Abstract
Brucellosis is a zoonosis of nearly worldwide distribution. Vaccination against this pathogen is an important control strategy to prevent the disease. Currently licensed vaccine strains used in animals are unacceptable for human use due to undesirable side effects and modest protection. Substantial progress has been made during the past 10 years toward the development of improved vaccines for brucellosis. In part, this has been achieved by the identification and characterization of live attenuated mutants that are safer in the host but still can stimulate an adequate immune response. In the present study, the identification and characterization of the mucR mutant (BMEI 1364) as a vaccine candidate for brucellosis was conducted. BALB/c mice were vaccinated intraperitoneally at a dose of 105 CFU with the mutant to evaluate safety and protective efficacy against intraperitoneal and aerosol challenge. All animals vaccinated with the vaccine candidate demonstrated a statistically significant degree of protection against both intraperitoneal and aerosol challenge. Safety was revealed by the absence of Brucella associated pathological changes, including splenomegaly, hepatomegaly, or granulomatous disease. These results suggest that the 16MΔmucR vaccine is safe, elicits a strong protective immunity, and should be considered as a promising vaccine candidate for human use.
INTRODUCTION
Brucella spp. are a diverse group of pathogens causing disease in many hosts, including humans. As the cause of brucellosis, Brucella abortus and B. melitensis are of particular importance to human health, with an estimated annual incidence of 500,000 human cases worldwide but predominantly in developing countries (31). Vaccination against brucellosis is an important control strategy to prevent the disease (29). Currently licensed vaccine strains implemented in the veterinary field are unacceptable for human use due to the undesirable side effects, antibiotic resistance, and lack of characterization (33, 37). Increased knowledge of protective immune responses to Brucella infection, along with the elucidation of the complete genomic sequence have provided better opportunities to understand, develop, and improve new vaccines against brucellosis.
Previous studies performed in this laboratory using signature-tagged mutagenesis permitted the identification of in vitro and in vivo virulence genes (1, 16, 40). Among these, a mucR (BMEI 1364) mutant was attenuated for survival in the mouse and macrophage model (40). The role of MucR protein in Brucella is unknown, but the function of the mucR gene has recently been identified in soil and plant bacteria such as Agrobacterium tumefaciencis and Sinorhizobium meliloti (19, 28). MucR is a transcriptional regulator that coordinates a diverse set of bacterial behaviors, including the control of exopolysaccharide production, which is important not only in bacterium-plant symbiosis but also in biofilm formation (4, 5).
In the present study, we conducted a series of experiments designed to characterize B. melitensis 16MΔmucR as a potential candidate vaccine against intraperitoneal and aerosol B. melitensis 16M challenge. Vaccination with the mutant did not induce systemic or local adverse reactions and significantly protected BALB/c mice against intraperitoneal and aerosol challenge.
MATERIALS AND METHODS
Mice.
Six- to eight-week-old female BALB/c mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and acclimated for 1 week prior to infection or vaccination. All experimental procedures and animal care were performed in compliance with the institutional animal care guidelines.
Bacterial strains.
Strains used in these experiments include B. melitensis 16MΔmucR (engineered for the present study and used as the vaccine candidate) and the virulent strain B. melitensis16M biovar 1(originally obtained from the American Type Culture Collection and reisolated by this lab from an aborted goat fetus) (24). Bacteria were grown on tryptic soy agar (TSA; Difco/Becton Dickinson) or Farrell's medium (TSA supplemented with Oxoid Brucella supplement) at 37°C with 5% CO2. For construction of the B. melitensis 16MΔmucR knockout, the medium was supplemented with kanamycin (100 μg/ml) or carbenicillin (100 μg/ml). Sucrose medium was utilized for sacB counterselection and unmarked gene deletion selection as previously described (25). Escherichia coli cultures utilized for the construction of the 16MΔmucR mutant were grown on Luria-Bertani (LB) (Difco/Becton Dickinson) plates or in LB broth overnight at 37°C with or without ampicillin (100 mg/liter) or kanamycin (100 mg/liter).
To prepare organism for animal infections, Brucella were harvested from the surface of the plates after 3 days of incubation using phosphate-buffered saline (PBS; pH 7.2). The bacteria were pelleted and resuspended to a final concentration based on optical density readings using a Klett meter and a standardized curve. Actual viable counts were confirmed by serial dilution, plating, and enumeration.
Construction of the B. melitensis 16MΔmucR deletion mutant.
