Abstract
To investigate if the primary function of the Agr system of Listeria monocytogenes is to monitor cell density, we followed Agr expression in batch cultures, in which the autoinducer concentration was uniform, and in biofilms. Expression was heterogeneous, suggesting that the primary function of Agr is not to monitor population density.
TEXT
Quorum sensing (QS) is the mechanism by which bacteria secrete signaling molecules called autoinducers that are sensed by neighboring cells in a population (30). The binding of these autoinducers to cognate receptors results in transcriptional regulation of gene expression. So far, for the species Listeria monocytogenes, one QS system, mediated by the agrBDCA operon, has been described (2, 7). Deletion of agrD or agrA results in impairment of major adaptive strategies, such as biofilm development (22, 23) and virulence (2, 21).
Historically, the term QS was coined to illustrate that accumulation of autoinducers enables a coordinated control of gene expression resulting in a population-wide phenotype switch when the population reaches a threshold or quorum (6, 8, 18). However, recent reports indicate that adaptive functions of QS can be diverse and are not limited to population density sensing (20).
For example, phenotypic heterogeneity of QS-regulated traits was reported in biofilms. Several subpopulations with distinct phenotypes organize Bacillus subtilis biofilms (13, 14). Extracellular DNA release during the sessile growth of Enterococcus faecalis is directed by a fratricidal mechanism triggered by a quorum-responsive subpopulation (26). Heterogeneity was also observed in QS-regulated bioluminescence of Vibrio harveyi (1).
Recent reports showed that confocal laser scanning microscopy (CLSM) associated with fluorescent reporter fusions may be used to trace the spatiotemporal expression of specific genes at a single-cell level within the overall biofilm structure (9, 12). When we traced Agr expression in biofilms, we detected green fluorescent protein (GFP) mainly in a network of elongated chains reminiscent of scaffoldings that surrounded densely populated microcolonies (22). This heterogeneous expression was surprising; indeed, maximum expression was expected within microcolonies, where the autoinducer concentration is maximum (19).
Thus, the question of whether the function of this QS system was primarily to monitor population density arose. In order to test this hypothesis, a Pagr-gfp fusion was integrated upstream of the agr locus of the L. monocytogenes EGD-e genetic background. This construct was designed to develop Agr expression reporters without affecting expression of the downstream agrBDCA operon (22).
We followed GFP fluorescence by flow cytometry and microscopy during growth in batch homogenized liquid cultures, which represents environmental conditions prone to facilitate responses to cell density (confined cultures and no diffusion). Cells were collected by centrifugation (10 min at 8,000 × g), washed, and diluted in 150 mM filtered NaCl solution before flow cytometry analysis. For each sample, at least 100,000 cells were analyzed on a Cyflow flow cytometer (Partec, Germany) operating a 20-mW solid-state laser (488 nm). Data containing the green fluorescent signals were collected by a fluorescein isothiocyanate (FITC) filter, and the photomultiplier voltage was set at 380 V.
Agr expression in confined liquid environments is heterogeneous.
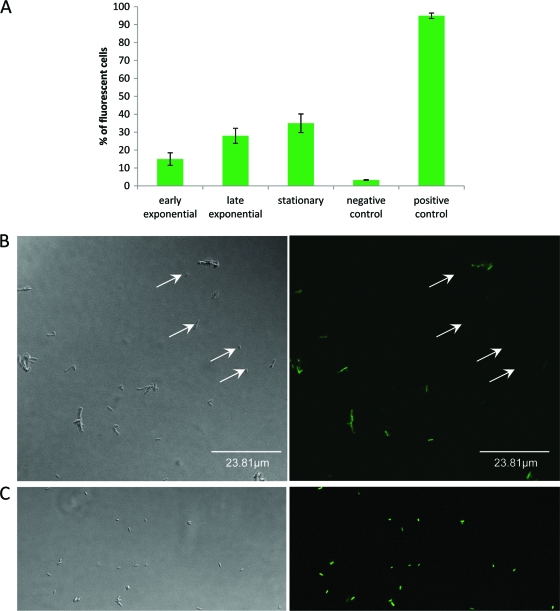
L. monocytogenes ARD009 (EGD-e background) was cultivated in tryptic soy broth (TSB) in a rotary incubator (150 rpm) at 25°C. Agr expression was low and represented approximately 15% of the total early-exponential-phase population (Fig. 1 A). It increased until late exponential phase (28%), and the subpopulation of cells expressing Agr (Agr-ON) stabilized to 35%. The percentage of viability was close to 100% during the length of the experiment (data not shown). Less than 4% of Agr-ON cells were detected when agrD and agrA mutants were used. The percentage of fluorescent cells of the positive control L. monocytogenes EGD-e(pNF8-GFP) was significantly higher throughout growth, and over 95% of fluorescent cells were detected by flow cytometry (Fig. 1A) as well as epifluorescence microscopy (Fig. 1C).
