Abstract
Resistance to schistosomiasis is associated with increased levels of serum parasite-specific IgE. IgE exerts its functions through its cellular receptors, FcεRI and FcεRII/CD23; however, its functional significance in humans requires further characterization. We previously reported that increased levels of CD23+ B cells correlate with resistance to schistosomiasis in hyperexposed populations and sought to define their potential function and relationship with IgE. We found that CD23+ B cells are a heterogeneous cell population with functional and phenotypic differences. Circulating CD23+ B cells are uniquely activated in schistosomiasis and express the CD23b isoform and CXCR5, the homing receptor for lymphoid follicles. High CXCR5 expression by CD23+ B cells was associated with the capacity to home to the cognate ligand CXCL13. CD23-bound IgE cross-linking increased surface expression of CXCR5, suggesting that CD23+ B cells home directly into the lymphoid follicles upon antigen capture. As human schistosomiasis is an intravascular parasitic infection associated with a high antigenic burden in the blood, circulating CD23+ B cells may play a role in the capture and shuttling of antigens directly to splenic follicles, highlighting a new role for circulating B cells. This function likely plays an important role in the development of protective immunity to infection with schistosomes.
INTRODUCTION
Resistance to schistosomiasis is associated with increased levels of serum parasite-specific IgE (10). The functional significance of IgE in humans requires further characterization, but the antibody may facilitate parasite attrition or immune responses (20, 21). IgE exerts its functions through its cellular receptors, FcεRI and FcεRII/CD23, which are expressed by a variety of cells (19). We previously reported that increased levels of CD23+ B cells correlate with resistance to schistosomiasis in hyperexposed populations (3, 34). CD23 is a 45-kDa type II membrane glycoprotein and contains an α-helical coiled-coil stalk region, which facilitates oligomerization of membrane-bound receptors (22). Trimerization of CD23 greatly increases the affinity of IgE to meet, or exceed, that of FcεRI (1.45 × 108 M−1) (28). CD23+ B cells preloaded with IgE circulate in the bloodstream, indicating a probable role for CD23-bound IgE in mediating some of the effector functions of IgE in schistosomiasis (34).
We demonstrated that CD23-bound, parasite-specific IgE induces kinase activation in B cells, but the role(s) of these signaling pathways in host resistance remains unclear (21). Indeed, the immunobiology of CD23 is highly complex. B cells express both isoforms of human CD23, CD23a and CD23b, which differ only in their cytosolic domains (42). CD23a is constitutively expressed by many cell types, including B cells, while CD23b is induced by exposure to certain factors, most notably interleukin-4 (IL-4) (14, 18). The gene for CD23 is located on chromosome 19, where the two isoforms are generated by individual promoters and alternative RNA splicing (11, 29). Functionally, the CD23 isoforms appear distinct as well. Whereas CD23b controls IgE-dependent cytotoxicity by macrophages (39), CD23a mediates endocytosis of bound ligands by B cells (25). This corresponds well to findings that the isoforms are associated with different signaling cascades; CD23b upregulates cyclic AMP (cAMP) and inducible nitric oxide synthase (iNOS) in macrophages, while CD23a mediates increased intracellular calcium (9, 30).
IgE bound to CD23 by B cells is thought to augment antigen presentation of captured antigens to T cells, but other roles, such as the transportation of immune complexes to splenic follicles, have been demonstrated in mice (23). However, although CD23b is inducible, the function of this isoform in human B cells is unknown. We therefore sought to better define the role of CD23+ B cells in human schistosomiasis. We demonstrate that circulating CD23+ B cells are uniquely activated and express CD23b, as well as CXCR5 (1). CXCR5 levels are generally increased by activated B cells upon receiving a positive signal from T cells. CXCR5 expression licenses the activated B cells to enter germinal centers to continue on a path of differentiation (37). Here, we provide evidence that CD23 plays a role in CXCR5 regulation to promote the capture and transportation of intravascular antigens directly into lymphoid follicles to augment immunity to schistosomiasis.
MATERIALS AND METHODS
Study area and population.
