Abstract
Living as a commensal, Candida albicans must adapt and respond to environmental cues generated by the mammalian host and by microbes comprising the natural flora. These signals have opposing effects on C. albicans, with host cues promoting the yeast-to-hyphal transition and bacteria-derived quorum-sensing molecules inhibiting hyphal development. Hyphal development is regulated through modulation of the cyclic AMP (cAMP)/protein kinase A (PKA) signaling pathway, and it has been postulated that quorum-sensing molecules can affect filamentation by inhibiting the cAMP pathway. Here, we show that both farnesol and 3-oxo-C12-homoserine lactone, a quorum-sensing molecule secreted by Pseudomonas aeruginosa, block hyphal development by affecting cAMP signaling; they both directly inhibited the activity of the Candida adenylyl cyclase, Cyr1p. In contrast, the 12-carbon alcohol dodecanol appeared to modulate hyphal development and the cAMP signaling pathway without directly affecting the activity of Cyr1p. Instead, we show that dodecanol exerted its effects through a mechanism involving the C. albicans hyphal repressor, Sfl1p. Deletion of SFL1 did not affect the response to farnesol but did interfere with the response to dodecanol. Therefore, quorum sensing in C. albicans is mediated via multiple mechanisms of action. Interestingly, our experiments raise the possibility that the Burkholderia cenocepacia diffusible signal factor, BDSF, also mediates its effects via Sfl1p, suggesting that dodecanol's mode of action, but not farnesol or 3-oxo-C12-homoserine lactone, may be used by other quorum-sensing molecules.
INTRODUCTION
Candida albicans is an opportunistic fungal pathogen of humans. In healthy individuals C. albicans resides in the gastrointestinal, vaginal, and oral tracts, where it is considered to be part of the normal flora. However, when an individual becomes immunocompromised as a consequence of age, cancer, chemotherapy, or trauma, C. albicans is able to invade the underlining mucosal surface and disseminate into the bloodstream causing systemic disease. Mortality rates associated with systemic candidiasis are reported to be 30% higher than those associated with bacterial infections. Thus, C. albicans is a medically relevant microbial pathogen (reviewed in references 2, 28, and 32).
The ability of C. albicans to undergo a morphological transition from yeast to hyphal forms is essential for virulence, with strains locked in either form displaying attenuated virulence in models for systemic candidiasis (35). This switch in morphology is governed by many host environmental signals acting through several well-established signaling cascades including the CEK1 mitogen-activated protein kinase (MAPK) pathway, the RIM101-dependent pathway, and the cAMP-dependent protein kinase A (cAMP/PKA) pathway (10, 27, 42). The cAMP/PKA pathway appears to be the most essential for morphogenesis, with an adenylyl cyclase mutant being defective for filamentation in response to the majority of stimulating cues (45).
In addition to host environmental signals, C. albicans must also adapt and respond to microorganisms from the microflora to successfully establish itself within a niche. Microorganisms communicate by a process known as quorum sensing (QS), where soluble chemical mediators termed quorum-sensing molecules (QSMs) or autoinducers (AIs) are secreted into the environment in a cell density-dependent manner (reviewed in references 23 and 50). Two distinct QSMs, the C. albicans self-generated sesquiterpene farnesol and the Pseudomonas aeruginosa secreted 3-oxo-C12-homoserine lactone (HSL), inhibit the yeast-to-hyphal transition in C. albicans morphogenesis (24, 25). Farnesol and 3-oxo-C12-HSL both contain a 12-carbon backbone, and they both appear to influence morphogenesis by inhibiting the Candida cAMP/PKA pathway (11). Dodecanol, a 12-carbon alcohol which is not a physiologically relevant QSM, also has activity against C. albicans filamentation (24), and it, too, appears to inhibit the cAMP/PKA pathway (11). Due to their similarities, dodecanol is routinely used as a substitute for 3-oxo-C12-HSL (11, 24). However, the exact mechanism by which these QSMs mediate their effects on C. albicans remains unknown. For example, additional pathways, including the MAPK- and Tup1p-dependent signaling cascades, have also been shown to play a role in QS (26, 46), suggesting that there is a considerable amount of cross talk between the systems or that QSMs can inhibit multiple processes contributing to filamentation.
Here, we demonstrate that farnesol, 3-oxo-C12-HSL, and dodecanol regulate morphogenesis in C. albicans through independent mechanisms mediated via the cAMP-dependent signaling cascade. Farnesol and 3-oxo-C12-HSL directly inhibit the adenylyl cyclase, Cyr1p, while dodecanol prevents cAMP-dependent hyphal development through a process dependent upon the transcriptional repressor SFL1. Dodecanol may serve as a model for the action of other quorum-sensing molecules, including the Burkholderia cenocepacia diffusible signal factor BDSF, also acting via Sfl1p.
MATERIALS AND METHODS
Strains and media.
C. albicans strains were maintained as glycerol stocks at −80°C and cultured on YPD medium (1% yeast extract, 1% Bacto peptone, 2% glucose, 2% agar) when required. Strains used for quorum-sensing assays were never more than 10 days old. To identify C. albicans components that are involved in mediating its response to QSMs, we systematically screened a C. albicans knockout library for strains with altered responses to either 150 μM farnesol (mixed isomers; Sigma F203) or 200 μM dodecanol (Sigma) in a plate-based filamentation assay (see below). This library consisted of 158 C. albicans nonessential transcription factor mutants (provided by D. Sanglard). The gene deletions in the library were constructed in the BWP17 strain either by UAU transposon mutagenesis (8), URA-blaster (16), or HIS3/ARG4 cassette technologies (17). Strain BWP17AHU was used as the parental control for these strains (20); other strains are listed in Table 1.
Table 1.
Strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| BWP17AHU | ura3::imm434/URA3 arg4::hisG/ARG4 his1::hisG/HIS1 | 20 |
| CAL2 | ura3::imm434/ura3::imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG sfl1::ARG4/sfl1::HIS1 | 33 |
| RAH20 | ura3::imm434/ura3::imm434 cyr1::hisG/cyr1::hisG (pSM2) | 21 |
| RAH40 | ura3::imm434/ura3::imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG sfl1::ARG4/sfl1::HIS1 (pSM2) | This study |
| RAH41 | ura3::imm434/ura3::imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG sfl1::ARG4/sfl1::HIS1 (pSM2-SFL1) | This study |
| RAH42 | ura3::imm434/ura3::imm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG sfl1::ARG4/sfl1::HIS1 (pGFP-SFL1) | This study |
Quorum-sensing assays were performed on Dulbecco's modified Eagle medium ([DMEM] 1.34% DMEM [Gibco], 3.57% HEPES supplemented to a final concentration of 2% glucose, pH 7) without bicarbonate and pyruvate and supplemented with 5% horse serum and either 150 μM farnesol (mixed isomer) or 200 μM dodecanol. Higher concentrations of QSMs were required to inhibit hypha formation due to the sequestering effects of the albumin, as previously reported (41). QSMs were diluted in 100% methanol (farnesol and dodecanol) or ethyl acetate (C12-HSL; Sigma) immediately prior to addition to the media. For cAMP spiking experiments, dibutyryl-cAMP (dbcAMP) was supplemented into DMEM agar to a final concentration of 10 mM. In brief, colonies from fresh YPD agar were serially diluted in water to the desired cell concentration and plated onto the plates to generate single colonies. Plates were incubated at 37°C for 24 h. Where CO2 was used to induce morphogenesis, strains were plated onto DMEM agar supplemented with methanol, 150 μM farnesol, and 200 μM dodecanol and incubated at 37°C in 5% (vol/vol) CO2.
