Abstract
Bacterial degradation pathways of fuel oxygenates such as methyl tert-butyl and tert-amyl methyl ether (MTBE and TAME, respectively) have already been studied in some detail. However, many of the involved enzymes are still unknown, and possible side reactions have not yet been considered. In Aquincola tertiaricarbonis L108, Methylibium petroleiphilum PM1, and Methylibium sp. strain R8, we have now detected volatile hydrocarbons as by-products of the degradation of the tert-alkyl ether metabolites tert-butyl and tert-amyl alcohol (TBA and TAA, respectively). The alkene isobutene was formed only during TBA catabolism, while the beta and gamma isomers of isoamylene were produced only during TAA conversion. Both tert-alkyl alcohol degradation and alkene production were strictly oxygen dependent. However, the relative contribution of the dehydration reaction to total alcohol conversion increased with decreasing oxygen concentrations. In resting-cell experiments where the headspace oxygen content was adjusted to less than 2%, more than 50% of the TAA was converted to isoamylene. Isobutene formation from TBA was about 20-fold lower, reaching up to 4% alcohol turnover at low oxygen concentrations. It is likely that the putative tert-alkyl alcohol monooxygenase MdpJ, belonging to the Rieske nonheme mononuclear iron enzymes and found in all three strains tested, or an associated enzymatic step catalyzed the unusual elimination reaction. This was also supported by the detection of mdpJK genes in MTBE-degrading and isobutene-emitting enrichment cultures obtained from two treatment ponds operating at Leuna, Germany. The possible use of alkene formation as an easy-to-measure indicator of aerobic fuel oxygenate biodegradation in contaminated aquifers is discussed.
INTRODUCTION
The extensive use of methyl tert-butyl ether (MTBE) and related compounds as fuel oxygenates has resulted in contamination of numerous groundwater sites in the United States and Europe (13, 26, 33, 51). Although MTBE is now banned in some countries and may be phased out in others (52), it will persist at polluted sites for a long time due to its poor biodegradability. Despite this enormous environmental prevalence, only a few strains capable of growth on MTBE, ethyl tert-butyl ether (ETBE), and tert-amyl methyl ether (TAME) have been found, and fuel oxygenate metabolism has not been elucidated in full detail (20, 30, 31, 46). Since the pioneering works of Salanitro et al. and Steffan et al. (42, 43, 48), it is generally agreed that aerobic MTBE degradation proceeds via a monooxygenase-catalyzed ether cleavage resulting in formation of tert-butyl alcohol (TBA). The latter is hydroxylated to the corresponding diol, 2-methyl-1,2-propanediol (MPD), which is further oxidized to 2-hydroxyisobutyric acid (2-HIBA). This branched carboxylic acid is then introduced into common metabolic routes after isomerization to 3-hydroxybutyric acid (39). Since ETBE shares the tert-butyl structure with MTBE, it should have a similar degradation path via TBA (8, 30). The biochemistry of TAME catabolism has not been elucidated in detail. It is likely that similar enzymatic steps to those for the other ethers are involved, with tert-amyl alcohol (TAA) being a central metabolite. Hydroxylation of the latter would result in three possible diol products, two of which could be further oxidized to carboxylic acids (2, 49). However, not all of these enzymatic steps involved in fuel oxygenate degradation have been identified unambiguously and characterized thoroughly. In addition, possible side reactions have not yet been considered.
Aquincola tertiaricarbonis L108 is an MTBE-metabolizing organism representing a new species within the Rubrivivax-Roseateles-Leptothrix-Ideonella-Aquabacterium branch of the Beta proteobacteria (28). The strain was isolated from an MTBE-contaminated aquifer in Germany (39) and is one of the few pure cultures which can completely degrade the fuel additive MTBE and related ethers (35). At other sites, in France and the United States, the closely related strains A. tertiaricarbonis CIP I-2052 (28, 38), Aquincola sp. strain ENV 736 (30), and Aquincola sp. strain S1B1 (16) have been isolated, indicating that the spread of this species is not locally restricted. Thus far, strain L108 is one of the most efficient aerobic MTBE degraders, showing growth at maximal rates of close to 0.05 h−1 and a biomass yield of 0.55 g g−1 on this substrate (35). Interestingly, strain L108 shares at least some MTBE-related enzymes with another well-studied MTBE degrader, Methylibium petroleiphilum PM1 (36). In both strains, a two-component cobalamin-dependent mutase seems to be involved specifically in 2-HIBA conversion, and the partial sequences of the mutase genes obtained from strain L108 show nearly 100% identity to the corresponding mdpO and mdpR genes found in strain PM1 (39). In addition, expression of the mdpJ and mdpK genes, encoding a putative oxygenase and its reductase, respectively, is increased at the mRNA level when strain PM1 grows on MTBE versus ethanol (20). Likewise, it has been found that the corresponding proteins are highly induced in strain L108 grown on TBA compared to lactate-grown cells (45), suggesting a role in TBA metabolism. Methylibium sp. strain R8 is also a PM1-like bacterium showing autarkic growth on MTBE and TAME. Besides a close phylogenetic relationship (40), strains PM1 and R8 share the same monooxygenase for attacking MTBE, encoded by the mdpA gene (22, 46), and show identical MTBE isotope fractionation patterns (40). In several studies, M. petroleiphilum PM1 or closely related strains were found to dominate the bacterial communities at MTBE-contaminated sites (7, 19, 23), indicating the importance of these bacteria for fuel oxygenate degradation.
To learn more about the biochemistry and physiology supporting the efficient degradation of fuel oxygenates by A. tertiaricarbonis L108, M. petroleiphilum PM1, and Methylibium sp. strain R8, gas samples from cultures grown on the relevant tertiary alkyl ethers and corresponding alcohols were analyzed for metabolites. Surprisingly, highly volatile C4 and C5 alkenes were emitted during growth and accumulated in the closed culture bottles. The TBA and TAA biodegradation steps turned out to be the sources of isobutene and isoamylene, respectively. Our results also show an oxygen dependence of alkene production, contributing maximally to alcohol conversion at low oxygen concentrations. Although formation of isoamylene from TAME and TAA is more pronounced than isobutene emissions from MTBE, ETBE, and TBA, the latter can also reach a few percentage points of total oxygenate conversion. Thus, the progress of fuel oxygenate degradation is easily monitored by analyzing gas samples by gas chromatography (GC). Furthermore, the alkene formation found in this study could be used as a sensitive indicator for evaluating natural attenuation of fuel oxygenates at contaminated field sites.
