Abstract
The microbial community in which a pathogen evolves is fundamental to disease outcome. Species interacting with a pathogen on the host surface shape the distribution, density, and genetic diversity of the inoculum, but the role of these species is rarely determined. The screening method developed here can be used to characterize pathogen-associated species affecting disease. This strategy involves three steps: (i) constitution of the microbial community, using the pathogen as a trap; (ii) community selection, using extracts from the pathogen as the sole nutrient source; and (iii) molecular identification and the screening of isolates focusing on their effects on the growth of the pathogen in vitro and host disease. This approach was applied to a soilborne plant pathogen, Phytophthora parasitica, structured in a biofilm, for screening the microbial community from the rhizosphere of Nicotiana tabacum (the host). Two of the characterized eukaryotes interfered with the oomycete cycle and may affect the host disease. A Vorticella species acted through a mutualistic interaction with P. parasitica, disseminating pathogenic material by leaving the biofilm. A Phoma species established an amensal interaction with P. parasitica, strongly suppressing disease by inhibiting P. parasitica germination. This screening method is appropriate for all nonobligate pathogens. It allows the definition of microbial species as promoters or suppressors of a disease for a given biotope. It should also help to identify important microbial relationships for ecology and evolution of pathogens.
INTRODUCTION
Before infecting a host, a pathogen evolves within a microbial community that colonizes the host surface and may form mixed-species biofilms (9, 10). This community is capable of affecting disease and exerting selection pressure on the pathogen and the host (18, 29, 30). Investigations of the pathogenesis of several infections are currently moving away from a reductionist paradigm toward the view of the microbial community as a pathogenic unit (19, 28). Despite the growing recognition that this community is a driving force for natural selection and pathogenicity, the role of each microorganism associated with a pathogen is rarely identified. Current studies tend to focus on community structure, species richness, and abundance (20). In the case of plant diseases, the studies are mainly focused on microbial species controlling host diseases in pathogen-suppressive soils in which the pathogen does not establish or persist. The correlation between the high density of the populations of some species within a microbial community and the high suppressiveness level of the soil suggests that these species may be involved in the disease suppressive process (6, 8). In most cases microbial species promoting host diseases remains to be identified.
In the present study the impact of rhizospheric microorganisms on the tobacco black shank disease caused by the soilborne pathogen Phytophthora parasitica was investigated. P. parasitica is a filamentous eukaryotic plant pathogen (3), a member of the oomycete group comprising several of the most devastating plant pathogens, causing diseases in natural ecosystems and in numerous economically important crops. This polyphagous species includes tobacco in its host range and causes the black shank disease. The infection cycle of P. parasitica may alternatively involve single cell behavior, via zoospore germination and germ tube penetration, or cell population dynamics of planktonic zoospores, through the formation of adherent microcolonies on the host surface that develop into large biofilms (13).
P. parasitica biofilm was used to study the interactions between the oomycete and the microorganisms from the rhizosphere of Nicotiana tabacum. This choice was based first on the principle that pathogens generally live in cooperative groups attached to surfaces. Biofilms contribute to pathogen virulence, as well as to the interaction dynamics with the host (5, 22, 25, 27, 31). They confer several advantages to pathogens favoring attachment on host surface, promoting virulence through aggregation, and providing protection against host defenses or biocide treatment (9). They also promote dissemination through transition from the aggregated to the planktonic lifestyle (10, 15). This choice was also conditioned by the fact that in the natural habitat biofilms constitute propitious niches for interactions between pathogens and other species (23).
To get an insight into the ecological mechanisms of disease regulation, we developed a screening method for characterizing the repertoire of pathogen-associated species affecting a disease. The approach involves the trapping of species associated with a pathogen, the identification of those capable of growing in this environment, and the assignment of ecosystemic functions in terms of pathological considerations.
MATERIALS AND METHODS
Plant material.
Nicotiana tabacum (cultivar Xanthi) plants were grown on compost (AGRI'OR) in a growth chamber at 24°C, with a 16-h photoperiod and at a light intensity of 100 μEm−2 s−1. The same compost was used for all of the experiments. Seeds were germinated in flowerpots (9 by 9 by 9.5 cm; SOPARCO). At 2 weeks postgermination, 25 plants were transferred individually into flowerpots. Fertilizer containing nitrogen (N), phosphorus (P), and potassium (K), (15:12:30) was applied once (100 ml per flowerpot), and the plants were then watered regularly and grown for 3 weeks. Leaves or roots were taken from 7- to 8-week-old plants.
Microbial strains.
Phytophthora parasitica strains 310, 329, and 408 were obtained from the Institut National de la Recherche Agronomique (INRA; Sophia-Antipolis, France) Phytophthora collection. Vorticella microstoma strain 30897 was purchased from the American Type Culture Collection (ATCC) collection of protists (LGC standards). The cells of the ciliate were cultivated for 3 to 4 days in V8 liquid medium at 24°C, with a 16-h photoperiod and at a light intensity of 100 μEm−2 s−1. The Phoma strain characterized during the present study is recorded in the National Collection of the Institut Pasteur (recording number CNCM I-4278).
Community constitution.
Microcolonies were prepared from strain 329, as previously described (13). Leaf pieces (5 by 0.5 cm) were inoculated in water for 3 h at 25°C with a suspension of P. parasitica zoospores (400 to 600 cells ml/μl). Microcolonies that formed on leaf surface were isolated and washed with water before incubation with the rhizospheric samples.
