Abstract
Bioremediation is an important approach to waste reduction that relies on biological processes to break down a variety of pollutants. This is made possible by the vast metabolic diversity of the microbial world. To explore this diversity for the breakdown of plastic, we screened several dozen endophytic fungi for their ability to degrade the synthetic polymer polyester polyurethane (PUR). Several organisms demonstrated the ability to efficiently degrade PUR in both solid and liquid suspensions. Particularly robust activity was observed among several isolates in the genus Pestalotiopsis, although it was not a universal feature of this genus. Two Pestalotiopsis microspora isolates were uniquely able to grow on PUR as the sole carbon source under both aerobic and anaerobic conditions. Molecular characterization of this activity suggests that a serine hydrolase is responsible for degradation of PUR. The broad distribution of activity observed and the unprecedented case of anaerobic growth using PUR as the sole carbon source suggest that endophytes are a promising source of biodiversity from which to screen for metabolic properties useful for bioremediation.
INTRODUCTION
Tremendous increases in the manufacture and consumption of plastics over recent decades have led to numerous ecological and economic concerns. The persistence of synthetic polymers introduced into the environment by human industry poses a major threat to natural ecological systems. The low cost and ease of manufacture have increased global plastic demand more than 150-fold, with the production of 1.5 million tons in 1950 and 245 million tons as of 2006 (21). Despite recognition of the persistent pollution problems posed by plastic, global production is still increasing, with the largest increases expected in developing nations. The sheer volume of plastics produced each year presents a problem for waste disposal systems. The scale of this problem and the recalcitrance of some polymers to degradation necessitate investigation into effective methods for biodegradation of plastics. By gaining an understanding of the mechanisms of polymer degradation, a more efficient technique for the biodegradation of plastic waste can be achieved. To accomplish this goal, researchers need greater knowledge of how compounds are metabolized by existing organisms, an investigation of new organisms with bioremediation potential, and the characterization of novel metabolic capabilities. A basic understanding of the biological processes that lead to biochemical degradation will advance the development of new bioremediation techniques.
Polyester polyurethane (PUR) is a plastic widely used in industry and manufacturing that has been shown to be susceptible to biodegradation (6, 10). The polymer is generated by the condensation of a polyisocyanate and a polyol. This results in a carbon polymer composed of a series of urethane linkages. Variations in the spacing between urethane linkages, as well as the nature of the substitutions, can change the properties of the resulting polymer from linear and rigid to branched and flexible. In a liquid suspension PUR appears milky white and completely opaque. Like other polyurethanes, this product is synthesized commercially for the manufacture of textiles and textile coatings.
Enzymatic degradation of PUR by both fungi (4, 5, 6, 19) and bacteria (11, 12, 14, 15, 17, 18, 23) has been demonstrated. Soil fungi comprise the majority of organisms screened for PUR degradation activity (4, 5). Fungi of the genera Alternaria, Aspergillus, Phoma, Pennicilium, Plectosphaerella, Geomyces, Nectria, and Neonectria were isolated with access to mixed nutrient sources from buried PUR samples (4). Geomyces pannorum was the most commonly isolated PUR-degrading organism with this method (4). Few organisms have been shown to degrade PUR as a sole carbon source. Aspergillus niger has some reported degradation activity; however, it was observed to be quite slow, with visible signs of degradation occurring only after 30 days (7).
Putative polyurethanases have been isolated and characterized from protein extracts of several organisms, including the bacteria Pseudomonas chlororaphis (25) and Comamonas acidovorans (1, 2), as well as the fungus Candida rugosa (8). The active enzymes have been classified as esterases (5, 13, 16), lipases (26), and proteases and ureases (19), suggesting degradation of the PUR substrate by cleavage of the ester bond.
In an effort to identify new organisms with novel metabolic capabilities for polymer degradation, we have embarked on an effort to explore the biological and chemical diversity of endophytes. This was achieved as part of an educational program to engage undergraduate students in discovery-based research (9, 28). Endophytes are hyperdiverse microorganisms, including bacteria and fungi, that live within the inner tissues of plants without causing overt disease symptoms (3). These organisms enter their hosts by penetrating exterior surfaces, and some play a key role in plant decomposition following host tissue death (3). Indeed, fungi such as these contribute to decomposition of lignocellulose polymers and are major contributors to the carbon cycle (3, 22, 24). The ability of these microorganisms to degrade a polymer as complex as lignocellulose would suggest that these organisms offer promise for their ability to degrade other complex polymers, such as those present in plastics. The unique biological niche of endophytes as endosymbionts of tissues rich in complex carbon polymers justifies the investigation of their wider metabolic capabilities. Each of the more than 300,000 land plant species on Earth potentially hosts multiple endophyte species. Only a small sampling of plants have been examined for their endophytic associations, yet many of these organisms can be readily cultured (3, 25, 28). Endophytes reach their greatest diversity in tropical forests. Individual trees can harbor hundreds of endophytic species, some of which are known but many of which are new to science (25).
In the current study, endophytes were isolated from plant stems collected in the Ecuadorian rainforest. A subset of these organisms was screened for their ability to degrade polyurethane. Several active organisms were identified, including two distinct isolates of Pestalotiopsis microspora with the ability to efficiently degrade and utilize PUR as the sole carbon source when grown anaerobically, a unique observation among reported PUR biodegradation activities.
MATERIALS AND METHODS
Plant sampling.
Woody plants of various families were collected in the Yasuni National Forest in the Ecuadorian Amazonian rainforest. Some plants were targeted for their purported ethnobotanical uses, while others were sampled randomly. A 10-cm stem sample was collected and stored in an air-tight polyethylene bag at 4°C. Herbarium samples were prepared and deposited in the Peabody Herbarium at Yale University and the Herbario Nacional del Ecuador (QCNE).
Endophyte isolation.
