Abstract
Colletotrichum gloeosporioides is a facultative plant pathogen: it can live as a saprophyte on dead organic matter or as a pathogen on a host plant. Different patterns of conidial germination have been recognized under saprophytic and pathogenic conditions, which also determine later development. Here we describe the role of CgRac1 in regulating pathogenic germination. The hallmark of pathogenic germination is unilateral formation of a single germ tube following the first cell division. However, transgenic strains expressing a constitutively active CgRac1 (CA-CgRac1) displayed simultaneous formation of two germ tubes, with nuclei continuing to divide in both cells after the first cell division. CA-CgRac1 also caused various other abnormalities, including difficulties in establishing and maintaining cell polarity, reduced conidial and hyphal adhesion, and formation of immature appressoria. Consequently, CA-CgRac1 isolates were completely nonpathogenic. Localization studies with cyan fluorescent protein (CFP)-CgRac1 fusion protein showed that the CgRac1 protein is abundant in conidia and in hyphal tips. Although the CFP signal was equally distributed in both cells of a germinating conidium, reactive oxygen species accumulated only in the cell that produced a germ tube, indicating that CgRac1 was active only in the germinating cell. Collectively, our results show that CgRac1 is a major regulator of asymmetric development and that it is involved in the regulation of both morphogenesis and nuclear division. Modification of CgRac1 activity disrupts the morphogenetic program and prevents fungal infection.
INTRODUCTION
Asymmetric growth is perhaps the most significant principle in development. For asymmetric growth to occur, a polar axis must be established, which defines cell and organ orientation and along which growth will take place. One of the most pronounced forms of cellular asymmetry coupled to growth is hyphal elongation in filamentous fungi (20).
Hyphal growth initiates with spore germination, which marks the transition from a resting state to active development. It is a crucial stage in the fungal life cycle, and therefore fungi have evolved mechanisms to ensure that spores will respond to specific signals that are indicative of favorable growth conditions. In most cases, free sugars are necessary and sufficient for germination (29). In plant pathogens, germination can also be induced by plant-derived substances or signals, such as contact with a solid hydrophobic surface or presence of cuticular waxes (7, 23, 30). In these species, spores have evolved different patterns of germination according to the signals that activate it (1, 12).
All types of spore germination are associated with cell polarization. First, a polar site is defined inside the cell (polarity establishment), and then growth occurs along the polar axis (polarity maintenance). These processes are regulated by specialized proteins: septins, which are present at the growth sites, serve as positional landmarks for recruitment and activation of the polarity-establishing machinery (15). The Spitzenkörper, an accumulation of vesicles present at sites of polarized growth, plays an important role in hyphal polarity, acting as a vesicle supply center for the growing tip (20). Actin and the microtubule cytoskeleton allow continuous flow of secreted vesicles to the growing tip, thereby enabling germ tube extension (36). Regulation of all these activities, particularly cytoskeleton organization and vesicle trafficking, involves Rho-type GTPases (22, 27, 41).
Rho GTPases constitute a distinct subfamily within the superfamily of Ras-related small GTPases. Similar to other types of small GTPases, Rho GTPases act as binary molecular switches. They are found in all eukaryotic cells and are involved in various aspects of cellular development, particularly morphogenesis, cell cycle, and vesicular trafficking (6, 42). The best-studied members of the Rho GTPase subfamily are RhoA, Rac1, and Cdc42 (43). Budding and fission yeasts lack Rac homologs, and the Cdc42 protein is essential for their survival (14). Filamentous fungi usually have homologs of both Cdc42 and Rac (most often termed Rac1). In most filamentous species, Cdc42 proteins play a relatively minor role, whereas Rac1 proteins are central players in fungal development, particularly in the regulation of polarized growth, pathogenicity, sporulation, and spore germination (5, 8–10, 26, 32, 34, 40). Due to the central role of Rac1, deletion mutants are either nonviable or severely defective in growth and development. To circumvent this limitation, constitutively active (CA) isoforms are commonly used in functional analyses of Rac1, as well as in other small GTPases. An amino acid substitution of Val for Gly in the GTP hydrolysis site of small GTPases gives rise to a constitutively activated phenotype because the mutated protein is unable to hydrolyze GTP and therefore constitutively interacts with downstream effectors (4). Because the transition between an active and inactive state is essential for proper activity of small GTPases, including Rac, these mutations are useful in the determination of Rac-regulated processes. For example, a CA-Rac strain of Colletotrichum trifolii had aberrant hyphal morphology and reduced rates of conidial germination (8). Interestingly, the CA-Rac mutation restored the defects in hyphal morphology that are observed in a strain that expresses a CA-Ras isoform, placing Rac1 downstream of Ras in this fungus. In Ustilago maydis, Rac1 is required for pathogenicity and normal hyphal morphology (26). Deletion of Rac1 in this fungus affected cellular morphology and hyphal growth, whereas overexpression of wild-type Rac1 induced filament formation in haploid cells. Expression of CA-Rac1 in this fungus was lethal. In Claviceps purpurea, Rac1 interacts with the kinase Cla4, and both proteins have an impact on growth, vegetative differentiation, and pathogenicity (32). In Magnaporthe oryzae, Rac1 is necessary for conidial formation and contributes to pathogenicity, since strains expressing CA-Rac display defects in infection-related growth (10). Deletion of Rac1 in Aspergillus fumigatus results in a reduced growth rate and abnormal conidial formation; however, it does not affect virulence (25). In Candida albicans, Rac1 is not necessary for viability or serum-induced hyphal growth, but it is essential for filamentous growth when cells are embedded in a matrix (3).
The fungus Colletotrichum gloeosporioides f. sp. aeschynomene (referred to herein as C. gloeosporioides) is pathogenic on the legume weed Aeschynomene virginica. It was previously shown that conidia (asexual spores) of C. gloeosporioides germinate in two different patterns, which were termed “saprophytic” (or nonpathogenic) and “pathogenic” (1). Pathogenic germination occurs on plants and can be induced by stimuli that are common to plant surfaces. It is characterized by instant activation of cell division and formation of a single germ tube, which later differentiates into an infection structure (appressorium). Although the morphogenetic events are closely associated with nuclear divisions, progression of the cell cycle is dispensable for completion of pathogenic germination, as blocking of the cell cycle does not prevent germination or appressorium formation (28).