MATERIALS AND METHODS
Bacterial strains and growth medium.
Aquincola tertiaricarbonis L108 and Methylibium sp. strain R8, previously isolated from an MTBE-contaminated aquifer at the Leuna test site, Germany (39), and from an MTBE-degrading enrichment culture (40) provided by E. Arvin (DTU, Denmark), respectively, were cultivated in liquid mineral salt medium (MSM [see the supplemental material for details]) containing MTBE at a concentration of 0.3 g liter−1. Methylibium petroleiphilum PM1, obtained from the American Type Culture Collection (ATCC BAA-1232), was grown under the same conditions as the other strains.
Chemicals.
Alkene GC standards of ≥99% purity (isobutene and the alpha, beta, and gamma isomers of isoamylene) and TAME (97% pure) were purchased from Sigma-Aldrich (Taufkirchen, Germany). MTBE (≥99% pure), ETBE (>97% pure), TBA (≥99% pure), 2-HIBA (>98% pure), and TAA (>99% pure) were purchased from Merck Schuchardt (Hohenbrunn, Germany). 2-Hydroxy-2-methylbutyric acid (2,2-HMB) (98% pure racemic mixture) was purchased from ABCR (Karlsruhe, Germany), 3-hydroxy-3-methylbutyric acid (3,3-HMB) (98% pure) was from Alfa Aeasar (Karlsruhe, Germany), and 2-methyl-1,2-propanediol (MPD) at the highest purity available was from Taros Chemicals (Dortmund, Germany).
Growth and resting-cell experiments.
Cultures were incubated at 30°C on rotary shakers. Bacterial cells used in experiments were pregrown on the respective substrates in closed glass bottles in up to 1 liter of culture medium and harvested by centrifugation at 13,000 × g at 4°C for 10 min. After being washed twice with MSM, cells were immediately used as an inoculum for growth or resting-cell experiments. For the latter, the cell concentration was adjusted to 1.35 g biomass (dry weight) per liter by dilution with MSM, whereas growth experiments were typically started with 30 to 60 mg biomass per liter. Degradation activities and relative alkene production in resting-cell experiments were determined by linear regression analysis of substrate decreases versus time and alkene formation versus substrate conversion, respectively (see Fig. S1 in the supplemental material). Substrates were normally added at 200 to 500 mg per liter. In cases of low degradation activity, the concentration was reduced to initial values of about 20 mg per liter. Bacteria were incubated in either 25 or 50 ml MSM in 120- or 240-ml glass serum bottles, respectively, sealed gas-tight with butyl rubber stoppers. In resting-cell experiments, oxygen concentrations were adjusted by mixing air with pure oxygen or nitrogen to the target value. During the experiments, concentrations were kept constant by adding appropriate amounts of oxygen. Volume reductions due to sampling of liquid and gas phases were balanced by adding appropriate gas volumes (air, oxygen, or nitrogen, depending on the experimental conditions). The data shown in this study represent the mean values and standard deviations (SD) for at least three replicate experiments.
Sampling and analytics.
Liquid samples were taken by puncturing the rubber stoppers with disposable syringes equipped with 0.6- by 30-mm Luer Lock needles. Biomass was monitored by measuring the optical density at 700 nm (OD700), using a multiplication factor of 0.54 for calculating dry biomass in g per liter (35). MTBE, ETBE, and TBA in liquid samples were quantified by headspace GC, applying an HP 6890 GC system (Agilent Technologies, Waldbronn, Germany) with an Optima Delta 3 column (60 m × 0.32 mm × 0.35 μm; Macherey-Nagel, Düren, Germany) and a flame ionization detector (FID). Ten-milliliter headspace vials were filled with 1 ml of a saturated aqueous NaCl solution and 1 ml of liquid sample. After incubation at 70°C for 20 min, 1 ml of gas phase was removed and injected into the GC system by using a G1888 autosampler. Helium was used as the carrier gas, and oven and detector temperatures were held at 50°C and 220°C, respectively. TAME and TAA were quantified by use of the same GC system, but sample incubation was at 95°C for 30 min, the detector temperature was 250°C, and the GC oven temperature program was set at 100°C for 2 min, increased from 100 to 180°C at a rate of 15°C per min, and finally kept at 180°C for 0.67 min. Degradation of diols and carboxylic acids was quantified using a high-performance liquid chromatography (HPLC) system with a refractive index (RI) detector as described elsewhere (34, 35), applying an eluent of 0.01 N sulfuric acid at 0.6 ml per min and a Nucleogel Ion 300 OA column (300 × 7.7 mm; Macherey-Nagel). The pH in liquid samples was monitored using a model 765 Calimatic pH meter (Knick, Berlin, Germany) with an Inlab 415 electrode (Mettler Toledo, Giessen, Germany). The oxygen concentration was measured in serum bottles equipped with PSt3 oxygen sensor spots by applying a Fibox 3 trace fiber optic device (both from PreSens, Regensburg, Germany) as previously described (22). The detection limit was 0.03% (vol/vol).
For quantification of alkenes, gas samples were taken with a 2.5-ml gas-tight syringe equipped with a Luer Lock push-pull valve (2.5MDR-VLL-GT; SGE Analytical Science, Ringwood, Australia) enabling use of disposable Luer Lock needles (0.6 × 30 mm). A 2-ml gas sample was added to 10-ml headspace vials already filled with 5 ml air and 5 ml deionized water. The same GC method as that described above for measuring MTBE was used. Peaks for isobutene and the three isoamylene isomers were clearly separated in GC chromatograms. Compounds in samples were identified according to the retention times of pure GC standards (see Fig. S2 and S3 in the supplemental material). In addition, assignments were verified by GC-mass spectrometry (MS) analysis (see Fig. S4 and S5 in the supplemental material).