Plasmid and strain construction.
The URA3 integration vector pSM2 (13) was used to complement uridine auxotrophies and was the base vector for the generation of integration cassettes. Integration of the cassettes was confirmed by diagnostic PCR, and expression levels were analyzed by reverse transcription-PCR (RT-PCR) (data not shown). To complement CAL2 (sfl1Δ ura3Δ) with a single copy of SFL1, a 4.4-kb fragment including the complete open reading frame of SFL1, 1.3 kb of upstream sequence, and 556 bp of downstream sequence was amplified from genomic DNA using the primers 5′-ggagaggcggccgcCTTTGCGCAGGAAATAGAGAAAG and 5′-ggagaggcggccgcCCAAGATTATAAATCGATTGCAT (lowercase indicates introduced restriction enzymes) and ligated into the pSM2 vector as an NotI fragment generating plasmid pSM2-SFL1. pSM2-SFL1 was integrated into the URA3 locus of CAL2 by standard heat shock procedures (55) generating strain RAH41.
To generate Sfl1p as a C-terminally tagged green fluorescent protein (GFP) fusion protein, GFP together with 60 bp of the ADH1 terminator was cleaved from the pGFP vector (4) as a SpeI-BamHI fragment and ligated into the C. albicans URA3 integration vector, pSM2, to generate plasmid pGFP-RH. The open reading frame of SFL1 (minus stop codon), including 1.3 kb of its native promoter, was cloned into the pGFP-RH plasmid as an NotI-SpeI fragment generating plasmid pGFP-Sfl1, which was integrated at the URA3 locus of CAL2, generating strain RAH42.
Activity testing of Cyr1p.
A fragment encoding amino acids 1166 to 1571 (containing the catalytic domain) of C. albicans Cyr1p was purified, and the catalytic activity was measured in the absence/presence of QSMs or alternative 12-carbon compounds as previously described (27). In brief, 100 ng of purified protein was incubated with the QSMs or solvent control in the presence of 50 mM Tris-HCl (pH 7.5), 10 mM ATP, and 10 mM MgCl2 and the indicated concentrations of NaHCO3 in a final volume of 100 μl. Reaction mixtures were incubated at 30°C for 30 min and stopped by the addition of 100 μl of 0.2 N HCl. The produced cAMP was measured as previously described (22) by a Correlate-EIA Direct cAMP enzyme immunoassay kit (Assay Designs), and data analyses were performed with GraphPad Prism, version 4.0a.
Localization of Sfl1p.
RAH42 was grown in yeast nitrogen base (YNB) medium (0.67% YNB without amino acids with ammonium sulfate [Difco], 2% sterile filtered glucose) for 16 h at 30°C and 150 rpm and diluted (optical density at 600 nm [OD600] of 0.1) in fresh YNB (yeast) and YNB medium supplemented with 5% horse serum (hyphae) and grown at 37°C and 150 rpm until mid-exponential phase (OD600 of 0.6); then, 1 μg/ml of 4′,6′-diamidino-2-phenylindole (DAPI) was added to the culture, which was incubated for a further 20 min. Then cells were harvested by centrifugation and washed three times with water. Cells were visualized using an Olympus IX-81 fluorescence microscope with a 150-W xenon-mercury lamp and an Olympus 150× Plan NeoFluor oil immersion objective. To determine the effects of QSMs on Sfl1p localization, cultures were supplemented with 150 μM farnesol and 200 μM dodecanol.
Coincubation of B. cenocepacia and C. albicans.
A total of 500 cells of C. albicans strains BWP17AHU, RAH40, and RAH41 were plated onto DMEM agar (pH 7) supplemented with 5% horse serum. Ten microliters of a 10-times-concentrated overnight culture (OD600 of 30) of B. cenocepacia strain J2315 (a cystic fibrosis clinical isolate [18]) was spotted onto the center of each plate, and the plate was incubated at 37°C for 24 h. Images were taken of C. albicans strains in close proximity to the B. cenocepacia and at distal sites.
Hydrogen peroxide sensitivity assay.
BWP17AHU, RAH40, and RAH41 were grown in YNB medium at 30°C for 16 h and then inoculated into 50 ml of YPD medium (containing a volume of methanol equivalent to that used to dissolve the QSMs) or YPD medium supplemented with either 50 μM farnesol or 66 μM dodecanol to an initial OD600 of 0.1. After 10 min of growth at 37°C, 5% horse serum was added to the cultures, and samples were incubated at 37°C for 2 h. Then, cultures were split into two equal volumes and centrifuged for 5 min at 3,500 rpm before being resuspended in 5 ml of YPD medium or 5 ml of YPD medium supplemented with 10 mM H2O2 and incubated at 30°C for 90 min. Cells were harvested by centrifugation (5 min at 3,500 rpm) and resuspended in 25 ml of water; then 50 μl of 1:100 and 1:1,000 dilutions was plated onto YPD medium. The number of CFU was determined after 24 h of incubation at 37°C.
Semiquantitative RT-PCR.
Overnight cultures of BWP17AHU in YPD medium were diluted in fresh YPD medium supplemented with 5% horse serum in the presence of either methanol (a volume equal to that used to dissolve QSM), 150 μM farnesol, or 200 μM dodecanol to an OD600 of 0.1. Cultures were grown at 37°C and 200 rpm for 4 h. Cells were harvested by centrifugation (5 min at 3,500 rpm) and immediately frozen in liquid nitrogen. Samples were homogenized using a Mikro dismembrator (2 min, 3,000 rpm), and total was RNA extracted with a Qiagen RNeasy kit following the recommended protocol for filamentous fungi. RT-PCR was performed with an iScript One-step RT-PCR kit with Sybr Green (Bio-Rad) using 30 ng of total RNA according to the manufacturer's recommendations. SFL1 was amplified with the primers (annealing temperature, 58°C) SFL1-F (TCAACACCAACTCACCCACCTACA) and SFL1-R (GTTGATTCGGCTCGATTGCGGAAA), and levels were normalized to ACT1.
RESULTS
Exogenous cAMP inhibits quorum sensing in response to physiological stimuli.
It has been previously shown that membrane-permeable cAMP (dbcAMP) can restore N-acetylglucosamine-induced hyphal formation in the presence of farnesol (11). To determine whether exogenous cAMP could also restore the filamentation response to host environmental signals blocked by QSMs, C. albicans was grown in medium containing 5% serum in the presence of farnesol and C12-HSL. Supplementation of medium with dbcAMP restored filamentation (Fig. 1A), confirming that exogenous cAMP can restore hyphal formation in response to host environmental cues.