The mutant was constructed as previously described with some modifications (25). The sequence upstream of the mucR gene (BMEI1364) was amplified from B. melitensis 16M by using the primer pair 5′-GCTCTAGAGCCCATCAACAACAGGACAAACGG-3′ (contains an XbaI site) and 5′-GGCGGCGCGCCTGGTTGCTCCGAACTATGCTG (contains an AscI site). The sequence downstream of mucR was amplified with the primer pair 5′-CCAGGCGCGCCGCCGCTGCGTATTTCATAATC (contains an AscI site) and 5′-GCTCTAGAGCCTTTGCAGGTTTTCCGTATCTTT (contains an XbaI site). These two products were ligated to one another via overlapping PCR via the AscI site (New England Biolabs) engineered between the two sequences. This overlap product was then ligated to pEX18Ap via the XbaI site (named pMMK40). A kanamycin resistance (Kanr) gene was subsequently ligated within the vector by the unique AscI site (plasmid pMMK44). This construct was used for electroporation into Brucella melitensis 16M. Potential marked deletion mutants were Kanr and carbenicillin sensitive (Carbs) and were verified by PCR and Southern blotting; the confirmed mutant was named 16MΔmucR::Kan. The unmarked deletion mutant was engineered by electroporation of pMMK40 into 16MΔmucR::Kan and selected on TSA plus carbenicillin at 100 μg/ml. Cointegrants with the phenotypes Kanr, Carbr, and sucrose sensitive (Sucs) were selected, indicating a cointegrant with a functional sacB gene. Bacteria were selected in the presence of sucrose for resolution of cointegration as previously described (25). All knockout candidates were verified by PCR and Southern blotting to demonstrate gene deletion and loss of the Kan cassette.
Evaluation of B. melitensis 16MΔmucR attenuation in mice.
Forty 6- to 8-week-old female BALB/c mice were used to evaluate the persistence and replication of the B. melitensis 16MΔmucR mutant. Mice were inoculated intraperitoneally with either (i) 106 CFU in 100 μl of 16MΔmucR or (ii) 106 CFU in 100 μl of the parental strain 16M. Groups of four mice were euthanized via carbon dioxide asphyxiation at 1, 3, 6, 9, or 12 weeks postinfection. At each time point, the spleens were harvested, weighed, and homogenized in 1 ml of peptone saline. Serial dilutions were prepared, and 100-μl aliquots of each dilution (including the undiluted organ) were plated in duplicate onto TSA plates. The levels of infection were expressed as means ± the standard errors of the mean (SEM) of individual log CFU/spleen values.
Immunization of mice for efficacy studies.
Six- to eight-week-old female BALB/c mice were distributed into three treatment groups and inoculated intraperitoneally with a single dose of B. melitensis 16MΔmucR. Treatment groups included doses of 105 CFU/mouse, 106 CFU/mouse, and PBS only as a control. Mice were housed for 20 weeks postvaccination under ABSL-3 conditions.
Vaccination efficacy against intraperitoneal challenge.
At 20 weeks postvaccination, mice (n = 5 per group) were challenged intraperitoneally using 6 × 105 CFU of B. melitensis 16M/mouse. At 1 week postchallenge, the mice were euthanized via CO2 asphyxiation. Spleens, lungs, and livers were collected, weighed, and homogenized in 1 ml of PBS and plated onto TSA or Farrell's medium plates to determine the total CFU/organ. Serial dilutions were performed, and aliquots were plated. The levels of infection were expressed as means ± the SEM of the individual log10 CFU/spleen, log10 CFU/liver, and log10 CFU/lung values.
Vaccination efficacy against aerosol challenge.
At 20 weeks postvaccination, groups of five mice were challenged with an aerosol chamber dose of 5 × 109 CFU of B. melitensis 16M/ml. At 4 weeks postchallenge, the mice were euthanized, and the lungs, livers, and spleens were removed, weighed, homogenized in 1 ml of PBS, serially diluted, and plated onto Farrell's medium to determine total CFU/organ. The levels of infection were expressed as means ± the SEM of the individual log 10 CFU/spleen, log10 CFU/liver, and log10 CFU/lung values. A group of three mice were euthanized directly after aerosol exposure to quantify the CFU/lung inhaled.
Histopathology.
Tissues from mice were assessed to determine the degree of pathology associated with aerosol challenge. Mice were either vaccinated with 16MΔmucR and subsequently aerosol challenged (2 weeks postchallenge) or not vaccinated (i.e., naive) and aerosol challenged (2 weeks postchallenge). The animals were euthanized by CO2 asphyxiation, and the spleens, lungs, livers, kidneys, and hearts were harvested, fixed in 10% buffered formalin, paraffin embedded, and stained with hematoxylin and eosin. The histological changes were assessed between groups. Slides were analyzed by a board-certified veterinary pathologist.