In order to gain molecular insight into pathogenic conidial germination, particularly the regulation of asymmetric germ tube formation following the first conidial cell division, we isolated and studied CgRAC1, a homolog of RAC1 in C. gloeosporioides. Here we show that conidial symmetry is already broken during the first cell division and that this early asymmetric development is regulated by CgRac1, which is also required for subsequent stages of pathogenic development. We also show that CgRac1 is involved in nuclear division during pathogenic germination and thus provides a possible link between morphogenesis and the cell cycle.
MATERIALS AND METHODS
Fungi, growth conditions, and transformation.
Emerson's YpSs (EMS), regeneration (REG), and pea extract (PE) media were prepared as previously described (1). Colletotrichum gloeosporioides f. sp. aeschynomene 3.1.3 wild-type and transgenic strains were cultured on EMS agar plates at 28°C with continuous light. Conidia were obtained from 5-day-old cultures by washing the plates with sterile water. To obtain mycelium, the fungus was cultured in 50 ml REG medium with constant agitation at 180 rpm, and the mycelium was collected after 48 h by centrifugation. Genetic transformation was performed by electroporation of germinated conidia according to the method of Robinson and Sharon (31). At least 16 independent hygromycin-resistant isolates were obtained from each type of transgenic strain and analyzed by PCR to confirm the presence of the transformed DNA.
DNA and RNA manipulations.
Genomic DNA was isolated according to the method of Barhoom and Sharon (2). Plasmid DNA was isolated using the GenElute plasmid miniprep kit (Sigma). Total RNA was extracted from dried mycelium using the GenElute mammalian total RNA miniprep kit (Sigma). For reverse transcriptase PCR (RT-PCR), cDNA sequences were generated with the Reverse-iT first-strand synthesis kit (ABgene), and PCR amplification was performed with the RT-PCR product and the appropriate primers using Taq DNA polymerase (Fermentas). Ex Taq polymerase (TaKaRa) was used for proofreading, according to the manufacturer's instructions.
Cloning of CgRAC1 and generation of transgenic strains.
Primers for amplification of CgRAC1 sequences were designed according to the CtRAC1 sequence of C. trifolii (GenBank accession no. AAP89013). Genomic and cDNA sequences of CgRAC1 were amplified by primers 1 and 2 (see Table S1 in the supplemental material for primer sequences). The PCR amplification products were cloned into the pTZ57R/T vector (Fermentas). The constitutive active (CA) form of CgRac1 was generated by site-directed mutagenesis according to the gene SOEing method (21). Primer 3, which carries a point mutation and insertion of an AatII restriction site, was used to produce the mutant clone from the vector pTZ57R-CgRac1. CA-CgRAC1 was excised from pTZ57R/T with NcoI/BamHI and introduced into pAHG4 and Ksh52-1 vectors between the Aspergillus nidulans GPDA promoter and TRPC terminator to form the pAHG4-CA-CgRac1 and Ksh-CA-CgRac1 vectors, respectively. The pAHG4 vector carries the double selection marker HPH-green fluorescent protein (HPH-GFP) fusion protein, whereas Ksh52-1 does not contain a selection marker and therefore was used in cotransformation with p57-gpd-HPH, which contains a hygromycin resistance cassette. For nuclear labeling, cotransformation was performed with pMF-280 (16), which carries the egfp gene fused to the C terminus of the histone E11-encoding gene hH1 from Neurospora crassa under the control of the N. crassa CCG-1 promoter and p57-gpd-HPH. To obtain GFP-labeled nuclei in a CA-CgRAC1 genetic background, simultaneous transformation was performed with the three vectors, pMF-280, Ksh-CA-CgRac1, and p57-gpd-HPH. For protein localization studies, an N-terminal cyan fluorescent protein (CFP)-CgRac1 fusion protein was generated. The cfp gene was amplified from pECFP-N1 (Clontech) by PCR using primers 4 and 5 and cloned into the pTZ57-CgRac1 vector in the EcoRI and XbaI sites. The CFP-CgRAC1 cassette was cut with NcoI and BamHI and inserted into Ksh52-1 to produce the Ksh-CFP-CgRac1 plasmid. A 1-kb fragment upstream of the CgRAC1 open reading frame (ORF) was amplified from genomic DNA using primers 6 and 7 and introduced at the NotI and NcoI sites of Ksh-CFP-CgRac1, replacing the GPDA promoter with the endogenous CgRAC1 promoter. CA-CgRAC1 was amplified by PCR from the pAHG4-CA-CgRac1 vector with primers 8 and 9 and inserted in place of the native CgRAC1 at the EcoRV and BamHI sites, yielding Ksh-CFP-CA-CgRac1.
Pathogenicity tests.
Whole-plant inoculations were performed by spraying 10-day-old A. virginica plants with conidial suspensions as previously described (1). Plants were sprayed to runoff with 103 to 105 conidia/ml and kept in a humid chamber for 24 h. Symptoms were evaluated after 5 to 7 days. Detached leaves (Pisum sativum cv. White sugar) and onion epidermis inoculations were performed according to the method of Nesher et al. (28). Detached pea leaves were inoculated with 5 to 10 μl of 102 to 104 conidia/ml with 0.01% Tween 20. For inoculation of wounded leaves, superficial scratches were made with a needle, and the leaves were inoculated with a 5-μl droplet containing conidia suspended in water without Tween 20.
Conidial adhesion and germination.
Germination and adhesion assays were performed as described by Chaky et al. (7). Droplets of the conidial suspension (106 conidia/ml in PE) were placed on the surface of a petri dish (hydrophobic) or on a glass slide (hydrophilic) and incubated at 28°C in a humid chamber. Conidia were counted every 30 min, washed by rinsing three times in water, and then counted again, and the percentage of attached conidia was calculated.