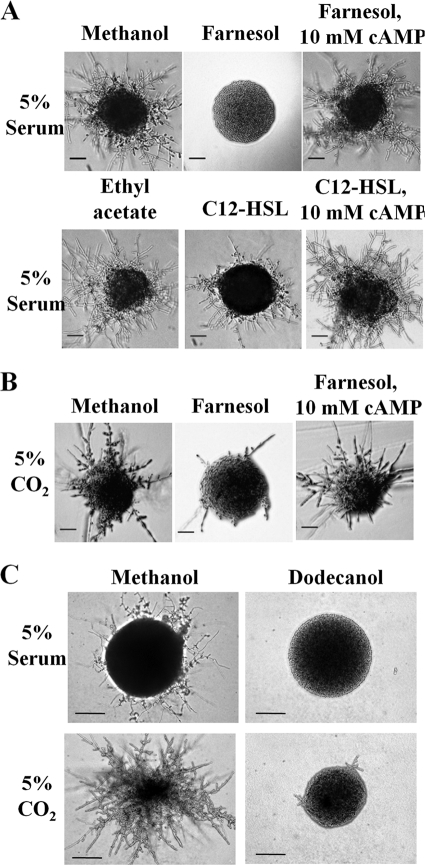
Fig. 1.
Farnesol, C12-HSL, and dodecanol modulate the cAMP/PKA pathway to inhibit morphogenesis in response to host environmental conditions. (A) C. albicans was inoculated onto DMEM agar, pH 7, supplemented with 5% horse serum and grown in the presence of methanol (vehicle control), 150 μM farnesol, 150 μM farnesol supplemented with 10 mM dbcAMP, ethyl acetate (vehicle control), 200 μM C12-HSL, and 200 μM C12-HSL supplemented with 10 mM dbcAMP and incubated at 37°C for 24 h. Scale bar, 500 μm. (B) C. albicans was inoculated onto DMEM agar, pH 7, supplemented with 5% CO2 in the presence of methanol (vehicle control), 150 μM farnesol, and 150 μM farnesol supplemented with 10 mM dbcAMP and incubated at 37°C for 24 h. Scale bar, 500 μm. (C) C. albicans was resuspended in water, serially diluted to obtain 200 CFU/plate, and incubated on DMEM agar, pH 7, supplemented with either methanol or 200 μM dodecanol in the presence of 5% horse serum or 5% CO2 and incubated at 37°C for 16 h. Scale bar, 100 μm.
As farnesol is hydrophobic, it is possible that it associates with cell membranes (51). Ras1p, a small GTPase usually attached to the cell membrane by a palmitoyl lipid moiety (36), is an essential component of both the MAPK and cAMP/PKA signaling cascades (15, 31). Therefore, one possible mode of action for farnesol-mediated inhibition of hyphal formation is that farnesol prevents Ras1p from dissociating from the membrane to activate downstream effectors (11, 40). To test this hypothesis, we explored the ability of farnesol to inhibit CO2-mediated hyphal induction. CO2 is also a host environmental factor which is a potent inducer of C. albicans morphogenesis; it has been shown to activate the cAMP/PKA cascade through carbonic anhydrase-dependent conversion into bicarbonate, which directly modulates the activity of the Candida adenylyl cyclase, Cyr1p (21, 27). Farnesol and dodecanol were able to inhibit CO2-mediated hyphal development when supplemented into the medium at the same concentrations as those used for other hypha-inducing cues (Fig. 1B and C). As Ras1p activity is not required for CO2 induced morphogenesis (27), we conclude that farnesol and dodecanol are able to exert their effects through the cAMP signaling pathway but via methods other than inhibiting Ras1p activity.
Dodecanol enhances C. albicans resistance to oxidative stress.
Previously, it has been shown that exposure to farnesol can enhance oxidative stress resistance of C. albicans (12, 57). To identify whether dodecanol is also able to induce oxidative stress resistance, we pretreated exponentially growing C. albicans cells with dodecanol prior to exposing the cells to oxidative stress. Addition of dodecanol resulted in a 2.6-fold increase in stress resistance, similar to that observed for farnesol (Fig. 2). Therefore, both farnesol and dodecanol can enhance C. albicans oxidative stress resistance.
Fig. 2.
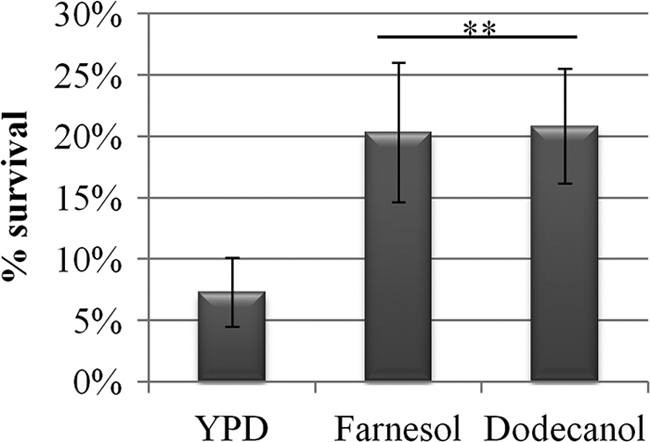
Farnesol and dodecanol enhance C. albicans oxidative stress resistance. C. albicans (BWP17AHU; wild type) was grown in YPD medium and 5% serum supplemented with solvent alone, 50 μM farnesol, or 66 μM dodecanol. Cultures were split in two, and one set was exposed to 10 mM H2O2 for 90 min, while the other set was maintained at 37°C. CFU were counted to determine cell survival, and data are expressed as percent survival of the untreated control samples. **, P < 0.05.
Farnesol and 3-oxo-C12-HSL, but not dodecanol, directly target Cyr1p.
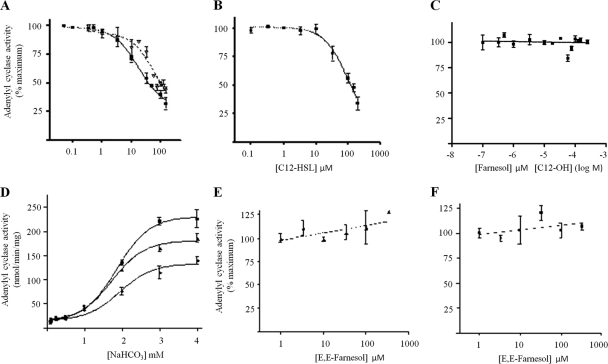
The adenylyl cyclase Cyr1p is an environmental multisensor that directly responds to host environmental cues, including serum, peptidoglycan, and elevated CO2 through different protein domains (14, 21, 27, 58). As the effects of quorum sensing in C. albicans occur through inhibition of cAMP signaling cascades and as addition of exogenous cAMP is sufficient to restore hyphal development (11), we decided to test whether QSMs directly target Cyr1p. While full-length, native Cyr1p has proven to be recalcitrant to purification, truncated recombinant Cyr1p (amino acids [aa]1166 to 1571) consisting almost exclusively of the catalytic domain has been successfully purified and was previously shown to be responsive to environmental concentrations of CO2 (27). This recombinant Cyr1p protein was directly inhibited by farnesol, with the bioactive (E,E) isomer displaying three times more potency than the isomeric mixture (50% inhibitory concentration [IC50] of 17 μM versus 60 μM) (Fig. 3A). These data strongly suggest that farnesol mediates its quorum-sensing effects by directly inhibiting the activity of Cyr1p.
Fig. 3.