For collection of soil samples from the rhizosphere, eight plants of similar size were chosen, and the roots were collected, together with the soil clinging to them. The eight samples were pooled together, mixed with sterile water (1/5 [wt/vol]), and filtered twice through a sieve with 100-μm pores. The resulting water suspension of microorganisms was rapidly decanted (5 min). The supernatant (5 ml) was placed in a 5.5-cm plastic petri dish, followed by incubation at 25°C in the presence of 10 to 20 freshly formed and water-washed P. parasitica microcolonies.
The kinetics mixed-species biofilm constitution was defined on the basis of four independent experiments and similar observations obtained by microscopy under white light. The early formation of bacterial colonies was detected by DAPI (4′,6′-diamidino-2-phenylindole) staining using an Axioplan fluorescence microscope (Carl Zeiss Microimaging, Inc., Germany).
Community selection.
After 3 days of incubation, the mixed-species biofilms obtained were rinsed three times in water and gently dissociated by mechanical trituration, consisting of 20 passes through the opening of a standard Pasteur pipette. The cell suspension obtained was spread on agar plates containing a Phytophthora extract as the sole nutrient source (Phytophthora crude extract, 10 g/liter; NaCl, 10 g/liter; agar 1.5% [wt/vol]) and incubated at 25°C. The Phytophthora crude extract was prepared from a 2-week-old mycelium of P. parasitica strain 329 (INRA). The mycelium was rinsed in water, ground to a fine powder in liquid nitrogen and freeze-dried. Eukaryotes were selected on plates supplemented with 30 μg of chloramphenicol/ml (in preliminary experiments, chloramphenicol appeared to be a more selective antibiotic than ampicillin and kanamycin for favoring growth of eukaryotes versus that of prokaryotes [data not shown]). Colonies appeared within 3 to 6 days. The colonies, which were morphologically different from those formed by P. parasitica, were isolated individually, transferred to 100-mm petri dishes, and expanded for mass cultures on V8 or malt agar.
Molecular identification.
For each isolate, boiled cells were used as templates for PCR amplification. The template was prepared by suspending the cells, spores, or mycelium in boiling water for 3 min, which was then rapidly chilled on ice and centrifuged at 10,000 × g for 3 min to remove debris. The supernatant (1 μl) was used for PCR amplification. The eukaryotic 18S rRNA gene was amplified with the forward primer EukA (5′-CTGGTTGATCCTGCCAG-3′) and the reverse primer EukB (5′-TGATCCTTCYGCAGGTTC-3′) (24). The PCR program included an initial denaturation at 94°C for 120 s, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 45 s, and extension at 72°C for 120 s.
FISH.
The probes Vortir339 (5′-Cy3-GACTGCCATGGTAGTCCAATACACT-3′) targeting Vorticella ciliates and Eukr560 (5′Cy5-5′-CGGCTGCTGGCACCAGACTTGCCCT-3′) targeting all eukaryotes were used. The sample preparation and hybridization conditions were essentially as described in a previous study (16). Mixed-species biofilms were fixed by incubation in a 4% (wt/vol) paraformaldehyde solution for 4 h at 4°C, dehydrated by sequential washes in 50, 75, and 100% (vol/vol) ethanol (30 min each), and rehydrated sequentially in the same solutions in reverse order. Subsequently, 2 ml of hybridization solution (900 mM NaCl, 20 mM Tris-HCl [pH 7.4], 0.1% [wt/vol] sodium dodecyl sulfate, 20% [wt/vol] formamide) containing a 1 μM concentration of probe was added to the samples, which were incubated overnight at 45°C. Biofilms were washed twice, for 15 min each time, in the hybridization solution at room temperature, placed on glass slides, and overlaid with an antifading reagent (VectaShield; Vector) before observation under a Zeiss LSM 510 Meta confocal microscope. Merged images showing fluorescence in situ hybridization (FISH) staining and light micrographs (differential interference contrast) were generated.
Screening of isolates for an effect on Phytophthora growth and plant disease.
Screening was performed in vitro by coincubating isolates and P. parasitica strains and in planta through coinfections. For the in vitro confrontations, P. parasitica strain 329 was first grown separately with each isolate on malt agar. An agar disk (5 mm in diameter) bearing the oomycete mycelium (strain 329) was placed on the right-hand part of a fresh petri dish containing malt agar; an agar disk carrying biological material for the isolate tested was placed on the left-hand part of the plate. The zone of growth inhibition seen around the disc corresponding to the isolate was used to evaluate the anti-oomycete activity.
The influence of isolates on the germination of P. parasitica cysts was also assayed on 10-well slides (Dominique Dutscher). A 20-μl suspension containing zoospores (400 cells from strain 310, 329, or 408/μl) was mixed with equal volumes of V8 medium and isolate-conditioned water filtrate (the cells were previously and briefly vortexed to ensure synchronized germination). The filtrate was prepared from four mycelial discs incubated in water (1 ml) for 1 h at 25°C. After centrifugation at 2,000 × g for 2 min, the supernatant was passed through a filter with 0.2-μm pores. The percentage germination was determined for two replicates, after incubation for 2 h at 25°C.