Statistical analysis.
Bacterial burden from mutant clearance and efficacy studies are expressed as the mean CFU ± the standard errors (SE) and are presented graphically as the log10 CFU of Brucella recovered per organ. Culture-negative organs were assigned a value of 4 CFU, which is below the limit of detection of 5 CFU/organ. Spleen weight data from kinetics studies are plotted as the mean spleen weights in mg ± the SE.
For the survival of 16MΔmucR in mice, a Student t test was performed to compare splenic colonization and weight of the knockout strain to the wild-type control group at each time point. Efficacy studies compared vaccinated and subsequently challenged mice to mice receiving PBS as a vaccine control that were challenged with the wild-type organism. In the intraperitoneal challenge study, statistical significance of differences between vaccinates were analyzed by analysis of variance (ANOVA) for each organ separately, followed by Tukey's honestly significant (HSD) post test comparing all groups to one another. In the aerosol protection studies a Student t test was performed for each organ separately to compare the vaccinees to naive mice. For all statistical analyses, P values of <0.05 were considered statistically significant.
RESULTS
Attenuation of 16MΔmucR in mice.
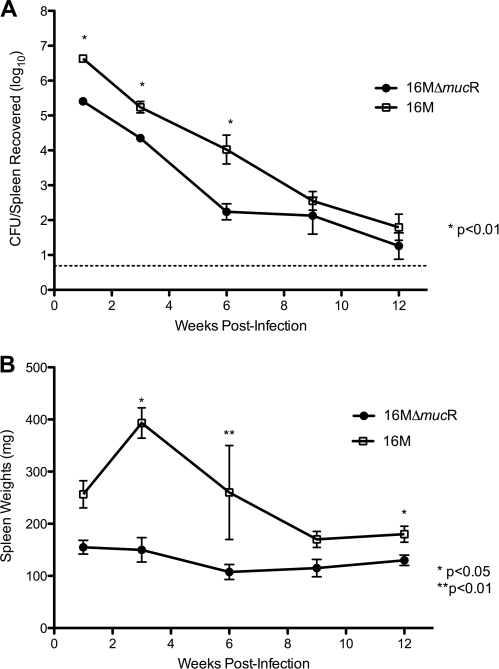
To determine the effect of the mucR gene deletion in vivo, mice were infected intraperitoneally with 106 CFU of B. melitensis 16MΔmucR/mouse. Compared to the wild-type strain 16M, the colonization of the spleen with 16MΔmucR was significantly reduced at 1, 3, and 6 weeks (P < 0.01) but not at 9 or 12 weeks (Fig. 1 A). Reduced splenic colonization by the 16MΔmucR mutant correlated with reduced spleen weights (Fig. 1B), indicating a reduced inflammatory response by the mutant. The spleen weights of mice infected with wild-type 16M were consistently higher.
Fig. 1.
Kinetics of clearance of 16MΔmucR from mice. Forty 6- to 8-week-old female BALB/c mice were used to evaluate the persistence and replication of 16MΔmucR. Mice were inoculated intraperitoneally with either (i) 106 CFU in 100 μl of 16MΔmucR or (ii) 106 CFU in 100 μl of the parental strain 16M. Groups of four mice were euthanized via carbon dioxide asphyxiation at 1, 3, 6, 9, or 12 weeks postinfection. At each time point, the spleens were harvested, weighed, and homogenized in 1 ml of peptone saline. Serial dilutions were prepared, and 100-μl aliquots of each dilution (including the undiluted organ) were plated in duplicate on TSA plates. (A) The levels of infection were expressed as means ± the SEM of individual log CFU/spleen values. (B) The spleen weights were measured and used to compare the mutant strain to the wild-type organism. Statistical significance is based upon a Student t test comparing the deletion mutant to the wild-type strain. The solid line at 0.69 logs represents the lower limit of detection (≥5 CFU).
Evaluation of immune protection provided by 16MΔmucR against intraperitoneal 16M challenge.