Microscopy.
Fluorescent and light microscopies were performed with a Zeiss AxioImager M1 microscope. Images were captured with a Zeiss AxioCam MRm camera and analyzed using the AxioVision Rel 4.5 software package. Confocal microscopy was performed with a Zeiss CLSM510 confocal microscope. Scanning electron microscopy was performed with a Hitachi S-3000N scanning electron microscope as described by Giesbert et al. (17). Samples were fixed in a glutaraldehyde solution and then dehydrated in ethanol. Critical-point drying was carried out with a K850 critical-point dryer (Emitech, Germany) and gold sputtered with vacuum sputter device K550X (Emitech).
Staining procedures.
Nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma) as previously described (1). Reactive oxygen species (ROS) were detected by staining with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen). Conidia were germinated on a glass slide for various lengths of time. H2DCFDA (10 mM stock solution in dimethyl sulfoxide [DMSO]) was added to a final concentration of 10 μM, and the samples were incubated for an additional 20 min at 28°C.
Sequence analysis.
Sequence analysis was performed using the Vector NTI Advance Suite 9 software package (InforMax, Inc.). Sequence searches were performed using BLAST algorithms. Multiple sequence alignments were performed using ClustalW.
RESULTS
Isolation and characterization of CgRAC1.
Genomic (GenBank accession no. EU364808) and cDNA (GenBank accession no. EU348861) clones of CgRAC1 were isolated and sequenced. The 199-amino acid (aa)-long predicted protein was highly homologous to Rac1 proteins from other fungi (see Fig. S1 in the supplemental material). All of the conserved motifs characteristic of Rac1 proteins were identified in the predicted CgRac1 sequence: GTP binding and hydrolysis sites (GDGAVGKT, residues 15 to 22, and DTAG, residues 62 to 65, respectively), effectors' interaction site (TVFDNY, residues 40 to 45), GDP-GTP exchange site (TKLD, residues 120 to 123), and plasma membrane association site (CTIL, residues 196 to 199). The GDP-GTP exchange site, TKLD, is unique to Rac proteins and differs from the motifs found in Cdc42 (TQXD) and the related Rho GTPase (NKXD). In order to study the regulation of pathogenic germination by CgRac1, we generated a strain that expresses a CA-CgRac1 isoform (G17 replaced with V) under the control of the strong Aspergillus nidulans GPDA promoter. Expression of the CA-CgRAC1 gene was verified in 16 independent hygromycin-resistant transgenic strains (not shown). The CA-CgRac1 colonies exhibited a typical morphology on plates, which distinguished them from wild-type cultures (Fig. 1 A). Detailed analyses were conducted with isolates CA19 and CA27, and the two strains showed similar phenotypes. For convenience, only results from strain CA27 are presented.
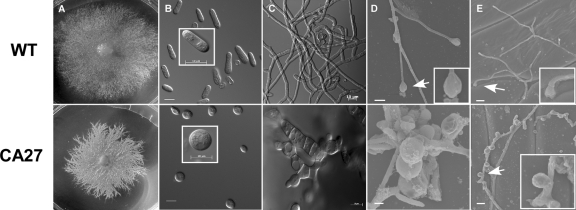
Fig. 1.
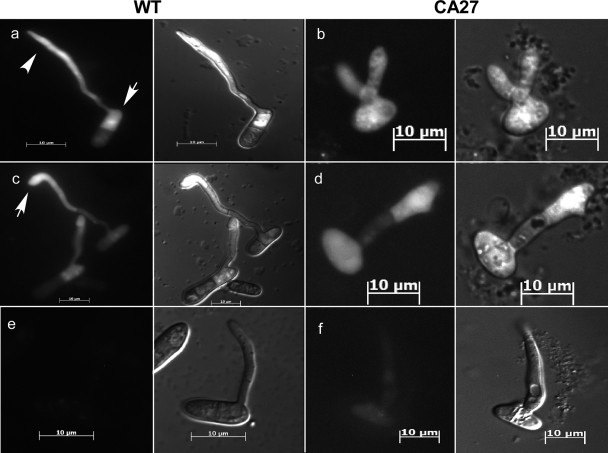
Morphology of CA-CgRac1 strains. (A) Colony morphology of wild-type (WT) and CA-CgRac1 (CA27) strains. Fungi were cultured for 7 days on EMS agar medium. Plates were incubated at 28°C with continuous fluorescent light. (B) Images of wild-type and CA-CgRac1 conidia (light microscope; magnification, ×1,000). Insets show enlarged images of a representative cell in each strain. For quantitative estimation of conidial morphology, the dimensions of 100 wild-type and CA-CgRac1 conidia were measured. Wild-type conidia had an average ratio between width and length of 0.333 ± 0.096. Only 7% of the conidia of the CA-CgRac1 strain had a wild-type phenotype (ratio = 0.333), and the rest had a higher ratio, with 56% of the conidia having a ratio greater than 0.6. (C) Images of hyphae following 24 h of germination on a glass slide (confocal microscope; magnification, ×630). Appressorium formation is shown on polypropylene after 24 h (D) and on onion epidermis after 16 h (scanning electron microscope) (E). Arrows point to appressoria (wild type) and appressorium-like structures (CA-CgRac1) that are presented as insets.
CgRac1 is important for growth and morphology.
The CA-CgRac1 strains exhibited reduced growth rate on plates (34 ± 2 mm) and produced less biomass in liquid culture (10 ± 2 mg) than the wild type (40 ± 2 mm and 15 ± 2 mg, respectively). In accordance with the role of Rac1 in determining cell polarity, cells of the CA-CgRac1 strains had polarity defects and a more rounded shape than wild-type cells. In particular, CA-CgRac1 conidia were swollen and had near-isometric dimensions (ratio between width and length, >0.6), unlike the polar conidia of the wild-type strain (ratio between width and length, 0.333 ± 0.096) (Fig. 1B). The CA-CgRac1 strains also formed swollen, round, hyphal cells (Fig. 1C), as well as a class of structures that resembled immature appressoria, but instead of a single appressorium at the end of a germ tube, these appressorium-like structures seemed to bud from random sites along the hyphae and formed bundles of cells (Fig. 1D and E).
