Abstract
The mercury-sensing regulatory protein, MerR (Tn21), which regulates mercury resistance operons in Gram-negative bacteria, was subjected to directed evolution in an effort to generate a MerR mutant that responds to Cd but not Hg. Oligonucleotide-directed mutagenesis was used to introduce random mutations into the key metal-binding regions of MerR. The effects of these mutations were assessed using a vector in which MerR controlled the expression of green fluorescent protein (GFP) and luciferase via the mer operator/promoter. An Escherichia coli cell library was screened by fluorescence-activated cell sorting, using a fluorescence-based dual screening strategy that selected for MerR mutants that showed GFP repression when cells were induced with Hg but GFP activation in the presence of Cd. Two Cd-responsive MerR mutants with decreased responses toward Hg were identified through the first mutagenesis/selection round. These mutants were used for a second mutagenesis/selection round, which yielded eight Cd-specific mutants that had no significant response to Hg, Zn, or the other tested metal(loid)s. Seven of the eight Cd-specific MerR mutants showed repressor activities equal to that of wild-type (wt) MerR. These Cd-specific mutants harbored multiple mutations (12 to 22) in MerR, indicating that the alteration of metal specificity with maintenance of repressor function was due to the combined effect of many mutations rather than just a few amino acid changes. The amino acid changes were studied by alignment against the sequences of MerR and other metal-responsive MerR family proteins. The analysis indicated that the generated Cd-specific MerR mutants appear to be unique among the MerR family members characterized to date.
INTRODUCTION
It is fundamentally important that we understand the interactions of metal ions in biological systems, as estimates indicate that 40% of all known proteins contain metal ions (25). The relationship between the structure of a protein and its binding specificity toward different metal ions has been the subject of active research but is still not well understood (10, 25). In bacteria, the metalloregulatory proteins play crucial roles in discriminating among metal ions, controlling the cellular levels of essential metals, and exporting/detoxifying metal(loid)s (17). The members of the metal-responsive MerR protein family are transcriptional activators that can be grouped on the basis of their metal-sensing preferences for Hg (MerR), Cu (CueR), Cd (CadR), Pb (PbrR), Zn (ZntR), and Au (GolS) (6, 21, 22). The archetypal and best-studied MerR family member is the mercuric ion-sensing protein, MerR, which was initially identified to be a regulator of the mercury resistance operons found in transposons Tn501 and Tn21 of the Gram-negative bacteria Pseudomonas aeruginosa and Shigella flexneri, respectively (2, 5, 26, 37). MerR functions as a homodimer and binds to the dyad operator (MerOP), the gene for which is located between −10 and −35 promoter elements of the mercury resistance operon (20). In the absence of Hg, MerR prevents transcription initiation by bending DNA (1) and forming an inactive complex with RNA polymerase (15, 20). In vitro, MerR binds one Hg ion per dimer; this provokes an allosteric change in the MerR protein that triggers unwinding of the spacer DNA, allowing transcription initiation of the mer operon (27). Cd and Zn can also activate the mer operon, but only when they are present at 100- and 1,000-fold higher concentrations, respectively, than the effective concentration of Hg (31). The three-dimensional (3D) structure of MerR has not yet been solved, but the crystal structures of ZntR and CueR (8), together with genetic, biochemical, and structural studies of MerR, have suggested that the MerR protein comprises an N-terminal helix-turn-helix DNA-binding domain (residues Ile10 to Arg29), a C-terminal Hg-binding domain (residues Cys82 to Cys126) containing a long helical region (Cys82 to Cys117), and the so-called coupling domain (Lys30-His81), which lies between the first two domains and is proposed to mediate inductive signaling from the metal-binding domain to the DNA-binding domain (33, 35, 38). Residues 80 through 128 have been shown to be necessary and sufficient for stable dimer formation and high-affinity Hg binding (43). The MerR homodimer binds Hg via three cysteine thiols that are conserved among all MerR proteins (19, 33, 40, 42).
In the present study, we constructed mutant libraries where metal-binding regions of MerR were randomized and screened the libraries for those mutants that were activated by Cd but not Hg. In the screening vector, a reporter gene encoding GFPmut2 (12) was placed under the control of MerOP, and either Hg or Cd was used to induce the mutant MerR library. On the basis of high or low green fluorescence intensity, rounds of fluorescence-activated cell sorting (FACS) were performed to enrich Cd-specific MerR mutants. As a result, we report on MerR mutants with a strict specificity for a single metal, Cd, showing that it is possible to remove the activity of MerR for its preferred metal while maintaining the protein's wild-type (wt) level repressor/activator functions.
MATERIALS AND METHODS
Construction of mutant merR library.
Escherichia coli XL1-Blue was used as the host for DNA cloning and library construction. The screening vector pKH100 contained gfpmut2 (12) and the firefly luciferase gene (luc) (13) under the control of MerOP. Details of the bacterial strain and construction of the screening vector are presented in the supplemental material.
Site-directed mutagenesis of mer was done using two different mutagenizing oligonucleotides (Fig. 1; see Table S1 in the supplemental material). In the first method, certain bases of oligonucleotides were doped with other bases, with the proportions designed to yield 79% of the original bases and 7% of each of the other bases. In the second method, selected positions of oligonucleotides were randomized with equal representation of the desired bases. Some of these mutagenized sites of oligonucleotides could potentially encode any amino acid, while some positions were limited to certain amino acids (Fig. 1; see Table S2 in the supplemental material). The utilized oligonucleotides were commercially synthesized by TAG Copenhagen or Oligomer. Details of the molecular cloning of the merR libraries are presented in supplemental material.
Fig. 1.
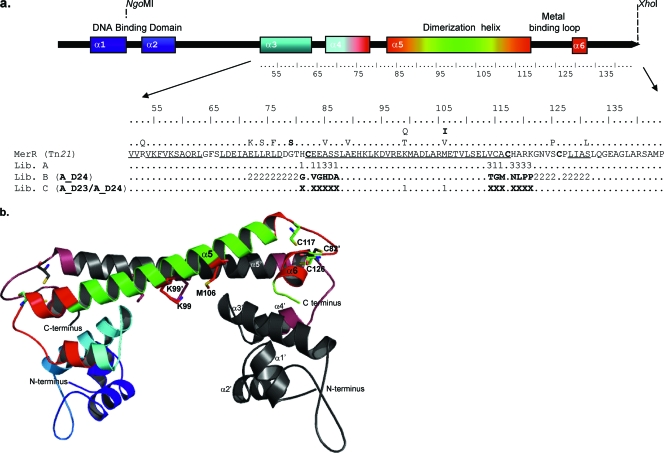
Amino acids targeted in each MerR mutant library and their positions in the model of MerR. (a) The schematic shows all six α helices of MerR (colored as described for panel b) and the positions of the restriction enzymes used during library construction. Helices α3, α4, α5, and α6 are underlined in the presented MerR sequence. The locations of the Cd-responsive single mutations and a double mutation (G79S and M106I [in boldface]) described by Caguiat et al. (7) are shown above the MerR sequence. The numbers represent the different mutational strategies used in library construction: 1, randomized amino acids; 2, doped amino acids; and 3, amino acids with limited changes. The parental amino acid sequences are shown as Xs. (b) A model of MerR generated using the crystal structure of CueR (8) as a template. The ribbon diagram shows one protomer in color and the other in gray. The residues targeted in our mutagenesis strategy are shown in red on the colored protomer and in raspberry on the gray protomer. Residues C82, K99, M106, C117, and C126 are presented in stick format in both protomers.