For in planta screening, parenchymatous leaf tissue was coinfected to prevent interference with the resident flora in the rhizosphere. We infiltrated the right-hand parts of five leaves from three tobacco plants with a suspension (100 μl) containing 500 zoospores of P. parasitica strain 329 (INRA Phytophthora collection) and 500 spores of each isolate. For each isolate, spore suspensions were prepared in water from two mycelial discs (5 mm in diameter) after incubation in water (1 ml, 15 min, room temperature) and counted using a Malassez chamber and calibration by dilution in water. The percentage of inoculated zones showing symptoms and the area damaged were determined 2 days after coinoculation. We assessed the influence of isolates on the disease by comparing the results to those for the left-hand parts of the leaves, which were inoculated with a suspension (100 μl) containing 500 P. parasitica zoospores. None of the isolates induced the development of symptoms on the plant when used alone for inoculation (data not shown). Data are expressed as the means ± the standard deviations (SD) of three independent experiments, and statistical analysis was carried out by performing Student t tests in Microsoft Excel 2003.
Dissemination assay.
Eight leaf pieces (5 by 0.5 cm) harboring or not P. parasitica microcolonies formed on their section (two to three microcolonies per centimeter) were incubated in water (10 ml) in the presence of cells of the V. microstoma strain 30897 (1,000 cells/ml) for 3 h at 25°C. The control without V. microstoma cells was adjusted with a volume of fresh and sterile V8 medium corresponding to the volume used for inoculation with the ciliate. After incubation leaf pieces with anchored ciliates, the cells were rinsed two times with 20 ml of water to eliminate contamination of the samples by circulating ciliate cells.
Dissemination assays were performed in a modified Boyden chamber (7). The apparatus consists of two-well chambers separated by a filter containing pores of 200 μm (Buisine) to allow migration of propagules of large size. The lower chamber was created into a petri dish (100 mm) pouring out 10 ml of a hot agar (2%) solution around the lower half of another petri dish (60 mm) used as the chamber mold. The lower chamber was filled with water (15 ml) and then covered with the filter. Eight leaf pieces were added to the upper chamber. The assembled chambers were incubated for 72 h at 25°C. Every 24 h a sample of 500 μl was taken from the lower chamber to count both Vorticella cells, using a Malassez chamber, and migrated propagules using a black shank disease assay. The disease assay was performed by infiltration of tobacco parenchymatous leaf tissue with 100 μl of inoculum from a 10-fold serial dilution (1, 1/10, and 1/100) of cell suspension. Two days later, the total number of inoculated zones showing symptoms was counted in order to determine the concentration of migrated propagules in the lower chamber. Each sample was tested in octuplicate. Statistical analysis was carried out on data obtained from three independent experiments by performing Student t tests in Microsoft Excel 2003.
Germination inhibition assay.
The antigerminative properties of the Phoma-conditioned water filtrate were tested on 10-well slides in vitro. A 20-μl suspension containing zoospores from oomycete strains or spores from fungi (400 to 1,000 spores/μl) was mixed with equal volumes of V8 medium and a serial dilution of the Phoma filtrate. Zoospores were previously and briefly vortexed to ensure synchronized germination. The germination percentage was determined for two replicates after incubation for 24 h at 25°C. Two parameters were determined from data: the minimum inhibitory dilution (MID), corresponding to the lowest dilution that inhibits 99.9% of germination for the tested microorganism (expressed as a dilution factor), and the half-inhibitory dilution (HID), corresponding to the dilution that inhibits 50% of germination for the tested organism (expressed as a percentage [vol/vol]).
Nucleotide sequence accession numbers.
Reported sequences are deposited in the GenBank databank. The accession numbers are given in Table 1.
Table 1.
Properties of eukaryotic isolatesa
| Isolate(s) |
P. parasitica growth |
Black shank disease |
Induction of plant symptoms | Molecular identification based on RNA 18S gene sequencing |
Spore morphology | ||||
|---|---|---|---|---|---|---|---|---|---|
| Inhibition | Enhancement | Suppression | Promotion | Genus | Nearest species (% identity) | GenBank accession no. | |||
| Ieuk1 | No | No | No | No | None | ND | ND | ND | |
| Ieuk2 | No | No | No | No | None | Penicillium | P. griseofulvum (98) | HM161744 | Round, fluorescent |
| Ieuk3 | Yes | No | Yes | No | None | Phoma | P. herbarum (98) | HM161743 | Round, smooth, brown |
| Ieuk4 | No | No | No | No | None | Poterioochromonas | P. malhamensis (99) | HM161745 | Elliptic, vacuolar |
| Ieuk5 | ND | ND | ND | ND | ND | Phytophthora | P. nicotianae (99) | HM161752 | |
| Ieuk6 | No | No | No | No | None | Candida | C. austromarina (99) | HM161746 | Round, green |
| Ieuk7 | No | No | No | No | None | Umbelopsis | U. isabellina (99) | HM161747 | Round, smooth, or spiny |
| Ieuk8 | No | No | No | No | None | Poterioochromonas | P. malhamensis (99) | HM161748 | Elliptic, vacuolar |
| Ieuk9 | ND | ND | ND | ND | ND | Phytophthora | P. nicotianae (99) | HM161752 | |
| Ieuk10 | ND | ND | ND | ND | ND | Phytophthora | P. nicotianae (99) | HM161752 | |
| Ieuk11 | No | No | No | No | None | ND | ND | ND | |
| Ieuk12 | No | No | No | No | None | Penicillium | P. phialosporum (98) | HM161749 | ND |
| Ieuk13 | No | No | No | No | None | Candida | C. lyxosophila (98) | HM161750 | ND |
| Ieuk14 | No | No | No | No | None | Umbelopsis | U. isabellina (99) | HM161751 | Round, smooth, or spiny |
| Ieuk15 | No | No | No | No | None | Candida | C. lyxosophila (98) | HM161753 | ND |
| Ieuk16 | No | No | No | No | None | Cyanidioschyzon | C. merolae (94) | HM161754 | ND |
| Ieuk17 to Ieuk19 | No | No | No | No | ND | ND | ND | ND | |
| Ieuk20 | No | No | No | No | None | Umbelopsis | U. isabellina (99) | HM161751 | ND |
| Ieuk21 to Ieuk31 | No | No | No | No | None | ND | ND | ND | |
| eukA | ND | Dissemination | ND | ND | ND | Vorticella | ND | Stalk, domed feeding zone | |
Each Ieuk isolate is annotated for its effect on P. parasitica growth in vitro, its impact on tobacco black shank disease when the plant was inoculated with both the isolate and the oomycete, its ability to induce plant symptoms when used alone for inoculation, the genus to which it belongs and the nearest species, based on closest match obtained with the BLAST algorithm, the GenBank accession number of the RNA 18S gene sequence, and the morphology of its spores. Two sets of 11 (Ieuk1 to Ieuk11) and 20 (Ieuk12 to Ieuk31) isolates were picked up from two independent experiments. ND, not determined.