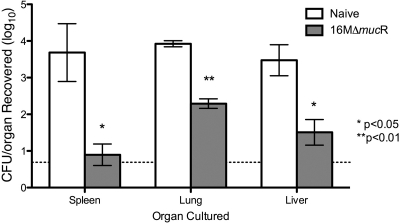
In order to determine the vaccination efficacy elicited by the 16MΔmucR mutant, the level of protection provided by the vaccine candidate was evaluated against intraperitoneal B. melitensis 16M wild-type challenge at 20 weeks postvaccination. Animals were euthanized at 1 week postchallenge because this time point corresponds to the highest bacterial load in the spleen based on previous studies (25). At 1 week postchallenge (21 weeks postvaccination), there was a statistically significant decrease in the splenic, hepatic, and pulmonary bacterial loads from the mice vaccinated with the 16MΔmucR mutant relative to those of the naive mice regardless of the vaccination dose, with a 4.14- to 4.75-log reduction in bacterial burden in the spleen (P < 0.001), 3.24- to 3.34-log reduction in the liver (P < 0.001), and 2.54- to 3.64-log reduction in the lungs (P < 0.001) (Fig. 2).
Fig. 2.
Protection against homologous intraperitoneal 16M challenge. Groups of five female BALB/c mice were vaccinated with 16MΔmucR at either 106 CFU/mouse or 105 CFU/mouse or left unvaccinated as naive controls. At 20 weeks postvaccination all animals were challenged with 6 × 105 CFU/mouse intraperitoneally. At 1 week postchallenge, mice were euthanized via CO2 asphyxiation, and the spleens, livers, and lungs were collected. The data are reported as the log10 recovery of Brucella from organs. The solid line at 0.69 logs represents the lower limit of detection (≥5 CFU). Statistical analysis was performed by ANOVA for each organ separately, followed by a Tukey's HSD post test comparing all groups to one another.
Evaluation of immune protection provided by 16ΔmucR against aerosol 16M challenge.
In order to determine the vaccination efficacy elicited by the 16MΔmucR mutant against a natural route of exposure, the level of protection provided by the vaccine candidate was evaluated against aerosol B. melitensis 16M wild-type challenge at 20 weeks postvaccination. For aerosol exposure studies, the 4-week postchallenge time point was chosen as the time point of peak splenic colonization (23). Mice that were euthanized within 1 h of aerosol exposure inhaled an average of 2.1 × 104 CFU/lungs, as determined by plating their lungs and enumerating the bacteria recovered.
When mice were challenged by the aerosol route, the 16MΔmucR vaccine candidate protected mice significantly in all organs plated. The mutant afforded a 2.79-log reduction in the bacterial burden in the spleen (P < 0.05), a 1.97-log reduction in the liver (P < 0.05), and a 1.63-log reduction in the lungs (P < 0.01) (Fig. 3).
Fig. 3.
Protection against homologous aerosol 16M challenge. Groups of five female BALB/c mice were vaccinated with 16MΔmucR at 105 CFU/mouse or left unvaccinated as naive controls. At 20 weeks postvaccination, all of the animals were challenged with an aerosol chamber dose of 5 × 109 CFU of 16M/ml. At 4 weeks postchallenge, the mice were euthanized via CO2 asphyxiation, and the spleens, livers, and lungs were collected. The data are reported as the log10 recovery of Brucella from organs. The solid line at 0.69 logs represents the lower limit of detection (<5 CFU). Statistical analysis was performed by using a Student t test comparing vaccinated to nonvaccinated mice for each organ separately.
Evaluation of histological changes in mice vaccinated with 16MΔmucR.
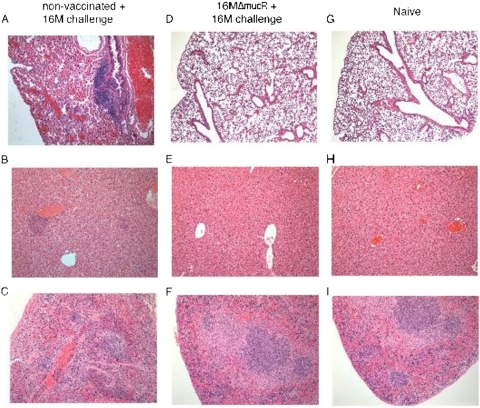
Histological analysis of the lungs, livers, and spleens of BALB/c mice inoculated with either 16MΔmucR (Fig. 4 D, E, and F) or naive PBS controls (Fig. 4A, B, and C) that were subsequently aerosol challenged with wild-type 16M and euthanized 2 weeks postchallenge was assessed to determine the degree of inflammation elicited by the challenge organism in animals that were vaccinated with the mutant. Histologically, the bronchiole-associated lymphoid tissue was prominent at 2 weeks postchallenge (Fig. 4A), and no significant changes were seen in mice vaccinated with the mucR mutant (Fig. 4D). Inflammatory foci in the liver of naive aerosol challenged mice were rare at 2 weeks postchallenge (Fig. 4B) and absent in mice vaccinated with the mucR mutant (Fig. 4E). Changes in the spleen of naive mice consisted of marked extramedullary hematopoiesis at 2 weeks postchallenge (Fig. 4C). There were no significant changes in the spleen of mice vaccinated with the mucR mutant (Fig. 4F). No significant changes were observed in the kidneys or hearts in vaccinated or aerosol exposed animals (data not shown).