CgRac1 regulates pathogenic germination.
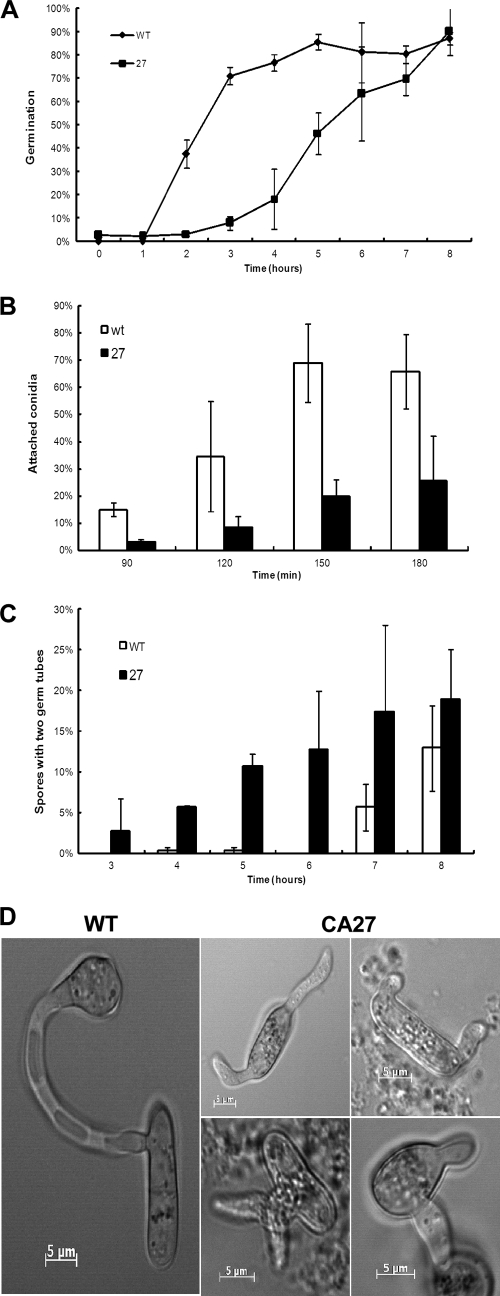
Conidia of the CA-CgRac1 strains showed delayed pathogenic germination: only 25% of conidia of the CA-CgRac1 strain germinated after 4 h on a hydrophobic surface in PE medium (conditions that induce pathogenic germination) compared with 75% germination rates after 4 h for the wild-type conidia (Fig. 2A) . Germination rates of the CA-CgRac1 strains reached wild-type levels after 8 h of incubation, suggesting that germination in the former was delayed but not blocked. CA-CgRac1 conidia were severely compromised in their ability to form a close contact with the surface, as revealed by an adhesion assay: after 150 min of incubation, less than 20% of the CA-CgRac1 conidia remained attached to the surface of a petri dish, compared to over 65% of the wild-type conidia (Fig. 2B). Close contact with the surface is essential for pathogenic germination, and therefore defects in adhesion might account for the low germination rates of CA-CgRac1 strains during the first hours. Nonpathogenic germination, which is slower but unaffected by surface contact, might account for the later increase in conidial germination in this strain (Fig. 2A).
Fig. 2.
Conidial germination in CA-CgRac1 strains. Conidia were suspended in PE medium, and 50-μl droplets of conidial suspension were applied to a glass slide and incubated in a humid chamber at 28°C. The percentages of conidial germination (A), attached conidia (B), and conidia that produced two germ tubes (C) were evaluated at time intervals during a period of up to 8 h. (D) Pictures of conidia after 5 h of incubation. Wild-type (WT) conidia produced a single germ tube that is attached to the surface and differentiate an appressorium. Conidia of CA-CgRac1 (CA27) strains are swollen; they produce two germ tubes that might not be attached to the surface and do not differentiate appressoria. Results in panels A to C are average results for two independent experiments, each with 3 replications per treatment. Mean values (A to C) and standard errors (A) are presented.
During pathogenic germination, the unicellular conidium divides, and then only one of the resulting cells germinates. Nuclear division in the other cell is arrested, and the cell remains inactive but can resume growth at a later stage (28). Remarkably, this pattern was disrupted in the CA-CgRac1 strain: up to 20% of the CA-CgRac1 conidia germinated by simultaneous formation of two germ tubes, one from each cell (Fig. 2C).
Taken together, these results show the importance of CgRac1 for proper morphogenesis during pathogenic germination. It might also be that cell cycle progression is altered in CA-CgRac1 strains because of the tight link between morphogenesis and nuclear division (28).
Appressorium formation is distorted in CA-CgRac1 mutants.
When germinated on leaves or a hard surface, C. gloeosporioides differentiates large melanized appressoria, which are necessary for penetration of the host cuticle. Appressorium formation represents a distinct morphogenetic stage and is associated with a switch from polar to isotropic growth. The cell cycle is also arrested, and nuclear division resumes only when a penetration peg is formed at the base of the appressorium (28). CA-CgRac1 strains failed to form normal appressoria. Instead, partly melanized, rounded cells were formed in different locations along the hyphae. Chains of single rounded cells or patches of several cells that seemed to bud from one another were observed (Fig. 1C and 3B). Scanning electron microscopy (SEM) observations showed that the cells might have lost topographical orientation, thus forming piles of such organs (Fig. 1D and E). These cells usually contained several nuclei, unlike normal appressoria, which always contain a single nucleus (see Fig. 4A). Thus, the CA-CgRac1 strains do not form normal appressoria, and the abnormal rounded cells, which might represent defective appressoria, seem to be nonfunctional.