Farnesol and 3-oxo-C12-homoserine lactone directly inhibit Cyr1p but not other soluble adenylyl cyclases. The activity of purified recombinant C. albicans Cyr1p (fragment encoding amino acids 1166 to 1571) was assayed with 5 mM ATP and 5 mM MgCl2 in the presence of the indicated concentrations of farnesol [▪, (E,E)-farnesol; ▴, isomeric mixture of farnesol] (A), C12-HSL (B), dodecanol (C), and of various bicarbonate concentrations [▪, solvent control; ▴,16.65 μM E,E-farnesol; ⧫, 50 μM E,E-farnesol] (D). (E) Activity of purified glutathione S-transferase (GST)-truncated rat sAC, in the presence of E,E-farnesol. (F) Activity of purified CyaC in the presence of E,E-farnesol. Data are expressed as percentage of activity in the presence of vehicle alone (ethanol for farnesol and dodecanol and DMSO for C12-HSL), and values are averages derived from four independent experiments, each performed in duplicate. Error bars represent standard error of the mean.
To determine whether farnesol inhibition is a general property of bicarbonate-responsive adenylyl cyclases, we tested both the mammalian and the cyanobacterial soluble adenylyl cyclase (sAC), CyaC. Neither of the two bicarbonate-responsive sACs was inhibited by farnesol (at concentrations up to 250 μM) (Fig. 3E and F). Therefore, farnesol inhibition is a unique property of the C. albicans adenylyl cyclase, Cyr1p.
As CO2 and farnesol directly impact on Cyr1p activity, the relationship between them was investigated. Farnesol and bicarbonate noncompetitively interacted with Cyr1p (Fig. 3D), suggesting that although they both bind within the catalytic domain, they bind different sites. In agreement with this, the strain CAI4-CYR11373, which has previously been shown to have reduced response to CO2 (21), was fully responsive to farnesol (data not shown). Therefore, QSMs bind to the catalytic domain of Cyr1p at a site distal to that of the CO2 binding site.
The QSM secreted by P. aeruginosa, 3-oxo-C12-HSL, also inhibits hyphal development in C. albicans (24). Therefore, we tested whether this QSM also inhibits the catalytic activity of Cyr1p. The commercially available bioactive analogue, C12 homoserine lactone (C12-HSL), directly inhibited the activity of recombinant Cyr1p (IC50 of 90 μM) (Fig. 3B). Therefore, we propose that farnesol and 3-oxo-C12-HSL mediate their effects by directly inhibiting the activity of Cyr1p, hence blocking the cAMP signaling response to environmental stimuli.
Farnesol and 3-oxo-C12-HSL share structural similarity; they both have 12-carbon backbones. Although in vivo assays have been performed with farnesol analogues (52), we decided to explore the generality of Cyr1p inhibition by other 12-carbon backbone molecules. We screened a number of selected 12-carbon molecules for their ability to directly modulate the activity of Cyr1p in vitro. However, the majority of the compounds tested did not have biological activity against Cyr1p activation (Table 2). These data did provide insight into the structure-activity relationship of the interaction, with the terminal hydroxyl group as well as the length of the carbon chain being important for both biochemical and biological activities. Most importantly, these data reveal a direct correlation between the inhibitory potential of Cyr1p protein and the ability to block filamentation.
Table 2.
Inhibition of Cyr1p activity
| Compound | IC50 Cyr1p activity | Range of effective concna | Reference |
|---|---|---|---|
| (E,E)-Farnesol | 17 μM | ∼1 μM | 25, 41 |
| Farnesol (isomer mix) | 60 μM | ∼3–5 μM | 52 |
| Farnesoic acid | Inert (>250 μM) | ∼40 μMb | 52 |
| Nerolidol | 100 μM | ∼60 μM | 25 |
| Geraniol | Inert (≫250 μM) | Inert | 25 |
| C12-HSL | 90 μM | ≤200 μM | 24 |
| C10-HSL | Inert (≫1 mM) | Inert (≫1 mM) | 24 |
| Decanol | Inert (≫250 μM) | Inert (≫1 mM) | 24 |
| Dodecanol | Inert (≫250 μM) | ≤200 μM | 24 |
| Tetradecanol | Inert (≫250 μM) | Inert (≫1 mM) | 24 |
| Dodecanoate | Inert (≫250 μM) | ||
| Decanoate | Inert (≫250 μM) |
Concentrations effective at inhibiting the yeast-to-hyphal transition in cell-based assays, which vary depending upon growth and assay conditions, especially the presence or absence of albumin and/or serum in medium. Where possible, we report the values determined in the absence of albumin or serum.
It is likely that the residual activity of farnesoic acid in vivo is due to conversion of farnesoic acid into farnesol via endogenous alcohol dehydrogenases.
Surprisingly, dodecanol, a 12-carbon alcohol, did not affect the activity of Cyr1p (IC50 of >200 μM) (Fig. 3C) even though dodecanol has been shown to have QSM activity against C. albicans hyphal formation through inhibition of cAMP signaling (11). These biochemical studies suggest that 3-oxo-C12-HSL is unique in its ability to mimic the actions of farnesol by binding to the farnesol inhibitory site in Cyr1p, and they demonstrate that although dodecanol is structurally related to farnesol and 3-oxo-C12-HSL (12-carbon backbone) and inhibits C. albicans hyphal development by inhibiting cAMP signaling, its mechanism of action is distinct.
Dodecanol inhibits hyphal development through Sfl1p.
To identify how dodecanol mediates its effects on hyphal development, we screened a library of C. albicans strains containing mutations in almost all of the known transcription factors on hypha-inducing medium in the presence or absence of 150 μM farnesol and 200 μM dodecanol. From the 158 strains tested, only strains containing a deletion in SFL1 displayed a dodecanol-specific, attenuated response to QSMs, with hyphal development inhibited by farnesol but not by dodecanol.
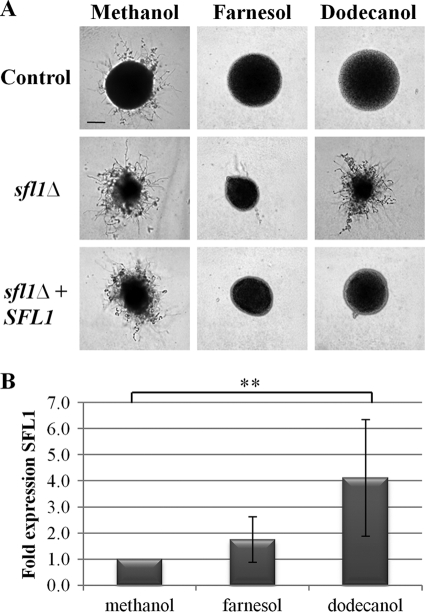
To confirm the involvement of SFL1 in quorum sensing, an independently constructed sfl1Δ strain, RAH40, was grown on hypha-inducing medium in the presence and absence of QSMs. RAH40 displayed an attenuated response to dodecanol, maintaining a filamentous phenotype in the presence of 200 μM dodecanol, but remained fully responsive to farnesol. Integration of a single copy of SFL1 under its native promoter (RAH41) was sufficient to restore the response to dodecanol (Fig. 4A).
Fig. 4.