Fig. 4.
Histological changes of organs associated with vaccination and challenge. BALB/c mice were vaccinated with 16MΔmucR at 105 CFU/mouse or left unvaccinated as naive controls. At 20 weeks postvaccination, all of the animals were challenged with an aerosol chamber dose of 5 × 109 CFU of 16M/ml. At 2 weeks postchallenge, tissues were collected for histology. The histology of the nonvaccinated but challenged animals (A, B, and C) were compared to vaccinated but challenged animals (D, E, and F). The lungs (A and D), livers (B and E), and spleens (C and F) were compared to determine the reduction in pathology afforded by vaccination with the 16MΔmucR mutant. Naive mice are depicted (G, H, and I) for comparison. Hematoxylin and eosin staining was used (×10 magnification).
DISCUSSION
Historically, the most efficacious vaccines against brucellosis have been live attenuated vaccines, as is the case for multiple currently licensed vaccine strains for animal use, including S19, Rev1, and RB51 (15, 37). Unfortunately, both S19 and Rev1 vaccines, which have been highly efficacious in controlling the disease in cattle and goats, respectively, have proven to be unsafe or have the capacity to cause adverse reactions in humans due to local and systemic reactions that in some cases resulted in the development of the disease (3, 38). Other vaccinology alternatives, including the use of subunit, recombinant proteins and DNA vaccines which might be safer for human use, though capable of eliciting both humoral and cellular immune responses to a certain degree, generally induce lower or no protection compared to the live attenuated vaccines in animal models (8–10, 14, 30, 32, 33, 36). As such, a live attenuated organism has been utilized as the vaccine type of choice for the prevention of brucellosis. An ideal Brucella vaccine would be one that persists long enough to generate a robust immune response without eliciting the undesired side effects such as splenomegaly or clinical signs of disease.
Previous investigations using signature-tagged mutagenesis in this laboratory have identified multiple candidate mutants that are attenuated for virulence and survival in the mouse and macrophage models, among these is disruption of the mucR locus in B. melitensis 16M (40). We previously demonstrated an in vitro and in vivo role for the mucR transposon mutant. The organism was found to be significantly attenuated for survival in vitro and in vivo when the gene was interrupted. To further characterize the role of mucR in regard to survival, protective efficacy, and safety in vivo, an unmarked gene deletion was created.
In the Gram-negative soil bacterium Sinorhizobium meliloti, a clear role for the mucR gene has recently been established (28). MucR has been identified as a transcriptional regulator with multiple functions that help in the establishment of symbiosis, including a key role in the control of exopolysaccharide biosynthesis, which is necessary for biofilm formation (4, 5, 35). The biofilm provides bacteria with a physical barrier against antibiotics, innate defense mechanisms from the host, and environmental stress conditions, including UV radiation, pH changes, and osmotic shock among others (11, 12). In order to ensure a successful symbiotic association, exopolysaccharide production and biofilm formation are tightly regulated and partially controlled by the mucR gene. Deletion of mucR in S. meliloti therefore results in deficiencies in the invasion or the establishment of symbiosis. Other established roles of mucR in this organism include an induction of increased expression of multiple operons required for nitrogen fixation and respiration, as well as numerous type IV secretion systems and putative transport-related genes, all of which are necessary for a successful symbiosis (28).
In the case of Brucella, the role of mucR is less well understood. Preliminary studies from this laboratory using microarray technology suggests that the mucR gene regulates exopolysaccharide biosynthesis, as well as genes involved in iron sequestration and storage, nitrogen metabolism, and stress response mechanisms. (J. Weeks, unpublished data). Although preliminary and still under investigation, all of these putative roles of MucR in Brucella explain to a certain degree the attenuation of the mutant strain observed in J774A macrophages and in mice. Recently, it has been reported that B. melitensis 16M produces an exopolysaccharide; studies suggested that Brucella may indeed be capable of biofilm formation (17). It is possible that MucR may play a role in biofilm formation through the regulation of exopolysaccharide synthesis.