Plant stems were surface sterilized by immersion in ethanol for 10 s followed by brief flaming. Outer layers of tissue were removed from the stems, and three sections of the inner tissues of each sample were plated on potato dextrose agar (PDA) (Difco), a 1:10 dilution of potato dextrose medium in water agar (WA), a 1:10 dilution of glycerol arginine medium (GAM) in WA, and WA plates (25). All plates were sealed with Parafilm and monitored every 2 to 3 days for growth. As growth of microbes was detected, fungal organisms were isolated by transferring a hyphal tip to fresh PDA plates. The plates were again wrapped with Parafilm and stored in plastic containers. Permanent stocks of each organism were made by growing the organisms on triple-autoclaved barley seeds and storing them at −80°C.
Sequencing and phylogenetic analysis.
Endophyte cultures were grown on PDA plates for 1 to 2 weeks at room temperature. DNA was extracted from approximately 100 mg of fungal material using the Qiagen DNeasy plant minikit. Approximately 10 ng of DNA was used as a template to amplify the internal transcribed spacer (ITS) region of ribosomal DNA (rDNA) by PCR as previously described (30). The primers used for ITS sequencing were ITS1 (5′-TCCGTAGGTGAACCTGCGGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′), corresponding to the forward and reverse primers (30). The amplified fragments were verified for length using agarose gel electrophoresis and purified using a QIAquick PCR purification kit (Qiagen). Sequencing was performed at the Yale University W. M. Keck Facility on Applied Biosystems 3730cL DNA Analyzer machines. Forward and reverse sequences for each organism were aligned using the programs Pregap4 and Gap4 (25). Endophyte sequences were aligned to organisms present in the GenBank database on 1 August 2008 using the Basic Local Alignment Search Tool (BLAST) of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/GenBank/index.html).
Initial PUR clearance screen.
Endophytes were first assayed for their ability to degrade PUR by growing them in the presence of Impranil DLF an anionic aliphatic aqueous PUR dispersion with 4% N-methyl pyrrolidone (NMP) (Bayer MaterialScience). Fifty-nine fungal endophytes were grown on solid PUR medium (PUR-A) containing 19 mM NaH2PO4, 33.5 mM K2HPO4, 7.6 mM (NH4)2SO4, 2.5 mM Na citrate, 250 μM MgSO4, 19 μM thiamine, 0.05% Casamino Acids, 147 μM FeCl3·6H2O, 14 μM ZnCl2·4H2O, 12 μM CoCl2·6H2O, 12 μM Na2MoO4·2H2O, 10 μM CaCl2·2H2O, 11 μM CuCl2, 12 μM MnCl2, 12 μM H3BO3, and 1.8 mM HCl. To 1 liter of this mixture was added 10 ml Impranil DLF and 15 g of agar. The polymer was added after autoclaving the medium to prevent deformation.
The solid medium screening assay followed the general method of Crabbe et al. (5). The PUR-A solid medium was added to sterile culture tubes in 10-ml aliquots. A 0.5-cm3 plug of fungus grown on PDA was added to each test tube using aseptic technique and allowed to grow undisturbed at 23°C. The bacterium Pseudomonas chlororaphis (ATCC 55729, from Gary Howard) and the fungus Aspergillus niger (from Gary Strobel, Montana State University) were used as positive controls for polyurethane degradation (6, 7, 13). PUR degradation was evidenced by a change in medium appearance from opaque to translucent. After 2 weeks of incubation, the depth of polyurethane clearance was measured from the top of the medium to the lowest point of visible clearance.
The liquid medium assay followed the method described by Gautam et al. (8). PUR-L liquid medium was prepared using the same recipe as for the solid PUR-A medium without agar. PUR-L medium was added to sterile culture tubes in 10-ml aliquots and inoculated with a 0.5-cm3 plug of fungus grown on PDA. At the end of 2 weeks, the liquid cultures were homogenized by vigorous shaking. A 1.5-ml portion of each culture was transferred to an Eppendorf tube and centrifuged for 1 min at 4,200 × g in an Eppendorf MiniSpin centrifuge to selectively pellet the fungal matter. The absorbance of the supernatant liquid was measured on a Varian Cary 50 Bio UV-visible spectrophotometer at a wavelength of 600 nm, with sterile water as a blank. Dilutions of PUR-L medium with sterile water were measured to construct a standard curve for converting absorbance to percent clearance. Samples were measured each day for 2 weeks for optical absorbance to determine a relative rate of clearance.
Sole carbon source assay.
Organisms identified as having PUR-degrading activity were tested for their ability to use PUR as the sole carbon source. For these studies, the substrate was Impranil DLN, which contains PUR suspended in only water (no N-methyl pyrrolidone is present). This isolates the Impranil as the sole source of carbon for metabolism and growth. The top five organisms from the initial activity screens were grown on Impranil DLN with no other carbon sources (PUR-Lmin). The fungal samples were washed prior to inoculation to remove all residual medium. These two considerations—the wash and the DLN substrate—ensure that the polymer is the sole source of carbon for fungal metabolism and growth. PUR-Lmin was prepared in a manner similar to that for the PUR-L medium but in the absence of sodium citrate, thiamine, Casamino Acids, or agar. The organism Aspergillus niger was tested as a basis for comparison.
Fungal cultures were grown for 1 week in potato dextrose broth (PDB). Stock cultures were homogenized by vigorous shaking, and 1 ml of each culture was centrifuged at 12,100 × g for 1 min. The supernatant was removed, and the fungal pellet was resuspended in 1 ml of the PUR-Lmin liquid medium. Samples were centrifuged and resuspended a second time to ensure removal of all residual PDB. The 1-ml sample of washed fungal material was added to sterile culture tubes to a final volume of 10 ml PUR-Lmin. The cultures were monitored for visual clearance of the opaque medium. Samples were measured every 2 days for 2 weeks for optical absorbance at 600 nm to determine an approximate rate of clearance. An increase in fungal mass correlating to PUR degradation was measured by lyophilizing mycelial mass from triplicate cultures containing minimal medium with and without PUR.
Anaerobic degradation of PUR.
Culture tubes containing 9-ml aliquots of PUR-Lmin medium and 1 ml of washed fungal inoculums were incubated under anaerobic conditions. The anaerobic environment was generated using a BD GasPak anaerobic chamber. Liquid cultures of each isolate were inoculated in duplicate. One set was placed inside the anaerobic chamber, and the control set was placed outside the chamber in an aerobic environment. Both sets were incubated at 25°C for the duration of the study. After 1 and 2 weeks, samples were removed and clearance was determined by absorption at 600 nm.
