase genes.
Genomic DNA was extracted from C. ruminicola as described previously (6). The PCR primers listed in Table 1 were used to amplify faeI, faeII, and faeIII. Restriction enzyme sites (Table 1) were designed for easy cloning of the faeI, faeII, and faeIII sequences into pET-15b, pET-28a, and pET-30a, respectively. PCR amplification was performed using Pfu DNA polymerase (Promega, Madison, WI) for 30 cycles of 95°C for 30 s, 51°C for 30 s, and 72°C for 3 min. PCR products were purified with a gel extraction kit (Omega Bio-Tek, Norcross, GA), ligated using T4 DNA ligase, and then used to transform Escherichia coli DH5α. Cloned genes were confirmed by sequencing, and the three plasmids containing Fae genes (faeI, faeII, and faeIII) were then transformed into E. coli BL21(DE3), Rosetta-gami 2(DE3), and Rosetta(DE3), respectively.
Table 1.
Primers used for overexpression and quantitative PCR of the feruloyl esterases
| Primera | Sequenceb |
|---|---|
| faeI-F | CCGCATATGGCAATTACGCCAGTAAT |
| faeI-R | CGGGATCCTTATTTAAGCACTACATTAGC |
| faeII-F | CGGGATCCATGAAAGAAATATCAGATGC |
| faeII-R | CCGCTCGAGTTATTTTGTATATTTATCAGC |
| faeIII-F | CCGCATATGGTAGGGGTAGAAAAG |
| faeIII-R | CGGGATCCTTGGCAAAGATTCTAACTT |
| Q16s-F | TACACACCGCCCGTCACAC |
| Q16s-R | CAGCCGCACCTTCCGATAC |
| QfaeI-F | ATACCACCAGCAGGATAT |
| QfaeI-R | TTCATTACCACCAATACCAT |
| QfaeII-F | ACACCAAGTAGAGATAGCAATA |
| QfaeII-R | GCCTACAACCTCATCACT |
| QfaeIII-F | AGTAGTTGGCAGAGGATT |
| QfaeIII-R | ATGGCATTGACCTTCTTC |
The Q prefix identifies the primers designed for quantitative PCR.
Restriction sites are underlined.
The recombinant E. coli strains were grown at 37°C in LB liquid medium supplemented with 50 μg·ml−1 ampicillin (for FaeI) and 50 μg·ml−1 kanamycin (for FaeII and FaeIII). When cultures reached an optical density at 600 nm of 0.4 to 0.6, a final concentration of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to induce the expression of Fae genes. After an additional 4 to 8 h of incubation, cells were collected by centrifugation at 6,000 × g, resuspended in 25 mM Tris-HCl (pH 7.4), and lysed by sonication, and then the cell lysates were purified by nickel affinity chromatography as described previously (41). The purified proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), stained with Coomassie blue and in-gel trypsin digestion, and analyzed by liquid chromatography-tandem mass spectrometry as described previously (6).
Characterization of the feruloyl esterases.
Endoglucanase, xylanase, and pectinase activities were assayed as previously described (33). One unit of enzyme activity was defined as the amount of enzyme that liberates 1 μmol of reducing sugars per minute. Cellobiohydrolase, β-glucosidase, β-xylosidase, α-arabinofuranosidase, and β-glucuronidase were assayed according to the methods of Adelsberger et al. (2). Lipase activity was determined using p-nitrophenyl (pNP) esters with various numbers of carbon atoms, from C2 to C16, in the acyl chain (Sigma, St. Louis, MO), as previously described (27). The reaction mixture contained 5 μl of 10 mM pNP esters in acetonitrile, 490 μl of 25 mM Tris-HCl (pH 7.4) buffer, and 5 μl enzyme in a final volume of 500 μl and was incubated at 37°C for 30 min. Lipase activity was calculated by measuring absorbance at 410 nm for pNP release. One unit of enzyme activity was defined as the amount of enzyme that liberates 1 μmol of pNP per minute.
Feruloyl esterase activity was measured against MFA and 5-o-caffeoyl quinic acid (chlorogenic acid [CGA]) as previously described (5) with the slight modifications of adding 5 μl protein to 195 μl buffer containing 10 mM substrate, incubating at 37°C for 30 min, and finally, adding 20 μl of 20% (vol/vol) formic acid to stop the reaction. The phenolic acid released was measured by high-performance liquid chromatography (HPLC) using a mobile phase of 10 mM sodium formate (pH 3.0) and 45% (vol/vol) methanol. One unit of feruloyl esterase activity was defined as the amount of enzyme that releases 1 μmol of phenolic acid per minute. All the assays were performed in triplicate.
The kinetic parameters of the enzymes were determined using the synthetic chemicals MFA, MCA, MpCA, and MSA (Apin Chemicals, Oxfordshire, United Kingdom) as model substrates. The kinetic constants were estimated using the Lineweaver-Burk plot. Protein concentrations were determined using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Rockford, IL) with bovine serum albumin as the calibration standard.
The pH and temperature profiles of the three feruloyl esterases were determined on MFA (10 mM) according to the methods described previously (32). The enzymes' stability against pH was determined based on the residual activity after incubation of the enzymes at 4°C for 16 h in 25 mM citrate-phosphate buffer (pH 3.0 to 7.0), 25 mM Tris-HCl (pH 7.0 to 9.0), and 25 mM glycine-NaOH (pH 9.0 to 11.0). To determine the temperature stability of the enzymes, residual activities were determined after incubation at various temperatures for 12 h at pH 7.0.
Western blot analysis for the cellular localization of FaeI, FaeII, and FaeIII.
Polyclonal antibodies against the three Faes were prepared at the Laboratory Animal Center, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, by injecting rats with purified Fae domains of the three recombinant proteins.