Sfl1p is required for quorum sensing in response to dodecanol but not farnesol. (A) C. albicans strains (control, BWP17AHU strain; sfl1Δ, RAH40 strain; and sfl1Δ % SFL1, RAH41 strain) were plated onto DMEM (pH 7) with 5% serum. Plates were supplemented with methanol, 150 μM farnesol, or 200 μM dodecanol and incubated at 37°C for 24 h. Scale bar, 100 μm. (B) Total RNA was extracted from BWP17AHU grown in YPD medium supplemented with 5% serum and either methanol, 150 μM farnesol, or 200 μM dodecanol for 4 h at 37°C, and SFL1 levels were determined by semiquantitative RT-PCR. **, P < 0.05.
To identify whether QSMs differentially regulate SFL1, semiquantitative RT-PCR was performed on total RNA extracted from the control strain exposed to methanol, 150 μM farnesol, and 200 μM dodecanol. SFL1 was upregulated in response to dodecanol, but not farnesol, compared to the solvent control (Fig. 4B), further confirming the specificity of SFL1 in the QS response to dodecanol.
Although SFL1 is described as a negative regulator of filamentation, its effects are more subtle than those of Tup1p as deletion of SFL1 does not result in constitutive filamentation (5, 33). Therefore, the observed phenotype in response to dodecanol is not a consequence of the strain being locked in hyphal growth but is specifically an inability to respond to dodecanol. Furthermore, the observation that RAH40 remains responsive to farnesol confirms that dodecanol and farnesol mediate their effects via distinct mechanisms.
Sfl1p is localized in the nucleus.
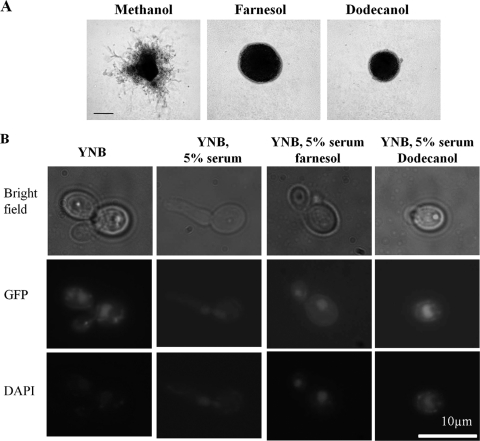
Previous studies have shown that Sfl1p is localized to the nucleus in both yeast and hyphal cells (33). However, other reports suggest that Sfl1p is not expressed in hyphae (5). To confirm the localization of Sfl1p in hyphal cells, we expressed Sfl1p as a C-terminal GFP fusion protein from the SFL1 promoter in the sfl1Δ strain (RAH42). To confirm that the GFP fusion protein was functional, the strain was screened on hypha-inducing medium in the presence and absence of QSMs. RAH42 was fully responsive to farnesol and dodecanol (Fig. 5A), confirming that the fusion protein is both expressed and functional. To analyze Sfl1p localization in both yeast and hyphal cells, RAH42 was grown in YNB medium at 30°C to generate yeast cells and in YNB medium supplemented with 5% serum at 37°C for hyphal cells. Sfl1p-GFP could be visualized in the nucleus of both yeast and hyphal cells (Fig. 5B). To determine whether Sfl1p localization is affected by the presence of QSMs, Sfl1p-GFP localization was assessed in the presence of 150 μM farnesol or 200 μM dodecanol. Sfl1p-GFP was localized in the nuclei of cells treated with QSMs, similar to the localizations in control samples (Fig. 5B), suggesting that these two QSMs do not influence the localization of Sfl1p.
Fig. 5.
Sfl1p is localized to the nucleus in yeast and hyphal cells in response to quorum-sensing molecules. (A) The sfl1Δ mutant carrying SFL1-GFP (RAH42) was plated onto DMEM agar (pH 7) with 5% serum. Plates were supplemented with either methanol, 150 μM farnesol, or 200 μM dodecanol and incubated at 37°C for 24 h. Scale bar, 100 μm. (B) C. albicans strains were grown in YNB medium and YNB medium supplemented with 5% serum in the presence or absence of farnesol and dodecanol for 2 h at 37°C. The nucleus was stained with DAPI.
Dodecanol may function as an analogue of a distinct QSM.
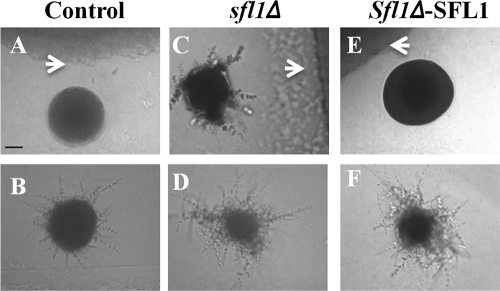
Although dodecanol is secreted at low levels during planktonic and biofilm growth by C. albicans and Candida dubliniensis (39) and has QS properties (i.e., the ability to inhibit hypha formation in C. albicans), it is currently not an established QSM. However, as a commensal C. albicans encounters many microorganisms which have developed QS systems, and it is possible that dodecanol is mimicking the activity of another biologically relevant QSM. Besides farnesol and 3-oxo-C12-HSL, other soluble molecules containing 12-carbon backbones have been shown to have QS activity against C. albicans hyphal development. For example, cis-2-dodecanoic acid (Burkholderia diffusible signal factor [BDSF]) secreted by B. cenocepacia is known to inhibit C. albicans hyphal development through an unknown mechanism (7). Therefore, we sought to identify whether Sfl1p was required for the response to B. cenocepacia and BDSF. Coincubation of C. albicans with B. cenocepacia J2315, a strain that has been shown to secrete BDSF (7), under hypha-inducing conditions confirmed that there may be a BDSF gradient around the bacteria which inhibited C. albicans hyphal development (Fig. 6A). This inhibition of morphogenesis was specific to areas surrounding B. cenocepacia as colonies distal to the bacterium were fully filamentous (Fig. 6B). However, coincubation of the sfl1Δ mutant in close proximity to B. cenocepacia did not inhibit hyphal development (Fig. 6C), and the extent of hyphal formation was similar to that in cells distal to the bacterium (Fig. 6D). Integration of a single copy of SFL1 was sufficient to restore the response to the diffusible factor produced by B. cenocepacia (Fig. 6E and F). Therefore, we conclude that B. cenocepacia and possibly BDSF inhibit C. albicans hyphal development through an SFL1-dependent signaling pathway similar to that of dodecanol.
Fig. 6.
Sfl1p is required for the response to B. cenocepacia. C. albicans was coincubated with B. cenocepacia (control, BWP17AHU strain [A and B]; sfl1Δ, RAH40 strain [C and D]; sfl1Δ-SFL1, RAH41 strain [E and F]) on DMEM agar, pH 7, supplemented with 5% serum for 24 h at 37°C. Images were taken of C. albicans cells in close proximity (top row) and distal to B. cenocepacia (bottom row). Arrowheads identify B. cenocepacia. Scale bar, 100 μm.
