Abstract
In this study, we evaluated the effect of soil clay content on RNA isolation and on quantitative reverse transcription-PCR (qRT-PCR) quantification of microbial gene transcripts. The amount of clay significantly altered RNA isolation yields and qRT-PCR analyses. Recommendations are made for quantifying microbial gene transcripts in soil samples varying in clay content.
TEXT
Real-time quantitative reverse transcription-PCR (qRT-PCR) is sensitive and specific, allowing one to detect and quantify low levels of transcripts, which makes it highly suited for the analysis of microbial gene expression in soil (4, 5, 17). However, only a few studies have reported the use of qRT-PCR to quantify microbial gene expression under different soil conditions (1, 2, 6, 10, 11).
One important factor limiting microbial gene expression studies in soil is inadequate isolation and purification of RNA (15). When high levels of clay particles are found, cell lysis during nucleic acid isolation may be incomplete and nucleic acids may not be separated from clay particles (20).
The heterogeneity of soil has led to the development of numerous RNA isolation protocols (15, 16, 19) and commercial kits, but no consensus has been reached as to which of them offer high efficiency under a large spectrum of soil conditions.
In this study, the effect of clay content on RNA isolation from soil and on subsequent microbial gene transcript quantification using qRT-PCR was evaluated. Pseudomonas sp. strain LBUM300, a previously characterized hydrogen cyanide producer, was used as a model organism (13).
Three agricultural soils, a sandy loam soil (low-clay soil; 13% clay), a silty clay soil (medium-clay soil; 41% clay), and a clay soil (high-clay soil; 68% clay), were sampled in Bouctouche, Normandin, and Lévis, Canada, respectively, and identified using the USDA soil classification system (18) (see Table S1 in the supplemental material).
For each soil, four 100-g samples were used; two were inoculated with 15 ml of 1 × 109 Pseudomonas sp. LBUM300 cells ml−1 grown in tryptic soy broth (TSB), and two nonspiked controls were inoculated with 15 ml of TSB. Following inoculations, one sample containing Pseudomonas sp. LBUM300 and one control were immediately stored at −80°C (day 0), while the other two samples were incubated at 25°C for 7 days and then stored at −80°C (day 7). Samples were lyophilized prior to RNA isolations.
For each harvesting time (days 0 and 7; destructive sampling) and each soil type, four independent replicate soil samples were used for RNA isolation by four commonly used protocols and with two commercial RNA isolation kits (Table 1). Residual coextracted DNA was eliminated from RNA samples as previously described (6). The hcnC gene transcript involved in hydrogen cyanide production was reverse transcribed and quantified by qRT-PCR, as per reference 6. Pseudomonas sp. LBUM300 populations were also quantified from the same soil samples using qPCR following DNA extraction, as per reference 12. RNA and DNA quantity and purity were verified by spectrophotometry. Sufficient coextracted DNA removal was verified by performing qPCR amplification of DNase-digested RNA that was not reverse transcribed.
Table 1.
Summary of RNA isolation protocols used in this studya
| RNA isolation protocol (reference) | Soil vol (g) | Method of cell disruptionb |
Nucleic acid separation solutionc | Nucleic acid precipitation solutiond | Speed to performe | |
|---|---|---|---|---|---|---|
| Solution and equipment | Bead-beating time(s) and speed(s) | |||||
| Bürgmann et al. (3) | 0.5 | 0.2% CTAB, 0.75 g of 0.1-mm silica beads | 45 s at 6 m s−1 | PCI | 20% PEG | + |
| FastRNA Pro Soil-Direct kit (Bio101) | 0.5 | 1 ml RNApro soil lysis solution (proprietary solution); lysing matrix tube | 40 s at 6 m s−1 | PC | 0.6 vol isopropanol | ++ |
| Griffiths et al. (8) | 0.5 | Lysing matrix E (Qbiogene); 5% CTAB | 30 s at 5.5 m s−1 | CI | 30% PEG | ++ |
| Hurt et al. (9), as modified by Gomes et al. (7) | 0.5 | 1% CTAB, 2% SDS; 0.4 g of 0.1-mm glass beads | 60 s at 5.5 m s−1 | CI | 0.6 vol isopropanol | + |
| Mo Bio RNA PowerSoil Total RNA isolation kit (MoBio) | 2.0 | Solution SR1 (proprietary; contains SDS); bead tube; vortex adaptor | Vortex at maximum speed for 15 min | PCI | 5 ml isopropanol (with column purification) | ++ |
| Peršoh et al. (14) | 0.5 | 0.4 M LiCl; 0.5 g of 0.5-mm, 0.3 g of 0.1-mm, and 1 4-mm glass bead; freeze-thaw cycles | 30 s at 4 m s−1 and 60 s at 5.5 m s−1 | PCI | 0.7 vol isopropanol | +++ |
The final volume eluted for all protocols was 100 μl. The Griffiths et al. and Peršoh et al. protocols, which normally elute RNA in 50 μl, were set to 100 μl. The RNeasy mini kit (Qiagen) was used with the Griffiths et al. and Hurt et al. protocols, as is recommended for protocols that require an additional purification step.