C. ruminicola H1 cells at late logarithmic growth phase were harvested by centrifugation at 6,000 × g and washed three times with phosphate-buffered saline (PBS). The cell suspension was lysed by sonication and centrifuged at 21,000 × g to obtain the cell fractions. The supernatant and pellet were used as the cytoplasm and cell debris fractions, respectively. Meanwhile, the cell-free culture medium was precipitated using 85% (wt/vol) ammonium sulfate, redissolved, and dialyzed against 25 mM PBS, which was then used as the extracellular fraction.
The three fractions were subjected to SDS-PAGE with 12% gel and then transferred to nitrocellulose membranes (Amersham, Little Chalfont, United Kingdom). Western blotting and immunodetection were performed as described previously (21); the nonspecific binding of antibodies was blocked by incubation with Tris-buffered saline and Tween 20 (TBST) with 5% skim milk. The membranes were then probed with a 1,000-fold dilution of one of the following polyclonal antibodies: rat anti-FaeI, rat anti-FaeII, or rat anti-FaeIII. Bound antibodies were visualized using goat anti-rat IgG (Cwbio, China) conjugated to horseradish peroxidase (HRP), followed by enhanced chemiluminescence (Amersham, Little Chalfont, United Kingdom) according to the manufacturer's instructions.
Quantitative PCR (qPCR) analysis of feruloyl esterase gene expression.
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. C. ruminicola H1 was grown in various test substrates and in two separate batches of culture for each substrate. Cells were harvested at early and late logarithmic phase and stationary phase, washed with excess RNase-free water, and then frozen in liquid nitrogen and ground three times with a glass rod, followed by the addition of TRIzol reagent. After incubation at 15 to 30°C for 5 min, chloroform was mixed with the lysate and the mixture centrifuged at 12,000 × g. RNA in the aqueous phase was then precipitated using isopropyl alcohol, washed with 75% ethanol, and air dried for 5 to 10 min. The pellet was then dissolved in RNase-free water, and the trace amount of DNA in the RNA preparation was removed by the addition of DNase. Approximately 2 μg of RNA was used in the standard reverse transcription reaction mixtures with random primers.
Real-time PCR oligonucleotide primers (Table 1) were designed using Beacon Designer 5.0 (Premier Biosoft, Palo Alto, CA) to obtain maximal amplification efficiency and sensitivity. The specificity of the primers was verified by evaluating the qPCR melting curve and PCR products by DNA gel electrophoresis. DNA for the standard curve was serially diluted 10-fold (10−2 to 10−8), and PCRs performed in triplicate.
Transcript quantification was performed using an ABI Prism 7000 sequence detection system (Applied Biosystems) with SYBR green SuperMix (Takara, Dalian, China), with the thermocycling program as follows: 40 cycles of 95°C for 10 s, 55.4°C for 30 s, and elongation at 72°C for 30 s. Each cDNA sample was processed in triplicate. The copy number in each cDNA sample was calculated according to the calibration curve generated by the gene PCR products.
Synergy of the three Faes with (hemi)cellulases.
Onozuka R-10 cellulase from Trichoderma viride (Yakult, Japan) or a xylanase from Thermomyces lanuginosus (Sigma, China) was added to 1.5 ml of reaction buffer (25 mM Tris, pH 7.0) containing 60 mg of maize cob with or without 75 μg of FaeI, FaeII, or FaeIII. After various time periods of incubation at 38°C, the amounts of ferulic acids and reducing sugars in reaction mixtures with different combinations were measured.
RESULTS
Three putative feruloyl esterase genes predicted in the genome of Cellulosilyticum ruminicola H1.
Previously, ORF18248 of C. ruminicola H1 was determined by enzymatic assay to encode an extracellular feruloyl esterase; however, feruloyl esterase activity was also detected in the cell extract of the bacterium. Attempting to identify more potential feruloyl esterase genes, we used the ORF18248 sequence as a probe to screen the draft genome and found that ORF18995 and ORF08358 had 36 and 15.6% sequence identity, respectively, to the deduced amino acids. Therefore, the three genes were named faeI, faeII, and faeIII. Based on the bioinformatics data, these genes were predicted to encode peptides of 566, 286, and 373 amino acid residues with isoelectric points of 5.59, 6.02, and 4.98, respectively.
To explore the protein structures of the Fae genes more extensively, we performed a Pfam analysis for all three proteins. These analyses predicted that all three proteins possess an esterase-lipase superfamily domain (Fig. 1). In addition, FaeI putatively possesses two other domains, ricin and a carbohydrate binding module (CBM2). A signal peptide of 32 amino acids was predicted at the N terminus of FaeI, suggesting that this protein is secreted. An unknown domain was predicted at the C terminus of FaeIII.
Fig. 1.
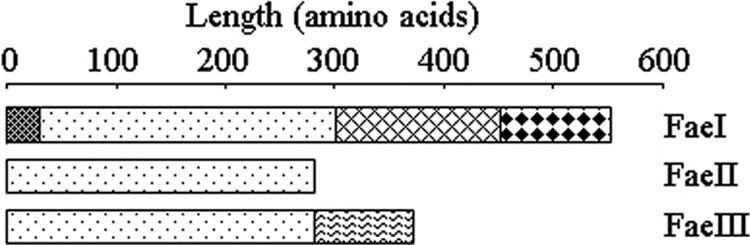
Domain organization of FaeI, FaeII, and FaeIII constructed based on Pfam analysis., signal peptide; , feruloyl esterase; , ricin; , carbohydrate binding module 2 (CBM2); , unknown domain. The predicted molecular masses of FaeI, FaeII, and FaeIII were 58 kDa, 31.5 kDa, and 42 kDa, respectively.