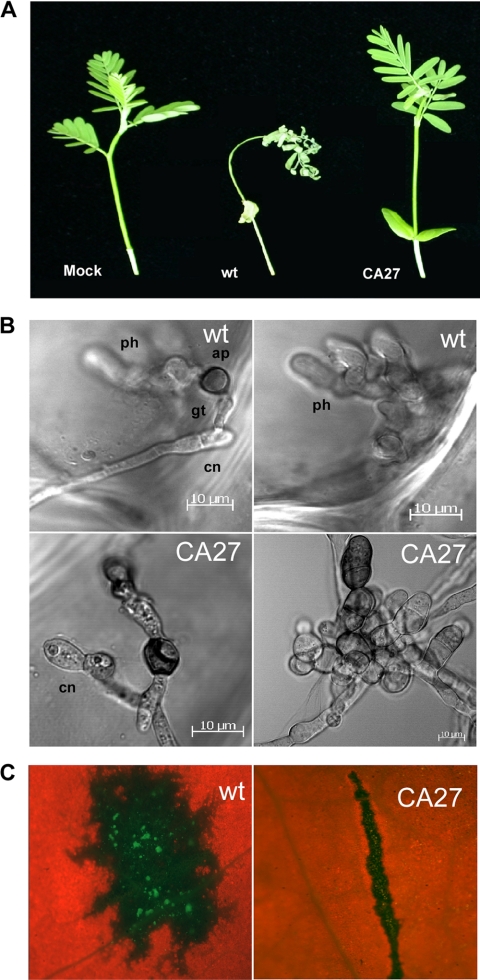
Fig. 3.
Infection assays. (A) Infection of whole plants. A. virginica plants were inoculated by spraying to runoff with 105 conidia/ml. Pictures were taken 7 days postinoculation. (B) Onion epidermis infection assays. Conidia of the wild type (wt) and CA-CgRac1 strain 27 (CA27) were applied to onion epidermis. Images were captured 24 h postinoculation. The top left panel shows a wild-type conidium (cn) that produces a germ tube (gt) and an appressorium (ap). The top right panel shows development of subcuticular primary hyphae (ph) underneath the appressorium. (C) Invasive growth assay of wounded pea leaves. Leaves were scratched using a needle, and conidia (103 conidia/ml) were applied to the wounded area. The inoculated leaves were incubated for 48 h in a humid chamber. Images were captured after 48 h using a fluorescent stereoscope.
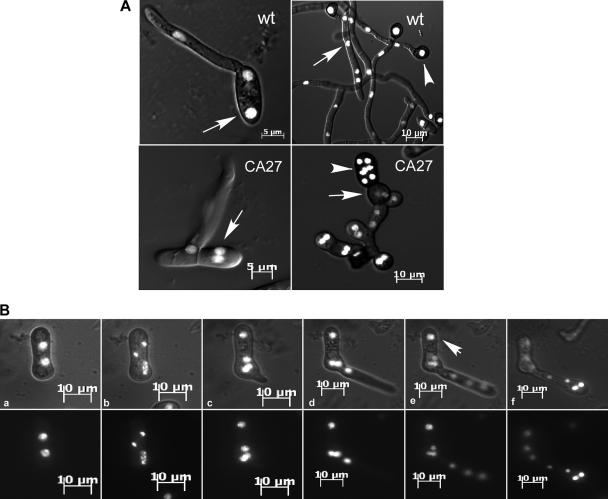
Fig. 4.
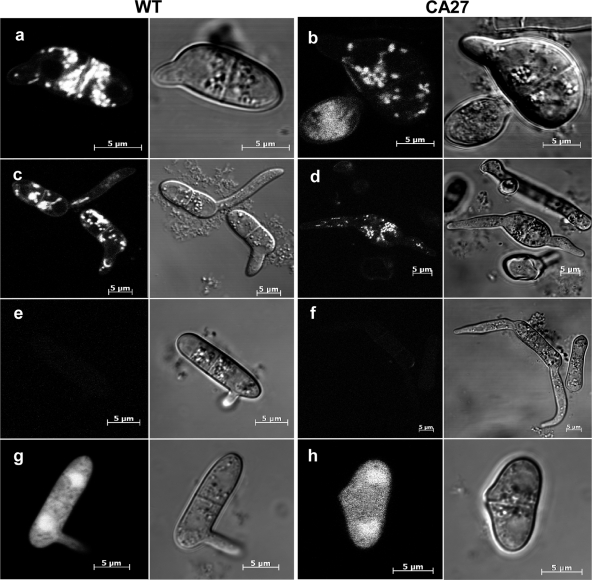
CgRac1 effect on cell cycle and nuclear division. (A) Nuclei division in germinating conidia and in developing hyphae. Cells were germinated for 6 h (left) and 24 h (right). In the wild type (wt), the nucleus does not divide in the conidial cell that does not germinate (top left, arrow) or in subapical hyphal cells (top right, arrow), and wild-type appressoria always contain a single nucleus (top right, arrowhead). In CA-CgRac1 (CA27) strains, the nuclei in the conidial cell that does not germinate (bottom left, arrow) and in subapical hyphal cells (bottom right, arrow) continue to divide, resulting in multinucleated cells. Appressorium-like structures may contain numerous nuclei (bottom right, arrowhead). (B) Live-cell imaging of nuclear division during pathogenic germination of CA-CgRac1 for 6 h after germination induction. Merged differential interference contrast (DIC) and GFP channels (top), GFP channel (bottom). Simultaneous divisions of all nuclei can be seen in the second (images a and b) and third (images d and e) mitoses. Following germination (images c and d), 3 of the 4 nuclei migrate into the elongating germ tube, and then all three nuclei divide (e). Three nuclei return to the original conidial cell, and the three others disperse along the germ tube. The time interval between images a and b and between images d and e is 7 min. Two nuclei in the cell did not germinate (arrow).
CgRac1 is necessary for fungal pathogenicity.
Infection assays showed that the CA-CgRac1 strains are unable to cause disease on A. virginica plants (Fig. 3A). The multiple defects in early stages of pathogenic development, particularly the reduced attachment of conidia and the abnormal appressoria, suggested that the mutants would be compromised in their ability to penetrate the host cuticle. On onion epidermis, the CA-CgRac1 strains developed abnormal, swollen hyphae and bundles of appressorium-like structures (Fig. 3B). These structures were compromised in their ability to penetrate the epidermal cell layer, as indicated by the absence of subcellular hyphae in the CA-CgRac1 strain, whereas abundant subepidermal primary hyphae developed in wild-type-inoculated samples (Fig. 3B). These results confirmed that the CA-CgRac1 strain is impaired in host penetration.
