Abstract
An improved strategy was developed for the high-density culture of Magnetospirillum gryphiswaldense strain MSR-1 and large-scale magnetosome production in both 7.5- and 42-liter autofermentors. By using a nutrient-balanced feeding strategy and the replacement of carbon and nitrogen sources to reduce accumulation of Na+ and Cl− ions, we reduced the factors that tend to inhibit cell growth, particularly the increase of osmotic potential. Semicontinuous culture was thereby achieved in the autofermentor for the first time. When the cells were harvested at 36 and 73 h, magnetosome yields (dry weight) as high as 168.3 and 83.5 mg/liter/day, respectively, were achieved. These values were, respectively, approximately 10 and 5 times higher than the yields achieved in previous studies and represent a significant improvement in magnetosome production efficiency.
INTRODUCTION
Biomineralized magnetosomes (chains of magnetite crystals found in prokaryotes) have attracted commercial interest because of their narrow size range, good dispersibility, and biomembrane enclosure. Previous studies have addressed a variety of applications and properties, including enzyme immobilization (5), gene delivery system (16), cell separation (19), drug carriers (11, 12), immunoassays (4, 14), protein and multisubunit enzyme complexes (7, 20), and use of microorganisms per se for mineral recovery (13). Because of the highly restrictive culture conditions for magnetotactic bacteria, in terms of the dissolved oxygen concentration (dO2) (3, 17), nutrients, etc., the yields of both magnetosomes and their host microorganisms under artificial culture tend to be low (10, 18). A long-standing research goal of our laboratory is improved large-scale production of cells and magnetosomes.
In a previous study using fed-batch culture techniques (10), we achieved maximal cell density (optical density at 565 nm [OD565] of 7.24, cell dry weight of 2.17 g/liter [0.87 g/liter/day], and magnetosome dry weight of 41.7 mg/liter [16.7 mg/liter/day]). Through further optimization of culture temperature, pH, dO2, and nutrients, we achieved an OD565 value of 12 in a 7.5-liter fermentor after 40 h of culture (unpublished data). In revising our previous feeding strategy, we focused on supplementation of carbon and nitrogen sources but ignored two possible factors that could inhibit cell growth: (i) nutrient limitation arising during fermentation process and (ii) the accumulation of Na+ and Cl− in a fermentor fed with sodium lactate and ammonium chloride. By replacing the carbon and nitrogen sources and using an optimally nutrient-balanced feeding strategy, in a 7.5-liter fermentor after 44 h, we achieved an OD565 of 30.4, a cell dry weight of 7.59 g/liter (3.8 g/liter/day), and a magnetosome dry weight of 225.53 mg/liter (112.77 mg/liter/day). In a larger (42-liter) fermentor, after 44 h, we achieved an OD565 of 42, a cell dry weight of 9.16 g/liter (4.58 g/liter/day), and a magnetosome dry weight of 356.52 mg/liter (178.26 mg/liter/day). The efficiency of magnetosome production in a 42-liter fermentor was 10.7 times higher than the previous maximal value. Based on these optimizations of feeding strategy, a semicontinuous culture was achieved successfully for the first time in a 7.5-liter fermentor.
MATERIALS AND METHODS
Bacterial strain.
M. gryphiswaldense strain MSR-1 (DSM6361) was purchased from Deutsche Sammlung von Mikro-organismen und Zellkulturen (Brunswick, Germany).
Culture medium 1.