Four parallel electroporations were performed to transform each merR library into XL1-Blue competent cells. After 1 h recovery in SOC medium (34), transformed cell cultures were paired and pooled. Small samples were plated on Luria agar (LA) plates supplemented with 100 μg ml−1 ampicillin (Amp) and 12.5 μg ml−1 tetracycline (Tet), and colonies were counted to determine the number of plasmid-carrying cells and the corresponding library diversity. The remaining bacteria were centrifuged at 600 × g for 15 min at 4°C and washed twice with HMM medium (23) supplemented with 0.5% (wt/vol) hydrolyzed casein, 1 μg ml−1 thiamine, 200 μg ml−1 Amp, and 12.5 μg ml−1 Tet. The washed cells were diluted to an optical density at 600 nm (OD600) of 0.5 with the same HMM medium and cultivated to an OD600 of 0.6 to 0.7. During cultivation, ampicillin was added hourly to maintain plasmid selection.
Induction of merR library and flow cytometric cell sorting.
Induction of merR-dependent gfpmut2 expression was performed in 1 ml containing 100 μl of 1 × 10−5 or 5 × 10−5 M HgCl2 (>99.5%; Riedel-de Haën) or 1 × 10−5 or 1 × 10−4 M CdCl2 (99%; Riedel-de Haën) and 900 μl of freshly cultivated transformant cells (OD600, 0.6 to 0.7). The concentrations of metals in the various experiments are shown in Table 1. After 2 h of metal induction, the cells were pelleted and washed twice with an equal volume of ice-cold phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4). Washed bacteria were stored overnight at 4°C, and each merR library was suspended with 1 ml of PBS.
Table 1.
Properties of the MerR libraries and parameters used in flow cytometric cell sorting
| MerR library | Diversitya | Backgroundb | Low-fluorescence screening (sort 1) |
High-fluorescence screening (sort 2) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [HgCl2]c (M) | Anal. cellsd | % cells chosene | Cell handlingf | [CdCl2] (M) | Anal. cellsg | % cells chosen | Cell handlingf | |||
| A | 1.0 × 106 | 6.0 × 104 | 1 × 10−5 | 2.2 × 107 | 8.3 | Cult. | 1 × 10−5 | 1.0 × 106 | 0.1 | Plate |
| B | 8.4 × 105 | 8.0 × 103 | 5 × 10−5 | 8.8 × 106 | 5.9 | Cult. | 1 × 10−4 | 2.0 × 106 | 0.5 | Plate |
| C | 4.0 × 106 | 7.5 × 104 | 5 × 10−5 | 4.3 × 106 | 6.0 | Cult. | 1 × 10−4 | 1.1 × 106 | 0.6 | Plate |
Library diversity was defined as the number of transformed cells obtained after electroporation.
Number of colonies on the ligation control plate (ligation reaction only with insert-free vector).
Metal concentration used for induction of the merR library.
Anal. cells, number of cells analyzed from the initial library.
Percentage of the sorted cells collected with the flow cytometer.
Method used to handle the cells after flow cytometric cell sorting: Cult., sorted cells were directly cultivated in liquid medium for the next sorting step; Plate, sorted cells were collected on filter papers that were then placed on LA plates.
Number of cells analyzed after the first round of sorting.
Flow cytometric sorting was performed with an argon laser (488 nm) in either a FACSVantage SE or a FACSCalibur (BDBiosciences) FACS apparatus on the basis of the technique described by Valdivia and Falkow (41) and Tang et al. (39). Bacteria were detected by forward and side scatter and green fluorescence using a 530/30-nm band-pass filter. The GFPmut2 used in this study has an excitation maximum at 481 nm and an emission maximum at 507 nm (12). Side scatter, forward scatter, and fluorescence data were collected with logarithmic amplification. The sorting parameters were set to discriminate on the basis of fluorescence intensity. The sorting mechanism of the FACSCalibur apparatus differed from that of the FACSVantage SE apparatus, in that the FACSCalibur system used a mechanical catcher tube to sort cells, while the FACSVantage system was a jet-in-air-type cell sorter. Approximately 5,000 events/s were analyzed with the FACSVantage SE apparatus. To obtain a 99% purity of sorted cells, the analysis rate of the FACSCalibur apparatus was set to not exceed 300 cells/s, as recommended (14); however, the actual sorting rates depended on the properties of the library and sorting gate used in each experiment.
The first sorting round was done with the higher-throughput FACSVantage flow cytometer, since the aim was to interrogate 10 times more cells than the number of initial transformed colonies (i.e., to sample each transformant clone ∼10 times) (Table 1). Subsequent sorting rounds involved the lower-throughput FACSCalibur apparatus, since the first sort considerably reduced the library size. After the FACSVantage sort, cells were plated on LA supplemented with 100 μg ml−1 Amp and 12.5 μg ml−1 Tet. After being sorted with the FACSCalibur apparatus, the sorted cells were in 300 to 500 ml of sheath fluid (sterile PBS). The cells were recovered using 45-μm-pore-size membrane filters in plastic filter holders (Schleicher & Schuell MicroScience GmbH), each filter was placed on an LA plate supplemented with 100 μg ml−1 Amp and 12.5 μg ml−1 Tet, and the cells were grown overnight on filter membranes. After each sorting experiment, approximately 30 to 52 colonies were further tested as described below. Low viability of the sorted cells was a problem throughout the study; the reasons for this are discussed in the supplemental material.
Preliminary testing of MerR mutants in vivo.
Isolated MerR mutants were grown on LA plates supplemented with 100 μg ml−1 Amp and 12.5 μg ml−1 Tet, and single colonies derived from sorted cells were separately inoculated to 2 ml of HMM medium. After cells were cultivated for 24 h at 37°C with shaking at 250 rpm, luminescence was measured using a microplate luminometer (Labsystems Luminoskan; Thermo Electron Corporation). Nonluminescent cultures were discarded. Cell density was observed by visual inspection (OD600, higher than approximately 0.3), and 10 ml of HMM medium was added to cultures that were determined to have a high cell density. Precultures with low cell densities were used directly. On white 96-well microtiter plates (Thermo Electron Corporation), 50 μl of standard metal solution (3 × 10−8, 1 × 10−7, and 3 × 10−7 M for HgCl2; 1 × 10−7, 3 × 10−7, 1 × 10−6, and 3 × 10−6 M for CdCl2) or water was mixed with 50 μl of bacterial suspension per well. The plates were incubated at 37°C for 2 h, and then 100 μl of 0.5 mM d-luciferin (in 0.1 M sodium citrate buffer, pH 5.0) was added to each reaction mixture (18). The samples were incubated for 5 min at room temperature and then analyzed with a Luminoskan plate luminometer (Thermo Electron Corporation). Mutants showing altered responses to the inducing metals were sequenced.
Characterization of MerR mutants in vivo.
For further in vivo testing of the metal specificities of the various MerR mutants, MerR mutants were grown overnight in HMM medium, diluted to an OD600 of 0.02 with HMM medium, and cultivated until the OD600 reached 0.10. For luminescence measurement, the culture was then diluted to an OD600 of 0.05, and measurements were performed as described above using HgCl2 and CdCl2 concentrations ranging from 6 × 10−10 to 2 × 10−5 M. MerR mutants were also tested with the following metals: Pb(CH3COO)2 (>99.5%), CuSO4·5H2O (99%), and AgNO3 (99%) (from J. T. Baker); ZnCl2 (>98%) and C4H4KO7Sb·0.5 H2O (99 to 103%) (from Fluka Chemica); NaAsO2 (92%), Na2HAsO4·7H2O (100.3%), AlCl3 (99%), and K2Cr2O7 (99%) (from Sigma-Aldrich); and CoCl2 (analytical grade; Riedel de Haën).