We inoculated wounded pea leaves to determine whether the CA-CgRac1 strain is also affected in invasive growth. Severe necroses developed in wounded leaves following inoculation with conidia (103 conidia/ml) of the wild-type strain (Fig. 3C). In contrast, no symptoms developed when leaves were inoculated with a similar concentration of CA-CgRac1 conidia. These results show that the CA-CgRac1 strain has defects in host penetration as well as in later stages of pathogenic development.
CgRac1 regulates nuclear and cell division.
Nuclear division was followed in transgenic strains expressing a histone-GFP (H1-GFP) fusion protein, which produces GFP-labeled nuclei. Normally, resting conidia of C. gloeosporioides contain a single nucleus and the cell cycle is arrested. Following induction of germination, the conidium divides, and then nuclear division continues in only one of the resulting cells (28). We found that cell cycle arrest was less stringently maintained during these developmental stages in the CA-CgRac1 strain than in the wild type. About 10% of conidia collected from CA-CgRac1 culture plates contained two or more nuclei, compared with less than 1% of wild-type conidia having more than a single nucleus. In accordance with the formation of two germ tubes instead of one, nuclear division progressed in both cells following the first cell division and all resulting nuclei progressed through the cell cycle, even when only a single germ tube was produced (Fig. 4A and B). A similar phenomenon was observed in the hyphae: all nuclei divided more than once, unlike in the wild type, in which only nuclei in apical cells of the hyphal tips divided. As a result, instead of having a single nucleus per compartment as in the wild type, hyphae and appressoria of the CA-CgRac1 strains were swollen and multinucleated (Fig. 4A).
Taken together, these results suggest that CgRac1 is necessary for resumption of both the cell cycle and growth during transitions between different developmental stages.
CgRac1 affects production and intracellular localization of ROS.
Levels and cellular localization of ROS were examined during conidial germination. In the wild type, ROS accumulated in the apical cell of elongating germ tubes forming a gradient toward the back of the cell (Fig. 5a). ROS also accumulated in the conidial cell in which germination occurred but not in the other cell that did not germinate. In CA-CgRac1 strains, the two conidial cells accumulated equal amounts of ROS, regardless of whether one or both of the cells germinated (Fig. 5b). ROS also accumulated in the germ tube, but unlike in the wild type, there was no gradient, and uniform staining was detected in all of the apical as well as subapical cells. These results show that production and cellular localization of ROS is disrupted in the CA-CgRac1 strain, which might account for some of the observed defects, especially in polarization, and hence loss of asymmetric germination.
Fig. 5.
Accumulation and cellular distribution of ROS during pathogenic germination. Conidia were germinated for 4 h on glass slides with PE medium and then stained with H2DCFDA. In the wild type (WT), ROS accumulated in the germinating cell and hyphal tips (a) and in developing appressoria (c). In the CA-CgRac1 (CA27) strain, ROS accumulated in both conidial cells and germ tubes (b). Staining was detected in both conidial cells, even when only one of the cells produced a germ tube (d). No signal was detected in unstained wild-type (e) or CA-CgRac1 (f) control samples.
Cellular localization of the CgRac1 protein.
Cellular localization of the CgRac1 protein was determined in the wild-type and CA-CgRac1 genetic backgrounds. In germinating conidia, CgRac1 was detected in rapidly moving vesicles, throughout the cytoplasm, and at the sites designated for polar growth, such as the germ tube and hyphal tips (Fig. 6). The signal was also visible in the septa. Surprisingly, no significant localization differences were observed between the wild-type and CA-CgRac1 strains, unlike the distribution of ROS, which was drastically altered in the CA-CgRac1 strain. This result suggests that CgRac1 activity is regulated by activation/inactivation of the protein and not by protein levels or subcellular localization. Measurement of transcript levels showed constant expression under various conditions (data not shown), further supporting this possibility.
Fig. 6.
Cellular localization of CgRac1. Protein localization was followed using a confocal microscope in transgenic strains expressing a CFP-Rac1 fusion in wild-type (CgRAC1) and CA-CgRAC1 genetic backgrounds. (a and b) In resting conidia and following the first cell division after 2 h of incubation, the protein is localized in small vesicles in both cells of the conidium. (c and d) Larger vesicles are observed during germination, and protein can be detected in the elongating germ tube after 3 h of incubation. Signal intensities and distributions are similar in the wild type (a and c) and CA-CgRac1 (b and d). No signal is detected in wild-type or CA-CgRac1 strains after 2 and 3 h of incubation, respectively (e and f), whereas an evenly distributed signal is observed throughout the cytoplasm in strains expressing a cytoplasmic GFP in wild-type and CA-CgRac1 backgrounds after 2 h of incubation (g and h).
DISCUSSION
Fungal Rac1 proteins are required for polarized growth, and hence, they are involved in and necessary for various processes, such as conidial germination, conidial formation, and pathogenesis (20). Here we showed that CgRac1 affects asymmetric fungal development by controlling both morphogenesis and cell cycle progression. Impairment of CgRac1 activity by the expression of a constitutive active form of the protein disrupts the developmental scheme during early stages of pathogenic development, and as a consequence, CA-CgRac1 strains are completely nonpathogenic.