RESULTS
Screening of P. parasitica-associated species affecting tobacco black shank disease.
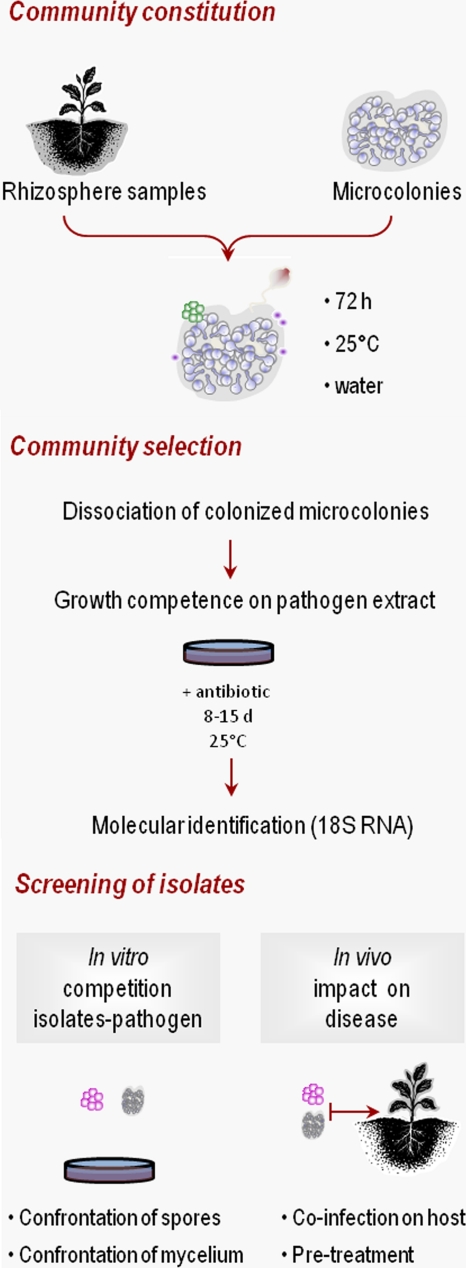
A three-stage strategy was developed in order to identify P. parasitica-associated species affecting tobacco black shank disease. The first step was the constitution of the community through the use of the pathogen as a trap for associated microorganisms in a natural habitat. Second, the microorganisms were selected on the basis of their ability to grow in the vicinity of the pathogen. The process was terminated by the identification of organisms affecting the host disease (Fig. 1).
Fig. 1.
Scheme of microbial community screening for pathogen-associated microorganisms affecting host disease. This represents an example approach to analysis of the rhizosphere community associated, in biofilms, with the plant pathogen P. parasitica.
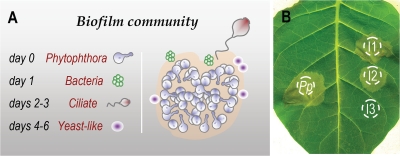
For the first step, P. parasitica biofilms were used to trap oomycete-associated microorganisms. We first formed mixed-species biofilms from P. parasitica microcolonies and microbial samples representative of the natural ecosystem. Microcolonies of P. parasitica were incubated with samples from the rhizosphere of Nicotiana tabacum. Based on four independent experiments, the kinetics of colonization appeared to consist of three main events. Invasion began with the formation of bacterial colonies, followed by the attachment of stalked ciliates (48 to 72 h) and the installation of yeast-like cells (96 to 144 h) (Fig. 2A). We then selected the microorganisms that survived and grew in the vicinity of the pathogen. Mixed-species biofilms were dissociated and spread onto agar plates containing a P. parasitica extract as the main nutrient source. Since P. parasitica biofilm secretes cyclic AMP (cAMP) (13), a lot of microorganisms should be in the vicinity of biofilm in response to cAMP as a chemotactic signal (12). So this step was performed to focus on microorganisms interacting with P. parasitica and able to growth on biofilm matrix.