Field samples and enrichment cultures.
Two aerated treatment pond systems (22) at the Leuna test site were sampled in April 2011. One pond was equipped with coconut fiber geotextile, and the other was equipped with polypropylene fleece geotextile, enabling biofilm formation. Samples of 1 cm by 1 cm of both textiles (C and P cultures, respectively) were incubated in 50 ml MSM in 240-ml serum bottles with MTBE under the same conditions as those described above for pure cultures. Oxygen concentrations of the liquid and gas phases were measured by again applying the Fibox 3 trace device and PSt3 oxygen sensor spots. In control experiments, cultures were pretreated by incubation at 65°C for 2 h.
PCR and sequencing.
DNA extraction from cells of strain R8 and the environmental enrichment cultures C and P was performed with a MasterPure DNA purification kit (Epicentre Biotechnologies). The resulting DNA was diluted 10-fold for PCR amplification of the mdpJK genes by using specific primers as previously described (see Table S1 in the supplemental material) (45). The amplification product was purified with a Wizard SV gel and PCR clean-up kit (Promega). Sequencing reactions were performed using a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems). The determination of the sequence was carried out via a model 3130XL genetic analyzer (Applied Biosystems). The sequence was determined twice and compared with the NCBI database. Similarities were evaluated using the BLAST alignment tool (1).
Nucleotide sequence accession numbers.
Complete mdpJK sequences obtained from DNA of strain Methylibium sp. strain R8 have been deposited in the GenBank/EMBL/DDBJ database under the accession numbers JN197272 and JN197273.
RESULTS
Detection of alkenes in cultures grown on fuel oxygenates.
In using GC to analyze gas samples taken from the headspace of cultures of strains L108, PM1, and R8 grown on the fuel oxygenates MTBE and TAME, we were surprised to detect the alkenes isobutene and isoamylene. On ETBE, only strain L108 showed alkene formation, and neither Methylibium strain grew on this oxygenate or degraded it. MTBE and ETBE cultures produced only isobutene, whereas isoamylene accumulated exclusively after growth on TAME (Table 1), with the beta isoform representing about 97% of the isoamylene, whereas gamma-isoamylene was present in traces and alpha-isoamylene was not detected. In sterile control experiments, the ether oxygenates and the alcohols TBA and TAA were stable, and dehydration to the corresponding alkenes did not occur even when we lowered the pH value to 5. Consequently, the drop in pH from 7.5 to values between 5 and 6 typically observed in growing cultures on MSM could not be responsible for alkene formation. Isobutene was formed not only in MTBE and ETBE cultures but also when cells were shifted to TBA. However, it was not detectable when strain L108 was grown on the other MTBE metabolites, MPD and 2-HIBA, or on unspecific substrates, such as lactic acid and fructose, suggesting that degradation of the alcohol TBA is the alkene-emitting step. In total, between 0.1 and 0.3% isobutene was produced from MTBE, ETBE, and TBA degradation. Isoamylene formation, on the other hand, was more significant and amounted to up to about 40% of the degraded TAME. A similar alkene production level also accompanied the degradation of the TAME intermediate TAA, in line with the observed isobutene formation from TBA. The possible TAME and TAA metabolites 3,3-HMB and 2,2-HMB (2), however, supported growth of strain L108 but did not produce alkenes. Generally, alkene formation from fuel oxygenates in cultures of strains L108 and R8 was more significant than that in PM1 cultures. In particular, independent of the amount of substrate degraded, TAME was converted to <5% isoamylene by strain PM1, whereas the other strains produced >20% alkene from this oxygenate.
Table 1.
Alkene formation during batch growth of A. tertiaricarbonis L108, M. petroleiphilum PM1, and Methylibium sp. strain R8 on various substrates
| Growth substrate | Strain | Amt of substrate turnover (μmol) | % Isobutene formationa | % Isoamylene formationb |
|---|---|---|---|---|
| MTBE | L108 | 74 ± 12 | 0.33 ± 0.07 | |
| R8 | 71 ± 3 | 0.25 ± 0.01 | ||
| PM1 | 56 ± 3 | 0.11 ± 0.01 | ||
| PM1 | 114 ± 17 | 0.12 ± 0.04 | ||
| ETBE | L108c | 25 ± 3 | 0.17 ± 0.09 | |
| TBA | L108 | 71 ± 7 | 0.29 ± 0.03 | |
| R8 | 89 ± 13 | 0.26 ± 0.03 | ||
| PM1 | 118 ± 3 | 0.16 ± 0.02 | ||
| MPD, 2-HIBA, lactic acid, or fructose | L108d | 30-120 | ≤0.03 | |
| TAME | L108 | 61 ± 18 | 38 ± 4 | |
| R8 | 68 ± 5 | 21 ± 1 | ||
| PM1 | 74 ± 4 | 4.5 ± 0.7 | ||
| PM1 | 26 ± 6 | 4.3 ± 0.2 | ||
| TAA | L108 | 104 ± 22 | 9 ± 1 | |
| R8 | 62 ± 15 | 22 ± 4 | ||
| R8 | 9 ± 2 | 27 ± 3 | ||
| PM1 | 130 ± 10 | 2.5 ± 0.8 | ||
| 3,3-HMB, 2,2-HMB, lactic acid, or fructose | L108d | 20-140 | ≤0.03 |
Percentage of substrate turnover.
Percentage of substrate turnover of beta and gamma isomers.
Not applicable for strains PM1 and R8.
Not tested with strains PM1 and R8.
Isoamylene accumulation during growth on TAA.