IR analysis of PUR degradation.
Infrared (IR) spectra of liquid PUR-Lmin suspensions were collected every 48 h using a Nicolet 6700 infrared spectrometer. A 50-μl portion of each sample was removed for each measurement and centrifuged for 60 s at 4,200 × g to settle out fungal material from the samples without significant sedimentation of the polymer. Liquid sample spectra were collected using deionized water as a background spectrum. Measurements were performed until complete degradation of PUR in the 10-ml culture was observed within 2 weeks.
Enzymatic characterization of putative polyurethanase.
An extracellular enzyme fraction was prepared by growing a 1-liter liquid culture of the most active organism, Pestalotiopsis microspora E2712A, in PUR-Lmin and PDB media. After 10 days of incubation at 30°C, the culture was filtered through a 0.22-μm filter into a sterile container. Crude extracellular extract was stored at 4°C. To test for the activity of an extracellular enzyme, 4 ml of extract was added to 6 ml of PUR-Lmin medium. Samples were incubated for 2 h in a rotary incubator at 30°C.
Three mechanism-based inhibitors were used to characterize the activity. Phenylmethylsulfonyl fluoride (PMSF), a serine hydrolase inhibitor, was added to an aliquot of enzyme extract and added to PUR-Lmin medium to a final concentration of 1 mM. Iodoacetate, a cysteine hydrolase inhibitor, was added to a final concentration of 10 μM. EDTA at a concentration of 5 mM was used as a metallohydrolase inhibitor. Extract that was heat treated at 98°C for 20 min served as a negative control. Samples were incubated in a rotary incubator at 30°C, and clearance was observed macroscopically after 2 h.
Based upon initial observations that a serine hydrolase was implicated in PUR degradation, activity-based probes were used to test for a serine hydrolase protein in crude cell-free filtrates. The probe molecules contain a fluorophosphonate moiety that reacts specifically with the active-site serines of enzymes in the serine hydrolase family and a tetramethylrhodamine (TMR) tag (20). Reactions of the crude protein extract with the probe were carried out as previously described (20). The resulting SDS-polyacrylamide gel was visualized using a Fujifilm FLA-5100 fluorescent bed scanner with excitation at 532 nm and a 570-nm emission filter. The same gel was silver stained to visualize all proteins in the samples.
Crude protein from cell-free filtrate of Pestalotiopsis microspora E2712A grown in 1-liter cultures of PUR-Lmin minimal medium and PDB rich medium were concentrated and purified to approximately 90% purity by gel filtration chromatography (data not shown) using a Superdex 200 column (GE Healthcare) in buffer comprised of 10 mM MES (morpholineethanesulfonic acid) (pH 5.5) and 50 mM NaCl. The ability of the purified protein to degrade PUR was assayed as described above.
RESULTS
Initial PUR clearance screen.
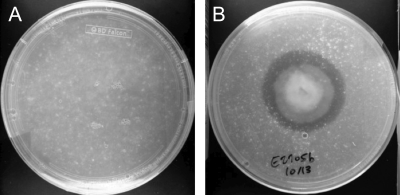
Impranil DLN, a polyester polyurethane (PUR), is an opaque milky suspension that becomes transparent upon degradation. Organisms capable of degrading this polymer display a zone of clearance around the growing culture (5). A collection of 59 fungal endophytic organisms isolated from plant samples in the Ecuadorian Amazon were screened for their ability to grow on and degrade polyester polyurethane using the PUR halo assay as the initial screen. Of the organisms screened, 18 organisms produced a halo of clearance such as that shown in Fig. 1. Two other organisms, identified by ITS sequencing as Guignardia mangiferae (E2702C) and Zopfiella karachiensis (E2719A), could grow on but not degrade PUR-A medium. These were used as negative controls in the subsequent studies. The host plants, isolation media, and identities of the 18 active fungi and two inactive control fungi are listed in Table 1.
Fig. 1.
Example of PUR-A plates initially used to screen for polyurethane-degrading activity after 2 weeks of fungal growth. (A) Negative control. (B) Pleosporales sp. strain E2705B.
Table 1.
Endophytes studied and the host plant species from which the endophytes were isolated
| Endophytea | Highest-homology organism (maximum % identity)b | Host plant | Isolation mediumc |
|---|---|---|---|
| E2524A | Alternaria sp. (100) | Annonaceae, Annona muricata | PDA |
| E2914A | Alternaria dauci (100) | Malvaceae, Malva alcea | 1:10 PDA |
| E2910B | Bionectria sp. (99) | Gesneriaceae, Drymonia semicordata | 1:10 PDA |
| E2705G | Plectosphaerella sp. (94) | Piperaceae, Piper arboretum | 1:10 PDA |
| E3432O | Edenia gomezpompae (99) | Fabaceae, Erythrina smithiana | 1:10 GAM |
| E2702C | Guignardia mangiferae (99) | Melastomataceae, Axinaea sodiroi | 1:10 PDA |
| E2611A | Lasiodiplodia sp. (100) | Moraceae, Naucleopsis oblongifolia | WA |
| E2711A | Pestalotiopsis microspora (100) | Monimiaceae, Siparuna aspera | 1:10 PDA |
| E2712A | Pestalotiopsis microspora (100) | Myrtaceae, Psidium guajava | 1:10 PDA |
| E2708A | Pestalotiopsis microspora (100) | Mimosaeae, Calliandra angustifolia | 1:10 PDA |
| E2911H | Pestalotiopsis microspora (100) | Commelinaceae, Dicrorisandra ulei | PDA |
| E3317B | Pestalotiopsis microspora (100) | Annonaceae, Annona muricata | WA |
| E3412F | Pestalotiopsis microspora (99) | Fabaceae, Lonchocarpus glabrescens | 1:10 PDA |
| E3314A | Pestalotiopsis sp. (100) | Sterculiaceae, Guazuma ulmifolia | 1:10 PDA |
| E2520A | Pestalotiopsis microspora (99) | Myrtaceae, Psidium acutangulum | PDA |
| E3432K | Phaeosphaeria sp. (95) | Fabaceae, Erythrina smithiana | PDA |
| E2104E | Nectria sp. (97) | Onagraceae, Fuschia hybrida | 1:10 PDA |
| E2705B | Pleosporales sp. (99) | Piperaceae, Piper arboretum | 1:10 GAM |
| E2812A | Pleosporales sp. (99) | Sterculiaceae, Theobroma kakau | WA |
| E2719A | Zopfiella karachiensis (99) | Myrtaceae, Psidium guajava | WA |
Each distinct fungal isolate was given a unique identification number.