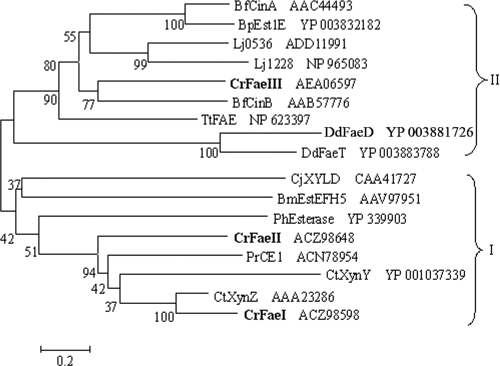
On the basis of the primary sequences, we conducted a phylogenetic analysis of the currently described bacterial feruloyl esterases. The results in Fig. 2 show that all the Faes can be grouped into two phyla; FaeI and FaeII were in one phylum, and FaeIII in the other. It was shown that FaeI and FaeII exhibit the highest sequence identities with XynZ from Clostridium thermocellum (60 and 40%, respectively); however, FaeIII showed the highest similarity to CinB from Butyrivibrio fibrisolvens (32%). Like the fungal Faes, the three feruloyl esterases were predicted to contain only esterase domains and not xylanase domains like GH10 from XynZ of Clostridium thermocellum.
Fig. 2.
Phylogenetic analysis of the bacterial feruloyl esterases. The tree was constructed with a consensus sequence of 280 amino acids from 17 proteins using MEGA 4.0 software. Proteins for the tree construction and the bacteria containing them are as follows: CtXynZ and CtXynY, xylanase Z and xylanase Y from Clostridium thermocellum (5); PrCE1, cinnamoyl esterase from Prevotella ruminicola (15); CrFaeI, CrFaeII, and CrFaeIII, feruloyl esterases from Cellulosilyticum ruminicola H1; BfCinA and BfCinB, cinnamoyl ester hydrolases from Butyrivibrio fibrisolvens (9, 10); Lj0536 and Lj1228, cinnamoyl esterases from Lactobacillus johnsonii (31); TtFAE, feruloyl esterase from Thermoanaerobacter tengcongensis (1); BpEst1E, feruloyl esterase from Butyrivibrio proteoclasticus (22); CjXYLD, esterase from Cellvibrio japonicus (17); PhEsterase, feruloyl esterase from Pseudoalteromonas haloplanktis (3); BmEstEFH5, ferulate esterase from Burkholderia multivorans (35); DdFaeD and DdFaeT, feruloyl esterases from Dickeya dadantii (25). Following the sequence names are the GenBank accession numbers. The numbers at the cluster nodes are the supporting percentages of bootstrap evaluation. Bar, 20% sequence divergence. The boldface shows the three feruloyl esterases in this study.
Sequence alignment of the three proteins with the bacterial feruloyl esterases described to date revealed that they all contain the characteristic Ser-His-Asp catalytic triad that exists in serine protease and esterase families of enzymes. By consulting the three-dimensional structures of bacterial feruloyl esterases, the putative triad positions of FaeI, FaeII, and FaeIII were determined to be Ser174-His262-Asp232, Ser163-His262-Asp231, and Ser106-His227-Asp197, respectively. The collective data support the idea that the three Faes are three different types of feruloyl esterases.
Characterization of the three overexpressed Faes from C. ruminicola H1.
To determine whether faeII and faeIII encode active feruloyl esterases, we cloned these genes and overexpressed the gene products in E. coli. The proteins, with 6×His tags, were purified using Ni2+ columns, and sizes were determined by SDS-PAGE to be as predicted (Fig. 3). The purified proteins, together with FaeI, were then used for enzymatic assay. Feruloyl esterase activities were detected for all three purified proteins on MFA; however, none exhibited activities on carboxymethyl cellulose, oat spelt xylan, pectin, p-nitrophenyl (pNP)-arabinopyranoside, pNP-glucopyranoside, pNP- xylopyranoside, pNP-cellobiose, or pNP-glucuronide, nor were protease activities detected (data not shown). Therefore, FaeI, FaeII, and FaeIII were determined to be authentic feruloyl esterases, consistent with the bioinformatics predictions.
Fig. 3.
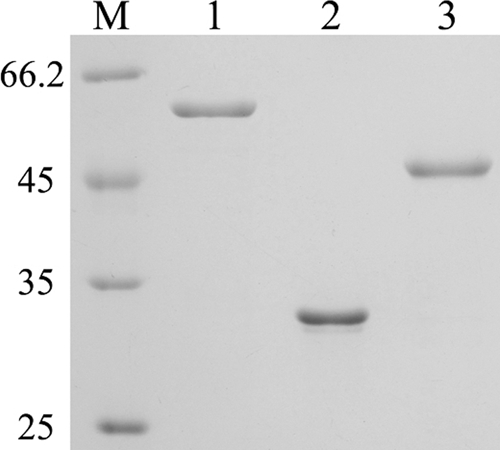
SDS-PAGE analysis of the purified FaeI, FaeII, and FaeIII. Lanes: M, protein standards (in kilodaltons); 1, FaeI; 2, FaeII; 3, FaeIII.
FaeI, FaeII, and FaeIII were characterized for their specific activity and substrate profiles using the model substrates MFA, MCA, MpCA, and MSA and p-nitrophenyl esters (pNP) with different numbers of carbon atoms in the acyl chain. All three proteins exhibited efficient hydrolysis of the four methyl hydroxycinnamates, with the highest levels of activity on MFA (Table 2). Moreover, FaeII and FaeIII but not FaeI were active on a natural compound, chlorogenic acid (CGA). The three proteins all released less ferulic acid from maize cob than from the model substrates, whereas, when extending the reaction time on maize cob, FaeI and FaeII showed higher specific activities than FaeIII. However, both FaeII and FaeIII exhibited higher activities against the model substrates than FaeI. Comparing the three Faes with described feruloyl esterases from fungi and bacteria, FaeII and FaeIII could be categorized as robust feruloyl esterases.