This study was approved by the Institutional Review Board of Boston University (BU IRB), the Scientific Steering Committee of the Kenya Medical Research Institute (KEMRI), and the National Ethics Review Committee of Kenya. The study was conducted along the shores of Lake Victoria, approximately 80 km from Kisumu city in western Kenya, with adult males exposed as car washers (n = 45) and fishermen (n = 10) (Table) 1. Occupationally exposed laborers have relatively longer contact with the lake water, raising their average rates of infection (26). Uninfected Kenyan subjects were recruited from KEMRI (n = 5).
Table 1.
Study population characteristics
| Group | No. | Age (yr) | No. of eggs/g feces (range) |
|---|---|---|---|
| Car washers | 45 | 25 ± 4.4 | 9.5 ± 16.9 (0–92) |
| Fishermen | 10 | 39.4 ± 12.4 | 479.3 ± 792.0 (4–2,880) |
Upon informed consent, peripheral blood was drawn into heparinized tubes for the experiments outlined below. Stool samples were examined for Schistosoma mansoni eggs and for other helminth ova by the modified Kato-Katz method (Vestergaard Frandsen; 2 slides each, 3 stool specimens obtained over several days). Subjects positive for S. mansoni were treated with 40 mg/kg praziquantel; those positive for other helminth ova were treated with 400 mg of albendazole as previously described (34).
Blood and tissue samples.
Peripheral blood (n = 12) was purchased from Source Leukocytes (NY Biologics) and was used to characterize and isolate circulating B cells from the unexposed/uninfected population. Fresh, surgically discarded tonsils (n = 10), peripheral lymph nodes (PLN; n = 3), and spleens (n = 2) were purchased from the Pathology Department at BU (Boston, MA) or from the National Disease Research Institute (Philadelphia, PA) and processed as previously described (15). Briefly, minced lymphoid tissues were gently homogenized and passed over a 70 μM cell strainer (Falcon) to obtain a single-cell suspension, followed by Ficoll gradient centrifugation to isolate mononuclear cells. B cells were isolated from mononuclear cells from tissues, or peripheral blood mononuclear cells (PBMC), by negative-selection magnetic beads, with a resulting 97 to 99% purity of CD19+ B cells (Miltenyi, Auburn, CA; Invitrogen, Carlsbad, CA). The CD23+ Ramos B cell line was purchased from the ATCC (Manassas, VA).
Flow cytometry.
B cells in fresh whole-blood samples were evaluated for surface expression of CD23 and CXCR5. Tubes with 100 μl/tube of heparinized whole blood were incubated with fluorescently labeled antibodies purchased from BD Pharmingen (San Jose, CA) at 4°C for 30 min. Red blood cells were lysed with 2 ml of fluorescence-activated cell sorter (FACS) lysing buffer (BD Pharmingen). Assessment of surface expression on B cells was performed with gates generated with anti-CD19 and the appropriate isotype controls for each sample. Other flow cytometry was performed using standard protocols (35).
CD23 gene expression.
Total RNA was extracted from ∼1 million purified B cells using a commercially available kit from Qiagen (Valencia, CA). The extracted RNA was treated with DNase and heated at 37°C for 30 min and then at 65°C for an additional 30 min. DNA-free mRNA was subjected to reverse transcriptase PCR (RT-PCR) with the SuperScript III one-step RT-PCR system with Platinum Taq DNA polymerase (Invitrogen) to determine CD23a and CD23b mRNA expression, with β-actin mRNA as a control. Primers for CD23a and -b were previously published (33). The DNA was labeled with 2 μl of EvaGreen dye (Biotium Inc., CA). PCR products were resolved by agarose gel electrophoresis to identify bands reflecting levels of CD23a and CD23b mRNA expression. Relative levels of expression of CD23a and CD23b were assessed with ImageJ (http://rsbweb.nih.gov/ij).
Cell culture.
Tonsil and Ramos B cells were cultured overnight with 20 ng/ml of IL-4 to upregulate nascent surface CD23. The following day, B cells were subjected to an IgE-binding protocol to load nascent CD23 molecules with IgE (21). B cells were rotated in Tris-buffered saline (TBS) buffer containing 2 mM CaCl2 with 20 μg of 4-hydroxy-3-nitrophenylacetyl (NP)-specific IgE (AbD Serotec, Oxford, United Kingdom). B cells loaded with antigen-specific IgE were stimulated with NP-bovine serum albumin (NP-BSA; Biosearch Technologies, Novato, CA) or anti-IgE (2 μg/ml; Sigma-Aldrich, St. Louis, MO). An isotype control was used at 2 μg/ml (eBioscience, San Diego, CA).