DNA sequence identity for each endophyte was determined by ITS-5.8s rDNA sequencing and comparison to sequences in the GenBank database.
PDA, potato dextrose agar; 1:10 PDA, 1:10 dilution of potato dextrose medium in water agar; 1:10 GAM, 1:10 dilution of glycerol arginine medium in water agar; WA, water agar.
Several organisms of the Pestalotiopsis genus were represented among the active organisms in the first PUR-A clearance screen. Based upon initial activity observed from this genus, all of the Pestalotiopsis isolates in the collection were screened for activity. Some organisms within the genus exhibited high activity (E2712A, E3317B, and E2711A), some exhibited moderate activity (E3314A and E2708A), and others exhibited no detectable activity (E4112A). This variable expression was observed under identical growth conditions for each organism. While it is notable that some Pestalotiopsis microspora strains demonstrated some of the highest levels of activity, the level was quite variable among the various isolates.
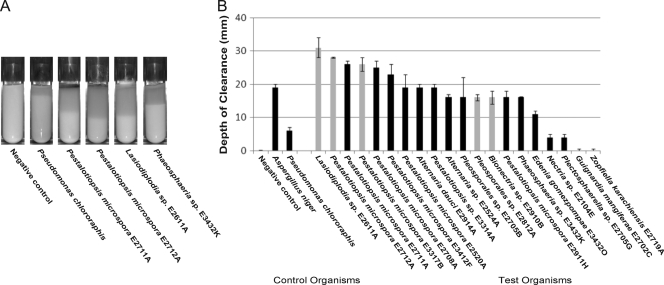
The active organisms from the plate clearance test were assayed in two additional PUR clearance assays, one using a solid medium and the second using a liquid medium. In the solid PUR-A clearance assay, fungal plugs were used to inoculate test tubes of PUR-A medium and vertical clearance was measured after 2 weeks of growth (Fig. 2). All of the 18 active fungi visibly cleared the PUR-A medium. Sixteen of these fungi demonstrated activity that was at least twice that of the organism Pseudomonas chlororaphis, a positive control for PUR degradation (13). The top clearing organism in this solid medium assay was identified by ITS sequencing as a Lasiodiplodia sp. (strain E2611A). Four of the six most active organisms belonged to the Pestalotiopsis genus, with high relatedness at the species level to Pestalotiopsis microspora. These top six organisms all cleared PUR more efficiently than the positive-control fungus Aspergillus niger. The two negative-control organisms, Guignardia mangiferae (E2702C) and Zopfiella karachiensis (E2719A), demonstrated no visible clearance of the Impranil substrate.
Fig. 2.
(A) Examples of PUR-A solid agar cultures at 2 weeks after inoculation, including Pseudomonas chlororaphis as a positive control. (B) Fungal cultures were grown in triplicate on solid PUR-A medium for 2 weeks. Depth-of-clearance measurements were made after 2 weeks of growth. The positive-control test organisms, Pseudomonas chlororaphis and Aspergillus niger, are represented at the left. The five highlighted organisms (Lasiodiplodia sp. strain E2611A, Pestalotiopsis microspora E2712A, Pestalotiopsis microspora E3317B, Pleosporales sp. strain E2812A, and Bionectria sp. strain E2910B) were selected for further screening. Error bars represent the standard deviation for each data set.
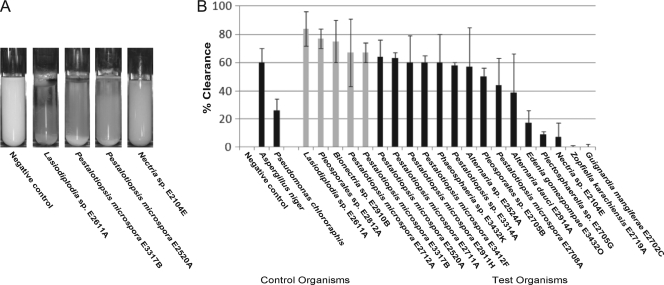
The relative rate of PUR clearance was also measured in a liquid culture assay. Optical absorbance at 600 nm, due to the scattering of the suspended polymer, was measured as an indication of the extent of clearance by the fungus. The liquid cultures were cleared to the point of visual transparency at the surface of the liquid culture, which extended down the length of the tube as time progressed (Fig. 3A). The relative order of the liquid clearance assay was different from that observed for the solid clearance assay (Fig. 2A). A Lasiodiplodia sp. (strain E2611A) was the most active organism in the liquid medium clearance screen, along with a Pleosporales sp. (strain E2812A) and a Bionectria sp. (strain E2910B), followed by Pestalotiopsis microspora (E2712A) and Pestalotiopsis microspora (E3317B). These organisms all cleared as well or better than Aspergillus niger, a positive fungal control, after 2 weeks of growth. Almost all of the active organisms tested showed more activity than the positive bacterial control Pseudomonas chlororaphis. The two negative-control organisms did not produce detectable clearance of the PUR-L liquid medium.
Fig. 3.
(A) Examples of PUR-L liquid cultures at 2 weeks after inoculation. (B) Fungal cultures were grown in triplicate in liquid PUR-L medium containing Impranil DLF for 2 weeks. Percent clearance was determined by a decrease in light scattering at 600 nm as measured by UV-visible spectrophotometry. Percent clearance measurements were taken after 2 weeks of growth. All data were normalized to the negative control. The positive-control test organisms, Pseudomonas chlororaphis and Aspergillus niger, are represented at the left. The 5 highlighted organisms (Lasiodiplodia sp. strain E2611A, Pestalotiopsis microspora E2712A, Pestalotiopsis microspora E3317B, Pleosporales sp. strain E2812A, and Bionectria sp. strain E2910B) were selected for further screening. Error bars represent the standard deviation for each data set.