Fig. 1.
Individual cell measurement of Agr expression during growth of L. monocytogenes ARD009 in homogenized liquid cultures incubated at 25°C. (A) Percentages of GFP fluorescent cells detected by flow cytometry. A positive GFP signal is detected in Agr-ON cells (cells expressing Pagr-gfp). (B) Phase-contrast microscopy and fluorescence microscopy of L. monocytogenes ARD009 cells expressing Pagr-gfp after 16 h of incubation at 25°C. (C) Phase-contrast microscopy and fluorescence microscopy of L. monocytogenes EGD-e(pNF8-GFP). White arrows show examples of Agr-OFF cells.
We then modified the growth medium in order to simulate a range of environmental conditions, including low nutrient levels (half-strength TSB), osmolarity (TSB plus 3% NaCl), and high glucose concentration (TSB plus 1% glucose or TSB plus 2% glucose). We also tested the rich medium brain heart infusion (BHI). The composition of the growth medium affected Agr expression. The stationary-phase Agr-ON subpopulation was significantly smaller in the rich medium BHI (23.1% ± 1.37%) and in TSB supplemented with glucose (11.1% ± 0.22%). On the other hand, nutrient limitation (half-strength TSB) increased Agr expression (37.3% ± 0.56%), while the percentage of Agr-ON cells did not significantly change upon the addition of 3% NaCl. As temperature is an important cue for the environmental adaptation of L. monocytogenes (10), we followed cultures incubated at 37°C. Agr expression was significantly higher at this temperature (48.5% ± 8.03%) than at 25°C. This effect of environmental cues on Agr expression suggests that Agr is involved in a complex regulatory network integrating various environmental cues, including temperature and the energy status of the cell. Indeed, interconnection of several regulons in a network was reported recently (3).
Agr expression is heterogeneous in other genetic backgrounds.
Considering the biodiversity of the species L. monocytogenes (4), we decided to investigate Agr expression during growth of other isolates from lineage I and lineage II (Table 1). Pagr-gfp fusions within five other genetic backgrounds were constructed. Agr expression was heterogeneous, indicating that this phenotype was not specific to the domesticated laboratory strain L. monocytogenes EGD-e. The percentages of Agr-ON cells significantly differed among genetic backgrounds and ranged from 15% to 73% and were different from the positive-control value (Table 1). Regardless of the genetic background, Agr expression was heterogeneous. This suggests that it may be a generic phenomenon in this species.
Table 1.
Percentages of Agr-ON cells detected after 24 h of growth in homogenized TSB cultures incubated at 25°Ca
| Strain | Genotype | Lineage | Origin of parental strain | Reference | % of Agr-ON cells |
|---|---|---|---|---|---|
| ARD009 | EGD-e::pGID128 Pagr-gfp | I | Reference strain/rabbit listeriosis outbreak (17) | 22 | 35 ± 5.2 |
| ARD010 | DG119D::pGID128 Pagr-gfp | I | EGD-e ΔagrD (23) | This study | 3.5 ± 0.25 |
| ARD011 | DG125A::pGID128 Pagr-gfp | I | EGD-e ΔagrA (23) | 22 | 3.3 ± 0.22 |
| DG303 | LO28::pGID128 Pagr-gfp | I | Healthy pregnant women (16) | This study | 15.1 ± 0.10 |
| DG305 | NV4::pGID128 Pagr-gfp | I | Minced beef (24) | This study | 68.4 ± 0.12 |
| DG304 | 12749::pGID128 Pagr-gfp | II | South Nation River sample (15) | This study | 67.2 ± 0.57 |
| DG306 | ScottA::pGID128 Pagr-gfp | II | Human listeriosis outbreak (31) | This study | 72.0 ± 0.13 |
| DG307 | 3E::pGID128 Pagr-gfp | II | Sink at a cheese-making plant (23) | This study | 73.5 ± 0.03 |
| EGD-e(pNF8-GFP) | EGD-e(pAT18Ω(PdltΩgfp-mut1)) | Reference strain/rabbit listeriosis outbreak (17) | 5 | 95 ± 1.50 |
Percentages were determined after cytometry analysis.
In this experimental setup, environmental cues, including autoinducer concentration, are uniform. Still, we did not observe a population-wide switch to the Agr-ON phenotype, but two subpopulations were detected; this heterogeneity suggests that the Agr system of L. monocytogenes may not be primarily dedicated to population density sensing but could mediate other adaptive functions (11, 20).