In some experiments, B cells were activated with one of the following: stimulatory anti-CD23 (2 μg/ml; eBioscience) to cross-link CD23, Fab anti-BCRμ (Jackson ImmunoResearch Inc.; 2 μg/ml), anti-CD40 (R&D Systems), Pam3CSK4 (InvivoGen, San Diego, CA; 1 μg/ml), soluble CD21 (XpressBio, Thurmont, MD; 1 μg/ml), schistosome egg antigen (SEA; 5 μg/ml), or schistosome adult worm antigen preparation (SWAP; 5 μg/ml) (generous gifts from W. Evan Secor, CDC, Atlanta, GA). B cells were also treated with recombinant IL-10 (rIL-10), rIL-4, rIL-2, rIL-7, and rIL-13 (eBioscience; 10 to 20 ng/ml).
Chemotaxis assay.
Purified B cells were untreated or treated for 18 h with IL-4 or anti-CD40, washed, and subjected to a chemotaxis assay. Assays for B cell chemotaxis were performed using 8-μm Costar Transwell plates (Corning). Bottom chambers contained recombinant human CXCL13 (rHuCXCL13; R&D Systems) at 1,000 ng/ml in cell culture media. B cells (5 × 105) were placed in the upper chamber and incubated for 4 h at 37°C. Cells that had migrated were enumerated and are presented as percentages reflecting the number of input cells that migrated over the number that migrated in the medium control.
Intracellular phospho-specific flow cytometry.
CD23 activation was assessed by phosphokinase activity with Phospho-Flow. B cells were cultured in the presence of stimuli for 10 min, fixed with paraformaldehyde, and permeabilized with BD Phosflow Perm buffer II (21). Cells were vortexed, incubated with fluorescently labeled anti-phospho-Syk (pY352) or -ZAP70 (Y319) (BD Pharmingen) at room temperature for 30 min in the dark, washed, and evaluated by flow cytometry.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism (GraphPad Software). A one-way analysis of variance with Dunn's posttest and the Mann-Whitney U test were used for multiple- or single-group comparisons, respectively. Possible correlations were examined using Spearman's rank correlation test. Group sample sizes differ among the tests because some patient samples were unavailable.
RESULTS
Schistosome antigens do not alter the surface expression of CD23.
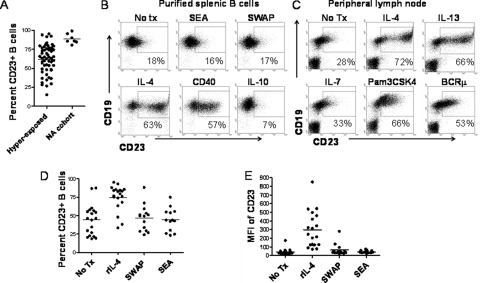
CD23 expression on B cells is high during the development of immunity to schistosomiasis (3, 34). We sought to better define host-parasite interactions that might lead to changes in levels of CD23+ B cells. In general, subjects with schistosomiasis have a larger range of CD23+ B cell percentages than North American populations, suggesting that schistosome infection may lower CD23 expression levels in some individuals (Fig. 1A). However, crude schistosome antigens, SEA and SWAP, did not directly alter the expression of surface CD23 on splenic (Fig. 1B) or peripheral lymph node (not shown) B cells from unexposed/uninfected donors. Further, schistosome antigens did not affect surface levels of CD23 on B cells from subjects hyperexposed to schistosomes (Fig. 1D and E).
Fig. 1.