Table 2.
Substrate specificities of FaeI, FaeII, and FaeIII
| Substratea | Sp act (U mg−1)b of: |
||
|---|---|---|---|
| FaeI | FaeII | FaeIII | |
| Maize cob | 0.05 ± 0.01 | 0.10 ± 0.00 | 0.06 ± 0.00 |
| Maize cobc | 2.03 ± 0.12 | 2.64 ± 0.18 | 0.63 ± 0.07 |
| MFA | 3.78 ± 0.10 | 38.76 ± 1.3 | 56.88 ± 0.49 |
| MCA | 1.47 ± 0.04 | 18.44 ± 0.54 | 32.58 ± 1.12 |
| MpCA | 1.1 ± 0.01 | 32.65 ± 0.67 | 34.19 ± 0.98 |
| MSA | 3.66 ± 0.01 | 29.04 ± 0.29 | 37.51 ± 0.67 |
| CGA | ND | 5.74 ± 0.07 | 21.73 ± 0.28 |
| pNP esters by chain length | |||
| C2 | 0.16 ± 0.01 | 0.36 ± 0.01 | 0.25 ± 0.01 |
| C4 | 0.29 ± 0.01 | 0.44 ± 0.01 | 0.16 ± 0.01 |
| C6 | 0.15 ± 0.03 | 0.31 ± 0.02 | 0.15 ± 0.01 |
| C8 | 0.07 ± 0.01 | 0.23 ± 0.02 | 0.06 ± 0.01 |
| C10 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 |
MFA, methyl ferulate; MCA, methyl cinnamate; MpCA, methyl p-coumarate; MSA, methyl sinapate; CGA, chlorogenic acid; C2, p-nitrophenyl (pNP)-acetate; C4, pNP-butyrate; C6, pNP-caproate; C8, pNP-octanoate; C10, pNP-caprate.
Values are given as means ± standard deviations. ND, not determined.
Activity calculated as ferulic acid (μmol) released per mg of protein in 6 h.
The three proteins only weakly hydrolyzed the ester bonds of pNP-acetate (C2), pNP-butyrate (C4), pNP-caproate (C6), pNP-octanoate (C8), and pNP-caprate (C10), but none were able to hydrolyze the ester bonds in acyl chain substrates with more than 12 carbon atoms (Table 2), thus excluding them as lipases.
We further characterized the kinetic activities of the three feruloyl esterases on model substrates. The results in Table 3 show that FaeI displayed the lowest levels of both substrate affinity and catalytic efficiency, resembling those of the Fae domain in XynZ from C. thermocellum and the Fae domain in XLYD from Cellvibrio japonicus. FaeIII displayed especially high affinity and activity on MFA (Km of 0.03 mM), whereas FaeII appeared to be more active on the other three model substrates, with approximately 5-fold more activity than FaeIII on MCA and MSA. To our knowledge, FaeIII is the feruloyl esterase with the highest activity so far reported.
Table 3.
Kinetic parameters of FaeI, FaeII, and FaeIII on model substratesa
| Substrate | FaeI |
FaeII |
FaeIII |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Km (mM) | kcat (s−1) | kcat/Km (s−1 mM−1) | Km (mM) | kcat (s−1) | kcat/Km (s−1 mM−1) | Km (mM) | kcat (s−1) | kcat/Km (s−1 mM−1) | |
| MFA | 4.78 ± 0.46 | 8.46 ± 0.55 | 1.77 | 0.36 ± 0.03 | 645.94 ± 14.75 | 1,794.28 | 0.03 ± 0.00 | 2,130.89 ± 33.28 | 71,029.64 |
| MCA | 2.50 ± 0.28 | 2.34 ± 0.22 | 0.94 | 0.16 ± 0.01 | 232.72 ± 6.41 | 1,454.49 | 1.06 ± 0.03 | 330.90 ± 4.06 | 312.17 |
| MpCA | 13.76 ± 0.82 | 7.78 ± 0.40 | 0.57 | 0.51 ± 0.03 | 310.51 ± 17.68 | 608.85 | 1.26 ± 0.08 | 538.62 ± 59.99 | 427.48 |
| MSA | 6.58 ± 0.38 | 12.70 ± 0.97 | 1.93 | 0.18 ± 0.01 | 753.63 ± 11.88 | 4,186.81 | 0.63 ± 0.03 | 566.97 ± 7.74 | 899.96 |
Values are given as means ± standard deviations or ratios; substrate abbreviations are defined in Table 2.
Table 4 lists the temperature and pH profiles of the three enzymes, which indicated that they are all mesophilic proteins but work optimally in a varied pH range from neutral to alkaline pH.
Table 4.
Temperature and pH profiles of FaeI, FaeII, and FaeIIIa
| Enzyme | Optimum temp (°C) | Temp stability range (°C) | Optimum pH | pH stability range |
|---|---|---|---|---|
| FaeI | 40 | ≤50 | 6–7 | 3–10 |
| FaeII | 35 | ≤45 | 8 | 4–9 |
| FaeIII | 40 | ≤50 | 9 | 5–10 |
To determine temperature profiles, the proteins were incubated for 16 h. To determine pH profiles, the proteins were incubated for 12 h at 4°C.
The three feruloyl esterase genes are induced distinctly.