Sole carbon source assay.
The five most active organisms were tested for their ability to degrade PUR in liquid culture using PUR as the sole carbon source (PUR-Lmin). For these studies we used Impranil DLN, which is equivalent to the Impranil DLF (an anionic aliphatic PUR dispersion in water) used for the previous studies but does not contain N-methyl pyrrolidone. If an organism grows in Impranil DLN, it can use PUR as the sole carbon source for metabolism and growth. All fungal inocula were washed with minimal medium to remove residual carbon and enzymes from the stock culture. The top five organisms from the two screen assays were tested for this activity, and all five were capable of PUR degradation activity under this minimal medium condition. Each fungus showed significant mycelial growth as monitored by visual inspection. For Pestalotiopsis microspora E2712A, cultures grown with PUR showed increased growth of 0.110 g ± 0.031 g of fungal material over that of a control culture grown under identical conditions with no PUR added. In addition, the positive-control fungus Aspergillus niger was able to slowly degrade PUR as a sole carbon source, as previously reported (7).
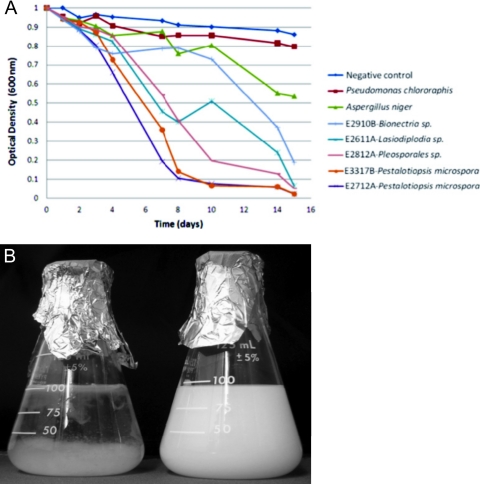
In order to compare the rates at which PUR was degraded under this condition, the top five organisms in the solid and liquid screen assays were grown in duplicate and tracked for 16 days (Fig. 4A). Two organisms, Pestalotiopsis microspora E2712A and E3317B, demonstrated the highest rate of PUR clearance. The approximate half time for clearance for these two organisms was 5 days. The cultures were visually transparent by the end of the 16-day period, consistent with complete degradation of the Impranil DLN (Fig. 4B). The only nontranslucent material remaining in the flask consisted of fungal growth. In comparison to other standards, the half time for clearance by the control organism Aspergillus niger was 15 days, and the bacterium Pseudomonas chlororaphis did not reach a state of half clearance within the 16-day time course. Experiments performed using Impranil DLF as the substrate showed that the presence of N-methyl pyrrolidone did not affect the growth rate for the organisms tested. This established that PUR alone is sufficient for fungal growth for these organisms. As a result, Impranil DLN was used for subsequent studies to characterize anaerobic growth and the nature of the degradation reaction.
Fig. 4.
(A) Degradation of PUR as a sole carbon source by the five most active organisms was monitored over a 16-day time course. Cultures containing PUR-Lmin medium with Impranil DLN as the sole carbon source were inoculated with washed fungal inoculums. The assay was performed in duplicate, and the values shown represent the averages for the two cultures at each time point. Aspergillus niger (green) and Pseudomonas chlororaphis (red) served as the positive controls. (B) Pestalotiopsis microspora E2712A (left) degrading PUR Impranil DLN as a sole carbon source after a 16-day time course. The remaining material in the flask is the result of fungal growth.
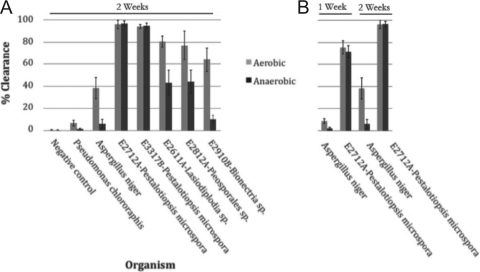
Anaerobic degradation of PUR.
Some of the organisms tested during the liquid culture assays, including Pestalotiopsis microspora E2712A and Pestalotiopsis microspora E3317B, were observed growing from the bottom of the culture tube rather than from the top. This suggested that these fungi are capable of anaerobic degradation of the polyurethane. To test this possibility, the assay using PUR-Lmin medium with Impranil DLN substrate was repeated under anaerobic conditions. The top five organisms from the previous assay were inoculated in triplicate and grown for 1 and 2 weeks in a BD GasPak anaerobic chamber. A corresponding set of cultures was grown outside the chamber, and the extents of clearance under the two conditions were compared (Fig. 5). The Pestalotiopsis microspora E2712A fungal isolate used in this assay showed equivalent rates of degradation under both anaerobic and aerobic conditions (Fig. 5B). Lasiodiplodia sp. strain E2611A and Pleosporales sp. strain E2812A showed diminished rates of clearance, while Bionectria sp. strain E2910B and the control Aspergillus niger showed negligible substrate degradation under anaerobic conditions. All organisms showed mycelial growth under anaerobic conditions as evinced by visual inspection.
Fig. 5.
(A) PUR degradation measurements of fungal cultures grown under aerobic and anaerobic conditions. Triplicate sets of fungal cultures were grown in PUR-Lmin medium containing Impranil DLN as the sole carbon medium under aerobic or anaerobic conditions. At the end of 2 weeks of growth, samples were removed from the sealed chamber and the percent clearance of the PUR in solution for each set of cultures was measured by decreased light scattering at a wavelength of 600 nm using UV-visible spectrophotometry The results for the controls Pseudomonas chlororaphis and Aspergillus niger are shown at the left. Two strains of Pestalotiopsis microspora (E2712A and E3317B) maintained polyurethanase activity when grown under anaerobic conditions, while Pseudomonas chlororaphis, Aspergillus niger, Lasiodiplodia sp. strain E2611A, Pleosporales sp. strain E2812A, and Bionectria sp. strain E2910B all showed significant reductions in the magnitude of PUR degradation activity. Error bars represent the standard deviation for each data set. (B) The rates of degradation were compared by measurement in triplicate of cultures of Aspergillus niger and Pestalotiopsis microspora E2712A after 1 week and 2 weeks of growth. Pestalotiopsis microspora E2712A shows equivalent rates of degradation activity under aerobic and anaerobic conditions. Error bars represent the standard deviation for each data set.