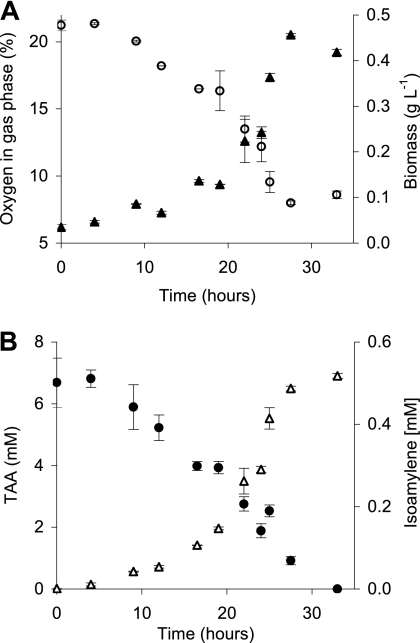
In TAA cultures of strain L108, isoamylene formation was studied in more detail. As expected, the strain grew well on TAA and the substrate was consumed within less than 2 days (Fig. 1). Within this period, the biomass concentration of the culture increased from 0.03 to around 0.45 g liter−1, indicating a maximal growth rate of 0.08 h−1, which is close to previously observed values (35). Due to the experimental design not allowing gas exchange with the environment, the oxygen concentration in the headspace of the culture bottles dropped from about 21% to <10% during aerobic degradation of 7 mM TAA. Initial isoamylene formation was low, representing only 3 to 5% of TAA conversion (Fig. 1B), e.g., within 19 h of incubation, only about 0.15 mM isoamylene was produced, while nearly 3 mM TAA was degraded. However, alkene production then increased significantly, as nearly 0.4 mM isoamylene was produced from the residual 4 mM TAA. The conversion thus rose to nearly 10% at the end of the experiment. Interestingly, the more pronounced alkene formation coincided with the lower oxygen concentrations in the headspace, indicating an oxygen dependence of this process. Throughout the experiment, the beta isoform was the dominant isoamylene species, representing about 96 to 97% of isomers produced (data not shown).
Fig. 1.
Isoamylene formation during batch growth of A. tertiaricarbonis L108 on TAA in a gas-tight incubation system. (A) Headspace oxygen concentrations (open symbols) and biomass dry weights (solid symbols). (B) TAA (solid symbols) and isoamylene (open symbols) concentrations. For a direct comparison with TAA conversion, isoamylene values are the sums of gamma and beta isomers and refer to concentrations in the liquid phase, although alkenes were found exclusively in the gas phase of the closed incubation bottles.
Oxygen dependence of alkene formation by resting cells.
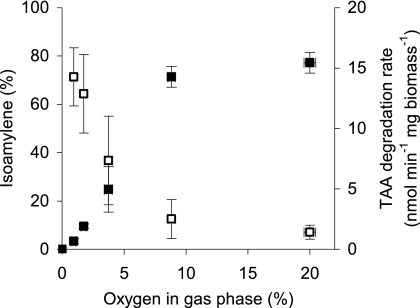
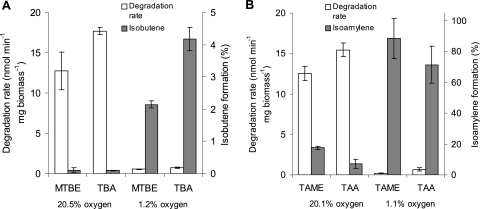
For precise study of the oxygen dependence of alkene formation from oxygenates, resting-cell experiments at different constant oxygen concentrations were performed. However, as it turned out to be difficult to maintain the dissolved oxygen at a constant level, only gas-phase oxygen was controlled. In similar experiments, it was already demonstrated that dissolved oxygen and headspace oxygen are not in equilibrium, likely due to the high oxidation activities exceeding diffusion rates (41). Nevertheless, under these conditions, cells of strain L108 showed constant substrate degradation and alkene formation rates, allowing exact quantification of these values (see Fig. S1 in the supplemental material). At 20% oxygen in the gas phase of the culture bottles, resting cells of strain L108 degraded TAA at about 15 nmol min−1 mg biomass−1 (Fig. 2). This rate decreased dramatically when we lowered the oxygen concentration, e.g., at 2% oxygen, TAA was consumed at <2 nmol min−1 mg biomass−1. Accordingly, TAA degradation was not detectable under anoxic conditions. Alkene production showed a similar dependence, with highest rates of 1 to 1.6 nmol min−1 mg biomass−1 between 9 and 20% oxygen and lower values when the oxygen concentration was reduced further. However, the relative share of isoamylene production from the total TAA conversion increased significantly with decreasing oxygen concentrations. While alkene production reached only about 7% at 20% oxygen, at oxygen concentrations of 1 and 2%, more than 50% of the TAA was converted to isoamylene. This trend was also observed with the other oxygenates tested, although absolute alkene emissions differed greatly depending on the substrate (Fig. 3). As expected, degradation of TAME gave high isoamylene formation values that were close to those obtained for TAA. In contrast, isobutene formation from MTBE and TBA was about 20-fold lower. At 20% oxygen, isobutene could hardly be detected and amounted to <0.2% of substrate conversion. When lower oxygen concentrations, in the 1% range, were applied, alkene production was more significant and reached values between 2 and 4%.
Fig. 2.
Isoamylene formation from TAA in resting-cell experiments with incubation of A. tertiaricarbonis L108 at various headspace oxygen concentrations. Substrate degradation rates (solid symbols) were normalized to biomass dry weights, and formation of isoamylene isomers (open symbols) is shown as percentages of TAA conversion.
Fig. 3.
Alkene formation from fuel oxygenates in resting-cell experiments with incubation of A. tertiaricarbonis L108 at headspace oxygen concentrations adjusted to values close to that of normal air and to 1% oxygen. (A) Degradation of MTBE and TBA. (B) Degradation of TAME and TAA. Degradation rates were normalized to biomass dry weights, and formation of alkenes is shown as percentages of substrate conversion. Headspace oxygen concentrations represent mean values with SD of <1% for the 20% range and <0.2% for the 1% range.
Isobutene formation in MTBE-degrading enrichment cultures.