The metal-dependent luminescence responses of the MerR mutants were fit by nonlinear regression to a sigmoidal dose-response curve (variable slope) using the Prism software package (GraphPad Software). The quality of fit was expressed as an r-squared (R2) value. The effective concentration of the metal that provoked the half-maximum response (the 50% effective concentration [EC50]), the activation efficiency (NLmax; which described the maximum luminescence response with respect to normalized luminescence, i.e., the maximum luminescence signal divided by the minimum luminescence signal), and the Hill coefficient (which described the steepness of the curve and indicated the sensitivity of the response [31]) were determined. The background luminescence value, which was obtained from uninduced cells and reflected the repressor activity of the MerR mutant, was expressed as relative light units (RLUs). The limit of determination (LOD) was calculated using the equation, (XB + 3SDB)/XB, where XB is the mean value of background luminescence and SDB is the standard deviation.
Construction of homology model of MerR and sequence alignments of mutants against MerR family members.
A 3D model of MerR was generated using the crystal structure of CueR (Protein Data Bank entry 1QO5 [8]) as a template. Details of the homology modeling of MerR (Tn21) are described in the supplemental material.
Metalloregulators of the MerR family proteins were classified on the basis of their metal preferences (8). Various numbers of sequences were selected from different species and used in multiple-sequence alignments (136 for MerR [Hg2+], 92 for CadR and PbrR [Cd2+/Pb2+], 64 for ZntR [Zn2+], and 72 for CueR, HmrR, PmtR, and GolS [Cu+, Ag+, and Au+]). The sequence alignments were done with Clustal W in BioEdit sequence analysis program (Tom Hall, Ibis Biosciences, Carlsbad, CA). See Fig. S4 to S7 in the supplemental material for the multiple-sequence alignments.
RESULTS AND DISCUSSION
First mutagenesis round, library A.
As a crystal structure is not yet available for MerR, we used homology modeling to predict the structure of MerR on the basis of that of CueR (8) and then used the predicted structure of MerR to design a mutagenesis strategy that avoided the introduction of amino acid changes that could reduce the functionality of the resulting mutant library (Fig. 1). Library A was constructed using randomized oligonucleotides in which six amino acids (His81, Glu83, Glu84, Ser87, Cys115, and Ala116) were fully randomized using an NNS scheme (where N is G, A, T, or C and S is G or S) around the metal-binding domain and near Cys82, Cys117, and Cys126, without actually mutating these essential metal-binding cysteines (19, 33, 35) (Fig. 1; see Table S2 in the supplemental material). According to our structural model, these positions were predicted to tolerate mutations without distorting the protein's tertiary structure. In addition, seven other amino acids specifically mutated in library A, Ala85, Ser86, Val114, His118, Ala119, Arg120, and Lys121, were potentially changed to the amino acids listed in Table S2 in the supplemental material. The DNA-binding domain was not targeted for mutagenesis in any of the constructed MerR mutant libraries, because two previous studies had indicated that the C-terminal metal-binding domain is essential for the metal selectivity of MerR family proteins (3, 9).
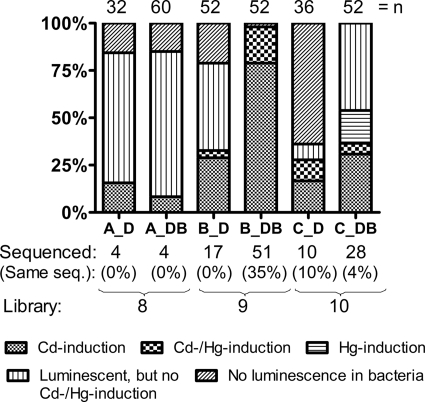
The mutated PCR products of mer were cloned into the screening vector to construct a system where each MerR mutant controlled the expression of GFPmut2 (12) via MerOP. In the first selection round, we induced library A with HgCl2 and selected the bacteria representing the lowest 8% of intensity of the green fluorescent signals (Table 1; see Fig. S1 in the supplemental material). This selection state was intended to exclude MerR mutants that were capable of responding to HgCl2. Furthermore, the self-circularizing vector can drive expression of the reporter genes (gfpmut2 and luc) even in the absence of MerR; however, this background expression is higher than that seen in the presence of MerR mutants that have maintained their repressor activities. Therefore, the first-round sorting of MerR mutants was also expected to exclude bacteria harboring only insert-free vectors. Sorted cells were plated, and the expression of the luciferase reporter gene (which was expressed after gfpmut2 was expressed in the screening vector) was used to screen for Cd-specific phenotypes among the sorted cells (Fig. 2; see Fig. S1 in the supplemental material). The cells could be divided into five different phenotypes, namely, MerR mutants having (i) a Cd-specific response, (ii) a Cd/Hg response, (iii) an Hg-specific response, (iv) continuous luminescence but no Cd or Hg response, and (v) no luminescence (Fig. 2). Four out of 32 selected MerR mutants showed specific responses toward Cd after the first round of the low-fluorescence sorting strategy (Fig. 2). Approximately 70% of the sorted cells showed constitutive luminescence, probably because they were not metal inducible and had repressor activity, which translates to constitutive fluorescence. Although the activator MerR mutants were excluded by the first round of negative sorting, the non-metal-inducible repressor mutants were not (expression data are not shown).
Fig. 2.
Properties of the cells sorted from libraries A, B, and C after the first and second rounds of FACS. Sorted cells were preliminarily tested with a luciferase assay. The letters given after the name of the library (A, B, and C) represent the sorting experiments, D represents the dimmest bacteria that were sorted from Hg-induced cells, and B represents the brightest bacteria that were sorted from Cd-induced cells. The data compare the percentages that each value contributed to the total. The total number of tested colonies is given as a number at the top of each column. The number of mutants sequenced is shown under the bar representing each sorting step. The percentage of mutants having identical sequences is shown in parentheses.
For the second sorting step, the sorted mutants after the first screening round were cultivated and induced with CdCl2, and then the brightest 0.1% of bacteria was enriched. The sorted MerR mutants theoretically either (i) had maintained their repressor function and could be transcriptionally activated only by CdCl2 or (ii) had maintained their repressor function and could be transcriptionally activated by CdCl2 and other metals, potentially including HgCl2, at concentrations greater than the concentration used in the first sorting round. Four out of 60 MerR mutants showed Cd-specific responses after completion of the preliminary testing protocol (Fig. 2).