Culture medium 1 was prepared by using a shaking flask and contained preculture medium composed of sodium lactate at 2.6 g/liter, NH4Cl at 0.4 g/liter, yeast extract at 0.1 g/liter, MgSO4·7H2O at 0.1 g/liter, K2HPO4·3H2O at 0.5 g/liter, and 0.5 ml of mineral elixir (pH 7.0). The mineral elixir (1 liter, pH 7.0) was composed of the following: nitrilotriacetic acid, 15 g; MgSO4·7H2O, 3 g; MnSO4·2H2O, 5.0 g; NaCl, 10.0 g; FeSO4·7H2O, 1.0 g; CoSO4·7H2O, 1.8 g; CaCl2·2H2O, 30.0 g; ZnSO4·7H2O, 1.8 g; CuSO4·5H2O, 0.1 g; KAl(SO4)2·12H2O, 0.2 g; H3BO3, 0.1 g; Na2MoO4·2H2O, 0.1 g; NiCl2·6H2O, 0.25 g; and Na2SeO3·5H2O, 3.0 mg.
Culture medium 2.
Culture medium 2 was prepared by using a shaking flask and contained 0, 40, 80, or 160 mM NaCl, respectively, plus 50 ml of shaking flask medium with an additional 0 g (positive control), 0.117 g, 0.234 g, or 0.468 g of NaCl, respectively.
Fermentation medium.
The fermentation medium (4.5 liters) was composed of the following: sodium lactate (70 to 80%), 6.0 g; NH4Cl, 1.0 g; MgSO4·7H2O, 0.12 g; yeast extract, 0.3 g; K2HPO4·3H2O, 0.3 g; and mineral elixir, 0.35 ml.
Feeding media.
Feed A was composed of the following: sodium lactate, 45.0 g; lactate (85 to 90%), 64.0 g; NH4Cl, 36.6 g; K2HPO4·3H2O, 3.0 g; MgSO4·7H2O, 1.2 g; FeCl3·6H2O, 2.0 g; yeast extract, 3.0 g; and mineral elixir, 3.5 ml. Feed B was composed of the following: sodium lactate, 45.0 g; lactate, 64.0 g; NH4Cl, 36.6 g; K2HPO4·3H2O, 6.0 g; MgSO4·7H2O, 2.4 g; FeCl3·6H2O, 2.0 g; yeast extract, 6.0 g; and mineral elixir, 7 ml. Feed C was composed of the following: lactate, 100.0 g; NH3·H2O (25 to 28%), 18.0 ml; K2HPO4·3H2O, 6.0 g; MgSO4·7H2O, 2.4 g; FeCl3·6H2O, 2.0 g; yeast extract, 6.0 g; and mineral elixir, 7 ml. All reagents were analytical grade.
Effect of NaCl concentration on cell growth.
A defined amount of cells was inoculated (10% [vol/vol]) into shaking flask medium and subjected to gradient NaCl concentrations as described above. Cells were cultured for 24 h, 30°C, and 100 rpm, and the OD565 values were measured.
Determination of the optimal dissolved oxygen concentration (dO2).
The dO2 was cascaded at 50, 30, 10, and 1%, and between 0 and 1% for 4 h in 7.5-liter fermentor (Bioflo 110; New Brunswick Scientific, NJ). The dO2 could not be accurately cascaded at values between 0 and 1% because of the limited accuracy of the cascade control.
Fed-batch culture in 7.5-liter fermentor.
The inoculum for the fermentation was cultured by three sequential transfers with 10% (vol/vol) inoculation, under the same conditions as for the shaking flask culture. Fermentation was conducted with a working volume of 5 liters after 10% (vol/vol) inoculation at 30°C, an initial airflow of 0.5 liter/min, and agitation at 200 rpm. The pH was maintained at 6.9 by automated supplementation of feeding medium. The dO2 was maintained between 0 and 1% by the regulation of airflow or agitation every 2 h, after which it decreased to 0% at 12 h. One bottle feeding medium was sufficient for MSR-1 growth to an OD565 of ∼20. The OD565, the osmotic potential, the magnetic response (Cmag), and the concentrations of lactate, NH4+, and Fe2+/Fe3+ ions were measured starting at 12 h, at intervals of 4 h until termination of the culture.
Semicontinuous culture in a 7.5-liter fermentor.