Schistosomes do not alter CD23 surface levels. (A) Circulating CD23+ B cell percentages were compared between subjects with schistosomiasis (n = 55) and North Americans (NA; n = 12). CD23+ B cells levels are higher in North Americans (P < 0.0001). (B) Purified splenic B cells were cultured in the presence of the indicated stimuli for 48 h or left untreated (No Tx). rIL-4 (20 ng/ml) and anti-CD40 (1 μg/ml) increase CD23, whereas crude schistosome antigens (SEA, 5 μg/ml; soluble egg antigen and SWAP, 5 μg/ml; soluble adult worm preparation) have no effect. rIL-10 (10 ng/ml) reduces basal levels of CD23. (C) Isolated peripheral lymph node cells were cultured in the presence of the indicated stimuli. rIL-4 (20 ng/ml), rIL-13 (10 ng/ml), anti-BCRμ (2 μg/ml), and Pam3CSK4 (TLR2 ligand; 1 μg/ml) increase CD23 levels, whereas IL-7 (20 ng/ml) has a null effect. (D and E) Purified circulating B cells from subjects with schistosomiasis were cultured in the presence of IL-4 and schistosome antigens (SEA and SWAP). IL-4 increased both the percentage (D) and the mean fluorescence intensity (MFI) (E) of CD23, but there was no effect from schistosome antigens (n = 13 to 19; P < 0.0001).
CD23 upregulation on B cells is induced by cytokines, including IL-4 (Fig. 1B and C), IL-13 (Fig. 1C), and IL-2 (not shown), as well as by CD40 stimulation and BCRμ cross-linking (Fig. 1B and C). IL-4 also induces an increase in CD23 on B cells from subjects with schistosomiasis (Fig. 1D and E). In contrast, IL-10, which is elevated in schistosomiasis (8), reduces basal levels of CD23 (Fig. 1B). IL-7, a B cell growth factor, has a null effect on CD23 surface levels (Fig. 1C).
Schistosomiasis, caused by intravascular parasites, greatly raises the systemic antigenic burden and Toll-like receptor (TLR) expression by B cells (38). Figure 1C demonstrates that TLR2 ligands also increase CD23 expression. Thus, CD23 levels are likely affected by many factors relevant to schistosomiasis.
CD23b is elevated relative to CD23a on B cells of adults exposed to S. mansoni.
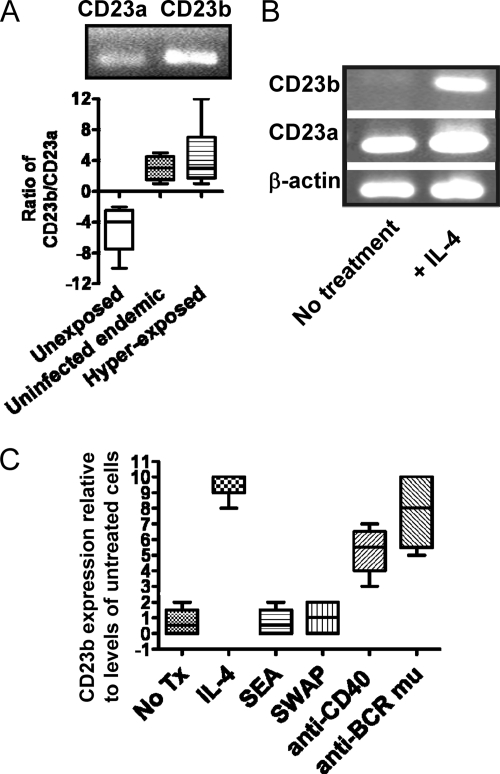
CD23 exists in two isoforms, detectable only intracellularly by mRNA expression levels. To determine which isoform of CD23 was dominant in schistosomiasis, purified B cells were subjected to RT-PCR for expression of CD23a and CD23b. Figure 2 demonstrates that CD23a is the predominant isoform expressed in uninfected/unexposed subjects (Fig. 2A, upper and lower panels). However, CD23b is higher in individuals hyperexposed to schistosomes (Fig. 2A, upper and lower panels), although a modest increase in CD23b levels was noted in Kenyan individuals who indicated no current infection with schistosomes (Fig. 2A, lower panel).
Fig. 2.