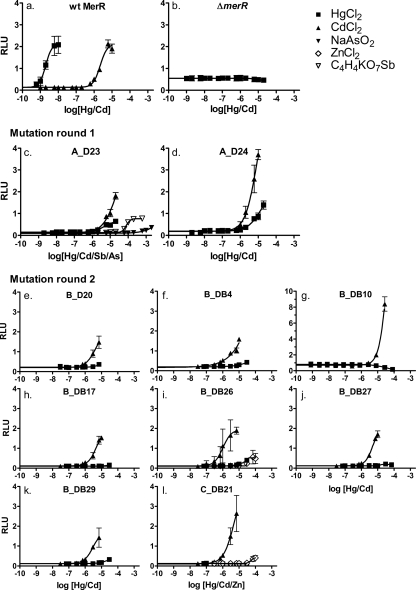
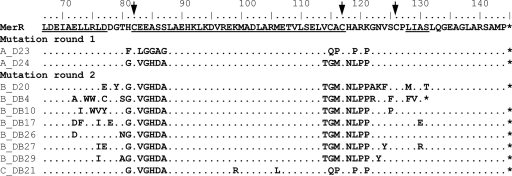
All of the MerR mutants that showed Cd-specific responses (i.e., a Cd-specific response or a Cd/Hg-specific response) in preliminary testing and had distinctive DNA sequences were then characterized for their responses to other metal(loid)s (Hg, Cd, Zn, Cu, Ag, Co, Cr, As, Sb, and Al), using subtoxic to toxic metal(loid) concentrations and a luciferase reporter gene assay. None of the tested mutants were completely Cd specific. Instead, they had lower-than-wt responses toward Hg. Only two mutants, A_D23 and A_D24, were more sensitive to Cd than Hg (Table 2 and Fig. 3). Mutants A_D23 and A_D24 had 1,600- and 3,600-fold less sensitive Hg responses, respectively, than the wt, whereas the Cd response remained unchanged in both mutants. MerR mutant A_D23 also responded weakly toward antimony and arsenic. In contrast, MerR mutant A_D24 responded only to Hg and Cd. In mutants A_D23 and A_D24, 10 and 13 amino acids, respectively, were changed in and near the metal-binding domain of MerR (Fig. 4).
Table 2.
Characteristics of wt MerR and the MerR mutants obtained from the first and second mutagenesis rounds
| MerR mutant or mutation round and MerR mutanta | Metal response | EC50 (M) | NLmax | Background luminescencec (RLU ± SDB) | Hill coefficient | R2 |
|---|---|---|---|---|---|---|
| wt merR | Hg | 2.0 × 10−9 | 18 | 0.12 ± 0.01 (1.3) | 2.5 | 0.930 |
| Cd | 2.2 × 10−6 | 18 | 2.2 | 0.869 | ||
| ΔmerR | Hg/Cd | NRd | NR | 0.53 ± 0.11 | NR | NR |
| Mutation round 1 | ||||||
| A_D23 | Hgb | 3.2 × 10−6 | 3.7 | 0.14 ± 0.07 (2.5) | 1.7e | 0.816 |
| Cdb | 4.9 × 10−6 | 7.8 | 1.2e | 0.892 | ||
| Sb | 7.9 × 10−5 | 5.4 | 3.7 | 0.941 | ||
| Asb | 1.1 × 10−3 | 2.5 | 1.0e | 0.948 | ||
| A_D24 | Hgb | 7.2 × 10−6 | 6.8 | 0.20 ± 0.13 (3.0) | 1.2e | 0.839 |
| Cd | 4.2 × 10−6 | 18 | 1.3e | 0.810 | ||
| Mutation round 2 | ||||||
| B_D20 | Hg | NDf | 1.8 | 0.20 ± 0.03 (1.5) | NR | NR |
| Cd | 2.7 × 10−6 | 7.4 | 1.3e | 0.912 | ||
| B_DB4 | Hg | ND | 2.4 | 0.18 ± 0.02 (1.3) | ND | ND |
| Cd | 3.8 × 10−6 | 9.0 | 0.6e | 0.942 | ||
| B_DB10 | Cd | 1.6 × 10−5 | 10 | 0.84 ± 0.20 (1.7) | 1.7e | 0.944 |
| B_DB17 | Cd | 3.7 × 10−6 | 15 | 0.10 ± 0.04 (2.2) | 1.6 | 0.955 |
| B_DB26 | Hg | 1.4 × 10−5 | 4.2 | 0.09 ± 0.02 (1.7) | ND | ND |
| Cd | 9.8 × 10−7 | 20 | 1.7 | 0.698 | ||
| Zn | 3.2 × 10−5 | 5.2 | 15 | 0.566 | ||
| B_DB27 | Hg | ND | 1.6 | 0.11 ± 0.01 (1.3) | ND | ND |
| Cd | 3.9 × 10−6 | 16 | 1.9 | 0.988 | ||
| B_DB29 | Hg | ND | 2.6 | 0.13 ± 0.06 (2.4) | ND | ND |
| Cd | 2.3 × 10−6 | 11 | 1.6 | 0.882 | ||
| C_DB21 | Cd | 2.7 × 10−6 | 25 | 0.11 ± 0.01 (1.3) | 1.3e | 0.857 |
| Zn | 5.0 × 10−5 | 3.8 | 4.1 | 0.734 |
In the mutant name, the initial character is the library name and the second and third characters after the underscore indicate the sorting criteria (D, dimmest; B, brightest) through one or two sorting rounds. For example, A_D23 is from library A and was obtained from the first round of collection of the dimmest bacteria.
Due to metal toxicity, luminescence decreased in the presence of concentrations above 1 × 10−5 M Hg and 1 × 10−4 M Cd, As, or Sb. The EC50 was calculated on the basis of the highest luminescence observed; we assumed that the curve fitted a nonlinear regression and followed a sigmoidal model.
The background luminescence value. Values are the mean (XB) from two to nine independent measurements ± the standard deviation (SDB). The LOD is shown in parentheses. NLmax values equal to or above the LOD were taken as representing a significant response toward the given metal.
NR, no response.
The Hill coefficient was extrapolated on the basis of the beginning of the curve.
ND, not determined.
Fig. 3.
Response curves of wt MerR and the Cd-specific MerR mutants found after the first and second mutation rounds. (a) wt MerR. (b) Vector with wt MerR deleted from the reporter gene plasmid. The curve shows continuous expression of the firefly luciferase gene from MerOP. The MerR mutants obtained after the first mutagenesis round were A_D23 (c) and A_D24 (d), and those obtained after the second mutagenesis round were B_D20 (e), B_DB4 (f), B_DB10 (g), B_DB17 (h), B_DB26 (i), B_DB27 (j), B_DB29 (k), and C_DB21 (l). The responses to HgCl2 and CdCl2 are shown for wt MerR and the MerR mutants; the detected responses to other metals are shown, while negative results are omitted.
Fig. 4.
Sequencing results from the MerR mutants beginning at residue L67. In the sequence of wt MerR, helices α4, α5, and α6 are underlined. The metal-binding cysteines are marked with black arrows above the wt MerR sequence.
Second mutagenesis round, libraries B and C.
Cd-responsive MerR mutants A_D23 and A_D24 were used for a second mutation round that generated libraries B and C (Fig. 1; see Fig. S1 in the supplemental material). To expand the mutated region of A_D24, we used doped oligonucleotides containing 79% of the original bases and 7% of each of the other bases for the construction of library B. Eighteen amino acids were targeted in MerR mutant A_D24; they were located in helix 4, in the loop prior to helix α5, and from Gly122 of the metal-binding loop to the end of helix α6. For the construction of library C, residues Lys99 and Met106 were fully randomized using both MerR mutants A_D23 and A_D24 as parents (Fig. 1). The library construction technique allowed formation of chimeras from A_D23 and A_D24, thereby further increasing the diversity of library C. Lys99 and Met106 were selected for mutagenesis, as they reside in the fifth long alpha helix, which constitutes the protomer interface, and these residues have been shown to play roles in the Cd response of MerR (7). The flow cytometric selection and testing strategies used for libraries B and C were the same as those described for library A (Table 1; see Fig. S1 in the supplemental material).