DISCUSSION
Colonization of host niches is the first step in establishing infection. In mammalian hosts the mucosal surface is lined with microbes which form the host's natural flora. The natural flora protects the host from infection through competition for space and nutrients. To gain a selective advantage, some microorganisms have developed quorum-sensing systems allowing them to overcome the natural flora. C. albicans is an opportunistic human pathogen and was the first eukaryote identified to have a QS system. In this system, farnesol is secreted into the environment in a biomass-dependent manner and, once reaching threshold concentrations, inhibits the yeast-to-hyphal transition (25). Farnesol is thought to allow the release of yeast cells from fungal biofilms, promoting the spread of infection (43). However, the mechanism by which this occurs is still unknown. We show here that farnesol can directly modulate the activity of the C. albicans adenylyl cyclase, reducing cAMP output to inhibit morphogenesis. Until recently Ras1p was suggested to be the mediator of QS in C. albicans. This hypothesis was based on the hydrophobicity of farnesol and on the fact that farnesol inhibits both the cAMP/PKA and Cek1p MAPK cascades, which are activated by the actions of Ras1p. However, we provide two lines of evidence that suggest that Ras1p is not the main target of farnesol. First, farnesol was able to inhibit morphogenesis induced through the actions of CO2, which does not require Ras1p, and, second, we have shown in vitro that farnesol is able to directly modulate the activity of Cyr1p. Therefore, we now propose a model (Fig. 7) whereby farnesol directly inhibits the catalytic activity of Cyr1p to reduce cytoplasmic cAMP levels and inhibit hyphal formation. In support of this model, strains carrying alleles leading to constitutive activation of Ras1p, which are hyperfilamentous due to continued activation of Cyr1p, remain fully responsive to farnesol (11).
Fig. 7.
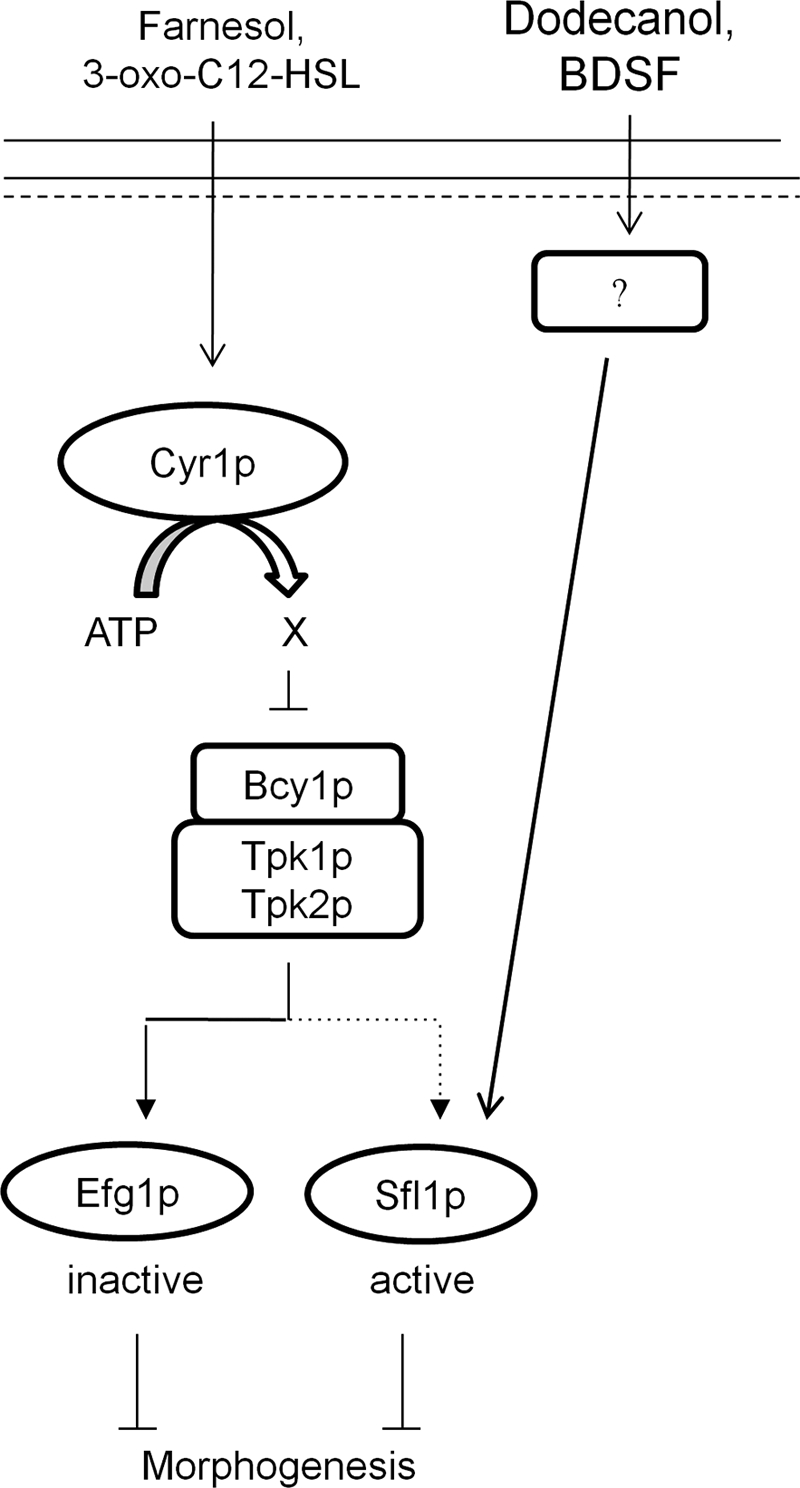
Schematic representation of how quorum-sensing molecules affect the C. albicans cAMP/PKA-dependent signaling cascade (see text for details).
C. albicans is also responsive to other QSMs including 3-oxo-C12-HSL, secreted by P. aeruginosa (24). In vitro biochemical studies confirm that this QSM also modulates the activity of Cyr1p directly, suggesting a conserved mechanism of action for QS in C. albicans. However, the 12-carbon alcohol dodecanol, which has QS activity against C. albicans, did not directly modulate the activity of Cyr1p even though its mechanism of action is dependent on the cAMP/PKA signaling cascade. However, as purification of full-length Cyr1p was unsuccessful, we cannot rule out that dodecanol inhibits the activity of Cyr1p at a site distal to that of the catalytic domain. This may include the leucine-rich region (LRR domain) which has previously been shown to directly interact with bacterial peptidoglycan to regulate cyclase activity (58). From our in vitro assays we also cannot rule out the involvement of effector proteins. For example, Cap1p has recently been shown to be required for cyclase activation under serum-inducing conditions (59). However, Cap1p is dispensable for Cyr1p activity in response to CO2 (59), suggesting that any role played by Cap1p in response to QSMs is likely to be minor.
Dodecanol appears to inhibit hyphal development through a cAMP-dependent pathway involving the transcriptional hyphal suppressor Sfl1p (Fig. 7). Therefore, Cyr1p may be only one of the receptors responsible for QS in C. albicans. In agreement with this, global transcriptional approaches and phenotypical screens have identified that the Cek1p MAPK, Tup1p, and the two-component histidine kinase cascade function in QS (26, 29, 46, 47). Although it is possible that QSMs affect multiple pathways, basal concentrations of cAMP are required for activation of these pathways, making it hard to deduce the role each pathway plays. Furthermore, as molecular mechanisms are dissected in detail, more cross talk between the pathways is emerging. For example, the cAMP/PKA pathway has recently been shown to regulate the phosphorylation of Hog1p (12). Therefore, inhibition of the cAMP/PKA pathway may impact the Cek1p-MAPK and Tup1p pathways through interactions which are currently unidentified. In agreement with this, Sfl1p is known to interact with Ssn6p to recruit the Ssn6p-Tup1p regulatory complex to promoters in Saccharomyces cerevisiae (9). Interactions between Sfl1p and the Ssn6p-Tup1p complex in C. albicans have not been studied. Therefore, it is possible that Tup1p's involvement in QS is due to disruption of the Sfl1p-Ssn6p-Tup1p complex. However, the tup1Δ mutant does not respond to either farnesol or dodecanol (26), suggesting that Tup1p plays another role in response to farnesol.