Fig. 2.
Biofilm community and in planta screening. (A) Illustration of a mixed-species biofilm after colonization of a P. parasitica microcolony. (B) For in planta screening, P. parasitica zoospores were used alone (Pp) or with spores from isolates Ieuk1, Ieuk2, and Ieuk3 (I1, I2, and I3) for inoculation. Only Ieuk3, corresponding to a Phoma species, suppressed the disease. The difference in the percentage area displaying symptoms between I3 and Pp was highly significant in a Student t test (P < 0.0001) in three independent experiments.
The microorganisms forming colonies were isolated with chloramphenicol to focus on the selection of eukaryotes. About 400 colonies grew in the presence of the antibiotic. From two independent experiments 50 clones were isolated representative of the morphological diversity of the colonies. The entire strategy was applied to two independent sets of 11 and 20 isolates, corresponding to those that underwent fast growth in the in vitro conditions tested (Table 1). The sequencing of 18S rRNA genes showed that the eukaryotes were mostly eumycetes, stramenopiles, red algae, and ciliates. We mixed each eukaryotic isolate with P. parasitica to identify the isolates with effects on plant disease. We coincubated hyphae and spores in vitro and coinfected plants with spores of the two species. Of 31 isolates (corresponding to at least nine species), only one (Phoma herbarum) affected oomycete growth and disease (Fig. 2B and Table 1). No disease symptoms were observed in the presence of each isolate alone, in the absence of the pathogen, except for isolates with 18S rRNA gene sequences identical to that of P. parasitica. Furthermore, among ciliates colonizing P. parasitica biofilms (and only studied at the first step of the present study, Fig. 2A), a Vorticella species was found to affect the oomycete cycle (eukA in Table 1).
A Vorticella species facilitates the dissemination of P. parasitica propagules.
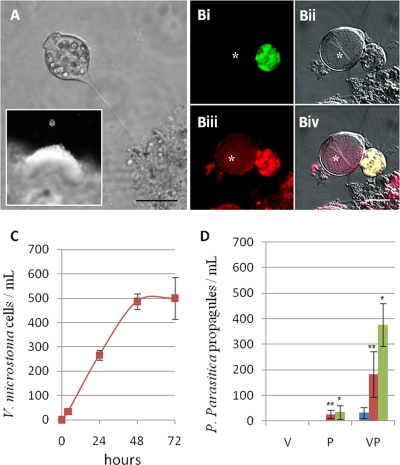
Based on the overall strategy, we characterized two types of interaction between P. parasitica and eukaryotes which might interfere with the disease cycle. We detected a mutualistic interaction involving a Vorticella species. This ciliate was initially identified on the basis of its morphological characteristics: about 120 to 150 μm in size, with a contractile stalk associated with a domed feeding zone (Fig. 3A). The identification was reinforced by a specific staining with a Vorticella probe by FISH. Double-labeling experiments were carried out with FISH probes specific for eukaryotes (Eukr560) and for the genus Vorticella (Vortir339). For all of the mixed-species biofilms analyzed, cells with the typical characteristics of the ciliate were double stained (Fig. 3Bi, Biii, and Biv). The other cells or structures present were either not stained or were stained with the Eukr560 probe only, as shown for the sporangium of P. parasitica (Fig. 3Bi and Biii). The action of the ciliate on the biofilm was much like that of a pollinator on a flowering plant. Interaction with the oomycete began with the attachment of the ciliate to a P. parasitica microcolony (Fig. 3A and see Movie S1, sequence 1, in the supplemental material). Once temporarily rooted in the biofilm, the ciliate probably fed on bacteria, small protozoa, or organic food (4, 26). The Vorticella cell then left the biofilm by swimming (see Movie S1, sequence 2, in the supplemental material), transporting with it material from the oomycete at the end of its stalk. This material could be large and included a P. parasitica sporangium (see Movie S1, sequences 3 and 4, in the supplemental material). In this way, each Vorticella cell swimming away from the biofilm facilitated the dissemination of P. parasitica propagules. An analysis of the video sequences showed that the Vorticella cells transporting oomycete material were able to reach speeds of up to 100 μm/s. These observations indicated that Vorticella could ensure rapid dissemination of the disease over large distances.
Fig. 3.
Vorticella-Phytophthora interaction. (A) Vorticella species anchored in a biofilm. The inset illustrates a larger view of the attachment of a ciliate cell to a microcolony. (B) Confocal laser scanning microscopy images of a ciliate cell and a P. parasitica sporangium anchored in a mixed-species biofilm (Bii). Double FISH staining was performed for Vorticella (Bi) and eukaryotic (Biii) 18S rRNAs. The Vorticella-specific probe decorated only the ciliate cell and did not stain the P. parasitica sporangium (*). The eukaryotic probe decorated both structures. Biv corresponds to the three merged images. Bars, 20 μm. (C) Kinetics of V. microstoma dissemination in a Boyden chamber. Leaf pieces harboring anchored V. microstoma cells and P. parasitica microcolonies were applied to the upper part of the chamber. At each time point V. microstoma cells concentration was determined in the lower part of the chamber. The data are expressed as means ± the SD of three independent experiments. (D) P. parasitica propagule dissemination in a Boyden chamber at 24 h (blue), 48 h (red), and 72 h (green). Three types of sources for inocula are shown: leaf pieces harboring anchored V. microstoma cells (V), leaf pieces harboring P. parasitica microcolonies (P), and leaf pieces harboring V. microstoma cells and P. parasitica microcolonies (VP). At each time point, the propagule concentration was determined in the lower part of the chamber by using a black shank disease assay. The data are expressed as means ± the SD of three independent experiments. The results were analyzed statistically by means of a Student t test. Significant differences were noted between P and VP at 48 (*, P = 0.03 [n = 3]) and 72 h (**, P = 0.02 [n = 3]).