The connection between cell cycle and morphogenesis in fungi is well established. In Saccharomyces cerevisiae, the cell cycle is tightly linked with morphogenesis: polarity establishment, polar growth, and isotropic expansion all take place during bud formation and depend on cell cycle progression (24). In M. oryzae, blockage of mitosis prevents completion of germination and appressorium formation (39). Genetic analysis has shown that commitment to form an appressorium is determined early in the cell cycle, between S and G2 (33). In U. maydis, spores expressing constitutive active Rac1 are arrested with a single nucleus, indicating that Rac1 might interfere with the mitotic cell cycle (26). During pathogenic germination in C. gloeosporioides, nuclear division always precedes the next morphogenetic event, suggesting a similar interplay between the cell cycle and developmental morphogenesis. However, morphogenesis continues when the cell cycle is blocked and activation of morphogenetic changes occurs before nuclear division (28). Furthermore, the developmental changes that take place during pathogenic germination are all preceded by arrest of the cell cycle, which resumes when new organs start to form. The developmental plan and coordination of cell cycle arrest with morphogenetic switches are disrupted in CA-CgRac1 strains, suggesting that CgRac1 is involved in regulating the exit from cell cycle arrest, an activity that is coordinated with resumption of growth when a certain developmental stage has been completed. Because in CA-CgRac1 strains the cell cycle is less tightly controlled and morphogenesis continues without the necessary adaptation time, multinucleate cells and malformed organs (e.g., multinucleated appressorium-like structures) are apparent.
Nuclear division in fungal hyphae can follow different patterns. In some species, such as A. nidulans, nuclei divide throughout the hypha in a coordinated manner (11), whereas in other species, such as Ashbya gossypii, nuclear division in the hypha is asynchronous (18). In C. gloeosporioides, only the nucleus that is closest to the hyphal tip (apical cell) divides, similar to the situation in C. albicans (19). Our results suggest that in pathogenic germination, a polar axis is established already after the first cell division. Accordingly, one of the resulting cells is defined as the apical side, and this cell will divide and produce the germ tube. The other cell, which represents the subapical side of the incipient hypha, does not continue to develop, and its nucleus is arrested. Because CgRac1 is essential for polarity, strains that express CA-CgRac1 lose the ability to form a polar axis, nuclei in both cells continue to divide, and both cells have the capacity to produce a germ tube. Notably, the effect of CgRac1 on the cell cycle is probably separate from its effect on morphogenesis, since in the CA-CgRac1 strain, both nuclei can divide, even when only one of the cells germinates.
ROS production and distribution are important for polarized growth and fungal pathogenicity (8, 13, 35, 38). In filamentous fungi, NADPH oxidase (NOX) and its associated protein complex, including Rac1, are a major source of ROS (37). In M. oryzae, ROS are found in apical cells and in appressoria (13). In C. gloeosporioides, ROS were most abundant in the hyphal tips, forming a gradient with lower concentrations toward the back of the hypha than toward the front (Fig. 5). In addition, ROS were highly abundant in developing organs, for example, in the germinating conidial cell and in appressoria. In the CA-CgRac1 strains, ROS were evenly distributed in the conidia, germ tubes, and short hyphae. ROS also accumulated to similar levels in both of the conidial cells following the first cell division and throughout germination. On the other hand, we could not determine significant differences in the localization of the CgRac1 protein in wild-type versus CA-CgRac1 strains. These results suggest that the protein levels and localization are similar in developing and arrested cells and that the activity of CgRac1 does not depend on the presence of the protein but rather on CgRac1-regulating elements such as guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). Further investigation will be needed to elucidate the molecular regulation of CgRac1 and its downstream effectors.
Supplementary Material
ACKNOWLEDGMENT
This research was supported by grant number 50/15-02 from the DFG.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 1 April 2011.
REFERENCES
- 1. Barhoom S., Sharon A. 2004. cAMP regulation of “pathogenic” and “saprophytic” fungal spore germination. Fungal Genet. Biol. 41:317–326 [DOI] [PubMed] [Google Scholar]
- 2. Barhoom S., Sharon A. 2007. Bcl-2 proteins link programmed cell death with growth and morphogenetic adaptations in the fungal plant pathogen Colletotrichum gloeosporioides. Fungal Genet. Biol. 44:32–43 [DOI] [PubMed] [Google Scholar]
- 3. Bassilana M., Arkowitz R. A. 2006. Rac1 and Cdc42 have different roles in Candida albicans development. Eukaryot. Cell 5:321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bishop A. L., Hall A. 2000. Rho GTPases and their effector proteins. Biochem. J. 348:241–255 [PMC free article] [PubMed] [Google Scholar]
- 5. Boyce K., Hynes M., Andrianopoulos A. 2003. Control of morphogenesis and actin localization by the Penicillium marneffei RAC homolog. J. Cell Sci. 116:1249–1260 [DOI] [PubMed] [Google Scholar]
- 6. Bustelo X., Sauzeau V., Berenjeno I. 2007. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 29:356–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaky J., Anderson K., Moss M., Vaillancourt L. 2001. Surface hydrophobicity and surface rigidity induce spore germination in Colletotrichum graminicola. Phytopathology 91:558–564 [DOI] [PubMed] [Google Scholar]
- 8. Chen C., Dickman M. 2004. Dominant active Rac and dominant negative Rac revert the dominant active Ras phenotype in Colletotrichum trifolii by distinct signalling pathways. Mol. Microbiol. 51:1493–1507 [DOI] [PubMed] [Google Scholar]
- 9. Chen C., Ha Y.-S., Min J.-Y., Memmott S. D., Dickman M. B. 2006. Cdc42 is required for proper growth and development in the fungal pathogen Colletotrichum trifolii. Eukaryot. Cell 5:155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen J., et al. 2008. Rac1 is required for pathogenicity and Chm1-dependent conidiogenesis in rice fungal pathogen Magnaporthe grisea. PLoS Pathog. 4:e1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clutterbuck A. 1970. Synchronous nuclear division and septation in Aspergillus nidulans. J. Gen. Microbiol. 60:133–135 [DOI] [PubMed] [Google Scholar]