CD23b expression predominates in schistosomiasis. (A) Purified B cells were subjected to RT-PCR to measure the expression of CD23b compared to that of CD23a. CD23a is the predominant isoform in B cells from unexposed/uninfected North American blood samples (n = 4). CD23b is the predominant isoform in both hyper-exposed (n = 5) (upper and lower panels) and uninfected (n = 8) (lower panel) Kenyan populations. (B) CD23a is constitutively expressed by B cells. IL-4 induces the expression of CD23b. Shown are data from Ramos B cells. (C) Quantified expression levels of CD23b in response to the indicated stimuli. No Tx, no treatment. Note that anti-CD40 does not induce CD23b expression in Ramos B cells. Shown is the effect on tonsil B cells (n = 4).
Whereas CD23a is constitutively expressed by B cells (apparently regardless of surface levels), CD23b is inducible, most notably by IL-4 (Fig. 2B). To determine whether schistosome antigens affected CD23 isoform expression, we incubated B cells with SEA or SWAP and found no effect on CD23b (Fig. 2C) or CD23a (not shown) mRNA levels (Fig. 2). In contrast, CD40 and BCRμ stimulation was a strong inducer of CD23b expression by B cells, as was IL-4 (Fig. 2C).
CD23+ B cells express CXCR5 in schistosomiasis.
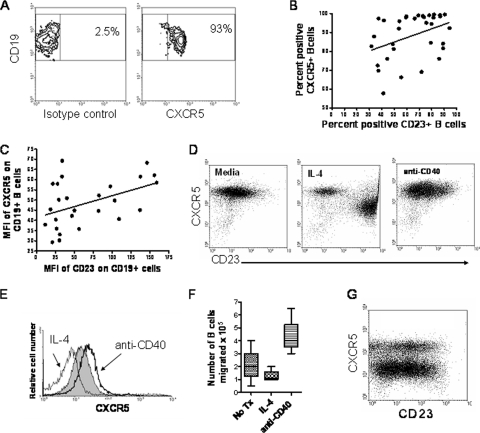
B cells that are stimulated through either the B cell receptor (BCR) or CD40 are thought to be retained in the lymphoid tissue and to not circulate in the blood (1). However, it was recently shown that CD23+ B cells play a role in the transportation of immune complexes from the blood to the follicular regions of the spleen (23). CXCR5 has a role in directing B cells to the lymphoid follicles, germinal centers (GC), and Peyer's patches (1). We therefore sought to evaluate expression levels of CXCR5 in schistosomiasis. CXCR5 expression was evident on B cells from individuals with schistosomiasis (Fig. 3A). Further, CXCR5 expression by B cells was correlated with expression of CD23 both by the proportions of cells that express CXCR5 and CD23 (Fig. 3B) and by the levels of CD23 and CXCR5 (mean fluorescence intensity [MFI]) (Fig. 3C). Thus, high levels of CD23, which arise during the development of resistance, are also associated with increased expression of CXCR5 (34).
Fig. 3.
CXCR5 expression and function by CD23+ B cells. (A) CXCR5 expression on CD19+ B cells in the fresh blood of an individual with schistosomiasis. (B) The percentage of CD23+ B cells correlates with the percentage of CXCR5+ B cells in schistosomiasis (n = 33, r = 0.39, P = 0.02). (C) The level (mean fluorescence intensity [MFI]) of CD23 correlates with the level of CXCR5 on B cells in schistosomiasis (n = 26, r = 0.45, P = 0.02). (D) IL-4 and anti-CD40 stimulate different populations of CD23+ CXCR5+ B cells. Tonsil B cells were treated for 18 h with 10 ng/ml of IL-4 or 1 μg/ml of stimulatory anti-CD40. Results are representative of 6 experiments with 6 tonsils. (E) IL-4 reduces CXCR5 levels, whereas anti-CD40 increases expression. Gray fill, untreated cells; gray line, IL-4 (10 ng/ml); black line, anti-CD40 (1 μg/ml). Results are representative of 6 experiments with 6 tonsils. (F) IL-4 reduces the chemotactic response to CXCL13, whereas CD40 stimulation increases the mobilization of B cells (4 tonsils; P = 0.03). (G) Ex vivo levels of CD23 and CXCR5 expression on CD19+ B cells from a tonsil.
CD40 stimulation induces high-expression CD23 (CD23high) CXCR5high B cells and mobilization to CXCL13.