Dissemination by a Vorticella species of P. parasitica propagules was demonstrated in vitro using a Boyden chamber assay. Eight leaf pieces harboring both P. parasitica microcolonies and anchored cells from the V. microstoma strain 30897 were deposited in the upper part of the chamber. The ciliate cells were found to migrate gradually to the lower chamber, reaching a cell density of 500 ± 84 cells/ml at 72 h (Fig. 3C). In these conditions migration properties of propagules causing tobacco black shank disease were also observed. In the lower chamber the migrated propagules increased with time and reached a concentration of 375 ± 83 propagules/ml at 72 h (VP in Fig. 3D). The detection of propagules in this chamber was dependent on Vorticella adhesion on P. parasitica microcolonies. The propagule concentration decreased drastically at each time point tested when preincubation with Vorticella cells was omitted, reaching 34 ± 27 at 72 h (P in Fig. 3D). No propagules could be detected when leaf pieces harbored anchored ciliates but not P. parasitica microcolonies in the upper chamber (V in Fig. 3D).
A Phoma species suppresses black shank disease.
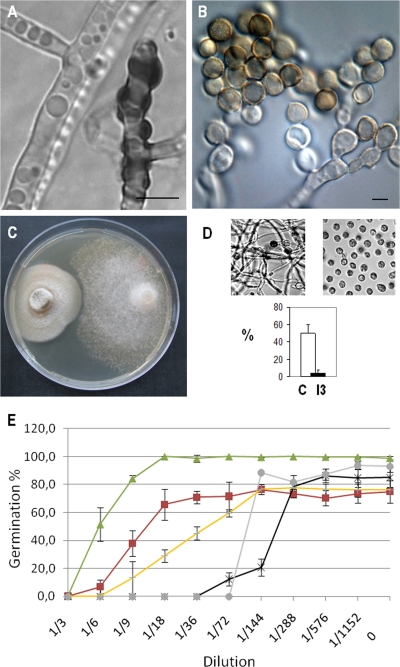
During the screening process, only one isolate (Ieuk3) representing a Phoma species was found to affect oomycete growth and disease (Fig. 2B and Table 1). An amensal interaction between this Phoma species and P. parasitica was characterized. The presence of this fungus was detrimental to P. parasitica, but its own growth was not affected by the presence of the oomycete (data not shown). The isolate was identified as most closely resembling P. herbarum, on the basis of the nucleotide sequence of the 18S rRNA gene for the closest match by BLAST analysis (1) (Table 1). The mycelium of the ascomycete sporulated laterally or by budding, forming aggregates of brown spores (Fig. 4 A and B), which strongly suppressed the development of black shank disease (Fig. 2B). After the inoculation of tobacco plants with a mixture of 500 spores from this Phoma species and 500 zoospores from P. parasitica, a mean of 95% ± 3% of the inoculated zones developed no symptoms, and no measurable area displaying disease symptoms could be identified. In these conditions, the inoculated parenchymatous tissue appeared to be healthy (Fig. 2B). In contrast, 100% of the zones inoculated with P. parasitica zoospores alone displayed disease symptoms within 48 h, over a mean area of 1.8 ± 0.3 cm2. Further investigations indicated that the growth of the oomycete was inhibited by the presence of the ascomycete in vitro. A clear zone of growth inhibition was observed around the P. parasitica strain 329 mycelium when the two microorganisms were incubated together on the same medium (Fig. 4C). The fungus produced a metabolic compound (or a mixture) preventing P. parasitica germination, as demonstrated by the effects of a Phoma-conditioned water filtrate, which reduced cyst germination by up to 90% for strain 329 (Fig. 4D). Similar results were obtained for two additional P. parasitica strains, with the germination rates of strains 310 and 408 reduced by 98 and 96%, respectively. These results suggest that this fungus may have broad-spectrum activity within the P. parasitica species.
Fig. 4.
Phoma-Phytophthora interaction. (A and B) Micrographs of brown spores emerging laterally or apically from the Phoma mycelium. (C) Agar plate showing a zone of inhibition of oomycete growth (right) by the mycelium of the Phoma isolate Ieuk3 (left). (D) Effect of Phoma-conditioned water filtrate on P. parasitica growth, measured as the percentage of cysts germinating (■, micrograph on the right) and compared to that for water treatment (□, micrograph on the left). Statistical analyses were performed with the Student t test (P < 0.001). Error bars denote means ± the SD. Bars, 10 μm. (E) Comparison of the effect of Phoma-conditioned water filtrate on germination of the P. parasitica strains 310 (gray) and 329 (black) and of the fungi Penicillium griseofulvum (red), Candida austromarina (green), and Botrytis cinerea (yellow). The data are means ± the SD (n = 4) of a representative experiment from three.