Mosel et al. have shown that farnesol is effective in inhibiting C. albicans morphogenesis at concentrations below 5 μM under certain conditions (41). However, our biochemical studies suggest that the IC50 of Cyr1p for farnesol is 17 μM. Although the IC50 may appear higher than concentrations found in vivo, our experiments were performed on a truncated protein in which several domains were absent that might be required for a full response to QS. Therefore, it might be possible that full-length Cyr1p would exhibit an IC50 nearer to in vivo concentrations. In addition, in in vivo situations QSMs appear to work by affecting several pathways (see above), so it may be possible that under these circumstances, where all components of every pathway are present, QS occurs at a much lower concentration.
Farnesol is toxic to a number of organisms as a result of the production and accumulation of reactive oxygen species (ROS) (34, 48, 49). ROS generation is due to inhibition of the electron transport chain (37, 38), suggesting that farnesol targets the mitochondria. Although it has been reported that farnesol is not toxic to C. albicans (11, 25), farnesol can still promote ROS generation (53) and have toxic side effects on C. albicans (30). Recently, it has been shown that farnesol can enhance resistance to ROS (12, 57). Initially the enhanced resistance was hypothesized to be a result of the elevated ROS inducing the expression of genes essential for ROS protection. However, Deveau et al. recently showed that the ROS generation by farnesol is not required for farnesol's impact on oxidative stress resistance (12). Here, we provide evidence that dodecanol, which has QSM activity against C. albicans, also enhanced oxidative stress resistance in C. albicans, suggesting that other QSMs may enhance C. albicans protection against ROS.
In the human host, potential outbreaks of C. albicans are controlled by circulating phagocytes, which kill the pathogen through the release of high concentrations of ROS. Therefore, infections resulting in an accumulation of fungal biomass, for example, in esophageal candidiasis, may be more resistant to phagocyte killing due to increased resistance to ROS provided by the elevated concentration of farnesol. Moreover, C. albicans infections can be a mixture of fungal and bacterial species. For example, in cystic fibrosis patients C. albicans is routinely isolated from the lungs along with P. aeruginosa and B. cenocepacia (3, 6, 19, 44), both of which produce QSMs with activity against C. albicans (7, 24). Therefore, the combination of QSMs present may affect the ability of the circulating phagocytes to kill C. albicans. In agreement with this, QSMs have been shown to inhibit macrophage ROS production and induction of macrophage apoptosis (1). Therefore, QSMs play essential roles in morphogenesis, stress resistance, and virulence through modulation of the C. albicans cAMP/PKA pathway. Further understanding of the molecular mechanisms of other QSMs (including the DSF, BDSF, and SDSF secreted by Xanthomonas campestris, B. cenocepacia, and Streptococcus species, respectively) on C. albicans morphogenesis will enhance our understanding of interkingdom communication and shed light on the effects QSMs may have on the immune response (7, 54, 56).
ACKNOWLEDGMENTS
We thank Jo Roobol and Adam Mannan for technical assistance in the early phase of this project.
This work was supported by grants from the Medical Research Council and the Biotechnology and Biological Sciences Research Council to F.A.M. and Wellcome Trust and National Institutes of Health to L.R.L. and J.B.
Footnotes
Published ahead of print on 10 June 2011.
REFERENCES
- 1. Abe S., et al. 2009. Suppression of anti-Candida activity of macrophages by a quorum-sensing molecule, farnesol, through induction of oxidative stress. Microbiol. Immunol. 53:323–330 [DOI] [PubMed] [Google Scholar]
- 2. Almirante B., et al. 2005. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 43:1829–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bakare N., Rickerts V., Bargon J., Just-Nübling G. 2003. Prevalence of Aspergillus fumigatus and other fungal species in the sputum of adult patients with cystic fibrosis. Mycoses 46:19–23 [DOI] [PubMed] [Google Scholar]
- 4. Barelle C. J., et al. 2004. GFP as a quantitative reporter of gene regulation in Candida albicans. Yeast 21:333–340 [DOI] [PubMed] [Google Scholar]
- 5. Bauer J., Wendland J. 2007. Candida albicans Sfl1 suppresses flocculation and filamentation. Eukaryot. Cell 6:1736–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bauernfeind A., et al. 1987. Qualitative and quantitative microbiological analysis of sputa of 102 patients with cystic fibrosis. Infection 15:270–277 [DOI] [PubMed] [Google Scholar]
- 7. Boon C., et al. 2008. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2:27–36 [DOI] [PubMed] [Google Scholar]
- 8. Bruno V. M., Mitchell A. P. 2004. Large-scale gene function analysis in Candida albicans. Trends Microbiol. 12:157–161 [DOI] [PubMed] [Google Scholar]
- 9. Conlan R., Tzamarias D. 2001. Sfl1 functions via the co-repressor Ssn6-Tup1 and the cAMP-dependent protein kinase Tpk2. J. Mol. Biol. 309:1007–1015 [DOI] [PubMed] [Google Scholar]
- 10. Csank C., et al. 1998. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect. Immun. 66:2713–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis-Hanna A., Piispanen A. E., Stateva L. I., Hogan D. A. 2008. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol. Microbiol. 67:47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deveau A., Piispanen A. E., Jackson A. A., Hogan D. A. 2010. Farnesol induces hydrogen peroxide resistance in Candida albicans yeast by inhibiting the Ras-cyclic AMP signaling pathway. Eukaryot. Cell 9:569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Barkani A., et al. 2000. Dominant active alleles of RIM101 (PRR2) bypass the pH restriction on filamentation of Candida albicans. Mol. Cell. Biol. 20:4635–4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang H.-M., Wang Y. 2006. RA domain-mediated interaction of Cdc35 with Ras1 is essential for increasing cellular cAMP level for Candida albicans hyphal development. Mol. Microbiol. 61:484–496 [DOI] [PubMed] [Google Scholar]
- 15. Feng Q., Summers E., Guo B., Fink G. 1999. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 181:6339–6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fonzi W. A., Irwin M. Y. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gola S., Martin R., Walther A., Dünkler A., Wendland J. 2003. New modules for PCR-based gene targeting in Candida albicans: rapid and efficient gene targeting using 100 bp of flanking homology region. Yeast 20:1339–1347 [DOI] [PubMed] [Google Scholar]
- 18. Govan J., et al. 1993. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet 342:15–19 [DOI] [PubMed] [Google Scholar]
- 19. Govan J. R., Brown A. R., Jones A. M. 2007. Evolving epidemiology of Pseudomonas aeruginosa and the Burkholderia cepacia complex in cystic fibrosis lung infection. Future Microbiol. 2:153–164 [DOI] [PubMed] [Google Scholar]