Semicontinuous culture was performed using the same control strategy as that described for the fed-batch culture, with the supplementation of Feed C. A 4.5-liter portion of broth was discharged at the late logarithmic growth phase (end of “first stage”), until 0.5 liter of broth remained; 4.5 liters of fresh fermentation medium were then added (beginning of the “second stage”).
Measurement of the cell density (OD565) and magnetic response (Cmag).
The OD565 was measured by a UV-VIS spectrophotometer (UNICO2100; UNICO Instrument Co., Ltd, Shanghai, China). The Cmag was calculated by measuring the maximum and minimum scattering intensities (8).
Concentrations of lactate, NH4+, and iron ions.
Sample supernatants at defined times were prepared by centrifugation (12,000 rpm, 2 min). The concentrations of lactate, NH4+, and Fe2+/Fe3+ ions in the supernatant were measured by using a BioSensor analyzer (SBA-40C; Institute of Biology, Shandong Academy of Sciences, China) (10), the indophenol blue method (6), and the ferrozine method (1), respectively.
Transmission electron microscopy (TEM).
A suspension of concentrated bacteria obtained by centrifugation was adsorbed onto a copper grid, washed twice with distilled water, dried, and viewed and recorded using a transmission electron microscope (H-8000; Hitachi, Japan) (10).
Osmotic potential.
The osmotic potential (in mmol/kg) of the supernatant as described above was measured with a vapor pressure osmometer (model 5520; Wescor, Inc., Logan, UT).
Dry weight of cells and magnetosomes.
The cell dry weight was measured after wet cells were dried at 70°C. The magnetosome dry weight was measured after the magnetosomes were purified by the magnetic absorption method described previously (15) and then dried at 70°C.
RESULTS AND DISCUSSION
Control strategy for dO2.
Magnetosomes can be synthesized under microaerobic or anaerobic conditions. However, low dO2 values (i.e., 0%) significantly inhibit cell growth. A dO2 control strategy was based on the measurement of Cmag and the observation of cell pellet color under a dO2 gradient in 7.5-liter fermentor. The cell pellet turned black when the dO2 decreased to a level between 0 and 1%. Cmag measurements revealed that the cells became responsive to external magnetic fields only when the dO2 fell below 1% (Table 1). Therefore, fermentations were performed with control of dO2 between 0 and 1% by regulation of airflow and agitation (rpm).
Table 1.
Effect of cascading dO2 values on magnetic response (Cmag)
| dO2 (%) | Cmag | Cell pellet color |
|---|---|---|
| 50 | 0 | White |
| 30 | 0 | White |
| 10 | 0 | White |
| 1 | 0 | White |
| <1 | 1.37 | Black |
Nutrient-balanced feeding strategy.
According to “Liebig's Law of the Minimum,” the biomass in a given system is usually restricted by the amount of one particular nutrient, while other nutrients are present in excess (2). We examined data from previous fermentation experiments and confirmed that such nutrient limitation phenomenon applied.
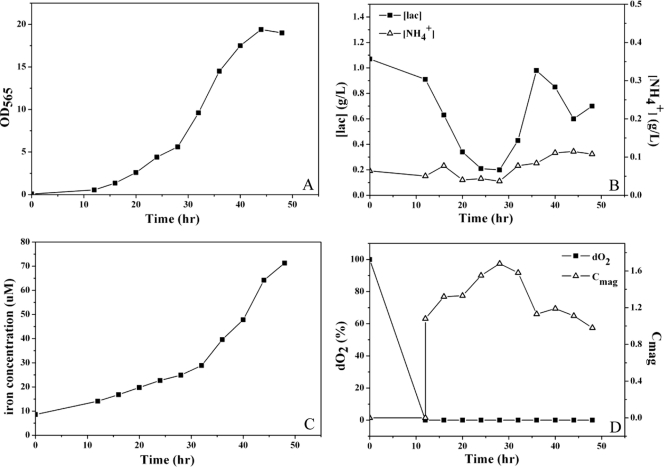
When the bacteria were grown in fed-batch culture with supplementation of feed A for 44 h, the OD565 reached a maximum of 12. When the cells were grown for the same time with feed B plus additional amounts of K2HPO4·3H2O, MgSO4·7H2O, yeast extract, and mineral elixir, the OD565 reached a maximum of 19.4 (Fig. 1A). The concentrations of lactate and NH4+ were maintained in the ranges 0.2 to 1.07 g/liter and 0.04 to 0.11 g/liter, respectively (Fig. 1B), which were favorable for growth. The concentration of Fe2+/Fe3+ was increased from 8.6 to 71.3 μM (Fig. 1C), whereby magnetosomes were synthesized, but cell growth was not suppressed. As the dO2 decreased to ∼0%, the Cmag increased rapidly, reaching a max imum of 1.68 at 28 h (Fig. 1D). The nutrient-balanced feeding strategy described above effectively extended the logarithmic growth phase of MSR-1 in fed-batch cultures.