Protective efficacy as a function of persistence has been previously evaluated by this laboratory (25). The construction and characterization of multiple deletion mutants in Brucella abortus and Brucella melitensis has led to the conclusion that a vaccine candidate needs to persist in the host long enough in order to mount a strong protective immune response (15, 25). This observation is apparent here, as well with the B. melitensis mucR mutant. Interestingly, the mucR mutant persists for at least for 12 weeks in mice, similarly to the wild-type 16M, but the degree of colonization is significantly reduced compared to the parental strain during the acute phase of the infection. This difference in colonization properties may explain the lack of gross and microscopic changes associated with infection. Lack of hepatic granuloma formation or splenomegaly associated with vaccination suggests that immunization with the mutant is safe and therefore superior to many other Brucella vaccines, including licensed ones (12). Most importantly, protection against the most common microscopic changes associated with the disease in mice, such as granulomatous hepatitis, granulomatous splenitis, or splenomegaly, was not observed, indicating that vaccination with the mucR mutant not only reduced the bacterial burden in multiple organs but also prevents against the development of Brucella-associated pathological changes. Lack of splenomegaly associated with vaccination has been previously demonstrated as a safety parameter in other Brucella vaccine candidates (2).
Protection against intraperitoneal challenge, observing the output of bacterial colonization in the spleen of mice, has been historically used as a means of evaluating Brucella vaccine efficacy (6, 27, 34, 39). Although this vaccination or challenge location does not reflect a natural route of infection, it has been extremely useful in determining the potential efficacy of vaccine candidates against brucellosis. Most importantly, it provides a reproducible and invariable means of comparing multiple vaccine candidate strains that had been studied for the past 30 to 50 years. When 16MΔmucR vaccinated mice were challenged against wild-type B. melitensis 16M, all of the animals demonstrated a statistically significant reduction in the bacterial burden in the spleen, lung, and liver regardless of the vaccination dose. The marked reduction in bacterial burden in the spleen conferred by the mutant is impressive and comparable to other live attenuated vaccine candidates tested by this laboratory and others (2, 20, 21, 25). Although an intraperitoneal challenge is of historical importance, a more logical approach is the use of an aerosol challenge route, not only because of the documented evidence of aerosol transmission of these organisms but also because of the potential threat of the use of Brucella as a bioterrorism agent (13, 18, 22, 26). It has been documented that 10 to 100 organisms are enough to cause disease in humans, and Brucella is therefore considered highly infectious when delivered by this route (7). Previous investigations performed by this laboratory have determined that BALB/c mice receiving an infectious dose of 5 × 109 CFU/ml added to the chamber nebulizer inhaled an average of 12,250 organisms per mouse (4.10 logs) and that tissue colonization reached a peak by 4 weeks postexposure (23). The high dose (100-fold more bacteria that actually needed to establish an infection) and the time postvaccination chosen to test efficacy provide us the means to evaluate the vaccine candidate efficacy under the most stringent conditions. Interestingly and importantly, the bacterial burdens in the spleen, liver, and lungs were markedly reduced in animals that received the vaccine, demonstrating the vaccine efficacy against an aerosol exposure. Gross and microscopic evaluation confirmed the protection against the pathological changes associated with the disease. As expected, the highest numbers of bacteria were isolated from the lung. It is possible that a diminished inflammatory response in the lungs masks the efficacy in reducing the bacterial colonization and, although high bacterial counts were observed, there were no significant gross or microscopic changes associated with the infection apart from an increased amount of bronchus-associated lymphoid tissue.
In the present study, the intraperitoneal vaccination with the live attenuated vaccine candidate 16MΔmucR was able to markedly enhance the bacterial clearance in the spleen, lungs, and liver using two different challenge routes. Most importantly, vaccination conferred protection against Brucella-associated pathological changes. Future studies to determine the correlates of immune protection and the function of MucR are under way.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants R01048496 and U54AI057156.
We thank Gabriel Gomez and Alfredo Wong-Gonzalez for technical assistance.
Footnotes
Published ahead of print on 27 June 2011.