The antigerminative properties of the Phoma-conditioned water filtrate was also investigated on three ascomycetes: Penicillium griseofulvum (Ieuk2), Candida austromarina (Ieuk6), and Botrytis cinerea. MIDs of 1:36 and 1:72 were found to completely inhibit the germination of P. parasitica strains for 24 h. Lower dilutions (ranging from 1:3 to 1:6) were required to observe the same effect on spores from the ascomycetes (Fig. 4E). The values of HIDs confirmed the rather higher antigerminative properties of the Phoma species on Phytophthora strains. The HID values were 1 and 1.5% for strains 310 and 329, while they were 3, 5, and 11% for U. isabellina, B. cinerea, and P. griseofulvum, respectively. Bacterial (Escherichia coli DH5α) and yeast (Saccharomyces cerevisiae JD53) growth was not impaired by exposure to Phoma filtrate at the lowest tested dilution (1:3) in vitro (data not shown).
DISCUSSION
The use of the ecosystem screening approach described here allows the characterization of species interacting with a pathogen and affecting host disease. A widely accepted system for classifying interactions between organisms has been developed by Odum (23). Interactions between two organisms are seen as having a negative effect (“−”), a positive effect (“+”), or a neutral effect (“0”) on each participant in the interaction. The extrapolation of this system may be proposed for the classification of biotic interactions involving a known pathogenic species, not in terms of the repercussion of the interaction on the two organisms, but in terms of the effects of the interaction on disease outcome. The species interacting with the pathogen would be considered to be promoters or suppressors of disease when they have positive or negative effects, respectively, on disease. For each of these species, and independently of the other species interacting with the pathogen studied, a disease index could be determined quantifying the intrinsic and individual influence of the species concerned on the disease. With the exhaustive characterization of most of the species affecting the disease, it would then become possible, for a given biotope, to calculate a community indicator of disease. This cumulative indicator would reflect the sum of individual indices weighted by the richness score for each species within the community. Its value would oscillate between two extremes: that for which all the biotic conditions are required for the occurrence of an epidemic and that for which these conditions would be most likely to prevent an epidemic. Thus, by combining studies on community function, such as this one, with metagenomics analyses providing a picture of a community structure, it should be possible to increase our ability to modify disease states through the use of crucial data defining the status of a biotic environment with respect to a disease and to forecast disease epidemics.
In the case presented here, the rhizospheric community screened was a mixed-species biofilm, the natural habitat of most microorganisms (9, 10). Two of the microorganisms trapped with the soilborne pathogen P. parasitica affected the biology of the pathogen and, for one of them, an interference with N. tabacum disease was demonstrated. These results constitute the first characterization, for a plant disease, of the influence of biocenotic relationships within an eukaryotic microbial community considered as the pathogenic unit.
A mobile unicellular organism, Vorticella, was identified as a disseminator of P. parasitica in vitro. Further work is required to establish whether the dissemination of oomycete propagules by Vorticella could contribute to disease propagation, if Vorticella may be a promoter of tobacco black shank disease in the field. For Phytophthora species such as P. parasitica, which produce zoospores with swimming motility in the soil (17), this alternative route of dissemination may be seen as secondary. However, it should be noted that the ciliate may adopt a rectilinear trajectory when transporting large amounts of pathogenic material (see Movie S1, sequence 2, in the supplemental material), and such trajectories are more efficient for long-distance exploration than the helical trajectory of zoospores (2). This mode of dissemination may predominate for nonmotile pathogens. In natural conditions, disseminator species such as Vorticella described here may increase the likelihood of the transported pathogen reaching a host of the appropriate genotype.
A Phoma species was identified as a suppressor of tobacco black shank disease. Experiments are needed to establish whether the strain may be a suitable organism for biological control of P. parasitica in the field. Nevertheless, its characterization using an ecosystem screening approach indicates that the application of this approach to other pathogens could be advantageous in diversifying material for biological control of some plant diseases.
Characterization of main suppressors should also help to study the evolution of pathogens. The presence of a suppressor species in the same habitat than Phytophthora limits the success of the oomycete. Within the rhizosphere, interspecific competition may also cause the displacement of Phytophthora species toward another habitat, such as the plant roots, the nearest alternative habitat. During evolution, competitive displacement may have resulted in the selection, within ancestral populations, of new genetic traits contributing to development of virulence in plants within the Phytophthora lineage (21). This would provide an example of a localized blow to what Darwin referred to as the “yielding surface” of nature, struck at the level of one of the 10,000 “wedges” packed closely together and representing different species (11, 14). The shock resulting from the blow—in this case, biotic competition—creates ripples extending outward over great distances, contributing here to the emergence of pathogenesis. In other words, some species may become pathogens to escape their competitors, with pathogenicity increasing the chances of survival for species subject to amensalism.
Supplementary Material
ACKNOWLEDGMENTS
We thank Julie Hopkins for revising the English. We also thank Véronique Saint-Ges for her constant encouragement and support.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 8 July 2011.