Fig. 1.
Fed-batch culture of M. gryphiswaldense strain MSR-1 cells in a 7.5-liter autofermentor supplemented with feed B (see the text). (A) Cell growth curve. The cell density (i.e., the OD565) reached a maximum of 19.4 at 44 h. (B) Concentrations of lactate (▪) and NH4+ (▵). Each was controlled at a low level suitable for cell growth. (C) The Fe2+/Fe3+ concentration in fermentor increased with time but remained within the cell tolerance range. (D) Relation of Cmag (▵) and dO2 (▪). The Cmag increased rapidly as dO2 declined to 0% at 12 h, but then decreased in the late logarithmic phase and the stationary phase.
We also formulated a nutrient-balanced feeding strategy in order to minimize possible growth-inhibitory effects of excessive amounts of nutrients. The results from repeated experiments showed that at least an “M” amount of a particular nutrient (one feeding bottle) was required for growth of MSR-1 cells to an OD565 of 20. We hypothesized that in order to reach an OD565 of “A”, a “B” amount of the nutrient would be required, according to the equation: B = (A/20)·M. The application of a nutrient-balanced feeding strategy, based on this equation, led to a 61.7% increase in the maximal OD565 in the present study.
Isosmotic culture.
The results of repeated fermentation experiments showed that an OD565 of ∼20 was the upper limit for a nutrient-balanced feeding strategy. Real-time examination by high-performance liquid chromatography (HPLC) showed the accumulation in culture broth of an unknown component (peak) during the fermentation process. We speculated that supplementation of sodium lactate and NH4Cl in culture medium led to the accumulation of Na+ and Cl− ions. Indeed, the unknown peak had the same HPLC retention time as NaCl.
Effect of NaCl concentration on cell growth in shaking flask culture.
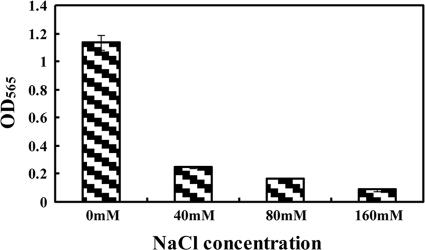
According to theoretical calculations, final concentrations of ∼89 mM Na+ and ∼157 mM Cl− were accumulated by the end of fermentation. The salt tolerance of MSR-1 in a shaking flask culture was evaluated by using a gradient of NaCl concentrations. Even a low concentration (40 mM [2.34 g/liter]) of NaCl significantly inhibited the cell growth. The OD565 at the end of fermentation was ∼22% that of positive control. Degree of growth inhibition was correlated with NaCl concentration (Fig. 2). These findings indicated that accumulation of Na+ and Cl− is the major factor inhibiting cell growth in fed-batch cultures.
Fig. 2.
Effect of NaCl (concentration gradient) on cell growth in a shaking flask culture.
Reversing inhibition of cell growth by replacement of C and N source.