In both enrichment cultures, inoculated with either coconut fiber (culture C) or polypropylene fleece (culture P) samples from the aerated treatment pond system at the Leuna test site (22), MTBE degradation started without any lag phase (see Fig. S6 and S7 in the supplemental material). Concomitant with ether degradation, isobutene formation was observed, amounting to about 0.5% of substrate conversion. In contrast, heat-treated cultures showed neither MTBE degradation nor isobutene formation. Compared to turnover of oxygenates by growing cultures of the pure strains tested in this study, degradation of MTBE by the C and P enrichments was slow, e.g., within 1 week of incubation, only about 0.5 to 1 mM MTBE was consumed. Throughout the experiments, dissolved oxygen concentrations were in good equilibrium with the gas-phase content (see Fig. S6 and S7 in the supplemental material), indicating that oxygen diffusion rates were higher than or equal to consumption rates under these conditions. Both MTBE degradation and isobutene formation stopped when oxygen concentrations dropped to values of 1% and under but could continue when additional oxygen was supplied through the gas phase.
Detection of mdpJK genes.
PCRs using mdpJK-specific primers and DNAs extracted from Methylibium sp. strain R8 cells and enrichment cultures C and P resulted in PCR products of the expected sizes. After sequencing, complete mdpJK genes (1,413 and 1,014 bp) were obtained for DNA from strain R8, showing 97 and 100% identity to the corresponding genes found in strains L108 (JN033363 and JN033364) and PM1 (Mpe_B0555 and Mpe_B0554), respectively. With PCR products from the enrichment cultures, about 95 and 60% of the complete mdpJ and mdpK genes, respectively, were sequenced, showing >99% identity to the mdpJK genes from strain L108. This high degree of sequence identity is not surprising, as it has already been found that not only mdpJ but also other genes related to MTBE degradation are obviously well conserved among MTBE- and TBA-metabolizing bacteria (3, 39, 45).
DISCUSSION
The betaproteobacterial strains A. tertiaricarbonis L108, M. petroleiphilum PM1, and Methylibium sp. strain R8 show alkene emission while degrading fuel oxygenates. Isobutene was formed exclusively from MTBE, ETBE, and TBA, whereas the beta- and gamma-isoamylene isomers were produced from TAME and TAA. Since degradation of other substrates, such as the MTBE intermediates MPD and 2-HIBA, by strain L108 is not accompanied by alkene formation, it can be concluded that the alkene-producing step is located at the stage of tertiary alcohol degradation.
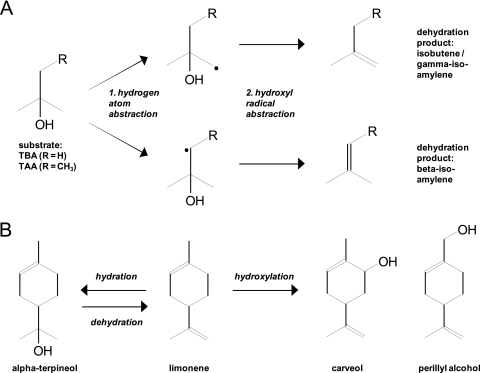
Although the emitted alkenes isobutene and isoamylene are structurally related to the corresponding tertiary alcohols TBA and TAA and can readily be produced from the latter by dehydration at elevated temperatures in the presence of an acid catalyst (6, 25), their formation under nearly neutral conditions at 30°C is surprising. In addition, acid-catalyzed dehydration of TAA would not lead to the observed isoform speciation with only 3% gamma-isoamylene, but about 20% of this alkene would be expected (10). Consequently, abiotic conversion can be excluded and an enzymatic dehydration reaction postulated.
There is general agreement that in the aerobic degradation of TBA and TAA, enzymatic monohydroxylation results in the formation of the corresponding diol compounds. On the basis of expression analysis, it was previously concluded that in A. tertiaricarbonis L108 and also M. petroleiphilum PM1, this step is catalyzed by the Rieske nonheme mononuclear iron oxygenase MdpJ and its reductase, MdpK (20, 45). In the present study, we could also detect the mdpJK genes in Methylibium sp. strain R8 and in the two MTBE-degrading enrichment cultures C and P, supporting their importance in fuel oxygenate metabolism. Moreover, 13C metagenomic (3) and metaproteomic (4) stable isotope probing (SIP) approaches have recently proven the relevance of MdpJ for TBA and MTBE degradation in mixed cultures. One of the most prominent members of the Rieske nonheme mononuclear iron oxygenase family is the naphthalene dioxygenase of Pseudomonas sp. strain NCIB 9816-4 (12). This enzyme not only is known to mono- and dihydroxylate aromatic compounds but also catalyzes hydrogen elimination reactions (29) similar to those catalyzed by di-iron center-containing fatty acid desaturases (47). Both monohydroxylation and elimination reactions actually follow similar mechanisms. Initial hydrogen abstraction from the substrate produces a radical intermediate, and then either a hydroxyl radical from the activated iron center is added (hydroxylation) or a second hydrogen abstraction occurs (elimination). The specificity of catalysis depends mainly on substrate structure and its mobility at the catalytic site of the enzyme (5, 53). Accordingly, formation of isobutene and isoamylene could be explained as a side reaction putatively catalyzed by the tertiary alcohol monooxygenase (Fig. 4 A). Hydrogen abstraction from TBA and TAA leads to tertiary alcohol radicals. Next, either a hydroxyl radical can be added, leading to the diol structure, or the tertiary alcohol group can be eliminated, resulting in alkene formation. Although this water removal from an aliphatic alcohol would be an unusual elimination reaction to be catalyzed by an oxygenase-like enzyme, our further findings also support this hypothesis. Both tertiary alcohol degradation and alkene formation by strain L108 show clear oxygen dependence. In line with the necessity of an oxygen-activated metal center for hydrogen abstraction (Fig. 4A), the reaction rates decrease with lowered oxygen concentrations. In addition, the relative share of alkene production in alcohol conversion is larger at low oxygen concentrations. This could be due to the fact that the water elimination reaction does not consume oxygen and thus might be favored when oxygen is limiting. Comparable oxygen-dependent dehydration catalysis of alcohols has been reported for limonene hydroxylases from Geobacillus stearothermophilus BR388 (9, 44) and Enterobacter cowanii 6L (54) that were not characterized further. The enzyme from strain BR388, for example, reversibly converts limonene to the tertiary alcohol alpha-terpineol, besides hydroxylating it to carveol and perillyl alcohol (Fig. 4B). However, the enzymatic step catalyzing the observed alkene emission from fuel oxygenates cannot be identified clearly, as the phenomenon has been studied only in whole-cell systems, and interaction with other enzymatic steps as well as the effects of the redox state or other physiological parameters on tertiary alcohol conversion might be relevant. The finding that strain PM1 shows lower alkene production than strains L108 and R8 might already indicate that besides MdpJ activity, other processes could be relevant. Further studies on cell-free systems and knockout mutants currently in progress in our lab will help to elucidate the underlying mechanism of bacterial alkene production from tertiary alcohols.