IR analysis of PUR degradation.
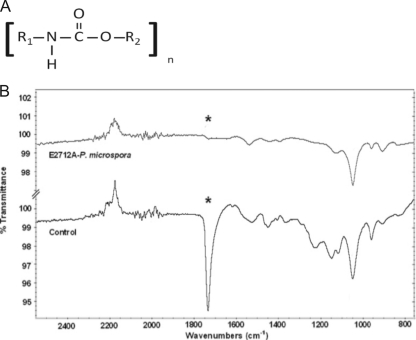
The mechanism of PUR degradation was initially investigated by infrared spectroscopy (16, 24). PUR samples of Impranil DLN display a large absorption peak at 1,735 cm−1 corresponding to the C(O)-O ester linkage in the polyurethane polymer (Fig. 6A). Fungal material was added to the PUR substrate and spectra collected throughout the course of the degradation experiment. A progressive reduction in the relative intensity of the peak at 1,735 cm−1 was observed and was accompanied by more subtle changes at other wave numbers. By the time the culture had became visually transparent, there was a complete loss of the absorbance peak at 1,735 cm−1 (Fig. 6B). Loss of this peak is consistent with hydrolysis of the ester bond in the urethane linkage.
Fig. 6.
(A) General chemical structure of the polyurethane molecule. R1 and R2 connect subsequent urethane monomers within the polymeric molecule. (B) Infrared spectra of PUR liquid medium containing Impranil DLN taken after 6 days of incubation with the fungus Pestalotiopsis microspora. The sample spectrum (top) and control spectrum (bottom) are shown together with a common scale. The strong peak denoted by an asterisk at 1,735 cm−1 in the control corresponds to the ester C=O stretch in the ethyl carbamate of the urethane motif. This peak disappears after degradation of the ester linkage by the polyurethanase enzyme.
Enzymatic characterization of putative polyurethanase.
Clearance zones in both the solid and liquid media occurred at a significant distance from the site of fungal growth, which is suggestive of an enzymatic activity that was extracellularly excreted. To explicitly test this possibility, clearance of the Impranil DLN substrate was tested using a cell-free filtrate of Pestalotiopsis microspora E2712A. Filtrate was prepared from a fungal culture grown in PUR-Lmin medium for 10 days. The crude filtrate from the PUR culture was able to completely clear the Impranil DLN in 1 h or less (data not shown). The active protein appears to be induced under PUR-Lmin growth conditions, as cell-free filtrate prepared from equivalent PDB liquid cultures did not exhibit any clearance activity (data not shown). This suggests that the activity is excreted, diffusible, and induced by cellular exposure to the polyurethane substrate.
The filtrate was next tested for the inhibition of the enzymatic activity responsible for the degradation activity using heat and small-molecule treatments. Heating the filtrates at 60°C and 80°C for 15 min had no detectable effect on substrate degradation activity. Treatment at 98°C for 20 min was required to inactivate the enzyme. The demonstration of heat-sensitive PUR biodegradation activity from a 0.22-μm-filtered extracellular extract indicates that a soluble and secreted extracellular protein is responsible for the enzymatic activity.
The enzyme responsible for PUR biodegradation was further characterized through the use of mechanism-based inhibitors. Phenylmethylsulfonyl fluoride (PMSF) specifically inactivates the active-site serine in serine hydrolases by covalent modification. Iodoacetate inactivates the active-site cysteine in cysteine hydrolases by a similar mechanism. EDTA sequesters metals and specifically inhibits the activity of metallohydrolases. PMSF, iodoacetate, and EDTA were used at inhibitory concentrations in cell-free filtrate of Pestalotiopsis microspora E2712A to monitor changes in the resulting activity of PUR clearance. Heat-treated extract was used as a negative control. Addition of PMSF, a serine hydrolase inhibitor, resulted in inactivation of the biodegradation activity. Addition of iodoacetate and EDTA had no effect on the rate or extent of activity (data not shown).
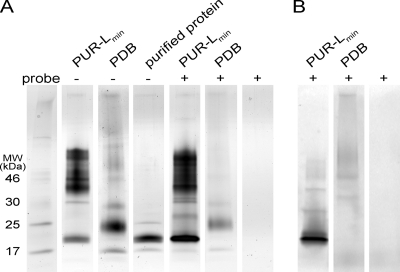
The reaction of the crude cell-free filtrate of Pestalotiopsis microspora E2712A with serine hydrolase inhibitor probes efficiently labeled one protein in the fraction grown in PUR-Lmin medium. Specific protein labeling was absent in the cell-free filtrate from PDB liquid culture, the condition in which no PUR degradation activity was observed (Fig. 7B). These results suggest that an approximately 21-kDa serine hydrolase-like enzyme is responsible for PUR degradation. Furthermore, growth in PUR-Lmin induced secretion of this enzyme (Fig. 7A). The 21-kDa protein was purified from the crude filtrate to approximately 90% purity, and it retained the ability to degrade PUR (data not shown).
Fig. 7.
Probing for serine hydrolases in crude protein from cell-free filtrate. SDS-PAGE analysis of proteins from a crude cell-free filtrate of Pestalotiopsis microspora E2712A grown in PUR-Lmin medium and PDB rich medium is shown. Cell-free filtrates were incubated with serine hydrolase activity-based probes prior to loading. (A) Protein bands were visualized by silver staining. (B) Labeled protein bands were visualized using a fluorescence scanner. This analysis shows that an approximately 21-kDa protein expressed exclusively under the induced PUR-Lmin growth condition is efficiently labeled by the serine hydrolase activity-based probe.