Total bead-beating times are displayed and can consist of multiple bead-beating rounds. The bead beater used in all protocols was a FastPrep instrument (Bio101). CTAB, cetyltrimethylammonium bromide.
PCI, phenol-chloroform-isoamyl alcohol (25:24:1); PC, phenol-chloroform (1:1); CI, chloroform-isoamyl alcohol (24:1).
PEG, polyethylene glycol.
Comparison of times required to perform RNA isolation from 4 samples in parallel. + indicates less than 4 h, ++ indicates between 4 and 8 h, and +++ indicates over 8 h.
Statistical analyses were performed on rank-transformed data using factorial analysis of variance (ANOVA) (with soil clay content, RNA isolation method, and time as factors) and the SAS software (SAS Institute, Cary, NC). A posteriori Tukey tests were performed to identify significant differences (P < 0.05). As time yielded a significant effect, data obtained at 0 and 7 days were analyzed separately.
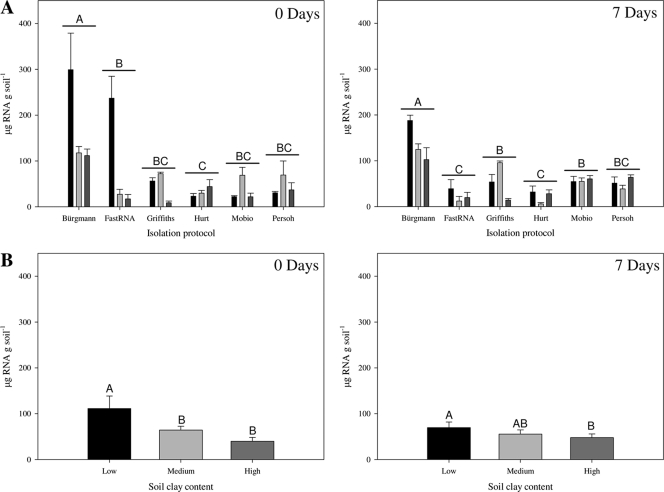
The highest level of RNA was isolated from the low-clay soil and was significantly greater than the amount of RNA isolated from the high-clay soil at both 0 (F5,54 = 15.54; P < 0.0001) and 7 (F5,54 = 21.02; P < 0.0001) days (Fig. 1). The method of Bürgmann et al. (3) isolated the largest amount of RNA. A significant interaction between soil type and RNA isolation method at 0 (F10,54 = 8.13; P < 0.0001) and 7 (F10,54 = 3.93; P = 0.0005) days suggests varied effects of clay content on the different RNA isolation methods. Following all extractions, RNA appeared clear and not brownish. A260/A280 ratios ranged from 1.4 to 2.0 and were lowest in high-clay soil.
Fig. 1.
Micrograms of total RNA isolated per gram of soil at 0 and 7 days postinoculation with Pseudomonas sp. LBUM300, as measured by spectrophotometry, with each RNA isolation method (A) and in each soil clay content (B). Black bars, light-gray bars, and dark-gray bars represent low-, medium-, and high-clay soil, respectively. Letters indicate significant differences at P < 0.05. All values are expressed as means ± standard errors of results from 4 and 24 separate RNA isolations (A and B, respectively).
Regardless of the soil's clay content, the protocol of Griffiths et al. (8) exhibited significantly higher levels of coextracted DNA than other methods (see Fig. S1 in the supplemental material). Therefore, due to the presence of high levels of coextracted DNA, which is likely to interfere with qRT-PCR results, and the inability to reduce these quantities even after a second round of DNase treatment (results not shown), we do not recommend the Griffiths et al. protocol for quantifying bacterial gene transcripts in soil.