Fig. 4.
Hydration/dehydration and hydroxylation of tertiary alcohol compounds catalyzed by oxygenase-like enzymes. (A) Proposed mechanism for the formation of isobutene from TBA and of beta- and gamma-isoamylene from TAA by an oxygen-dependent oxygenase-like enzyme from A. tertiaricarbonis L108. (B) Formation of terpineol, carveol, and perillyl alcohol from limonene by limonene hydroxylase from Geobacillus stearothermophilus BR388 (9, 44).
The discovery of dehydration reactions in bacterial fuel oxygenate degradation could be used for evaluating natural attenuation at contaminated sites. Recently, compound-specific stable isotope analysis (CSIA) for identification and quantification of in situ biodegradation of MTBE and related compounds has been proposed (27, 40, 50, 56). However, this method requires special analytical equipment. In addition, interpretation of H/D and 12C/13C fractionation patterns is far from trivial. Although differentiation between anaerobic and aerobic biodegradation has been demonstrated in several studies applying two-dimensional CSIA, isotope discrimination in aerobic bacterial degradation shows significant variation and can also lead to very low fractionation values, complicating detection in the field (22, 40). On the other hand, the measurement of isobutene emissions resulting from MTBE and ETBE biodegradation could be an alternative, or at least CSIA-supporting, procedure for identifying aerobic biodegradation. Particularly in the case of low isotope fractionation (22, 40), a combination of CSIA and alkene measurement might result in more reliable predictions of biodegradation activities. Although alkene formation from TAME and TAA was more pronounced, it could be expected that in contaminated aquifers typically possessing only low oxygen contents, isobutene production from MTBE, ETBE, and TBA would reach a few percentage points of oxygenate turnover. Since alkenes are highly volatile, they might be easily detectable in gas samples from contaminated sites showing ongoing biodegradation. Natural alkene sources are mainly emissions from hydrocarbon reservoirs and hydrothermal sites (11) as well as from burning and pyrolysis of organic materials (21, 55). In addition, traces of isobutene could be produced from side reactions occurring in the metabolism of isovaleric acid and 3,3-HMB (14, 15). Most important, of course, isobutene and isoamylene are also present in oil derivatives such as gasoline (24). Consequently, other alkene-emitting processes have to be considered in analyzing gas samples from gasoline-contaminated aquifers. At least at older sites where plumes are already depleted of volatile and easily biodegradable hydrocarbons, monitoring isobutene emissions might be useful for evaluating MTBE and ETBE biodegradation, even though alkene biodegradation might occur. Hydroxylation of isobutene results in the corresponding epoxide or unsaturated alcohol (17, 18, 37). Consequently, these compounds may also indicate fuel oxygenate biodegradation. In this context, CSIA could also be employed for elucidating the origin of the alkenes and their metabolites, as discrimination of heavy isotopes could be expected in the enzymatic processes responsible for alkene formation and degradation.
Thus far, alkene formation from oxygenates has been demonstrated only for A. tertiaricarbonis L108, M. petroleiphilum PM1, and Methylibium sp. strain R8. If this dehydration is really a side reaction of the putative tertiary alcohol monooxygenase MdpJ, other PM1-like strains should also be capable of emitting isobutene and isoamylenes from TBA and TAA, respectively. However, it is not clear whether other types of monooxygenases involved in tert-alkyl alcohol degradation, such as the AlkB-like enzymes found in MTBE- and TBA-degrading Mycobacterium austroafricanum strains (32), also catalyze water elimination reactions besides hydroxylations.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the UFZ within the CITE program.
We thank C. Schumann (UFZ) for technical assistance, B. Würz (UFZ) for excellent analytical advice, and F. Löper (UFZ) for sampling the aerated treatment pond systems at the Leuna test site.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 8 July 2011.