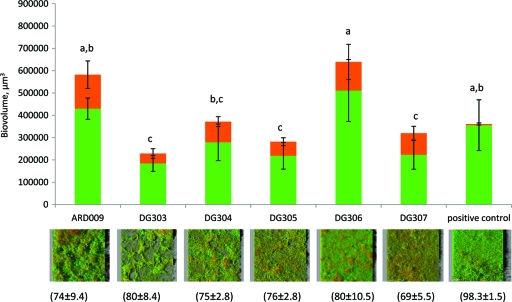
The relative ratio of Agr-ON to non-Agr-expressing (Agr-OFF) subpopulations was strain dependent. We wondered what these ratios would be during sessile growth. We cultivated and monitored biofilms in BST FC 81 flow cells (Biosurface Technologies Corporation, Bozeman, MT) for 24 h at 25°C as described previously (22). Briefly, flow chambers were inoculated with cultures grown overnight in TSB medium and left to rest for 1 h to allow bacterial adhesion prior to TSB circulation at 10 ml/h. Biofilms were observed on a Leica TCS SP2 AOBS CLSM (at the MIMA2 INRA microscopic platform) fitted with a 63×/1.2-numerical-aperture water immersion objective lens (Fig. 2). Strains DG303, DG304, DG305, and DG307 developed structures similar to those we reported previously regarding biofilms of L. monocytogenes EGD-e. Ball-shaped microcolonies were linked together by chains of elongated cells organized as a network at the apex of the biofilm (22). The biofilms of strain DG306 were less structured. Biovolumes (μm3) are indicators of the ability of the strains to grow on surfaces. Strains DG306 and ARD009 produced large amounts of biofilm; the biovolume of biofilms produced by DG304 was intermediate, while DG303, DG305, and DG307 were poor colonizers (Fig. 2). Whatever the strain tested, Agr expression was heterogeneous. The percentages of Agr-ON cells ranged from 69% ± 5.5% to 80% ± 10.5%, but these differences were not statistically significant (Fig. 2). These percentages were higher than those in liquid cultures. The percentage of fluorescent cells of the control EGD-e(pNF8-GFP) was always significantly higher and close to 100% whatever the growth condition (biofilms or liquid cultures). Our results differ from those in reports on expression of other QS systems. Population-wide expression of las and rhl was observed during sessile growth of Pseudomonas aeruginosa (25), and Veening et al. proposed that induction of all ComA-regulated genes could be homogenous in populations of B. subtilis (27).
Fig. 2.
Biovolumes of Agr-ON (green bars) and Agr-OFF (orange bars) cells in biofilms of six Pagr-gfp reporter strains grown for 24 h at 25°C under flowing conditions. The percentages of Agr-ON cells are presented in parentheses. Letters indicate groups of biovolumes identified after analysis of variance (P < 0.05).
In conclusion, our results indicate that in L. monocytogenes, population density sensing may not be the sole function of the Agr system. The rationale of generating heterogeneity through the Agr system has to be investigated. The bet-hedging strategy (28, 29) is an appealing theory; the generation of offsprings with different phenotypes could facilitate survival of clonal populations to ever-changing environmental conditions. This phenomenon could indeed drive ubiquity in the species L. monocytogenes. It will be of particular interest to decipher the adaptive function of the Agr system considering the various habitats colonized by this species. To this end, further in situ investigations are required.
Acknowledgments
We thank C. Arnould (Centre de Microscopie, INRA/UB) for technical assistance in confocal microscopy image capture of liquid cultures. We also thank Cécile Révelin for her advice in optimization of cytometry setups.
Footnotes
Published ahead of print on 1 July 2011.