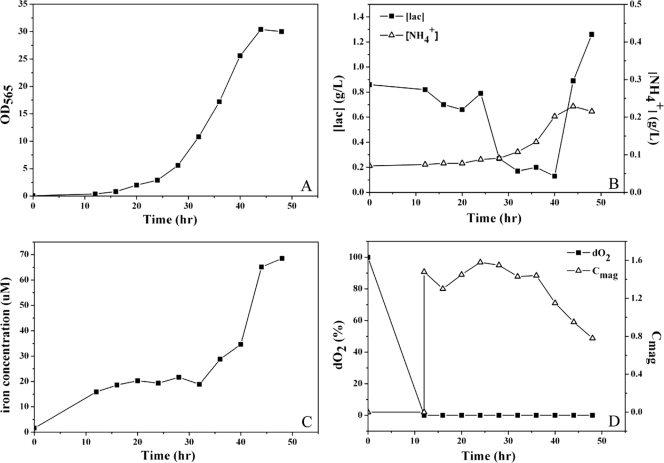
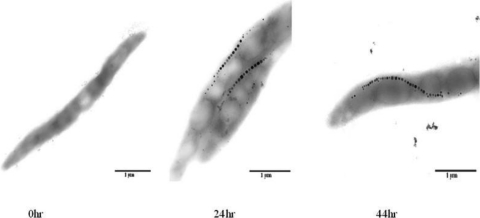
When fed-batch culture was supplemented with feed C for 44 h, the OD565 reached a maximum of 30.4, an increase of 56.7% compared to culture with feed B (Fig. 3A). Lactate and NH4+ concentrations were maintained in the growth-promoting ranges of 0.17 to 1.26 g/liter and 0.07 to 0.23 g/liter, respectively (Fig. 3B). Fe2+/Fe3+ concentration increased from 1.59 to 68.53 μM (Fig. 3C), which allowed magnetosome formation without cell growth inhibition. The Cmag reached a maximum of 1.58 at 24 h (Fig. 3D). TEM showed that no magnetosome synthesis occurred at the initial stage of fermentation (dO2 × 100%), whereas mature magnetosome chains were formed at later stages (24 to 44 h) (Fig. 4).
Fig. 3.
Fed-batch culture of MSR-1 cells in 7.5 L fermentor supplemented with Feed C. (A) Cell growth curve. The OD565 reached a maximum of 30.4 at 44 h. (B) Concentrations of lactate (▪) and NH4+ (▵). Each was controlled at a low level suitable for cell growth. (C) The Fe2+/Fe3+ concentration was sufficient for magnetosome formation without an inhibitory effect on cell growth. (D) Relation of Cmag (▵) and dO2 (▪). The Cmag increased initially and then declined in late logarithmic phase.
Fig. 4.
TEM images of MSR-1 cells at 0, 24, and 44 h in fed-batch cultures supplemented with feed C. No magnetosome formation occurred at 0 h. Mature magnetosome chains with linear arrangements were observed from 24 to 44 h. Bar, 1.0 μm.
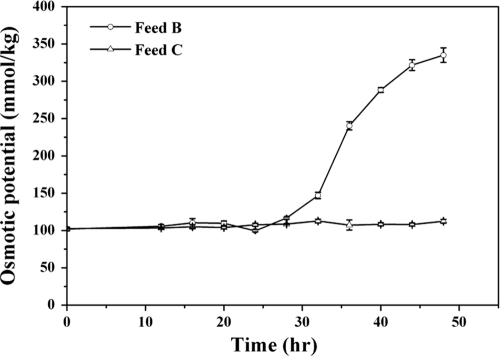
Osmotic potentials of fed-batch cultures supplemented with feed B versus feed C.
An increase in the osmotic potential is harmful to the bacteria and may result from the accumulation of Na+ and Cl− in the culture medium. Osmotic potential was compared for fed-batch cultures supplemented with feed B and feed C (Fig. 5). In cultures with feed B, the osmotic potential of broth increased ∼3-fold. In contrast, supplementation with feed C led to an isosmotic culture.
Fig. 5.