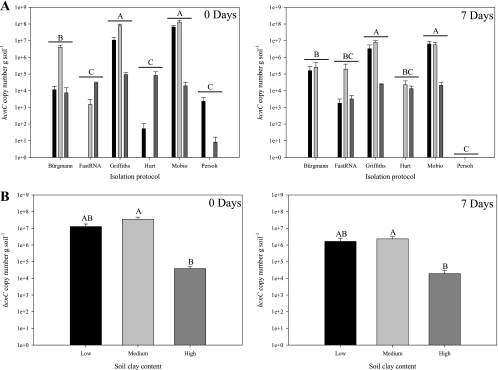
When hcnC gene transcripts were quantified, the medium-clay soil led to the largest amounts, while significantly lower levels of transcripts were detected in the high-clay soil (F2,54 = 4.07 [P = 0.02] and F2,54 = 5.43 [P = 0.007] for 0 and 7 days, respectively) (Fig. 2 B). Higher hcnC gene transcript levels were detected by the methods of Griffiths et al., Mo Bio, and Bürgmann et al. than by the other methods, and a significant interaction between soil type and RNA isolation method was observed for 0 (F10,54 = 13.36; P < 0.0001) and 7 (F10,54 = 3.23; P < 0.002) days (Fig. 2A). No qRT-PCR detection of hcnC gene transcripts was observed in nonspiked controls (results not shown).
Fig. 2.
Copy numbers of hcnC gene transcripts expressed by each RNA isolation protocol (A) and for each soil clay content (B) for 0 and 7 days postinoculation with Pseudomonas sp. LBUM300. Black bars, light-gray bars, and dark-gray bars represent soil of low, medium, and high clay content, respectively. Letters indicate significant differences at P < 0.05. All values are means ± standard errors of results from 4 and 24 isolations (A and B, respectively), expressed in hcnC copy numbers per gram of soil.
A direct link between the quantity of total RNA isolated and hcnC gene transcripts detected was not observed. The difference is most likely not due to the quantities of indigenous microbial RNA content, as these values were not different between soils (results not shown). Hence, for qRT-PCR quantification of microbial transcripts under soil conditions, the purity of the RNA seems more important than the quantity extracted.
An expression ratio (number of transcripts/number of gene copies) was also calculated (data not shown) and gave similar results for the remaining best-performing methods (Mo Bio and Bürgmann et al.). However, the expression ratio in the high-clay soil was significantly lower, except by the Bürgmann et al. protocol at day 0. As expression ratios should not vary at day 0 between the different soil types because equal amounts of Pseudomonas sp. LBUM300 in similar physiological states are present, this suggests that the Bürgmann et al. protocol is more robust than the Mo Bio kit under a wide range of soil clay contents.
To our knowledge, this is the first study investigating the effect of clay content on RNA isolation methods and microbial gene transcript quantification in soil. Based on the results obtained, the Bürgmann protocol appears to be the most robust and recommendable for extracting RNA and allowing microbial gene transcript quantification in soils varying in clay content. Despite the versatility of this protocol, one should keep in mind that all RNA isolation methods investigated were negatively affected by high-clay soils.
Supplementary Material
Acknowledgments
We kindly thank Martin Chantigny for supplying the medium- and high-clay soil samples.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 1 July 2011.