REFERENCES
- 1. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amberg A., Rosner E., Dekant W. 2000. Biotransformation and kinetics of excretion of tert-amyl-methyl ether in humans and rats after inhalation exposure. Toxicol. Sci. 55:274–283 [DOI] [PubMed] [Google Scholar]
- 3. Aslett D., Haas J., Hyman M. 2011. Identification of tertiary butyl alcohol (TBA)-utilizing organisms in BioGAC reactors using 13C-DNA stable isotope probing. Biodegradation doi: 10.1007/s10532-011-9455-3. [DOI] [PubMed] [Google Scholar]
- 4. Bastida F., et al. 2010. Elucidating MTBE degradation in a mixed consortium using a multidisciplinary approach. FEMS Microbiol. Ecol. 73:370–384 [DOI] [PubMed] [Google Scholar]
- 5. Behrouzian B., Buist P. H. 2002. Fatty acid desaturation: variations on an oxidative theme. Curr. Opin. Chem. Biol. 6:577–582 [DOI] [PubMed] [Google Scholar]
- 6. Boyd R. H., Taft R. W., Wolf A. P., Christman D. R. 1960. Studies on the mechanism of olefin-alcohol interconversion. The effect of acidity on the O18 exchange and dehydration rates of t-alcohols. J. Am. Chem. Soc. 82:4729–4736 [Google Scholar]
- 7. Bruns M. A., Hanson J. R., Mefford J., Scow K. M. 2001. Isolate PM1 populations are dominant and novel methyl tert-butyl ether-degrading bacterial in compost biofilter enrichments. Environ. Microbiol. 3:220–225 [DOI] [PubMed] [Google Scholar]
- 8. Chauvaux S., et al. 2001. Cloning of a genetically unstable cytochrome P-450 gene cluster involved in degradation of the pollutant ethyl tert-butyl ether by Rhodococcus ruber. J. Bacteriol. 183:6551–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheong T. K., Oriel P. 2000. Cloning and expression of the limonene hydroxylase of Bacillus stearothermophilus BR388 and utilization in two-phase limonene conversions. Appl. Biochem. Biotechnol. 84-86:903–915 [DOI] [PubMed] [Google Scholar]
- 10. Church J. M., Whitmore F. C., McGrew R. V. 1934. The ozonolysis of purely aliphatic olefins. The behavior of the five simplest normal alkyl radicals in the dehydration of tertiary alcohols. J. Am. Chem. Soc. 56:176–184 [Google Scholar]
- 11. Darling W. G. 1998. Hydrothermal hydrocarbon gases. 1. Genesis and geothermometry. Appl. Geochem. 13:815–824 [Google Scholar]
- 12. Ferraro D. J., Gakhar L., Ramaswamy S. 2005. Rieske business: structure-function of Rieske non-heme oxygenases. Biochem. Biophys. Res. Commun. 338:175–190 [DOI] [PubMed] [Google Scholar]
- 13. Fraile J., et al. 2002. Monitoring of the gasoline oxygenate MTBE and BTEX compounds in groundwater in Catalonia (Northeast Spain). Sci. World J. 2:1235–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fukuda H., et al. 1994. Reconstitution of the isobutene-forming reaction catalyzed by cytochrome P450 and P450 reductase from Rhodotorula minuta: decarboxylation with the formation of isobutene. Biochem. Biophys. Res. Commun. 201:516–522 [DOI] [PubMed] [Google Scholar]
- 15. Gogerty D. S., Bobik T. A. 2010. Isobutene formation from 3-hydroxy-3-methylbutyrate by diphosphomevalonate decarboxylase. Appl. Environ. Microbiol. 76:8004–8010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golart K. L. 2007. Physiology and enzymology of aerobic MTBE and TBA biodegradation. Ph.D. thesis. North Carolina State University, Raleigh, NC [Google Scholar]
- 17. Hicks K. A. 2002. Alternative substrates for estimating TCE-degrading capabilities of toluene-oxidizing bacteria. M.S. thesis. North Carolina State University, Raleigh, NC [Google Scholar]
- 18. Hou C. T., Patel R., Laskin A. I., Barnabe N., Barist I. 1983. Epoxidation of short-chain alkenes by resting-cell suspensions of propane-grown bacteria. Appl. Environ. Microbiol. 46:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hristova K., Gebreyesus B., Mackay D., Scow K. M. 2003. Naturally occurring bacteria similar to the methyl tert-butyl ether (MTBE)-degrading strain PM1 are present in MTBE-contaminated groundwater. Appl. Environ. Microbiol. 69:2616–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hristova K. R., et al. 2007. Comparative transcriptome analysis of Methylibium petroleiphilum PM1 exposed to the fuel oxygenates methyl tert-butyl ether and ethanol. Appl. Environ. Microbiol. 73:7347–7357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikan R., et al. 1988. Light hydrocarbons and volatile compounds produced during the thermal treatment of melanoidins and humic substances. Org. Geochem. 12:273–279 [Google Scholar]
- 22. Jechalke S., et al. 2011. Linking low-level stable isotope fractionation to expression of the cytochrome P450 monooxygenase-encoding ethB gene for elucidation of methyl tert-butyl ether biodegradation in aerated treatment pond systems. Appl. Environ. Microbiol. 77:1086–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kane S. R., et al. 2001. Aerobic biodegradation of methyl tert-butyl ether by aquifer bacteria from leaking underground storage tank sites. Appl. Environ. Microbiol. 67:5824–5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaplan I. R., Galperin Y., Lu S.-T., Lee R.-P. 1997. Forensic environmental geochemistry: differentiation of fuel-types, their sources and release time. Org. Geochem. 27:289–317 [Google Scholar]
- 25. Knifton J. F., Sanderson J. R., Stockton M. E. 2001. Tert-butanol dehydration to isobutylene via reactive distillation. Catal. Lett. 73:55–57 [Google Scholar]
- 26. Kolb A., Püttmann W. 2006. Comparison of MTBE concentrations in groundwater of urban and nonurban areas in Germany. Water Res. 40:3551–3558 [DOI] [PubMed] [Google Scholar]
- 27. Kuder T., Philp P. 2008. Modern geochemical and molecular tools for monitoring in-situ biodegradation of MTBE and TBA. Rev. Environ. Sci. Biotechnol. 7:79–91 [Google Scholar]
- 28. Lechner U., et al. 2007. Aquincola tertiaricarbonis gen. nov., sp. nov., a tertiary butyl moiety-degrading bacterium. Int. J. Syst. Evol. Microbiol. 57:1295–1303 [DOI] [PubMed] [Google Scholar]
- 29. Lee K., Gibson D. T. 1996. Toluene and ethylbenzene oxidation by purified naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4. Appl. Environ. Microbiol. 62:3101–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lopes Ferreira N., Malandain C., Fayolle-Guichard F. 2006. Enzymes and genes involved in the aerobic biodegradation of methyl tert-butyl ether (MTBE). Appl. Microbiol. Biotechnol. 72:252–262 [DOI] [PubMed] [Google Scholar]