Osmotic potential of fed-batch culture supplemented with feed B or feed C.
Automated feeding mode.
Feeding mode is a crucial factor in high-density culture. Previous studies of M. gryphiswaldense strain MSR-1 indicated that relatively low concentrations of C and N are suitable for growth. In order to maintain low C and N concentrations, it is necessary to establish an automated feeding mode. Results from repeated fermentation experiments showed that MSR-1 growth rate is directly related to the rate of increase of pH in broth. By regulating the pH through the proper adjustment of lactate and NH4+ levels in the feeding medium, we successfully established an automated supplementation of nutrients and pH regulation for optimal cell growth in 7.5- or 42-liter fermentor.
Excessively high levels of nutrients in culture medium will lead to excessive accumulation of metabolites and inhibit cell growth. The maintenance of proper, low concentrations of each nutrient during a long fermentation process is difficult. A successful automated feeding mode can be established only by carefully investigating relationships between the supplementation rate of various nutrients and the cell growth rate.
Semicontinuous culture.
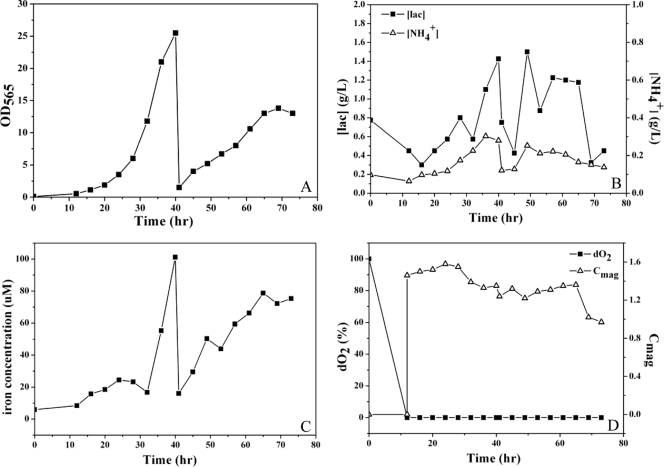
Based on the findings described above, we established a semicontinuous culture supplemented with feed C in 7.5-liter fermentor. Because mature magnetosome chains formed starting at 24 h and to ensure the growth of cells in the second stage, the first stage was ended at late logarithmic phase (OD565 = 25.5) and not at the stationary phase. The OD565 of the second stage reached a maximum of 13.8 at 69 h (Fig. 6A). The interval between the two stages was 1 h. The concentrations of lactate (0.3 to 1.5 g/liter) and NH4+ (0.06 to 0.3 g/liter) in the fermentor were suitable for growth (Fig. 6B), and the concentration of Fe2+/Fe3+ (increasing from 5.9 to 101.2 μM) allowed magnetosome synthesis without the inhibition of cell growth (Fig. 6C). In the first stage, the Cmag reached a maximum of 1.58 at 24 h and then decreased to 1.35 at the end. In the second stage, the Cmag reached a maximum of 1.36 and then declined to 0.97 (Fig. 6D).
Fig. 6.
Semicontinuous culture of MSR-1 cells in a 7.5-liter fermentor. (A) The maximal OD565 was 25.5 at the end of stage 1 and 13.8 at the end of stage 2. (B) Carbon (▪) and nitrogen (▵) sources were supplied at concentrations suitable for cell growth. (C) The Fe2+/Fe3+ concentration increased during both stages. (D) The Cmag (▵) increased initially and decreased later during each stage. The dO2 (▪) was controlled at 0% starting at 12 h.
Purification of magnetosomes.
In fed-batch cultures of MSR-1 in a 7.5-liter fermentor using an optimized feeding strategy, the OD565 reached 30.4 after 44 h. The dry weight yields (production) of cells and magnetosomes were 7.59 g/liter (3.8 g/liter/day) and 225.53 mg/liter (112.77 mg/liter/day), respectively.