REFERENCES
- 1. Allen C. A., Adams L. G., Ficht T. A. 1998. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect. Immun. 66:1008–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arenas-Gamboa A. M., Ficht T. A., Kahl-McDonagh M. M., Gomez G., Rice-Ficht A. C. 2009. The Brucella abortus S19 ΔvjbR live vaccine candidate is safer than S19 and confers protection against wild-type challenge in BALB/c mice when delivered in a sustained-release vehicle. Infect. and Immun. 77:877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashford D. A., et al. 2004. Adverse events in humans associated with accidental exposure to the livestock brucellosis vaccine RB51. Vaccine 22:3435–3439 [DOI] [PubMed] [Google Scholar]
- 4. Bahlawane C., Baumgarth B., Serrania J., Ruberg S., Becker A. 2008. Fine-tuning of galactoglucan biosynthesis in Sinorhizobium meliloti by differential WggR (ExpG)-, PhoB-, and MucR-dependent regulation of two promoters. J. Bacteriol. 190:3456–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bahlawane C., McIntosh M., Krol E., Becker A. 2008. Sinorhizobium meliloti regulator MucR couples exopolysaccharide synthesis and motility. Mol. Plant-Microbe Interact. 21:1498–1509 [DOI] [PubMed] [Google Scholar]
- 6. Bosseray N., Plommet M. 1990. Brucella suis S2, Brucella melitensis Rev 1, and Brucella abortus S19 living vaccines: residual virulence and immunity induced against three Brucella species challenge strains in mice. Vaccine 8:462–468 [DOI] [PubMed] [Google Scholar]
- 7. Bossi P., et al. 2004. Bichat guidelines for the clinical management of brucellosis and bioterrorism-related brucellosis. Eur. Surveill. 9:E15–E16 [PubMed] [Google Scholar]
- 8. Cassataro J., et al. 2005. A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic response. Infect. Immun. 73:6537–6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cassataro J., et al. 2002. Immunogenicity of the Brucella melitensis recombinant ribosome recycling factor-homologous protein and its cDNA. Vaccine 20:1660–1669 [DOI] [PubMed] [Google Scholar]
- 10. Cespedes S., Andrews E., Folch H., Onate A. 2000. Identification and partial characterisation of a new protective antigen of Brucella abortus. J. Med. Microbiol. 49:165–170 [DOI] [PubMed] [Google Scholar]
- 11. Costerton J. W. 1995. Overview of microbial biofilms. J. Indust. Microbiol. 15:137–140 [DOI] [PubMed] [Google Scholar]
- 12. Costerton J. W., Lewandowski Z., Caldwell D. E., Korber D. R., Lappin-Scott H. M. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711–745 [DOI] [PubMed] [Google Scholar]
- 13. Davos D. E., Cargill C. F., Kyrkou M. R., Jamieson J. A., Rich G. E. 1981. Outbreak of brucellosis at a South-Australian abattoir. 2. Epidemiological investigations. Med. J. Aust. 2:657–660 [PubMed] [Google Scholar]
- 14. Delpino M. V., Estein S. M., Fossati C. A., Baldi P. C., Cassataro J. 2007. Vaccination with Brucella recombinant DnaK and SurA proteins induces protection against Brucella abortus infection in BALB/c mice. Vaccine 25:6721–6729 [DOI] [PubMed] [Google Scholar]
- 15. Ficht T. A., Kahl-McDonagh M. M., Arenas-Gamboa A. M., Rice-Ficht A. C. 2009. Brucellosis: the case for live, attenuated vaccines. Vaccine 27(Suppl. 4):D40–D43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ficht T. A., Pei J., Kahl-McDonagh M. 2010. In vitro mutagenesis of Brucella species. Methods Mol. Biol. 634:15–35 [DOI] [PubMed] [Google Scholar]
- 17. Godefroid M. Brucella melitensis 16M produces a mannan and other extracellular matrix components typical of a biofilm. FEMS Immunol. Med. Microbiol. 59:364–377 [DOI] [PubMed] [Google Scholar]
- 18. Guihot A., Bossi P., Bricaire F. 2004. Bioterrorism with brucellosis. Presse Med. 33:119–122(In French.) [DOI] [PubMed] [Google Scholar]
- 19. Hay I. D., Remminghorst U., Rehm B. H. 2009. MucR, a novel membrane-associated regulator of alginate biosynthesis in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 75:1110–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong P. 2005. Utilization of the persistent nature of Brucella in the development of vaccines. Ph.D. dissertation. Texas A&M University, College Station, TX [Google Scholar]