As CD23b+ CXCR5+ B cells appeared to be a unique subset of activated circulating B cells, we sought to determine the stimuli that are potentially necessary to generate these cells in vivo. IL-4 is a strong inducer of surface CD23 (Fig. 1B and C) and CD23b (Fig. 2B and C) expression, and a hallmark of helminthiasis is the presence of indicators of increased IL-4 production, such as IgE and eosinophilia (16, 32). However, IL-4 reduces CXCR5 expression (Fig. 3D, middle panel, and E). In addition, whereas BCRμ cross-linking induced CD23 (Fig. 1C), CXCR5 levels were reduced (see Fig. 4C), consistent with B cells requiring a signal from T cells to enter the GC. In contrast, CD40 stimulation induced both CD23b (Fig. 2C) and CXCR5 (Fig. 3E) on B cells.
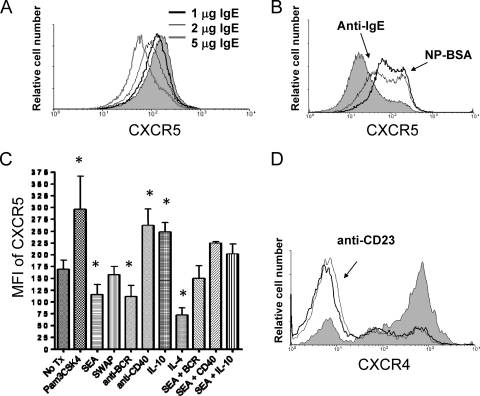
Fig. 4.
CD23-bound IgE cross-linking increases CXCR5 but reduces CXCR4 expression. (A) Increasing levels of exogenous IgE reduce surface levels of CXCR5 on IL-4-treated tonsil B cells. B cells were incubated for 18 h and evaluated by flow cytometry. Gray fill, untreated B cells. Results are representative of 3 experiments with 3 tonsils. (B) CD23-bound IgE cross-linking increases surface levels of CXCR5. NP-specific IgE was cross-linked by NP-BSA (thick black line) or anti-IgE (thin black line). Gray fill, untreated B cells. NP-BSA in the absence of IgE or an isotype control did not affect CXCR5 levels (not shown). Results are representative of 4 experiments with 4 tonsils. (C) Effect of B cell stimuli on CXCR5 levels. Tonsil B cells were treated with the indicated stimuli for 18 h, and CXCR5 levels were assessed by flow cytometry. Pam3CSK4 (TLR2 ligand), anti-CD40, and IL-10 increased levels of CXCR5, whereas SEA, anti-BCR, and IL-4 reduced levels. SWAP had no effect on CXCR5 surface levels (4 tonsils; *, P < 0.05 compared to untreated [No Tx] B cells). (D) CD23 cross-linking reduces CXCR4 expression on Ramos B cells. Gray line, 1 μg/ml anti-CD23; thick black line, 5 μg/ml anti-CD23; gray fill, untreated cells. Results are representative of 6 separate experiments. Similar results were obtained with tonsil B cells.
Although CXCR5 is expressed by most B cells, there appears to be a threshold level required for the ability to respond to chemokine (1). To test if experimentally generated CXCR5+ B cell populations had functional differences, we tested their potential to migrate to the cognate chemokine CXCL13 in chemotaxis assays. CD40-stimulated B cells migrated toward CXCL13, whereas IL-4-treated B cells demonstrated reduced chemotaxis compared to untreated B cells (Fig. 3F). Inflamed tonsils contain several populations of CXCR5+ and CD23+ B cells, indicating the physiological significance of the experimentally generated B cells in the lymphoid tissues (Fig. 3G). These results suggest that CD23+ B cells represent several distinct populations of cells with differing CD23 isoform expression levels, homing potentials, and functions.
CD23 cross-linking increases CXCR5 expression.
CD23-bound IgE is thought to play a role in antigen capture for presentation to T cells (17). This process is thought to be mediated by CD23a through an immunoreceptor tyrosine-based inhibitory motif (ITIM)-like motif that allows internalization of the captured antigen (27). The CD23b internal nucleotide sequence differs from that of CD23a, and there is no known role for CD23b in human B cells. As mentioned above, recent results suggest an important function for CD23+ B cells in immune complex transport to the follicles in mice (23). In schistosomiasis, antigen capture and transport to the follicles via CXCR5 are likely highly relevant processes in the context of a chronic intravascular infection. Thus, we speculated that there is an additional role for CD23b-bound IgE cross-linking in directing B cells toward the follicles.