In the first stage of semicontinuous culture in a 7.5-liter fermentor, the OD565 reached 25.5 in 40 h, and the dry weight yields of cells and magnetosomes were 6.24 g/liter (3.74 g/liter/day) and 280.49 mg/liter (168.29 mg/liter/day). In the second stage, the OD565 reached 13.8 in 28 h, and the dry weight yields of cells and magnetosomes were 3.28 g/liter (2.46 g/liter/day) and 111.39 mg/liter (83.54 mg/liter/day).
In further experiments, we performed fermentation in a 42-liter fermentor by enlarging the optimized feeding strategy. In single-batch culture, the OD565 reached a maximum of 42 in 44 h, and dry weight yields of cells and magnetosomes were 9.16 g/liter (4.58 g/liter/day) and 356.52 mg/liter (178.26 mg/liter/day). This finding shows that the optimized feeding strategy can be applied for industrial-scale production.
Outlook for culture of strain MSR-1.
This is the first report of a semicontinuous culture method for M. gryphiswaldense strain MSR-1. Overall, the yields of both cells and magnetosomes were greatly increased, resulting in significant improvement of the production efficiency. However, the productivity of both cells and magnetosomes was lower in the second stage than in the first stage. Clearly, there is opportunity for further improvement of the culture strategy.
One possible reason for the lower productivity in second stage is the instability of certain genes in this bacterial strain, particularly certain transposase genes located in the so-called “magnetosome island” of the genome (9), resulting in the instability of magnetosome synthesis. Further investigation of such instability—and possible ways to overcome it—is needed. At this time, the difference in productivity of single batch culture versus semicontinuous culture is minor, being based mainly on the fact that semicontinuous culture does not require repeated fermentor sterilization time. Significant increase in production efficiency for semicontinuous culture will come when we find conditions for greater stability of cell growth and magnetosome synthesis. This is a major goal of our future studies.
The optimal conditions for both MSR-1 cell growth and magnetosome synthesis are provided by a microaerobic environment with constant, low concentrations of each required nutrient. The microaerobic condition provides a sufficient level of electron receptors and thereby induces magnetosome formation. The low nutrient concentrations reduce the accumulation of metabolites in the fermentor and thereby avoid the inhibition of cell growth. The constant concentrations of each nutrient or salt satisfy the nutritional requirements and also create an isosmotic environment. The present study provides a new culture method for magnetotactic bacteria, with enhanced magnetosome production.
ACKNOWLEDGMENTS
We thank Zhizhong Gong, Junna He, Gang Zhang, and Xu Wang, China Agricultural University, for helpful discussions and technical assistance. We are also grateful to Steve Anderson for English editing of the manuscript.
This study was supported by the Chinese High Technology Research and Development Program (grant 2007AA021805) and the Chinese National Natural Science Foundation (grant 30970041).
Footnotes
Published ahead of print on 1 July 2011.