The FACS-based dual screening strategy yielded 29% and 79% Cd-specific mutants from library B after the first (low-fluorescence) and the second (high-fluorescence) screening steps, respectively, and 17% and 31% Cd-specific mutants from library C after the first and the second screening steps, respectively (Fig. 2). Sequencing of the selected mutants revealed that some had identical genotypes, indicating that library diversity decreased during the selection process (Fig. 2).
Properties of Cd-specific MerR mutants.
Characterization of the MerR mutants obtained from libraries B and C for their responses toward other metal(loid)s revealed that we had generated eight Cd-specific MerR mutants (Fig. 3). None of the eight Cd-specific mutants responded significantly to any other metal(loid). Two of the mutants (B_DB10 and B_DB17) had strictly Cd-specific responses, while five (B_D20, B_DB4, B_DB26, B_DB27, and B_DB29) showed barely detectable responses to the highest nontoxic concentration of HgCl2. MerR mutants B_DB26 and C_DB21 also showed weak responses toward high concentrations of ZnCl2 (Fig. 3). The Cd-specific mutants from library B harbored 2 to 9 mutations in addition to the 13 parental ones in clone A_D24, while the single Cd-specific mutant from library C, C_DB21, had 12 mutations in total (Fig. 4). None of the Cd-specific mutants showed a higher Cd sensitivity than that of wt MerR, perhaps suggesting that the high CdCl2 concentration used for library induction did not favor the selection of more sensitive Cd-responsive MerR mutants. It is also possible that as the Cys residues were not mutated in MerR and Hg has a stronger affinity to thiolates than Cd, these could pose difficulties to get a higher affinity for Cd in comparison to the wt with Hg.
To study the repressor activity of the mutants, we constructed a reporter plasmid with deletion of the MerR regulatory protein (ΔmerR; Table 2 and Fig. 3b) and compared the uninduced luminescence level from the ΔmerR strain with those from the wt and mutant MerR reporter strains. The expression of firefly luciferase from the ΔmerR strain was ∼4-fold higher than that from the wt merR strain, while repression from merR mutant B_DB10 was ∼7-fold higher than that from the wt merR strain (Table 2). All of the other Cd-specific mutants had repressions that ranged from wt levels to a maximum 1.7-fold decrease in repression.
MerR responds ultrasensitively (a response that rises more rapidly than the concentration increase of the inducer would predict) to its inducing metals both in vivo (11) and in vitro (31). Ultrasensitivity, which is expressed as the Hill coefficient (e.g., it is >2 for wt MerR) (31), results from Hg binding to the MerR-RNA polymerase complex (1, 31). We found that the Hill coefficients were from 0.6 to 1.9 for all of the Cd-responsive MerR mutants, potentially indicating that this repressor-to-activator transition is less efficient in the mutants than in wt MerR. Notably, MerR mutants B_DB17, B_DB26, and B_DB27 had the highest Hill coefficients among the mutants (from 1.6 to 1.9) and also showed wt-level repressor activities (background luminescence from 0.09 to 0.11, where that for the wt was 0.12) and NLmaxs (from 15 to 20, where that for the wt was 18) (Table 2). Mutants B_D20, B_DB4, and C_DB21 had the lowest Hill coefficients (from 0.6 to 1.3).
Caguiat et al. (7) identified 11 Cd-responsive MerR mutants, all of which had single or double mutations largely located close to the metal-binding pocket. None of these identified Cd-responsive mutants were Cd specific (7) but had retained the normal wt Hg responses and were defective in repression, indicating that they had relaxed inducer requirements rather than altered metal specificities. The metal sensitivities of these Cd-responsive mutants were not measured. These mutants also seemed to have gain-of-function mutations, as their Cd responses were severalfold higher than the wt Cd response. Our A_D24 and B_DB10 mutants showed higher signals toward Cd than wt MerR (Fig. 3), although the sensitivity was not higher than that of wt MerR. Therefore, our Cd-specific MerR mutants could be classified as loss-of-function mutants, in that their mercury sensitivities had been decreased by at least 10,000-fold. Comparison of the characteristics between our mutants and the mutants of Caguiat et al. (7) is relatively difficult, as there were differences between our protocol and their protocol and the experimental procedures; e.g., a difference in cell amounts and/or cultivation medium can affect metal toxicity. A sequence comparison of the previously reported Cd-responsive and Cd-specific MerR mutants (7) with those reported herein revealed that there was only one mutation common to both studies: mutation S125P in B_DB10 of the present study (Fig. 1 and 4).
Role of K99 and M106 in Cd-specific response.
The Cd-specific mutant C_DB21 arose through the combination of mutants A_D23 and A_D24 (Fig. 4). In addition, C_DB21 had additional mutations in residues K99R and M106L; these mutations modified it in the direction of the CadR proteins, which have highly conserved Arg and Leu residues in the corresponding positions (Table 3; see Fig. S5 in the supplemental material). The Cd-responsive (but nonspecific) MerR mutant C_DB10 arose from A_D23 and also had additional mutations, K99R and M106L (see Fig. S2 and S3 in the supplemental material). The metal specificity of C_DB10 was not significantly different from that of the parental mutant, A_D23, except that A_D23 was 10-fold less sensitive to Sb. This indicates that mutations K99R and M106L did not induce significant changes in the metal specificity of MerR. Our findings further suggest that the Cd specificity of C_DB21 was based on the formation of chimeras from A_D23 and A_D24 rather than mutations K99R and M106L in the dimerization helix.
Table 3.