DISCUSSION
Endophytes isolated from Ecuadorian Amazonian plant samples were screened for their ability to degrade polyester polyurethane (PUR). Almost half of the organisms displayed some activity in the initial plate clearance assay. Eighteen active and two inactive endophytes were further characterized. Eight of the most active organisms belonged to the Pestalotiopsis genus. The current literature reports some fungi with the ability to degrade PUR (4–6, 8), although these studies have focused primarily on organisms isolated from soil samples. This is the first study that demonstrates PUR degradation by endophytic fungi. The broad distribution of activity suggests that endophytes might be a promising source of biodiversity in which to test for activities important for bioremediation.
All active fungi were identified as Ascomycota, with a cluster of organisms belonging to the class Dothidiomycetes and the order Pleosporales. A large portion of the active fungi belonged to the class Sordariomycetes, including those identified as Pestalotiopsis sp. strains.
Although robust activity was observed among several Pestalotiopsis sp. isolates, not all of them demonstrated equivalent levels of activity. Of the nine isolates tested, three were highly active (E2712A, E3317B, and E2711A), five were moderately active (E3314A, E2520A, E2911H, E3412F, and E2708A), and one was inactive (E4112A). This variability in activity among distinct Pestalotiopsis microspora isolates suggests that there are genetic differences among the organisms.
The genus Pestalotiopsis is grouped in the Xylariales order and comprises several known plant pathogens. The fungus is not host specific and causes rot and disease in a wide variety of plant species (29), although these isolates were all endophytic and the plants showed no pathogenic symptoms. Pestalotiopsis microspora isolates have previously been shown to have a propensity for horizontal gene transfer. In one notable case, a Pestalotiopsis microspora strain isolated as a fungal endophyte from the taxol-producing plant Taxus wallachiana had acquired the ability to synthesize taxol (27). Such a propensity for horizontal gene transfer may have contributed to the ability of a subset of these isolates to degrade polyester polyurethane as a sole carbon substrate, or it may reflect a significant level of phenotypic diversity among the genus.
There are no previous reports of members of the genus Pestalotiopsis having biodegradation activity. We found that two isolates of Pestalotiopsis microspora (E2712A and E3317B) were able to degrade PUR when grown anaerobically with Impranil DLN serving as the sole carbon source. For these two organisms, the level of activity was the same when grown under either aerobic or anaerobic conditions. This is in contrast to the control fungus Aspergillus niger, which showed substantially less activity when grown anaerobically. This observation may have practical significance in that fungal growth on and metabolism of PUR by Pestalotiopsis microspora could be used in anaerobic fermentation systems.
The enzyme produced by Pestalotiopsis microspora that is responsible for PUR degradation appears to be a member of the serine hydrolase family. Furthermore, activity extended throughout the medium at a distance well removed from the areas of fungal growth. This suggests that the enzyme responsible for degradation is extracellular, secreted, and diffusible. In comparison to an inactive cell-free filtrate from a fungal culture grown in rich medium, we found that the polyurethanase is inducible when Pestalotiopsis microspora E2712A is grown in minimal PUR-Lmin medium containing a suspension of Impranil DLN. By using activity-based probes, the active enzyme was identified as a serine hydrolase with an approximate molecular mass of 21 kDa. The protein was shown to be able to degrade PUR after subsequent purification, showing that activity is independent of other components of the culture filtrate.
Polyurethanases have previously been isolated and characterized from protein extracts of several organisms, including the bacteria Pseudomonas chlororaphis (24) and Comamonas acidovorans (1, 2), as well as the fungus Candida rugosa (8). The active enzymes have been classified (17) as esterases (5, 13, 16), lipases (24), and proteases and ureases (19), suggesting degradation of the PUR substrate by cleavage of the ester bond. The IR analysis and molecular inhibition of PUR degradation by Pestalotiopsis microspora suggest that ester hydrolysis by a serine hydrolase is responsible for PUR biodegradation.
This investigation established the robust polyurethane degradation activity under anaerobic conditions in which the synthetic polymer served as the only carbon source for the fungus. A cell extract of the active culture containing a critical serine hydrolase is able to clear the polymer in under 1 h using the PUR concentrations reported here. This work establishes that endophytes are a useful source of biodiversity with potential application for bioremediation. The relative ease with which organisms can be isolated and screened makes this a highly accessible and environmentally relevant project for engaging undergraduate students in scientific research. It is possible that activities against other, more recalcitrant polymers could be discovered using this abundant source of biodiversity.
ACKNOWLEDGMENTS
This project was supported by an HHMI Professor's grant and NSF grant OISE 853408 to S.A.S. J.R.R and M.V. were supported with a fellowship from the Arnold and Mabel Beckman Foundation.
We thank Benjamin Cravatt from the Scripps Institute for his generosity in supplying the serine hydrolase activity-based probes used in the enzymatic characterization described in this work. We also thank Ian Suydam for helpful discussion. We also thank Bayer Materials Science for donating the Impranil DLF and DLN polyurethane suspension used as the substrate in these experiments. We thank the Yale Peabody Herbarium and the Herbario Nacional del Ecuador (QCNE) for cataloging the voucher specimens. We also thank Colección de Endófitos Quito Católica for cataloguing the microorganisms in their living culture collections. We thank the Pontificia Universidad Católica del Ecuador, Museo Ecuatoriano de Ciencias Naturales, and the government of Ecuador. This research was possible because of the kind permission of the Ministerio del Ambiente of Ecuador.