- 12. Doehlemann G., Berndt P., Hahn M. 2006. Different signalling pathways involving a Gα protein, cAMP and a MAP kinase control germination of Botrytis cinerea conidia. Mol. Microbiol. 59:821–835 [DOI] [PubMed] [Google Scholar]
- 13. Egan M., Wang Z., Jones M., Smirnoff N., Talbot N. 2007. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl. Acad. Sci. U. S. A. 104:11772–11777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Etienne-Manneville S. 2004. Cdc42—the centre of polarity. J. Cell Sci. 117:1291–1300 [DOI] [PubMed] [Google Scholar]
- 15. Fischer R., Zekert N., Takeshita N. 2008. Polarized growth in fungi—interplay between the cytoskeleton, positional markers and membrane domains. Mol. Microbiol. 68:813–826 [DOI] [PubMed] [Google Scholar]
- 16. Freitag M., Hickey P., Raju N., Selker E., Read N. 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41:897–910 [DOI] [PubMed] [Google Scholar]
- 17. Giesbert S., Lepping H. B., Tenberge K. B., Tudzynski P. 1998. The xylanolytic system of Claviceps purpurea: cytological evidence for secretion of xylanases in infected rye tissue and molecular characterization of two xylanase genes. Phytopathology 88:1020–1030 [DOI] [PubMed] [Google Scholar]
- 18. Gladfelter A., Hungerbuehler A., Philippsen P. 2006. Asynchronous nuclear division cycles in multinucleated cells. J. Cell Biol. 172:347–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gow N. 1997. Germ tube growth of Candida albicans. Curr. Top. Med. Mycol. 8:43–55 [PubMed] [Google Scholar]
- 20. Harris S. 2006. Cell polarity in filamentous fungi: shaping the mold. Int. Rev. Cytol. 251:41–77 [DOI] [PubMed] [Google Scholar]
- 21. Horton R., Cai Z., Ho S., Pease L. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8:528–535 [PubMed] [Google Scholar]
- 22. Jaffe A., Hall A. 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21:247–269 [DOI] [PubMed] [Google Scholar]
- 23. Kolattukudy P., Rogers L., Li D., Hwang C., Flaishman M. 1995. Surface signaling in pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 92:4080–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lew D. 2003. The morphogenesis checkpoint: how yeast cells watch their figures. Curr. Opin. Cell Biol. 15:648–653 [DOI] [PubMed] [Google Scholar]
- 25. Li H., et al. 2011. The small GTPase RacA mediates intracellular reactive oxygen species production, polarized growth, and virulence in the human fungal pathogen Aspergillus fumigatus. Eukaryot. Cell 10:174–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahlert M., Leveleki L., Hlubek A., Sandrock B., Bölker M. 2006. Rac1 and Cdc42 regulate hyphal growth and cytokinesis in the dimorphic fungus Ustilago maydis. Mol. Microbiol. 59:567–578 [DOI] [PubMed] [Google Scholar]
- 27. Momany M. 2002. Polarity in filamentous fungi: establishment, maintenance and new axes. Curr. Opin. Microbiol. 5:580–585 [DOI] [PubMed] [Google Scholar]
- 28. Nesher I., Barhoom S., Sharon A. 2008. Cell cycle and cell death are not necessary for appressorium formation and plant infection in the fungal plant pathogen Colletotrichum gloeosporioides. BMC Biol. 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Osherov N., May G. 2001. The molecular mechanisms of conidial germination. FEMS Microbiol. Lett. 199:153–160 [DOI] [PubMed] [Google Scholar]
- 30. Podila G. K., Rogers L. M., Kolattukudy P. E. 1993. Chemical signals from avocado surface wax trigger germination and appressorium formation in Colletotrichum gloeosporioides. Plant Physiol. 103:267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robinson M., Sharon A. 1999. Transformation of the bioherbicide Colletotrichum gloeosporioides f. sp. aeschynomene by electroporation of germinated conidia. Curr. Genet. 36:98–104 [DOI] [PubMed] [Google Scholar]
- 32. Rolke Y., Tudzynski P. 2008. The small GTPase Rac and the p21-activated kinase Cla4 in Claviceps purpurea: interaction and impact on polarity, development and pathogenicity. Mol. Microbiol. 68:405–423 [DOI] [PubMed] [Google Scholar]
- 33. Saunders D., Dagdas Y., Talbot N. 2010. Spatial uncoupling of mitosis and cytokinesis during appressorium-mediated plant infection by the rice blast fungus Magnaporthe oryzae. Plant Cell 22:2417–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scheffer J., Chen C., Heidrich P., Dickman M. B., Tudzynski P. 2005. A CDC42 homologue in Claviceps purpurea is involved in vegetative differentiation and is essential for pathogenicity. Eukaryot. Cell 4:1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Segmüller N., et al. 2008. NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol. Plant Microbe Interact. 21:808–819 [DOI] [PubMed] [Google Scholar]
- 36. Steinberg G. 2007. Hyphal growth: a tale of motors, lipids, and the Spitzenkörper. Eukaryot. Cell 6:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takemoto D., Tanaka A., Scott B. 2007. NADPH oxidases in fungi: diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal Genet. Biol. 44:1065–1076 [DOI] [PubMed] [Google Scholar]
- 38. Tanaka A., Takemoto D., Hyon G., Park P., Scott B. 2008. NoxA activation by the small GTPase RacA is required to maintain a mutualistic symbiotic association between Epichloë festucae and perennial ryegrass. Mol. Microbiol. 68:1165–1178 [DOI] [PubMed] [Google Scholar]
- 39. Veneault-Fourrey C., Barooah M., Egan M., Wakley G., Talbot N. 2006. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science 312:580–583 [DOI] [PubMed] [Google Scholar]
- 40. Virag A., Lee M., Si H., Harris S. 2007. Regulation of hyphal morphogenesis by Cdc42 and Rac1 homologues in Aspergillus nidulans. Mol. Microbiol. 66:1579–1596 [DOI] [PubMed] [Google Scholar]
- 41. Wendland J., Philippsen P. 2001. Cell polarity and hyphal morphogenesis are controlled by multiple rho-protein modules in the filamentous ascomycete Ashbya gossypii. Genetics 157:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wennerberg K., Rossman K., Der C. 2005. The Ras superfamily at a glance. J. Cell Sci. 118:843–846 [DOI] [PubMed] [Google Scholar]
- 43. Zohn I., Campbell S., Khosravi-Far R., Rossman K., Der C. 1998. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene 17:1415–1438 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.