Sequence alignments between Cd-specific MerR mutants and MerR family members presented in the supplemental material
| Mutation or region and mutationa | Mutant(s) | % of mutations in sequence alignment |
|||
|---|---|---|---|---|---|
| MerR (136)b | CadR/PbrR (92) | ZntR (64) | CueR/HmrR/PmtR/GolS (72) | ||
| E72A | B_DB4 | 7.4 | 32.6 | 0.0 | 5.4 |
| E72D | B_DB17/26 | 5.9 | 0.0 | 6.3 | 16.2 |
| L73I | B_DB10 | 0.0 | 0.0 | 0.0 | 0.0 |
| L73F | B_DB17 | 0.0 | 0.0 | 1.6 | 4.1 |
| L74W | B_DB4 | 0.0 | 0.0 | 0.0 | 0.0 |
| R75W | B_DB4/10 | 0.0 | 0.0 | 0.0 | 0.0 |
| L76I | B_DB17/27/29 | 2.2 | 0.0 | 96.9 | 0.0 |
| L76V | B_DB10 | 12.5 | 1.1 | 0.0 | 0.0 |
| Loop from residues D77 to H81c | |||||
| D77E | B_D20/D_DB27 | 28.7 | |||
| D77C | B_DB4 | 0.0 | |||
| D77Y | B_DB10 | 0.0 | |||
| D78E | B_DB17 | 5.9 | |||
| G79Y | B_D20 | 0.0 | |||
| T80S | B_DB4 | 5.1 | |||
| T80N | B_DB26 | 0.0 | |||
| T80A | B_DB29 | 10.3 | |||
| H81G | All | 1.5 | |||
| E83V | All | 0.7 | 0.0 | 1.6 | 1.4 |
| E84G | All | 1.5 | 13.0 | 0.0 | 0.0 |
| A85H | All | 0.0 | 0.0 | 0.0 | 0.0 |
| S86D | All | 0.0 | 0.0 | 0.0 | 0.0 |
| S87A | All | 14.7 | 48.9 | 31.3 | 45.9 |
| K99R | C_DB21 | 14.0 | 95.7 | 20.3 | 55.4 |
| M106L | C_DB21 | 16.9 | 100.0 | 1.6 | 16.2 |
| V114T | B_D20, B_DB4/10/17/26/27/29 | 1.5 | 0.0 | 1.6 | 0.0 |
| C115G | B_D20, B_DB4/10/17/26/27/29 | 3.7 | 2.2 | 0.0 | 1.4 |
| C115Q | C_DB21 | 14.7 | 16.3 | 3.1 | 4.1 |
| A116M | B_D20, B_DB4/10/17/26/27/29 | 0.0 | 0.0 | 0.0 | 0.0 |
| A116P | C_DB21 | 0.0 | 0.0 | 0.0 | 0.0 |
| Metal-binding loop from residues H118 to S125d | |||||
| H118N | All B mutants | 2.9 | |||
| A119L | All B mutants | 1.5 | |||
| A119P | C_DB21 | 0.0 | |||
| R120P | All B mutants | 3.7 | |||
| K121P | All | 4.4 | |||
| G122A | B_D20 | 4.4 | |||
| G122R | B_DB4 | 2.2 | |||
| N123K | B_DB10 | 2.2 | |||
| N123Y | B_DB29 | 0.0 | |||
| V124F | B_D20 | 0.7 | |||
| V124Y | B_DB27 | 6.6 | |||
| S125F | B_DB4 | 0.0 | |||
| S125P | B_DB10 | 0.0 | |||
| L128M | B_D20 | 3.7 | 0.0 | 0.0 | 0.0 |
| L128F | B_DB4 | 0.0 | 0.0 | 0.0 | 0.0 |
| I129V | B_DB4 | 2.9 | 22.8 | 0.0 | 0.0 |
| A130R | B_DB17 | 0.0 | 7.6 | 0.0 | 4.1 |
| A130E | B_DB27 | 21.3 | 5.4 | 71.9 | 18.9 |
| S131T | B_D20 | 19.1 | 0.0 | 7.8 | 4.1 |
Numbering of the residues is based on that of MerR from Tn21. Highly conserved amino acids of MerR proteins are highlighted in boldface.
Data in parentheses are the number of sequences used in the multiple-sequence alignment.
The loop lengths of the MerR-like proteins were from 5 to 7 residues. CadR/PbrR, ZntR, and CueR/HmrR/PmtR/GolS were 2, 3, and 2 residues longer than MerR (Tn21), respectively. CadR/PbrR and CueR/HmrR/PmtR/GolS loop sequences contained conserved P and R, respectively. After the loop of CueR/HmrR/PmtR/GolS, there is an S instead of metal-binding C82. H and P residues were present in the loops of ZntR-like proteins.
The motifs in the metal-binding loop were CysX8Cys for MerR, CysX5–9Cys for CadR/PbrR, CysX9Cys (which has C and H residues involved in Zn binding) for ZntR, and CysXGlyX4Asp/GluCys (in which there is a high degree of conservation of the D following the G) for CueR/HmrR/PmtR/GolS.
The other Cd-responsive MerR mutant, C_DB36, was descended from A_D24 and had the additional mutations K99E and M106F (see Fig. S2 and S3 in the supplemental material) in the dimerization helix. The metal response of this mutant differed from that of parental strain A_D24 as follows: C_DB36 was repressor deficient, was at least 10-fold less sensitive toward Hg, and, additionally, responded to Sb and As. A long helix (α5), the so-called coiled coil region of MerR, forms five heptads, each with a, b, c, d, e, f, and g positions indicating amino acid locations; notably, K99 and M106 are both in d positions (36). In the study of Caguiat et al. (7), 4 of 13 randomly selected or screened Cd(II)-responsive MerR mutants had mutations of K99 or M106. On the basis of this, the authors suggested that interface positions could be involved in the metal-specific response of MerR. Furthermore, it has been observed that in natural coiled coils having hydrophobic residues, the d position forms the helix interface between two subunits, and the presence of polar and charged residues deep within the hydrophobic core of coiled coils can affect a protein's behavior (36). In the model of MerR, K99 and K99′ are found in very close proximity to one another (Fig. 1b), and polar substitutions of these residues have been associated with decreased repressor activity in vivo (7). It has also been proposed that during metal-binding-provoked movement, M106 interacts with the L63′ residue of the other protomer's helix α4; this could cause a shift toward the DNA-binding domain. In the absence of metal, in contrast, M106 may stabilize DNA binding (36). Therefore, the two moderately radical substitutions (K99E and M106F) in C_DB36 could affect the protein's DNA-binding properties and metal specificity in a manner unlike that seen for C_DB21 and C_DB10, which had less radical mutations (K99R and M106L).
Fine adjustment of Cd selectivity in MerR mutants.
Cd-specific mutant B_DB17 (Fig. 3) had 18 amino acid changes (Fig. 4). Mutant B_DB34 had the same amino acid changes, with the exception of the Leu-to-Phe mutation at residue 73 (see Fig. S3 in the supplemental material), which is situated in the coupling domain at the end of helix α4. The metal responses of these mutants were different. B_DB34 was Cd responsive but not Cd specific, as it strongly responded to Hg and noticeably responded to several other metals (Sb, Zn, Cr, and As) (see Fig. S2 in the supplemental material). In addition, it was repressor deficient, giving a clearly higher background signal than B_DB17 in the absence of metal. This shows that a single relatively conserved type of amino acid change could have a significant effect on the MerR metal response. Mechanistically, it is notable that the side chain of residue 73 is buried within the metal coordination site, meaning that the structure of the coordination site is likely to be affected by the replacement of Leu with the larger Phe residue. It is tempting to speculate that the properties of the metals in question could be responsible for the mutation-induced change in the metal response, as the ionic radius of Cd(II) is 103 pm, while that of Hg(II) is 112 pm. Alternatively, the altered metal specificity could be related to the coordination preferences of the metals, with Cd(II) being more stably bound by the mutants.
Since protein tertiary structure preferences dictate the final geometry of a bound metal (16, 30), it seems logical to surmise that Cd-specific MerR mutants having a large number of amino acid changes could coordinate Cd(II) differently than wt MerR, with some of the mutations being responsible for making small, Hg-excluding changes to the metal-binding pocket. It is also possible that the coordination of a metal and subsequent specific conformational change or steric hindrance are responsible for metal specificity, as concluded by Rensing (32). The basis for selective metal ion recognition by MerR, which binds Hg highly selectively over Cd or Zn, is not yet fully understood (31), even though we do know the essential role of cysteines in Hg binding (19, 33, 35). It is also noteworthy that metal ion recognition preferences differ even between two Cd-responsive CadR proteins from Pseudomonas putida and Pseudomonas aeruginosa (at its cognate promoter PcadA) with the induction profiles Cd ≫ Pb > Zn and Cd ≫ Zn > Hg, respectively (4, 24). Giedroc and Arunkumar (17) have suggested that relatively subtle modifications in the metal-binding pocket could lead to the evolution of MerR family regulatory proteins, e.g., CadR and PbrR, which preferentially detect Cd and Pb over Zn, respectively, or MerR, which preferentially detects Hg over Cd. The X-ray crystallographic structures of two MerR family members, CueR and ZntR (3, 8, 29), indicate that the metal receptor sites are buried in a loop at the dimer interface, which holds the key factors for metal ion selectivity. The mechanisms of metal coordination can differ among the various members of the family. For example, ZntR binds four zinc atoms per dimer, and each Zn(II) atom is bound in a tetrahedral coordination environment (8, 28). In contrast, CueR binds two Cu(I) or Ag(I) ions and forms linear two-coordinate geometries in both cases (8), whereas Hg(II)-MerR forms an unusual tricoordinated complex (40), even though the preferred coordination geometry for Hg(II) is two ligands. Cd(II) and Zn(II) compete for MerR binding, and they may either form a trigonal thiolate complex or adopt higher coordinations (40). Unfortunately, the coordination geometries of Cd(II) are not yet known for wt MerR. In the future, it could prove very useful to examine the coordination environment of all the Cd-specific mutants described in this work.