REFERENCES
- 1. Anetzberger C., Pirch T., Jung K. 2009. Heterogeneity in quorum sensing-regulated bioluminescence of Vibrio harveyi. Mol. Microbiol. 73:267–277 [DOI] [PubMed] [Google Scholar]
- 2. Autret N., Raynaud C., Dubail I., Berche P., Charbit A. 2003. Identification of the agr locus of Listeria monocytogenes: role in bacterial virulence. Infect. Immun. 71:4463–4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaturongakul S., et al. 2011. Transcriptomic and phenotypic analyses identify coregulated, overlapping regulons among PrfA, CtsR, HrcA, and the alternative sigma factors sigmaB, sigmaC, sigmaH, and sigmaL in Listeria monocytogenes. Appl. Environ. Microbiol. 77:187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doumith M., et al. 2004. New aspects regarding evolution and virulence of Listeria monocytogenes revealed by comparative genomics and DNA arrays. Infect. Immun. 72:1072–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fortineau N., et al. 2000. Optimization of green fluorescent protein expression vectors for in vitro and in vivo detection of Listeria monocytogenes. Res. Microbiol. 151:353–360 [DOI] [PubMed] [Google Scholar]
- 6. Fuqua W. C., Winans S. C., Greenberg E. P. 1994. Quorum sensing in bacteria—the luxR-luxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176:269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garmyn D., Gal L., Lemaitre J. P., Hartmann A., Piveteau P. 2009. Communication and autoinduction in the species Listeria monocytogenes: a central role for the agr system. Commun. Integr. Biol. 2:371–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hooshangi S., Bentley W. E. 2008. From unicellular properties to multicellular behavior: bacteria quorum sensing circuitry and applications. Curr. Opin. Biotechnol. 19:550–555 [DOI] [PubMed] [Google Scholar]
- 9. Ito A., May T., Taninchi A., Kawata K., Okabe S. 2009. Localized expression profiles of rpoS in Escherichia coli biofilms. Biotechnol. Bioeng. 103:975–983 [DOI] [PubMed] [Google Scholar]
- 10. Johansson J., et al. 2002. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell 110:551–561 [DOI] [PubMed] [Google Scholar]
- 11. Keller L., Surette M. G. 2006. Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 4:249–258 [DOI] [PubMed] [Google Scholar]
- 12. Lenz A. P., Williamson K. S., Pitts B., Stewart P. S., Franklin M. J. 2008. Localized gene expression in Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 74:4463–4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez D., Vlamakis H., Kolter R. 2009. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol. Rev. 33:152–163 [DOI] [PubMed] [Google Scholar]
- 14. López D., Vlamakis H., Losick R., Kolter R. 2009. Paracrine signaling in a bacterium. Genes Dev. 23:1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyautey E., et al. 2007. Distribution and characteristics of Listeria monocytogenes isolates from surface waters of the South Nation River watershed, Ontario, Canada. Appl. Environ. Microbiol. 73:5401–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mengaud J., Geoffroy C., Cossart P. 1991. Identification of a new operon involved in Listeria monocytogenes virulence—its 1st gene encodes a protein homologous to bacterial metalloproteases. Infect. Immun. 59:1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murray E. G. D., Webb R. E., Swann M. B. R. 1926. A disease of rabbits characterized by a large mononuclear leucocytosis, caused by a hitherto undescribed bacillus Bacterium monocytogenes (n. sp.). J. Pathol. Bacteriol. 29:407–439 [Google Scholar]
- 18. Ng W. L., Bassler B. L. 2009. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 43:197–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parsek M. R., Greenberg E. P. 2005. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 13:27–33 [DOI] [PubMed] [Google Scholar]
- 20. Platt T. G., Fuqua C. 2010. What's in a name? The semantics of quorum sensing. Trends Microbiol. 18:383–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riedel C. U., et al. 2009. AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes. Mol. Microbiol. 71:1177–1189 [DOI] [PubMed] [Google Scholar]
- 22. Rieu A., et al. 2008. Listeria monocytogenes EGD-e biofilms: no mushrooms but a network of knitted chains. Appl. Environ. Microbiol. 74:4491–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rieu A., Weidmann S., Garmyn D., Piveteau P., Guzzo J. 2007. agr system of Listeria monocytogenes EGD-e: role in adherence and differential expression pattern. Appl. Environ. Microbiol. 73:6125–6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rousseaux S., Olier M., Lemaitre J. P., Piveteau P., Guzzo J. 2004. Use of PCR-restriction fragment length polymorphism of inlA for rapid screening of Listeria monocytogenes strains deficient in the ability to invade Caco-2 cells. Appl. Environ. Microbiol. 70:2180–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sauer K., Camper A. K., Ehrlich G. D., Costerton J. W., Davies D. G. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas V. C., et al. 2009. A fratricidal mechanism is responsible for eDNA release and contributes to biofilm development of Enterococcus faecalis. Mol. Microbiol. 72:1022–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Veening J. W., Hamoen L. W., Kuipers O. P. 2005. Phosphatases modulate the bistable sporulation gene expression pattern in Bacillus subtilis. Mol. Microbiol. 56:1481–1494 [DOI] [PubMed] [Google Scholar]
- 28. Veening J. W., Smits W. K., Kuipers O. P. 2008. Bistability, epigenetics, and bet-hedging in bacteria. Annu. Rev. Microbiol. 62:193–210 [DOI] [PubMed] [Google Scholar]
- 29. Veening J. W., et al. 2008. Bet-hedging and epigenetic inheritance in bacterial cell development. Proc. Natl. Acad. Sci. U. S. A. 105:4393–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waters C. M., Bassler B. L. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21:319–346 [DOI] [PubMed] [Google Scholar]
- 31. Wiedmann M., et al. 1997. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect. Immun. 65:2707–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]