As the three feruloyl esterases exhibited substrate specificity, we determined the induction of gene expression on different substrates by means of qPCR. The quantification of the gene transcript levels per cell was calibrated by using the 16S rRNA gene as a reference. The 16S rRNA gene of strain H1 seemed to express constitutively in the tested substrates, as the threshold cycle (CT) values of the gene copy numbers have standard deviations (SD) as low as 0.5 (data not shown), indicating it as a suitable reference gene. The results in Table 5 show that faeI, faeII, and faeIII were all expressed at basal levels in cellobiose culture in all growth phases, while they were all moderately upregulated in pectin culture, with faeI showing the greatest effects (about 5- to 24-fold). The pectin induction of the three genes seemed to vary with the growth phase, with faeI having the highest expression at stationary phase and faeII and faeIII at late log phase. Xylose and xylan seemed to affect the expression of faeI significantly, with levels 22- to 30-fold higher than the basal level during all growth phases. However, xylose and xylan only exerted a medium effect on the expression of faeII, with xylose but not xylan displaying growth phase-dependent induction. Neither xylose nor xylan showed an effect on the expression of faeIII.
Table 5.
Transcript levels of the three feruloyl esterase genes in C. ruminicola H1 grown on various carbon sources and with hydroxycinnamic acids
| Substrate | Gene transcription at indicated growth phasea |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
faeI |
faeII |
faeIII |
|||||||
| Early log | Late log | Stationary | Early log | Late log | Stationary | Early log | Late log | Stationary | |
| Cellobiose | 1.0 ± 0.0 | 1.4 ± 0.0 | 1.0 ± 0.3 | 1.1 ± 0.1 | 1.0 ± 0.0 | 0.9 ± 0.3 | 1.7 ± 0.1 | 1.5 ± 0.4 | 0.8 ± 0.1 |
| Xylose | 22.5 ± 1.6 | 23.1 ± 1.7 | 25.2 ± 1.7 | 1.3 ± 0.2 | 3.5 ± 0.7 | 6.4 ± 1.6 | 0.3 ± 0.0 | 1.6 ± 0.2 | 0.7 ± 0.2 |
| Xylan | 25.5 ± 2.7 | 29.3 ± 2.7 | 30.2 ± 2.4 | 3.2 ± 0.7 | 2.6 ± 0.4 | 3.0 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.0 | 1.2 ± 0.0 |
| Pectin | 5.2 ± 0.2 | 12.0 ± 1.1 | 24.3 ± 1.6 | 1.2 ± 0.3 | 9.3 ± 1.0 | 6.9 ± 1.1 | 0.6 ± 0.1 | 5.2 ± 0.5 | 3.9 ± 0.9 |
| Cellobiose + ferulic acid | 1.1 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.0 | 1.2 ± 0.1 | 1.5 ± 0.3 | 1.8 ± 0.2 | 1.3 ± 0.1 |
| Cellobiose + pCA | 1.0 ± 0.0 | 2.3 ± 0.2 | 2.0 ± 0.3 | 0.9 ± 0.0 | 0.9 ± 0.1 | 1.3 ± 0.0 | 1.7 ± 0.1 | 2.1 ± 0.2 | 1.6 ± 0.1 |
The transcription rate is the mean ± standard deviation of the specific gene copy number/1,000 16S rRNA copies.
We also tested the effects of ferulic acid and p-coumaric acid on the expression of the three feruloyl esterase genes, as they are present in wheat bran and maize cob. The results indicated that they did not change the expression of the three genes significantly, except that p-coumaric acid induced increases in the expression of faeI and faeIII of approximately 64% and 40%, respectively.
The three feruloyl esterases distribute in different cellular fractions.
As feruloyl esterases are expected to have extracellular action, while a signal peptide was only predicted in FaeI among the three Faes, we determined the cellular locations of the three proteins in C. ruminicola H1. Western blot analysis with antisera against the catalytic domains of the three enzymes was performed for the supernatant and cell debris of lysed cell pellet and spent cell-free culture of the cellobiose-grown strain H1 (Fig. 4). FaeI and FaeIII were detected mostly in the spent cell-free culture and supernatant of lysed cells, respectively, while only a weak signal of FaeII was detected in the cell debris of the lysed cell pellet. These data indicate that FaeI, FaeIII, and FaeII could be extracellular, cytoplasmic, and cell-associated proteins, respectively.
Fig. 4.
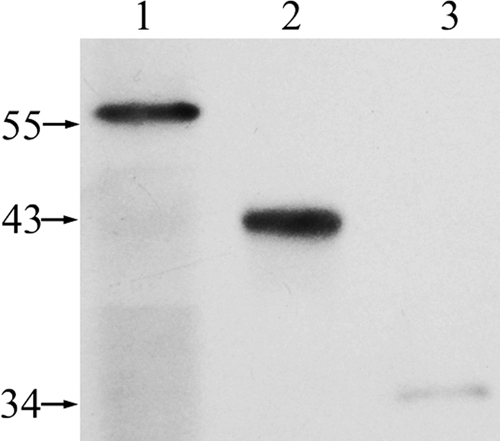
Western blot analysis and immunodetection of FaeI, FaeII, and FaeIII in different cellular fractions. Sizes of protein standard markers are shown in kilodaltons. Lanes: 1, proteins precipitated by 85% (wt/vol) ammonium sulfate from spent cell-free culture; 2, supernatant of cell lysate; 3, cell debris of cell lysate.
Although the apparent molecular masses of FaeI and FaeIII on the SDS-PAGE gel agreed with the predicted sizes, FaeII was observed to be 3 to 4 kDa larger than predicted. This size difference may be attributable to protein modifications or attachment of other components in the cell wall debris to the protein. The faint signal of FaeII in the Western blot assay could be attributed to weak binding to the cell, so that the enzyme was removed by excess washing of the cell pellet during cell fraction preparation.
Synergistic effects of the three feruloyl esterases with cellulase and xylanase on natural hemicellulose hydrolysis.