REFERENCES
- 1. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appiah A. A., van West P., Osborne M. C., Gow N. A. 2005. Potassium homeostasis influences the locomotion and encystment of zoospores of plant pathogenic oomycetes. Fungal Genet. Biol. 42:213–223 [DOI] [PubMed] [Google Scholar]
- 3. Attard A., et al. 2008. Strategies of attack and defense in plant-oomycete interactions, accentuated for Phytophthora parasitica Dastur (syn. P. nicotianae Breda de Haan). J. Plant Physiol. 165:83–94 [DOI] [PubMed] [Google Scholar]
- 4. Bhamrah H. S., Juneja K. 2002. An introduction to protozoa. J. L. Kumar/Anmol Publication Pvt., Ltd., New Delhi, India [Google Scholar]
- 5. Blankenship J. R., Mitchell A. P. 2006. How to build a biofilm: a fungal perspective. Curr. Opin. Microbiol. 9:588–594 [DOI] [PubMed] [Google Scholar]
- 6. Borneman J., Becker J. O. 2007. Identifying microorganisms involved in specific pathogen suppression in soil. Annu. Rev. Phytopathol. 45:153–172 [DOI] [PubMed] [Google Scholar]
- 7. Boyden S. V. 1962. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 115:453–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cook R. J., Baker K. F. 1983. The nature and practice of biological control of plant pathogens. American Phytopathological Society, St. Paul, MN [Google Scholar]
- 9. Costerton J. W., Stewart P. S., Greenberg E. P. 1999. Bacterial biofilms: a common cause of persistent infections. Science 21:1318–1322 [DOI] [PubMed] [Google Scholar]
- 10. Danhorn T., Fuqua C. 2007. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 61:401–422 [DOI] [PubMed] [Google Scholar]
- 11. Darwin C. 1859. On the origin of species by means of natural selection, or the preservation of favored races in the struggle for life. John Murray, London, England [Google Scholar]
- 12. Devreotes P. 1982. Chemotaxis, p. 117–168. In Loomis W. F. (ed.), Development of Dictyostelium discoideum. Academic Press, Inc., New York, NY [Google Scholar]
- 13. Galiana E., Fourré S., Engler G. 2008. Phytophthora parasitica biofilm formation: installation and organization of microcolonies on the surface of a host plant. Environ. Microbiol. 10:2164–2171 [DOI] [PubMed] [Google Scholar]
- 14. Gould S. J. 2002. The structure of evolutionary theory. Belknap (Harvard University Press), Cambridge, MA [Google Scholar]
- 15. Hall-Stoodley L., Stoodley P. 2005. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 13:7–10 [DOI] [PubMed] [Google Scholar]
- 16. Ishii S., Shimoyama T., Hotta Y., Watanabe K. 2008. Characterization of a filamentous biofilm community established in a cellulose-fed microbial fuel cell. BMC Microbiol. 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Judelson H. S., Blanco F. A. 2005. The spores of Phytophthora: weapons of the plant destroyer. Nat. Rev. Microbiol. 3:47–58 [DOI] [PubMed] [Google Scholar]
- 18. Kent A. D., Triplett E. W. 2002. Microbial communities and their interactions in soil and rhizosphere ecosystems. Annu. Rev. Microbiol. 56:211–236 [DOI] [PubMed] [Google Scholar]
- 19. Kuramitsu H. K., He X., Lux R., Anderson M. H., Shi W. 2007. Interspecies interactions within oral microbial communities. Microbiol. Mol. Biol. Rev. 71:653–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mao-Jones J., Ritchie K. B., Jones L. E., Ellner S. P. 2010. How microbial community composition regulates coral disease development. PLoS Biol. 8:e1000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morris C. E., et al. 2009. Expanding the paradigms of plant pathogen life history and evolution of parasitic fitness beyond agricultural boundaries. PLoS Pathog. 5:e1000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris C. E., Monier J. M. 2003. The ecological significance of biofilm formation by plant-associated bacteria. Annu. Rev. Phytopathol. 41:429–453 [DOI] [PubMed] [Google Scholar]
- 23. Odum E. P. 1971. Fundamentals of ecology, 3rd ed. W. B. Saunders, Philadelphia, PA [Google Scholar]
- 24. Petroni G., Dini F., Verni F., Rosati G. 2002. A molecular approach to the tangled intrageneric relationships underlying phylogeny in Euplotes (Ciliophora, Spirotrichea). Mol. Phylogenet. Evol. 22:118–130 [DOI] [PubMed] [Google Scholar]
- 25. Ramey B. E., Koutsoudis M., von Bodman S. B., Fuqua C. 2004. Biofilm formation in plant-microbe associations. Curr. Opin. Microbiol. 7:602–609 [DOI] [PubMed] [Google Scholar]
- 26. Ravva S. V., Sarreal C. Z., Mandrell R. E. 2010. Identification of protozoa in dairy lagoon wastewater that consume Escherichia coli O157:H7 preferentially. PLoS One 5:e15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rigano L. A., et al. 2007. Biofilm formation, epiphytic fitness, and canker development in Xanthomonas axonopodis pv. citri. Mol. Plant-Microbe Interact. 20:1222–1230 [DOI] [PubMed] [Google Scholar]
- 28. Siqueira J. F., Jr., Rocas I. N. 2009. Community as the unit of pathogenicity: an emerging concept as to the microbial pathogenesis of apical periodontitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 107:870–878 [DOI] [PubMed] [Google Scholar]
- 29. Stecher B., Hardt W. D. 2008. The role of microbiota in infectious disease. Trends Microbiol. 16:107–114 [DOI] [PubMed] [Google Scholar]
- 30. Wolinska J., King K. C. 2009. Environment can alter selection in host-parasite interactions. Trends Parasitol. 25:236–244 [DOI] [PubMed] [Google Scholar]
- 31. Yao J., Allen C. 2007. The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host. J. Bacteriol. 189:6415–6424 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.