As CD23 correlates with CXCR5 in schistosomiasis, we tested whether CD23 cross-linking affected surface CXCR5. Tonsil or Ramos B cells were treated with IL-4 to upregulate nascent CD23 and were experimentally bound by NP-specific IgE. We found that increasing levels of monomeric IgE exposure reduced CXCR5 surface expression (Fig. 4A). However, cross-linking NP-specific, cell-bound IgE with NP-BSA or anti-IgE increased CXCR5 levels, suggesting that antigen capture may drive B cells directly into the follicles and demonstrating the importance of antigen in mediating the effect (Fig. 4B).
Effect of schistosome antigens on CXCR5.
We recently reported that schistosome antigens reduced B cell activation levels (21). Interestingly, schistosome egg antigens, but not adult worm antigens, reduced surface levels of CXCR5 on B cells (Fig. 4C). These results suggest that the homing receptor CXCR5 is a target of immunoevasive tactics, highlighting the potential importance of the receptor in generating immunity to schistosomiasis.
IL-10 was a strong stimulator of CXCR5 and was able to overcome the effects of SEA (Fig. 4C). However, CD23 levels are reduced in response to IL-10 (Fig. 1B), perhaps supporting a role for other activation mechanisms, such as through CD40, in generating CD23high CXCR5high B cells. The TLR2 ligand also increased both CXCR5 and CD23 expression, illustrating another possible stimulator of the activated CD23+ B cells in schistosomiasis (Fig. 4C) (15). For comparison, CXCR4 expression, which homes activated B cells to an area of the lymphoid tissue involved in memory B cell and plasma cell differentiation, was reduced upon CD23 cross-linking (Fig. 4D).
CD23 ligation with CD21 activates B cells in a manner similar to that of CD23 cross-linking.
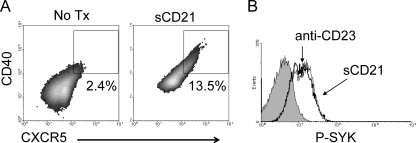
We reported that CD23 cross-linking is an important mediator in B cell signaling, particularly of Syk activation (21). Besides the role that CD23-bound IgE plays, B cells have been shown to transport immune complexes to follicular dendritic cells (FDC) in the GC in a CD21-dependent manner (12). In contrast to mouse CD23, human CD23 possesses a C-terminal tail that binds CD21 (2). CD21 is the type 2 complement receptor and can also exist in a soluble form (sCD21) which binds to complement-coated pathogen molecules. Because CD23b appears not to have an endocytosis signal, we hypothesized that this isoform may have a role in responding to CD21 ligation. CD23 is known to interact with CD21 on two sites of CD21. For these studies, we used a polypeptide of rHuCD21 spanning amino acids 30 to 280 (out of 1,092) and 1 to 2 of the short consensus repeats (SCR); this polypeptide contains the necessary binding sites for CD23 but does not cross-link CD23 molecules. B cells were treated with IL-4 to induce CD23b expression, followed by stimulation with soluble rHuCD21. Interestingly, CD23 ligation with sCD21 induced CXCR5 expression (Fig. 5A) as well as a strong phospho-Syk response (Fig. 5B) in B cells, similar to the effect mediated by CD23 cross-linking (21).
Fig. 5.
sCD21 activates B cells. (A) Tonsil B cells were treated for 18 h with IL-4 and washed and replated with 2 μg/ml of sCD21 for an additional 18 h. sCD21 induced the expression of CXCR4 as well as CD40. (B) sCD21 (thick black line) and 2 μg/ml anti-CD23 (thin black line) induce the phosphorylation of Syk in Ramos B cells, as do untreated B cells (gray fill).