- 20. Goyard S., et al. 2008. The Yak1 kinase is involved in the initiation and maintenance of hyphal growth in Candida albicans. Mol. Biol. Cell 19:2251–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall R. A., et al. 2010. CO2 acts as a signalling molecule in populations of the fungal pathogen Candida albicans. PLoS Pathog. 6:e1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hess K. C., et al. 2005. Soluble adenylyl cyclase mediates multiple specific signaling events in sperm required for fertilization. Dev. Cell 9:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hogan D. A. 2006. Talking to themselves: autoregulation and quorum sensing in fungi. Eukaryot. Cell 5:613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hogan D. A., Vik A., Kolter R. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 54:1212–1223 [DOI] [PubMed] [Google Scholar]
- 25. Hornby J. M., et al. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kebaara B. W., et al. 2008. Candida albicans Tup1 is involved in farnesol-mediated inhibition of filamentous growth induction. Eukaryot. Cell 7:980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klengel T., et al. 2005. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr. Biol. 15:2021–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klevay M. J., et al. 2008. Therapy and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diagn. Microbiol. Infect. Dis. 60:273–277 [DOI] [PubMed] [Google Scholar]
- 29. Kruppa M., et al. 2004. The two-component signal transduction protein Chk1p regulates quorum sensing in Candida albicans. Eukaryot. Cell 3:1062–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langford M. L., Hasim S., Nickerson K. W., Atkin A. L. 2010. Activity and toxicity of farnesol towards Candida albicans are dependent on growth conditions. Antimicrob. Agents Chemother. 54:940–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leberer E., et al. 2001. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol. Microbiol. 42:673–687 [DOI] [PubMed] [Google Scholar]
- 32. Leroy O., et al. 2009. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit. Care Med. 37:1612–1618 [DOI] [PubMed] [Google Scholar]
- 33. Li Y., Su C., Mao X., Cao F., Chen J. 2007. Roles of Candida albicans Sfl1 in hyphal development. Eukaryot. Cell 6:2112–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu P., et al. 2010. Farnesol induces apoptosis and oxidative stress in the fungal pathogen Penicillium expansum. Mycologia 102:311–318 [DOI] [PubMed] [Google Scholar]
- 35. Lo H.-J., et al. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939–949 [DOI] [PubMed] [Google Scholar]
- 36. Lobo S., Greentree W. K., Linder M. E., Deschenes R. J. 2002. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 277:41268–41273 [DOI] [PubMed] [Google Scholar]
- 37. Machida K., Tanaka T. 1999. Farnesol-induced generation of reactive oxygen species dependent on mitochondrial transmembrane potential hyperpolarization mediated by F0F1-ATPase in yeast. FEBS Lett. 462:108–112 [DOI] [PubMed] [Google Scholar]
- 38. Machida K., Tanaka T., Fujita K.-I., Taniguchi M. 1998. Farnesol-induced generation of reactive oxygen species via indirect inhibition of the mitochondrial electron transport chain in the yeast Saccharomyces cerevisiae. J. Bacteriol. 180:4460–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martins M., et al. 2007. Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryot. Cell 6:2429–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGeady P., Logan D. A., Wansley D. L. 2002. A protein-farnesyl transferase inhibitor interferes with the serum-induced conversion of Candida albicans from a cellular yeast form to a filamentous form. FEMS Microbiol. Lett. 213:41–44 [DOI] [PubMed] [Google Scholar]
- 41. Mosel D. D., Dumitru R., Hornby J. M., Atkin A. L., Nickerson K. W. 2005. Farnesol concentrations required to block germ tube formation in Candida albicans in the presence and absence of serum. Appl. Environ. Microbiol. 71:4938–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Odds F. C. 1988. Candida and candidosis. A review and bibliography, 2nd ed. W. B. Saunders, London, United Kingdom. [Google Scholar]
- 43. Ramage G., Saville S. P., Wickes B. L., Lopez-Ribot J. L. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reik R., Spilker T., Lipuma, J. J. 2005. Distribution of Burkholderia cepacia complex species among isolates recovered from persons with or without cystic fibrosis. J. Clin. Microbiol. 43:2926–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rocha C. R. C., et al. 2001. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell 12:3631–3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Román E., et al. 2009. The Cek1 MAPK is a short-lived protein regulated by quorum sensing in the fungal pathogen Candida albicans. FEMS Yeast Res. 9:942–955 [DOI] [PubMed] [Google Scholar]
- 47. Sato T., Watanabe T., Mikami T., Matsumot T. 2004. Farnesol, a morphogenetic autoregulatory substance in the dimorphic fungus Candida albicans, inhibits hyphae growth through suppression of a mitogen-activated protein kinase cascade. Biol. Pharm. Bull. 27:751–752 [DOI] [PubMed] [Google Scholar]
- 48. Savoldi M., et al. 2008. Farnesol induces the transcriptional accumulation of the Aspergillus nidulans apoptosis-inducing factor (AIF)-like mitochondrial oxidoreductase. Mol. Microbiol. 70:44–59 [DOI] [PubMed] [Google Scholar]
- 49. Semighini C., Hornby J., Dumitru R., Nickerson K., Harris S. 2006. Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol. Microbiol. 59:753–764 [DOI] [PubMed] [Google Scholar]
- 50. Shank E., Kolter R. 2009. New developments in microbial interspecies signaling. Curr. Opin. Microbiol. 12:205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shchepin R., Dumitru R., Nickerson K. W., Lund M., Dussault P. H. 2005. Biologically active fluorescent farnesol analogs. Chem. Biol. 12:639–641 [DOI] [PubMed] [Google Scholar]
- 52. Shchepin R., et al. 2003. Quorum sensing in Candida albicans: probing farnesol's mode of action with 40 natural and synthetic farnesol analogs. Chem. Biol. 10:743–750 [DOI] [PubMed] [Google Scholar]
- 53. Shirtliff M. E., et al. 2009. Farnesol-induced apoptosis in Candida albicans. Antimicrob. Agents Chemother. 53:2392–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vílchez R., et al. 2010. Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). ChemBioChem. 11:1552–1562 [DOI] [PubMed] [Google Scholar]
- 55. Walther A., Wendland J. 2003. An improved transformation protocol for the human fungal pathogen Candida albicans. Curr. Genet. 42:339–343 [DOI] [PubMed] [Google Scholar]
- 56. Wang L.-H., et al. 2004. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol. Microbiol. 51:903–912 [DOI] [PubMed] [Google Scholar]
- 57. Westwater C., Balish E., Schofield D. 2005. Candida albicans-conditioned medium protects yeast cells from oxidative stress: a possible link between quorum sensing and oxidative stress resistance. Eukaryot. Cell 4:1654–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xu X.-L., et al. 2008. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host & Microbe 4:28–39 [DOI] [PubMed] [Google Scholar]
- 59. Zou H., Fang H.-M., Zhu Y., Wang Y. 2010. Candida albicans Cyr1, Cap1 and G-actin form a sensor/effector apparatus for activating cAMP synthesis in hyphal growth. Mol. Microbiol. 75:579–591 [DOI] [PubMed] [Google Scholar]