Evolutionary aspects of Cd-specific MerR mutants.
In order to determine whether the mutations of our Cd-specific MerR variants exist in nature, the amino acids from Asp68 to the end of the protein were aligned with the equivalent sequences of MerR family proteins from different species: MerR, CadR/PbrR, ZntR, and CueR/HmrR/PmtR/GolS (see Fig. S4 to S7 in the supplemental material). Table 3 shows an amino acid sequence comparison of our Cd-specific mutants against the various MerR family proteins. This analysis excluded the loop structures, which were compared only among the MerR-like proteins, as the loop lengths differed significantly among the other MerR family proteins. Amino acid changes were classified into three categories: new mutations, where a mutation did not exist in any other of the aligned sequences; low-frequency mutations, where a mutation was found in less than 10% of sequences; and existing mutations, where a mutation was found at the aligned position in more than 10% of the sequences. Mutations found from the Cd-specific MerR mutants harbored 37% new mutations compared to MerR proteins and 53 to 58% compared to other family members. CadR/PbrR proteins had 73% and the other family members had 80 to 85% of mutations that were new or low-frequency mutations. The mutations were largely derived from parental sequence A_D24 (nine in library B and five in library C), as library A was quite randomly mutated. All of the Cd-specific mutants from libraries B and C also had one to five additional new mutations in their sequences in comparison to MerR proteins. Furthermore, it is interesting that the largest amount of mutations (27% were existing mutations) occurred in the aligned sequences of CadR/PbrR proteins compared to other family members (15 to 18%). Thus, such changes can favor a Cd-specific response. Also, our analysis of these mutations revealed that the structure of MerR appears to tolerate radical amino acid changes fairly well, especially near and within the metal-binding loop structures.
Among the conserved amino acids of MerR, we targeted 10 for mutagenesis. The total number of amino acids changes (18 to 22) was higher in the Cd-specific mutants that had mutations in 4 or 5 of the conserved amino acids (B_D20, B_DB4, B_DB10, and B_DB17) than in those having mutations in only 2 or 3 of the conserved amino acids (B_DB26, B_D27, B_DB29, and C_DB21, which had 12 to 17 amino acid changes) (Table 3). This suggests that the conserved residues are important to the structure of MerR, and additional mutations were needed to compensate for the structural and functional changes induced by mutations in these conserved residues. We also found that the amino acid residues after the last α helix (α6) did not seem to be critical for the functionality of MerR proteins, as Cd-specific mutant B_DB4 had a stop codon at position Ser131. Our sequence alignment also supported this observation, as many of the MerR-like proteins had tails of only one to three amino acids after helix α6 (see Fig. S4 in the supplemental material).
We believe that the Cd-specific MerR mutants described herein are unique among the MerR family members characterized to date. There appear to be few similarities in the loop structures of the Cd-specific mutants and other MerR family proteins, and the involved amino acid residues vary widely. However, the Cd-specific mutants and the other MerR family metalloregulatory proteins do have one interesting feature in common: they all have a Pro(s) in the two loop structures of the binding region, whereas parental MerR (Tn21) and several other MerR proteins do not. Our Cd-specific MerR mutants had two to three Pro residues in their metal-binding loops. Similarly, the CadR proteins have a highly conserved Pro in the loop of the N-terminal end of the dimerization helix or in the metal-binding loop (Table 3; see Fig. S5 in the supplemental material), while ZntR and CueR each have a Pro in one of their loop structures. Because Pro has a five-membered pyrrolidine ring that connects the α carbon to the preceding amide nitrogen, it imposes rigid constraints on the conformation of the peptide backbone. The introduction of two to three Pro residues within an eight-residue metal-binding loop could therefore conceivably cause significant changes in loop conformation and dynamics, resulting in a mutant with altered metal-binding properties (i.e., Cd specificity). The metal-binding loop (CysX8Cys) of the MerR proteins is followed by a proline (Pro127) instead of the Gly (or Ala) seen in the CadR proteins (see Fig. S4 and S5 in supplemental material). Interestingly, even though this Pro was targeted by our mutagenesis procedure, none of the Cd-specific MerR mutants had mutations in this residue. This, along with its conserved nature, indicates that this particular Pro is likely to play an important role in the metal-binding functionality of MerR.
Supplementary Material
ACKNOWLEDGMENTS
The work was financially supported by the Academy of Finland (grants 201677 and 207258 to Marko P. Virta).