- 31. Lopes Ferreira N., Labbé D., Monot F., Fayolle-Guichard F., Greer C. W. 2006. Genes involved in the methyl tert-butyl ether (MTBE) metabolic pathway of Mycobacterium austroafricanum IFP 2012. Microbiology 152:1361–1374 [DOI] [PubMed] [Google Scholar]
- 32. Lopes Ferreira N., et al. 2007. n-Alkane assimilation and tert-butyl alcohol (TBA) oxidation capacity in Mycobacterium austroafricanum strains. Appl. Microbiol. Biotechnol. 75:909–919 [DOI] [PubMed] [Google Scholar]
- 33. Moran M. J., Zogorski J. S., Squillace P. J. 2005. MTBE and gasoline hydrocarbons in ground water of the United States. Ground Water 43:615–627 [DOI] [PubMed] [Google Scholar]
- 34. Müller R. H., Rohwerder T., Harms H. 2007. Carbon conversion efficiency and limits of productive bacterial degradation of methyl tert-butyl ether and related compounds. Appl. Environ. Microbiol. 73:1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Müller R. H., Rohwerder T., Harms H. 2008. Degradation of fuel oxygenates and their main intermediates by Aquincola tertiaricarbonis L108. Microbiology 154:1414–1421 [DOI] [PubMed] [Google Scholar]
- 36. Nakatsu C. H., et al. 2006. Methylibium petroleiphilum PM1T gen. nov., sp. nov., a new methyl tert-butyl ether (MTBE) degrading methylotroph of the beta-Proteobacteria. Int. J. Syst. Evol. Microbiol. 56:983–989 [DOI] [PubMed] [Google Scholar]
- 37. Owens C. R., Karceski J. K., Mattes T. E. 2009. Gaseous alkene biotransformation and enantioselective epoxyalkane formation by Nocardioides sp. strain JS614. Appl. Microbiol. Biotechnol. 84:685–692 [DOI] [PubMed] [Google Scholar]
- 38. Piveteau P., Fayolle F., Vandecasteele J. P., Monot F. 2001. Biodegradation of tert-butyl alcohol and related xenobiotics by a methylotrophic bacterial isolate. Appl. Microbiol. Biotechnol. 55:369–373 [DOI] [PubMed] [Google Scholar]
- 39. Rohwerder T., Breuer U., Benndorf D., Lechner U., Müller R. H. 2006. The alkyl tert-butyl ether intermediate 2-hydroxyisobutyrate is degraded via a novel cobalamin-dependent mutase pathway. Appl. Environ. Microbiol. 72:4128–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosell M., et al. 2007. Variation in 13C/12C and D/H enrichment factors of aerobic bacterial fuel oxygenate degradation. Environ. Sci. Technol. 41:2036–2043 [DOI] [PubMed] [Google Scholar]
- 41. Rosell M., et al. 2010. Evaluation of the effects of low oxygen concentration on stable isotope fractionation during aerobic MTBE biodegradation. Environ. Sci. Technol. 44:309–315 [DOI] [PubMed] [Google Scholar]
- 42. Salanitro J. P. 1995. Understanding the limitations of microbial metabolism of ethers used as fuel octane enhancers. Curr. Opin. Biotechnol. 6:337–340 [Google Scholar]
- 43. Salanitro J. P., Diaz L. A., Williams M. P., Wisniewski H. L. 1995. Isolation of a bacterial culture that degrades methyl t-butyl ether. Appl. Environ. Microbiol. 60:2593–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Savithiry N., Cheong T. K., Oriel P. 1997. Production of α-terpineol from Escherichia coli cells expressing thermostable limonene hydratase. Appl. Biochem. Biotechnol. 63-65:213–220 [DOI] [PubMed] [Google Scholar]
- 45. Schäfer F., et al. 2007. Growth of Aquincola tertiaricarbonis L108 on tert-butyl alcohol leads to the induction of a phthalate dioxygenase-related protein and its associated oxidoreductase subunit. Eng. Life Sci. 7:512–519 [Google Scholar]
- 46. Schmidt R., Battaglia V., Scow K., Kane S., Hristova K. R. 2008. Involvement of a novel enzyme, MdpA, in methyl tert-butyl ether degradation in Methylibium petroleiphilum PM1. Appl. Environ. Microbiol. 74:6631–6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shanklin J., Guy J. E., Mishra G., Lindqvist Y. 2009. Desaturases: emerging models for understanding functional diversification of diiron-containing enzymes. J. Biol. Chem. 284:18559–18563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Steffan R. J., McClay K., Vainberg S., Condee C. W., Zhang D. 1997. Biodegradation of the gasoline oxygenates methyl tert-butyl ether, ethyl tert-butyl ether, and tert-amyl methyl ether by propane-oxidizing bacteria. Appl. Environ. Microbiol. 63:4216–4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sumner S. C. J., et al. 2003. Characterization of metabolites and disposition of tertiary amyl methyl ether in male F344 rats following inhalation exposure. J. Appl. Toxicol. 23:411–417 [DOI] [PubMed] [Google Scholar]
- 50. van Breukelen B. M. 2007. Extending the Rayleigh equation to allow competing isotope fractionating pathways to improve quantification of biodegradation. Environ. Sci. Technol. 41:4004–4010 [DOI] [PubMed] [Google Scholar]
- 51. van Wezel A., Puijker L., Vink C., Versteegh A., de Voogt P. 2009. Odour and flavour thresholds of gasoline additives (MTBE, ETBE and TAME) and their occurrence in Dutch drinking water collection areas. Chemosphere 76:672–676 [DOI] [PubMed] [Google Scholar]
- 52. Weaver J. W., Exum L. R., Prieto L. M. 2010. Gasoline composition regulations affecting LUST sites. EPA 600/R-10/001. Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 53. Whittle E. J., Tremblay A. E., Buist P. H., Shanklin J. 2008. Revealing the catalytic potential of an acyl-ACP desaturase: tandem selective oxidation of saturated fatty acids. Proc. Natl. Acad. Sci. U. S. A. 105:14738–14743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang E. J., Park Y. J., Chang H. C. 2007. Cloning of four genes involved in limonene hydroxylation from Enterobacter cowanii 6L. J. Microbiol. Biotechnol. 17:1169–1176 [PubMed] [Google Scholar]
- 55. Yokelson R., et al. 2007. Emissions from forest fires near Mexico City. Atmos. Chem. Phys. Discuss. 7:6687–6718 [Google Scholar]
- 56. Zwank L., et al. 2005. New evaluation scheme for two-dimensional isotope analysis to decipher biodegradation processes: application to groundwater contamination by MTBE. Environ. Sci. Technol. 39:1018–1029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.