Footnotes
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Akutsu Y., Nakajima-Kambe T., Nomura N., Nakahara T. 1998. Purification and properties of a polyester-polyurethane degrading enzyme from Comamonas acidovorans TB-35. Appl. Environ. Microbiol. 64:62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen B. A., Hilliard N. P., Howard G. T. 1999. Purification and characterization of a soluble polyurethane degrading enzyme from Comamonas acidovorans. Int. Biodeterior. Biodegrad. 43:37–41 [Google Scholar]
- 3. Bacon C., White J. 2000. Microbial endophytes. Marcel Dekker, New York, NY [Google Scholar]
- 4. Cosgrove L., McGeechan P. L., Robson G. D., Handley P. S. 2007. Fungal communities associated with degradation of polyester polyurethane in soil. Appl. Environ. Microbiol. 73:5817–5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crabbe J. R., Campbell J. R., Thompson L., Walz S. L., Schultz W. W. 1994. Biodegradation of a colloidal ester-based polyurethane by soil fungi. Int. Biodeterior. Biodegrad. 33:103–113 [Google Scholar]
- 6. Darby R. T., Kaplan A. T. 1968. Fungal susceptibility of polyurethanes. Appl. Microbiol. 16:900–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Filip Z. 1979. Polyurethane as the sole nutrient source for Aspergillus niger and Cladosporium herbarum. Eur. J. Appl. Microbiol. Biotechnol. 7:277–280 [Google Scholar]
- 8. Gautam R., Bassi A. S., Yanful E. K. 2007. Candida rugosa lipase-catalyzed polyurethane degradation in aqueous medium. Biotechnol. Lett. 29:1081–1086 [DOI] [PubMed] [Google Scholar]
- 9. Hanauer D. I., Jacobs-Sera D., Pedulla M. L. 2006. Teaching scientific inquiry. Science 314:1880–1881 [DOI] [PubMed] [Google Scholar]
- 10. Howard G. T. 2002. Biodegradation of polyurethane: a review. Int. Biodeterior. Biodegrad. 49:245–252 [Google Scholar]
- 11. Howard G. T., Blake R. C. 1998. Growth of Pseudomonas fluorescens on a polyester-polyurethane and the purification and characterization of a polyurethanase-protease enzyme. Int. Biodeterior. Biodegrad. 42:213–220 [Google Scholar]
- 12. Howard G. T., Hilliard N. P. 1999. Use of Coomassie blue-polyurethane interaction in screening of polyurethanase proteins and polyurethanolytic bacteria. Int. Biodeterior. Biodegrad. 43:23–30 [Google Scholar]
- 13. Howard G. T., Ruiz C., Hilliard N. P. 1999. Growth of Pseudomonas chlororaphis on a polyester-polyurethane and the purification and characterization of a polyurethanase-esterase enzyme. Int. Biodeterior. Biodegrad. 43:7–12 [Google Scholar]
- 14. Howard G. T., Vicknair J., Mackie R. I. 2001. Sensitive plate assay for screening and detection of bacterial polyurethanase activity. Lett. Appl. Microbiol. 32:211–214 [DOI] [PubMed] [Google Scholar]
- 15. Kay M. J., Morton L. H. G., Prince E. L. 1991. Bacterial degradation of polyester polyurethane. Int. Biodeterior. Biodegrad. 27:205–222 [Google Scholar]
- 16. Kay M. J., McCabe R. W., Morton L. H. G. 1993. Chemical and physical changes occurring in polyester polyurethane during biodegradation. Int. Biodeterior. Biodegrad. 31:209–225 [Google Scholar]
- 17. Nakajima-Kambe T., Shigeno-Akutsu Y., Nomura N., Onuma F., Nakahara T. 1999. Microbial degradation of polyurethane, polyester polyurethanes, and polyether polyurethanes. Appl. Microbiol. Biotechnol. 51:134–140 [DOI] [PubMed] [Google Scholar]
- 18. Oceguera-Cervantes A., et al. 2007. Characterization of the polyurethanolytic activity of two Alicycliphilus sp. strains able to degrade polyurethane and N-methylpyrrolidone. Appl. Environ. Microbiol. 73:6214–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pathirana R. A., Seal K. J. 1984. Studies on polyurethane deteriorating fungi. Int. Biodeterior. Biodegrad. 20:163–168 [Google Scholar]
- 20. Patricelli M. P., Giang D. K., Stamp L. M., Burbaum J. J. 2001. Direct visualization of serine hydrolase activities in complex proteomes using fluorescent active site-directed probes. Proteomics 1:1067–1071 [DOI] [PubMed] [Google Scholar]
- 21. PlasticsEurope January 2008. The compelling facts about plastics, an analysis of plastics production, demand and recovery for 2006 in Europe. PlasticsEurope, Brussels, Belgium. http://www.plasticsrecyclers.eu/docs/press%20release/080123CfaPpdfVersion.pdf
- 22. Ramirez-Coronel M. A., Viniegra-Gonzalez G., Darvill A., Augur C. 2003. A novel tannase from Aspergillus niger with β-glucosidase activity. Microbiology 149:2941–2946 [DOI] [PubMed] [Google Scholar]
- 23. Rowe L., Howard G. T. 2002. Growth of Bacillus subtilis on polyurethane and the purification and characterization of a polyurethanase-lipase enzyme. Int. Biodeterior. Biodegrad. 50:33–40 [Google Scholar]
- 24. Shah A. A., Hassan F., Hameed A., Ahmed S. 2008. Biological degradation of plastics: a comprehensive review. Biotechnol. Adv. 26:246–265 [DOI] [PubMed] [Google Scholar]
- 25. Smith S. A., et al. 2008. Bioactive endophytes warrant intensified exploration and conservation. PLoS One 3:e3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stern R. V., Howard G. T. 2000. The polyester polyurethane gene (pueA) from Pseudomonas clororaphis encodes lipase. FEMS Microbiol. Lett. 185:163–168 [DOI] [PubMed] [Google Scholar]
- 27. Strobel G., et al. 1996. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology 142:435–440 [DOI] [PubMed] [Google Scholar]
- 28. Strobel S. A., Strobel G. 2007. Plant endophytes as a platform for discovery-based undergraduate science education. Nat. Chem. Biol. 3:356–359 [DOI] [PubMed] [Google Scholar]
- 29. Uchida J. Y. 2004. Pestalotiopsis diseases, p. 27–28. In Elliott M. L., Broschat T. K., Uchida J. Y., Simone G. W. (ed.), Diseases and disorders of ornamental palms. American Phytopathological Society, St. Paul, MN [Google Scholar]
- 30. White T. J., Bruns T., Lee S., Taylor J. W. 1990. Amplification and direct sequencing of fungal rRNA genes for phylogenetics, p. 315–322. In Innis M. A., Gelfand D. H., Sninsky J. J., White T. J. (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, NY [Google Scholar]