Based on the observations described above, we tested the possible synergistic actions between each of the three feruloyl esterases and cellulase or xylanase in a pairwise analysis. The results in Table 6 show that the addition of either FaeI or FaeII increased xylanase activity (37% and 27% more reducing sugars were released, respectively, than xylanase alone), but FaeIII did not. Similarly, FaeI slightly elevated cellulase activity, by 17%; however, both FaeII and FaeIII reduced cellulase activity by 34%. These data further suggested that distinct working patterns are employed by the three feruloyl esterases from C. ruminicola. Conversely, both cellulase and xylanase promoted the activities of the three feruloyl esterases by 2 to 40%, by showing the highest synergism with FaeIII.
Table 6.
Amounts of ferulic acids or reducing sugars released from maize cob by feruloyl esterases of strain H1 with cellulase or xylanasea
| Enzyme | Material released | Amt released with: |
|||
|---|---|---|---|---|---|
| No enzyme | FaeI | FaeII | FaeIII | ||
| Fae alone | Ferulic acid | 0.0 | 2.0 ± 0.1 | 2.6 ± 0.2 | 0.6 ± 0.1 |
| Cellulase | Ferulic acid | 0.0 | 2.5 ± 0.1 | 2.7 ± 0.1 | 0.9 ± 0.1 |
| Reducing sugar | 61.8 ± 3.4 | 72.3 ± 6.0 | 40.8 ± 3.0 | 40.4 ± 2.6 | |
| Xylanase | Ferulic acid | 0.0 | 2.2 ± 0.1 | 2.7 ± 0.1 | 0.8 ± 0.0 |
| Reducing sugar | 19.9 ± 1.5 | 27.2 ± 2.1 | 25.3 ± 2.0 | 19.0 ± 1.7 | |
Released materials are calculated as 1 μmol of released ferulic acid or reducing sugar per mg of protein in 6 h of incubation.
DISCUSSION
Typically, one or two Fae genes are identified in each bacterial strain that produces these enzymes (5, 9, 10, 15, 25). In this study, we show that in the rumen bacterium C. ruminicola H1 there are three active feruloyl esterases, which share such a low level of amino acid sequence identity in the catalytic domains that we “hit” them through re-Blasting the candidates, which were obtained by screening the genome of strain H1 with CGSCsYakCAS ORF18248 as a probe, against the GenBank database. In combination with the differing architectures of the three proteins, this presents a rare example of a bacterial strain that possesses three different feruloyl esterase genes.
The three feruloyl esterases differ in substrate specificity, cellular localization, and substrate regulation, suggesting that they play distinct roles in lignocellulose degradation by the rumen bacterium. As an extracellular enzyme, the CBM-borne FaeI could be the pioneer in the hydrolysis of the ferulic acid ester bonds in hemicelluloses, as it not only displays relatively higher activity on maize cob but also is the only one induced significantly by the plant cell wall polysaccharides xylan and pectin. FaeII was detected as a cell-associated protein, though no signal peptide was detected; it could be translocated by other mechanisms, such as a type III secretory system, which exists in the genome of strain H1. FaeII can attack the ester bonds either in natural polysaccharides or in oligosaccharides produced by FaeI, since it shows activities both on maize cob and model substrates, while FaeIII can hydrolyze the ester bonds only in oligomers, for it is not active on natural materials, which is consistent with its intracellular location.
We propose that the three feruloyl esterases work in concert for the complete hydrolysis of ferulate esters in natural hemicelluloses by attacking the ester bonds in different polymers. This is also supported by the fact that FaeI and FaeII exert synergistic effects with cellulase and xylanase on oligosaccharides released from natural hemicelluloses, but FaeIII does not.
Phylogenetic analysis based on the primary sequences of bacterial feruloyl esterases suggests that there are two types of esterases (Fig. 2). XYLD from Cellvibrio japonicus, of type I in this study, was previously classified as type C, while XynZ and XynY from C. thermocellum corresponded to the tentative type E assigned by Crepin et al. (8). By comparing the characterization of the two types of bacterial Faes, we found that the majority of type I Faes are secreted proteins, possessing a signal peptide, and contain a CBM or bifunctional domains like XynZ (C. thermocellum) (5), which displays much higher enzymatic activity on ferulated oligosaccharides than on the ferulate esters with a small moiety. Moreover, XYLD (C. japonicus) showed a higher affinity for ferulated oligosaccharides with longer chain lengths (17), and similar results were found for the ferulate esterase from Burkholderia multivorans, EstEFH5 (35). A CBM is a stretch of amino acid sequences appended to carbohydrate-active enzymes, and it carries the catalytic domains to attach to the substrates and, thus, enhance enzyme activity. We did find that CBM2-borne FaeI binds to Avicel (6), and the deletion of CBM2 reduced the activity of FaeI to 60% of the activity of the intact protein (data not shown). Therefore, we postulate that the type I bacterial Faes play a central role in disrupting natural materials by cleaving the ferulate esters in synergy with hemicellulases.
Most previous studies show that it is the (hemi)cellulases that assist Faes in ferulic acid release (1, 11, 12, 14, 18, 19, 28, 29, 30); however, in the present study, we found that xylanase does not assist FaeI and FaeII in the release of ferulic acid from maize cob, except for the stimulation of FaeIII. In contrast, FaeI and FaeII, the type I bacterial Faes, rather than FaeIII, the type II bacterial Fae, promote the activity of xylanase; similar synergistic effects were also observed previously (39, 44).
The alkaline feruloyl esterases are potentially useful in industrial applications, especially in pulp treatment; however, only a very limited number of such kinds of feruloyl esterases are currently available (1, 16, 34). The alkaline working pH range of FaeII and FaeIII determined in this study makes them attractive candidates for industrial feruloyl esterases.
ACKNOWLEDGMENTS
This study was supported by grants from the Chinese Academy of Sciences (grant KSCX1-YW-11B1), National High Technology Program (863 Program) of China (grant 2007AA021301), and the State Key Laboratory of Microbial Resources.