REFERENCES
- 1. Andria V., Reinchenauer T. G., Sessitsch A. 2009. Expression of alkane monooxygenase (alkB) genes by plant-associated bacteria in the rhizosphere and endosphere of Italian ryegrass (Lolium multiflorum L.) grown in diesel contaminated soil. Environ. Pollut. 157:3347–3350 [DOI] [PubMed] [Google Scholar]
- 2. Baelum J., et al. 2008. Direct analysis of tfdA gene expression by indigenous bacteria in phenoxy acid amended agricultural soil. ISME J. 2:677–687 [DOI] [PubMed] [Google Scholar]
- 3. Bürgmann H., Widmer F., Sigler W. V., Zeyer J. 2003. mRNA extraction and reverse transcription-PCR protocol for detection of nifH gene expression by Azotobacter vinelandii in soil. Appl. Environ. Microbiol. 69:1928–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bustin S. A., Benes V., Nolan T., Pfaffl M. W. 2005. Quantitative real-time RT-PCR—a perspective. J. Mol. Endocrinol. 34:597–601 [DOI] [PubMed] [Google Scholar]
- 5. Bustin S. A., Mueller R. 2005. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin. Sci. 109:365–379 [DOI] [PubMed] [Google Scholar]
- 6. DeCoste N. J., Gadkar V. J., Filion M. 2011. Relative and absolute quantitative real-time PCR-based quantifications of hcnC and phlD gene transcripts in natural soil spiked with Pseudomonas sp. strain LBUM300. Appl. Environ. Microbiol. 77:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomes N. C. M., Costa R., Smalla K. 2008. Rapid simultaneous extraction of DNA and RNA from bulk and rhizosphere soil, p. 159–169 In Kowalchuck G. A., de Bruijn F. J., Head I. M., Akkermans A. D. L., van Elsas J. D. (ed.), Molecular microbial ecology manual, vol. 1 Springer, Dordrecht, The Netherlands [Google Scholar]
- 8. Griffiths R. I., Manefield M., Whiteley A. S., Bailey M. J. 2008. DNA and RNA extraction from soil, p. 149–158 In Kowalchuck G. A., de Bruijn F. J., Head I. M., Akkermans A. D. L., van Elsas J. D. (ed.), Molecular microbial ecology manual, vol. 1 Springer, Dordrecht, The Netherlands [Google Scholar]
- 9. Hurt R. A., et al. 2001. Simultaneous recovery of RNA and DNA from soils and sediments. Appl. Environ. Microbiol. 67:4495–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacobsen C. S., Holben W. E. 2007. Quantification of mRNA in Salmonella sp. seeded soil and chicken manure using magnetic capture hybridization RT-PCR. J. Microbiol. Methods 69:315–321 [DOI] [PubMed] [Google Scholar]
- 11. Kolb S., Knief C., Dunfield P. F., Conrad R. 2005. Abundance and activity of uncultured methanotrophic bacteria involved in the consumption of atmospheric methane in two forest soils. Environ. Microbiol. 7:1150–1161 [DOI] [PubMed] [Google Scholar]
- 12. LeBlanc P. M., Hamelin R. C., Filion M. 2007. Alteration of soil rhizosphere communities following genetic transformation of white spruce. Appl. Environ. Microbiol. 73:4128–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paulin M. M., et al. 2009. Transcriptional activity of antifungal metabolite-encoding genes phlD and hcnBC in Pseudomonas spp. using qRT-PCR. FEMS Microbiol. Ecol. 68:212–222 [DOI] [PubMed] [Google Scholar]
- 14. Peršoh D., Theuerl S., Buscot F., Rambold G. 2008. Towards a universally adaptable method for quantitative extraction of high-purity nucleic acids from soil. J. Microbiol. Methods 75:19–24 [DOI] [PubMed] [Google Scholar]
- 15. Saleh-Lakha S., et al. 2005. Microbial gene expression in soil: methods, applications and challenges. J. Microbiol. Methods 63:1–19 [DOI] [PubMed] [Google Scholar]
- 16. Sayler G. S., Fleming J. T., Nivens D. E. 2001. Gene expression monitoring in soils by mRNA analysis and gene lux fusions. Curr. Opin. Biotechnol. 12:455–460 [DOI] [PubMed] [Google Scholar]
- 17. Sharkey F. H., Banat I. M., Marchant R. 2004. Detection and quantification of gene expression in environmental bacteriology. Appl. Environ. Microbiol. 70:3795–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soil Survey Division Staff, U.S. Department of Agriculture 1993. Soil survey manual. Soil Conservation Service. U.S. Department of Agriculture handbook 18. U.S. Department of Agriculture, Washington, DC [Google Scholar]
- 19. Sorensen J., Nicolaisen M. H., Ron E., Simonet P. 2009. Molecular tools in rhizosphere microbiology—from single-cell to whole-community analysis. Plant Soil. 321:483–512 [Google Scholar]
- 20. van Elsas J. D., Duarte G. F., Rosado A. S., Smalla K. 1998. Microbiological and molecular biological methods for monitoring microbial inoculants and their effects in the soil environment. J. Microbiol. Methods 32:133–154 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.