- 21. Hong P. C., Tsolis R. M., Ficht T. A. 2000. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 68:4102–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jamieson J. A., Rich G. E., Kyrkou M. R., Cargill C. F., Davos D. E. 1981. Outbreak of brucellosis at a South-Australian abattoir. 1. Clinical and serological findings. Med. J. Aust. 2:593–596 [PubMed] [Google Scholar]
- 23. Kahl-McDonagh M. M., Arenas-Gamboa A. M., Ficht T. A. 2007. Aerosol infection of BALB/c mice with Brucella melitensis and Brucella abortus and protective efficacy against aerosol challenge. Infect. Immun. 75:4923–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kahl-McDonagh M. M., et al. 2006. Evaluation of novel Brucella melitensis unmarked deletion mutants for safety and efficacy in the goat model of brucellosis. Vaccine 24:5169–5177 [DOI] [PubMed] [Google Scholar]
- 25. Kahl-McDonagh M. M., Ficht T. A. 2006. Evaluation of protection afforded by Brucella abortus and Brucella melitensis unmarked deletion mutants exhibiting different rates of clearance in BALB/c mice. Infect. Immun. 74:4048–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller C. D., Songer J. R., Sullivan J. F. 1987. A twenty-five year review of laboratory-acquired human infections at the National Animal Disease Center. Am. Ind. Hyg. Assoc. J. 48:271–275 [DOI] [PubMed] [Google Scholar]
- 27. Montaraz J. A., Winter A. J. 1986. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect. Immun. 53:245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mueller K., Gonzalez J. E. Complex regulation of symbiotic functions is coordinated by MucR and quorum sensing in Sinorhizobium meliloti. J. Bacteriol. 193:485–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nicoletti P. 1990. Vaccination against Brucella. Adv. Biotechnol. Processes 13:147–168 [PubMed] [Google Scholar]
- 30. Onate A. A., et al. 2003. A DNA vaccine encoding Cu,Zn superoxide dismutase of Brucella abortus induces protective immunity in BALB/c mice. Infect. Immun. 71:4857–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pappas G., Akritidis N., Bosilkovski M., Tsianos E. 2005. Brucellosis. N. Engl J. Med. 352:2325–2336 [DOI] [PubMed] [Google Scholar]
- 32. Pasquevich K. A., et al. 2009. Immunization with recombinant Brucella species outer membrane protein Omp16 or Omp19 in adjuvant induces specific CD4+ and CD8+ T cells as well as systemic and oral protection against Brucella abortus infection. Infect. Immun. 77:436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perkins S. D., Smither S. J., Atkins H. S. 2010. Towards a Brucella vaccine for humans. FEMS Microbiol. Rev. 34:379–394 [DOI] [PubMed] [Google Scholar]
- 34. Plommet M., Bosseray N. 1977. Checking of anti-Brucella vaccines by counting the Brucella in the spleen of intraperitoneally innoculated, vaccinated or unvaccinated, mice J. Biol. Standardization 5:261–274 [DOI] [PubMed] [Google Scholar]
- 35. Rinaudi L. V., Sorroche F., Zorreguieta A., Giordano W. Analysis of the mucR gene regulating biosynthesis of exopolysaccharides: implications for biofilm formation in Sinorhizobium meliloti Rm1021. FEMS Microbiol. Lett. 302:15–21 [DOI] [PubMed] [Google Scholar]
- 36. Saez D., Guzman I., Andrews E., Cabrera A., Onate A. 2008. Evaluation of Brucella abortus DNA and RNA vaccines expressing Cu-Zn superoxide dismutase (SOD) gene in cattle. Vet. Microbiol. 129:396–403 [DOI] [PubMed] [Google Scholar]
- 37. Schurig G. G., Sriranganathan N., Corbel M. J. 2002. Brucellosis vaccines: past, present, and future. Vet. Microbiol. 90:479–496 [DOI] [PubMed] [Google Scholar]
- 38. Spink W. W., Hall J. W., III, Finstad J., Mallet E. 1962. Immunization with viable Brucella organisms: the results of a safety test in humans. Bull. World Health Organ. 26:409–419 [PMC free article] [PubMed] [Google Scholar]
- 39. Winter A. J., et al. 1996. Protection of BALB/c mice against homologous and heterologous species of Brucella by rough strain vaccines derived from Brucella melitensis and Brucella suis biovar 4. Am. J. Vet. Res. 57:677–683 [PubMed] [Google Scholar]
- 40. Wu Q., Pei J., Turse C., Ficht T. A. 2006. Mariner mutagenesis of Brucella melitensis reveals genes with previously uncharacterized roles in virulence and survival. BMC Microbiol. 6:102. [DOI] [PMC free article] [PubMed] [Google Scholar]