We are grateful for the assistance of P. Terho and M. Korkeamäki (Cell Imagining Core facility of Turku). We also thank Taina Tyystjärvi for critical reading of the manuscript.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Ansari A. Z., Chael M. L., O'Halloran T. V. 1992. Allosteric underwinding of DNA is a critical step in positive control of transcription by Hg-MerR. Nature 355:87–89 [DOI] [PubMed] [Google Scholar]
- 2. Barrineau P., et al. 1984. The DNA sequence of the mercury resistance operon of the IncFII plasmid NR1. J. Mol. Appl. Genet. 2:601–619 [PubMed] [Google Scholar]
- 3. Brocklehurst K. R., et al. 1999. ZntR is a Zn(II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Mol. Microbiol. 31:893–902 [DOI] [PubMed] [Google Scholar]
- 4. Brocklehurst K. R., Megit S. J., Morby A. P. 2003. Characterisation of CadR from Pseudomonas aeruginosa: a Cd(II)-responsive MerR homologue. Biochem. Biophys. Res. Commun. 308:234–239 [DOI] [PubMed] [Google Scholar]
- 5. Brown N. L., Ford S. J., Pridmore R. D., Fritzinger D. C. 1983. Nucleotide-sequence of a gene from the Pseudomonas transposon Tn501 encoding mercuric reductase. Biochemistry 22:4089–4095 [DOI] [PubMed] [Google Scholar]
- 6. Brown N. L., Stoyanov J. V., Kidd S. P., Hobman J. L. 2003. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 27:145–163 [DOI] [PubMed] [Google Scholar]
- 7. Caguiat J. J., Watson A. L., Summers A. O. 1999. Cd(II)-responsive and constitutive mutants implicate a novel domain in MerR. J. Bacteriol. 181:3462–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Changela A., et al. 2003. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 301:1383–1387 [DOI] [PubMed] [Google Scholar]
- 9. Checa S. K., et al. 2007. Bacterial sensing of and resistance to gold salts. Mol. Microbiol. 63:1307–1318 [DOI] [PubMed] [Google Scholar]
- 10. Chen P. R., He C. 2008. Selective recognition of metal ions by metalloregulatory proteins. Curr. Opin. Chem. Biol. 12:214–221 [DOI] [PubMed] [Google Scholar]
- 11. Condee C. W., Summers A. O. 1992. A mer-lux transcriptional fusion for real-time examination of in vivo gene expression kinetics and promoter response to altered superhelicity. J. Bacteriol. 174:8094–8101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cormack B. P., Valdivia R. H., Falkow S. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33–38 [DOI] [PubMed] [Google Scholar]
- 13. deWet J. R., Wood K. W., De Luca M. 1985. Cloning of firefly luciferase cDNA and the expression of active luciferse in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 80:7870–7873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferrari B. C., Oregaard G., Sorensen S. J. 2004. Recovery of GFP-labeled bacteria for culturing and molecular analysis after cell sorting using a benchtop flow cytometer. Microb. Ecol. 48:239–245 [DOI] [PubMed] [Google Scholar]
- 15. Franz B., O'Halloran T. V. O. 1990. DNA distortion accompanies transcriptional activation by the metal-responsive gene-regulatory protein MerR. Biochemistry 29:4747–4751 [DOI] [PubMed] [Google Scholar]
- 16. Ghosh D., Lee K. H., Demeler B., Pecoraro V. L. 2005. Linear free-energy analysis of mercury(II) and cadmium(II) binding to three-stranded coiled coils. Biochemistry 44:10732–10740 [DOI] [PubMed] [Google Scholar]
- 17. Giedroc D. P., Arunkumar A. I. 2007. Metal sensor proteins: nature's metalloregulated allosteric switches. Dalton Trans. 29:3107–3120 [DOI] [PubMed] [Google Scholar]
- 18. Hakkila K., Maksimow M., Karp M., Virta M. 2002. Reporter genes lucFF, luxCDABE, gfp, and dsred have different characteristics in whole-cell bacterial sensors. Anal. Biochem. 301:235–242 [DOI] [PubMed] [Google Scholar]
- 19. Helmann J. D., Ballard B. T., Walsh C. T. 1990. The MerR metalloregulatory protein binds mercuric ion as a tricoordinate, metal-bridged dimer. Science 247:946–948 [DOI] [PubMed] [Google Scholar]
- 20. Heltzel A., Gambill D., Jackson W. J., Totis P. A., Summers A. O. 1987. Overexpression and DNA-binding properties of the mer-encoded regulatory protein from plasmid NR1 (Tn21). J. Bacteriol. 169:3379–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hobman J. L. 2007. MerR family transcription activators: similar designs, different specificities. Mol. Microbiol. 63:1275–1278 [DOI] [PubMed] [Google Scholar]
- 22. Hobman J. L., Wilkie J., Brown N. L. 2005. A design for life: prokaryotic metal-binding MerR family regulators. Biometals 18:429–436 [DOI] [PubMed] [Google Scholar]
- 23. LaRossa R. A., Smulski D. R., Van Dyk T. K. 1995. Interaction of lead nitrate and cadmium chloride with Escherichia coli K-12 and Salmonella typhimurium global regulatory mutants. J. Ind. Microbiol. 14:252–258 [DOI] [PubMed] [Google Scholar]
- 24. Lee S. W., Glickmann E., Cooksey D. A. 2001. Chromosomal locus for cadmium resistance in Pseudomonas putida consisting of a cadmium-transporting ATPase and a MerR family response regulator. Appl. Environ. Microbiol. 67:1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lovell T., Himo F., Han W., Noodleman L. 2003. Density functional methods applied to metalloenzymes. Coordin. Chem. Rev. 238:211–232 [Google Scholar]
- 26. Misra T. K., et al. 1984. Mercuric ion-resistance operons of plasmid R100 and transposon Tn501: the beginning of the operon including the regulatory region and the first two structural genes. Proc. Natl. Acad. Sci. U. S. A. 81:5975–5979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Halloran T. V., Frantz B., Shin M. K., Ralston D. M., Wright J. G. 1989. The MerR heavy metal receptor mediates positive activation in a topologically novel transcription complex. Cell 56:119–129 [DOI] [PubMed] [Google Scholar]
- 28. Outten C. E., Outten F. W., O'Halloran T. V. 1999. DNA distortion mechanism for transcriptional activation by ZntR, a Zn(II)-responsive MerR homologue in Escherichia coli. J. Biol. Chem. 274:37517–37524 [DOI] [PubMed] [Google Scholar]
- 29. Outten F. W., Outten C. E., Hale J., O'Halloran T. V. 2000. Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal MerR homologue, CueR. J. Biol. Chem. 275:31024–31029 [DOI] [PubMed] [Google Scholar]
- 30. Pennella M. A., Giedroc D. P. 2005. Structural determinants of metal selectivity in prokaryotic metal-responsive transcriptional regulators. Biometals 18:413–428 [DOI] [PubMed] [Google Scholar]
- 31. Ralston D. M., O'Halloran T. V. 1990. Ultrasensitivity and heavy-metal selectivity of the allosterically modulated MerR transcription complex. Proc. Natl. Acad. Sci. U. S. A. 87:3846–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rensing C. 2005. Form and function in metal-dependent transcriptional regulation: dawn of the enlightenment. J. Bacteriol. 187:3909–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ross W., Park S. J., Summers A. O. 1989. Genetic analysis of transcriptional activation and repression in the Tn21 mer operon. J. Bacteriol. 171:4009–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 35. Shewchuk L. M., et al. 1989. Transcriptional switching by the MerR protein: activation and repression mutants implicate distinct DNA and mercury(II) binding domains. Biochemistry 28:2340–2344 [DOI] [PubMed] [Google Scholar]
- 36. Song L. Y., Teng Q., Phillips R. S., Brewer J. M., Summers A. O. 2007. 19F-NMR reveals metal and operator-induced allostery in MerR. J. Mol. Biol. 371:79–92 [DOI] [PubMed] [Google Scholar]
- 37. Stanisich V. A., Bennett P. M., Richmond M. H. 1977. Characterization of a translocation unit encoding resistance to mercuric ions that occurs on a non conjugative plasmid in Pseudomonas aeruginosa. J. Bacteriol. 129:1227–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Summers A. O. 1992. Untwist and shout: a heavy metal-responsive transcriptional regulator. J. Bacteriol. 174:3097–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang S.-Y., Fazelinia H., Cirino P. C. 2008. AraC regulatory protein mutants with altered effector specificity. J. Am. Chem. Soc. 130:5267–5271 [DOI] [PubMed] [Google Scholar]
- 40. Utschig L. M., Bryson J. W., O'Halloran T. V. 1995. Mercury-199 NMR of the metal receptor site in MerR and its protein-DNA complex. Science 268:380–385 [DOI] [PubMed] [Google Scholar]
- 41. Valdivia R. H., Falkow S. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367–378 [DOI] [PubMed] [Google Scholar]
- 42. Wright J. G., Tsang H. T., Pennerhahn J. E., Ohalloran T. V. 1990. Coordination chemistry of the Hg-MerR metalloregulatory protein: evidence for a novel tridentate Hg-cysteine receptor site. J. Am. Chem. Soc. 112:2434–2435 [Google Scholar]
- 43. Zeng Q., Stalhandske C., Anderson M. C., Scott R. A., Summers A. O. 1998. The core metal-recognition domain of MerR. Biochemistry 37:15885–15895 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.