Footnotes
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Abokitse K., Wu M., Bergeron H., Grosse S., Lau P. C. 2010. Thermostable feruloyl esterase for the bioproduction of ferulic acid from triticale bran. Appl. Microbiol. Biotechnol. 87:195–203 [DOI] [PubMed] [Google Scholar]
- 2. Adelsberger H., Hertel C., Glawischnig E., Zverlov V. V., Schwarz W. H. 2004. Enzyme system of Clostridium stercorarium for hydrolysis of arabinoxylan: reconstitution of the in vivo system from recombinant enzymes. Microbiology 150:2257–2266 [DOI] [PubMed] [Google Scholar]
- 3. Aurilia V., Parracino A., Saviano M., Rossi M., D'Auria S. 2007. The psychrophilic bacterium Pseudoalteromonas haloplanktis TAC125 possesses a gene coding for a cold-adapted feruloyl esterase activity that shares homology with esterase enzymes from gamma-proteobacteria and yeast. Gene 397:51–57 [DOI] [PubMed] [Google Scholar]
- 4. Benoit I., Danchin E. G., Bleichrodt R. J., de Vries R. P. 2008. Biotechnological applications and potential of fungal feruloyl esterases based on prevalence, classification and biochemical diversity. Biotechnol. Lett. 30:387–396 [DOI] [PubMed] [Google Scholar]
- 5. Blum D. L., Kataeva I. A., Li X. L., Ljungdahl L. G. 2000. Feruloyl esterase activity of the Clostridium thermocellum cellulosome can be attributed to previously unknown domains of XynY and XynZ. J. Bacteriol. 182:1346–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cai S., et al. 2010. Cellulosilyticum ruminicola, a newly described rumen bacterium that possesses redundant fibrolytic-protein-encoding genes and degrades lignocellulose with multiple carbohydrate-borne fibrolytic enzymes. Appl. Environ. Microbiol. 76:3818–3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cornu A., Besle J. M., Mosoni P., Grenet E. 1994. Lignin-carbohydrate complexes in forages—structure and consequences in the ruminal degradation of cell-wall carbohydrates. Reprod. Nutr. Dev. 34:385–398 [DOI] [PubMed] [Google Scholar]
- 8. Crepin V. F., Faulds C. B., Connerton I. F. 2004. Functional classification of the microbial feruloyl esterases. Appl. Microbiol. Biotechnol. 63:647–652 [DOI] [PubMed] [Google Scholar]
- 9. Dalrymple B. P., Swadling Y. 1997. Expression of a Butyrivibrio fibrisolvens E14 gene (cinB) encoding an enzyme with cinnamoyl ester hydrolase activity is negatively regulated by the product of an adjacent gene (cinR). Microbiology 143(Pt. 4):1203–1210 [DOI] [PubMed] [Google Scholar]
- 10. Dalrymple B. P., Swadling Y., Cybinski D. H., Xue G. P. 1996. Cloning of a gene encoding cinnamoyl ester hydrolase from the ruminal bacterium Butyrivibrio fibrisolvens E14 by a novel method. FEMS Microbiol. Lett. 143:115–120 [DOI] [PubMed] [Google Scholar]
- 11. de Vries R. P., Kester H. C., Poulsen C. H., Benen J. A., Visser J. 2000. Synergy between enzymes from Aspergillus involved in the degradation of plant cell wall polysaccharides. Carbohydr. Res. 327:401–410 [DOI] [PubMed] [Google Scholar]
- 12. de Vries R. P., et al. 1997. The faeA genes from Aspergillus niger and Aspergillus tubingensis encode ferulic acid esterases involved in degradation of complex cell wall polysaccharides. Appl. Environ. Microbiol. 63:4638–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Vries R. P., Visser J. 2001. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol. Mol. Biol. Rev. 65:497–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Vries R. P., Visser J. 1999. Regulation of the feruloyl esterase (faeA) gene from Aspergillus niger. Appl. Environ. Microbiol. 65:5500–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dodd D., et al. 2009. Biochemical analysis of a beta-d-xylosidase and a bifunctional xylanase-ferulic acid esterase from a xylanolytic gene cluster in Prevotella ruminicola 23. J. Bacteriol. 191:3328–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donaghy J. A., Bronnenmeier K., Soto-Kelly P. F., McKay A. M. 2000. Purification and characterization of an extracellular feruloyl esterase from the thermophilic anaerobe Clostridium stercorarium. J. Appl. Microbiol. 88:458–466 [DOI] [PubMed] [Google Scholar]
- 17. Faulds C. B., Ralet M. C., Williamson G., Hazlewood G. P., Gilbert H. J. 1995. Specificity of an esterase (XYLD) from Pseudomonas fluorescens subsp. cellulosa. Biochim. Biophys. Acta 1243:265–269 [DOI] [PubMed] [Google Scholar]
- 18. Faulds C. B., Williamson G. 1993. Ferulic acid esterase from Aspergillus niger: purification and partial characterization of two forms from a commercial source of pectinase. Biotechnol. Appl. Biochem. 17(Pt. 3):349–359 [PubMed] [Google Scholar]
- 19. Faulds C. B., Williamson G. 1995. Release of ferulic acid from wheat bran by a ferulic acid esterase (FAE-III) from Aspergillus niger. Appl. Microbiol. Biotechnol. 43:1082–1087 [DOI] [PubMed] [Google Scholar]
- 20. Fazary A. E., Ju Y. H. 2007. Feruloyl esterases as biotechnological tools: current and future perspectives. Acta Biochim. Biophys. Sin. (Shanghai) 39:811–828 [DOI] [PubMed] [Google Scholar]
- 21. Gallagher S., Winston S. E., Fuller S. A., Hurrell J. G. 2008. Immunoblotting and immunodetection, unit 8.10. In Current protocols in immunology. John Wiley and Sons, New York, NY: [DOI] [PubMed] [Google Scholar]