REFERENCES
- 1. Dailey H. J., Lascelles J. 1977. Reduction of iron and synthesis of protoheme by Spirillum itersonii and other organisms. J. Bacteriol. 129:815–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Egli T., Zinn M. 2003. The concept of multiple-nutrient-limited growth of microorganisms and its application in biotechnological processes. Biotechnol. Adv. 22:35–43 [DOI] [PubMed] [Google Scholar]
- 3. Heyen U., Schüler D. 2003. Growth and magnetosome formation by microaerophilic Magnetospirillum strains in an oxygen-controlled fermentor. Appl. Microbiol. Biotechnol. 61:536–544 [DOI] [PubMed] [Google Scholar]
- 4. Li A., et al. 2010. Rapid separation and immunoassay for low levels of Salmonella in foods using magnetosome-antibody complex and real-time fluorescence quantitative PCR. J. Sep. Sci. 33:3437–3443 [DOI] [PubMed] [Google Scholar]
- 5. Matsunaga T., Kamiya S. 1987. Use of magnetic particles isolated from magnetotactic bacteria for enzyme immobilization. Appl. Microbiol. Biotechnol. 26:328–332 [Google Scholar]
- 6. Miller R. H., Keeney D. R., Page A. L. 1982. Methods of soil analysis. 2. Chemical and microbiological properties. American Society of Agronomy/Soil Science Society of America, Madison, WI [Google Scholar]
- 7. Ohuchi S., Schüler D. 2009. In vivo display of a multisubunit enzyme complex on biogenic magnetic nanoparticles. Appl. Environ. Microbiol. 75:7734–7738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scheffel A., et al. 2006. An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature 440:110–114 [DOI] [PubMed] [Google Scholar]
- 9. Schübbe S., et al. 2003. Characterization of a spontaneous nonmagnetic mutant of Magnetospirillum gryphiswaldense reveals a large deletion comprising a putative magnetosome island. J. Bacteriol. 185:5779–5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun J. B., et al. 2008. High-yield growth and magnetosome formation by Magnetospirillum gryphiswaldense MSR-1 in an oxygen-controlled fermentor supplied solely with air. Appl. Microbiol. Biotechnol. 79:389–397 [DOI] [PubMed] [Google Scholar]
- 11. Sun J. B., et al. 2008. Preparation and anti-tumor efficiency evaluation of doxorubicin-loaded bacterial magnetosomes: magnetic nanoparticles as drug carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol. Bioeng. 101:1313–1320 [DOI] [PubMed] [Google Scholar]
- 12. Sun J. B., et al. 2007. In vitro and in vivo antitumor effects of doxorubicin loaded with bacterial magnetosomes (DBMs) on H22 cells: the magnetic bio-nanoparticles as drug carriers. Cancer Lett. 258:109–117 [DOI] [PubMed] [Google Scholar]
- 13. Tanaka M., Arakaki A., Staniland S. S., Matsunaga T. 2010. Simultaneously discrete biomineralization of magnetite and tellurium nanocrystals in magnetotactic bacteria. Appl. Environ. Microbiol. 76:5526–5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wacker R., et al. 2007. Magneto immuno-PCR: a novel immunoassay based on biogenic magnetosome nanoparticles. Biochem. Biophys. Res. Commun. 357:391–396 [DOI] [PubMed] [Google Scholar]
- 15. Xiang L., et al. 2007. Purified and sterilized magnetosomes from Magnetospirillum gryphiswaldense MSR-1 were not toxic to mouse fibroblasts in vitro. Lett. Appl. Microbiol. 45:75–81 [DOI] [PubMed] [Google Scholar]
- 16. Xiang L., et al. 2007. Bacterial magnetic particles (BMPs)-PEI as a novel and efficient non-viral gene delivery system. J. Gene Med. 9:679–690 [DOI] [PubMed] [Google Scholar]
- 17. Yang C., Takeyama H., Tanaka T., Matsunaga T. 2001. Effects of growth medium composition, iron sources and atmospheric oxygen concentrations on production of luciferase-bacterial magnetic particle complex by a recombinant Magnetospirillum magneticum AMB-1. Enzyme Microb. Technol. 29:13–19 [DOI] [PubMed] [Google Scholar]
- 18. Yang C., Takeyama H., Matsunaga T. 2001. Iron feeding optimization and plasmid stability in production of recombinant bacterial magnetic particles by Magnetospirillum magneticum AMB-1 in fed-batch culture. J. Biosci. Bioeng. 91:213–216 [DOI] [PubMed] [Google Scholar]
- 19. Yoshino T., et al. 2008. Magnetic cell separation using nano-sized bacterial magnetic particles with reconstructed magnetosome membrane. Biotechnol. Bioeng. 101:470–477 [DOI] [PubMed] [Google Scholar]
- 20. Yoshino T., Matsunaga T. 2005. Development of efficient expression system for protein display on bacterial magnetic particles. Biochem. Biophys. Res. Commun. 338:1678–1681 [DOI] [PubMed] [Google Scholar]