- 22. Goldstone D. C., et al. 2010. Structural and functional characterization of a promiscuous feruloyl esterase (Est1E) from the rumen bacterium Butyrivibrio proteoclasticus. Proteins 78:1457–1469 [DOI] [PubMed] [Google Scholar]
- 23. Gubitz G. M., Stebbing D. W., Johansson C. I., Saddler J. N. 1998. Lignin-hemicellulose complexes restrict enzymatic solubilization of mannan and xylan from dissolving pulp. Appl. Microbiol. Biotechnol. 50:390–395 [Google Scholar]
- 24. Guglielmetti S., et al. 2008. Bacterial cinnamoyl esterase activity screening for the production of a novel functional food product. Appl. Environ. Microbiol. 74:1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hassan S., Hugouvieux-Cotte-Pattat N. 2011. Identification of two feruloyl esterases in Dickeya dadantii 3937 and induction of the major feruloyl esterase and of pectate lyases by ferulic acid. J. Bacteriol. 193:963–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hermoso J. A., et al. 2004. The crystal structure of feruloyl esterase A from Aspergillus niger suggests evolutive functional convergence in feruloyl esterase family. J. Mol. Biol. 338:495–506 [DOI] [PubMed] [Google Scholar]
- 27. Hu Y., et al. 2010. Novel lipolytic genes from the microbial metagenomic library of the South China Sea marine sediment. FEMS Microbiol. Ecol. 72:228–237 [DOI] [PubMed] [Google Scholar]
- 28. Koseki T., Hori A., Seki S., Murayama T., Shiono Y. 2009. Characterization of two distinct feruloyl esterases, AoFaeB and AoFaeC, from Aspergillus oryzae. Appl. Microbiol. Biotechnol. 83:689–696 [DOI] [PubMed] [Google Scholar]
- 29. Koseki T., et al. 2010. Characterization of a chimeric enzyme comprising feruloyl esterase and family 42 carbohydrate-binding module. Appl. Microbiol. Biotechnol. 86:155–161 [DOI] [PubMed] [Google Scholar]
- 30. Kroon P. A., Williamson G. 1996. Release of ferulic acid from sugar-beet pulp by using arabinanase, arabinofuranosidase and an esterase from Aspergillus niger. Biotechnol. Appl. Biochem. 23(Pt. 3):263–267 [PubMed] [Google Scholar]
- 31. Lai K. K., Lorca G. L., Gonzalez C. F. 2009. Biochemical properties of two cinnamoyl esterases purified from a Lactobacillus johnsonii strain isolated from stool samples of diabetes-resistant rats. Appl. Environ. Microbiol. 75:5018–5024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moukouli M., Topakas E., Christakopoulos P. 2008. Cloning, characterization and functional expression of an alkalitolerant type C feruloyl esterase from Fusarium oxysporum. Appl. Microbiol. Biotechnol. 79:245–254 [DOI] [PubMed] [Google Scholar]
- 33. Murashima K., Kosugi A., Doi R. H. 2002. Determination of subunit composition of Clostridium cellulovorans cellulosomes that degrade plant cell walls. Appl. Environ. Microbiol. 68:1610–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rakotoarivonina H., Hermant B., Chabbert B., Touzel J. P., Remond C. 2011. A thermostable feruloyl-esterase from the hemicellulolytic bacterium Thermobacillus xylanilyticus releases phenolic acids from non-pretreated plant cell walls. Appl. Microbiol. Biotechnol. 90:541–552 [DOI] [PubMed] [Google Scholar]
- 35. Rashamuse K. J., Burton S. G., Cowan D. A. 2007. A novel recombinant ethyl ferulate esterase from Burkholderia multivorans. J. Appl. Microbiol. 103:1610–1620 [DOI] [PubMed] [Google Scholar]
- 36. Rumbold K., Gübitz G., Prior Bernard A. 2004. Microbial feruloyl esterases, p. 255–270In Saha B. C., Hayashi K.(ed.), Lignocellulose biodegradation. ACS Symposium Series 889. American Chemical Society, Washington, DC [Google Scholar]
- 37. Saulnier L., Thibault J. F. 1999. Ferulic acid and diferulic acids as components of sugar-beet pectins and maize bran heteroxylans. J. Sci. Food Agric. 79:396–402 [Google Scholar]
- 38. Schubot F. D., et al. 2001. Structural basis for the substrate specificity of the feruloyl esterase domain of the cellulosomal xylanase Z from Clostridium thermocellum. Biochemistry 40:12524–12532 [DOI] [PubMed] [Google Scholar]
- 39. Selig M. J., Knoshaug E. P., Adney W. S., Himmel M. E., Decker S. R. 2008. Synergistic enhancement of cellobiohydrolase performance on pretreated corn stover by addition of xylanase and esterase activities. Bioresour. Technol. 99:4997–5005 [DOI] [PubMed] [Google Scholar]
- 40. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 41. Tong H., Chen W., Shi W., Qi F., Dong X. 2008. SO-LAAO, a novel l-amino acid oxidase that enables Streptococcus oligofermentans to outcompete Streptococcus mutans by generating H2O2 from peptone. J. Bacteriol. 190:4716–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williamson G., Kroon P. A., Faulds C. B. 1998. Hairy plant polysaccharides: a close shave with microbial esterases. Microbiology 144(Pt. 8):2011–2023 [DOI] [PubMed] [Google Scholar]
- 43. Wong D. W. 2006. Feruloyl esterase: a key enzyme in biomass degradation. Appl. Biochem. Biotechnol. 133:87–112 [DOI] [PubMed] [Google Scholar]
- 44. Zeng W., Chen H. 2009. Synergistic effect of feruloyl esterase and cellulase in hydrolyzation of steam-exploded rice straw. Sheng Wu Gong Cheng Xue Bao 25:49–54 [PubMed] [Google Scholar]