DISCUSSION
CD23 expression by B cells is associated with the development of resistance to schistosomiasis (3, 34). We previously demonstrated that CD23-bound IgE augments B cell responses to schistosome antigens, thereby identifying a possible function of IgE in resistance (21). Here we show that CD23-bound IgE may be important in influencing B cell homing mechanisms. Because soluble or complexed antigens must be transported to the lymphoid follicles by specialized cells, specific subpopulations of macrophages and marginal-zone B cells are required for antigen transportation to and within the lymph node structures (7, 12, 40). In mice, CD23+ B cells were also shown to bind IgE immune complexes in blood. Capture of the IgE, but not IgG2a, immune complexes induced rapid homing of the B cells directly into the follicular areas of the spleen (23). Antigens complexed by IgE have been shown to have potent immunostimulatory effects through CD23+ B cells, similar to those of an adjuvant (17). Direct trafficking of antigens into the follicles by CD23+ B cells resulted in augmentation of T cell organization in the T cell-B cell borders of the T cell zone and an overall enhanced immune response to the CD23-transported antigen (23). These observations are likely clinically relevant in the context of schistosomiasis. Here, we present evidence that circulating CD23+ B cells in humans also transport immune complexes directly into the lymphoid follicles, a process which may play a role in the Th2-mediated immunity associated with resistance to schistosomiasis (24, 32).
We found that circulating B cells in schistosomiasis expressed a predominance of CD23b and that surface CD23 levels were correlated with expression of CXCR5. CXCR5 expression licenses activated B cells to enter germinal centers in response to a cognate chemokine, CXCL13, produced by follicular dendritic cells in the GC (1). Therefore, CXCR5 expression is generally regulated by T cells through CD40-CD40L interactions in the B cell areas of the lymphoid tissues (5). Experimentally generated CD23b+ CXCR5high B cells readily responded to CXCL13 in chemotaxis assays, suggesting that populations of CD23+ B cells in schistosomiasis have a propensity to traffic into lymphoid follicles. Cross-linking of CD23 also enhanced CXCR5 expression, demonstrating that CD23-bound IgE-mediated capture of antigen may itself increase follicular homing mechanisms in the absence of T cell help or regulation. Why CD23+ B cells would play an important role in the IgE-mediated transport of antigen is not clear, but their specific functions may include concentrating antigen in certain regions of the tissue or initiating steps in CD23-mediated antigen presentation to T cells upon arrival to the GC.
Whereas the CD23a-associated endocytosis signal is thought to be important in antigen presentation by CD23+ B cells, the role of CD23b remains undefined in human B cells (33). Thus, the inducible CD23b isoform likely has other roles during an immune response. It is possible that the lack of an endocytosis signal allows for the efficient transport of antigen without ensuing internalization by the B cell. On the other hand, both CD23a and -b were shown to transport antigens within human gastrointestinal epithelial cells, a process which requires internalization of the IgE-antigen complex (33, 41). Sessile cells, like mucosal epithelium, may utilize mechanisms of immune complex shuttling different than those used by B cells, and this requires further characterization. CD23b likely also has a role in complement-mediated transportation of immune complexes by B cells through the CD21-binding C-terminal tail, a process which increases CXCR5 surface expression as well.
B cell migration toward the germinal center normally initiates a pathway of differentiation to memory B cells or plasma cells (4). However, B cells activated by CD23-bound IgE will likely not class switch and differentiate because the antigen captured is noncognate for the B cell receptor. This supposition is illustrated by our observation that CD23 cross-linking reduced CXCR4 surface levels, which induces plasmablasts to home to the outer edges of the GC, where they differentiate into plasma cells and memory B cells (36). Nevertheless, two-photon laser scanning microscopy demonstrated that naïve follicular mantle B cells continually visit the GC (13). Fooksman et al. speculated that occasional antigen-specific B cells would recognize cognate antigen and join in the preexisting germinal center. Our results, and those of Hjelm et al., suggest that some of the follicular mantle B cells, all of which are CD23+ in mice, may have transported antigen into the GC (23). This hypothesis may explain the lack of clonal relationship between B cell populations found in the dark zone containing germ line-encoded V regions and those expressed by B cells in the GC, as well as the lack of clonal relationship between the two in human tonsils (31). And, once B cells have released their cargo, they may no longer be bound by the chemokines in the FDC region, which is consistent with our observation that IgE monomers reduce CXCR5 surface levels, and the cells subsequently reenter the circulation.
We have demonstrated that CD23-mediated signaling is dominant over that mediated by BCRμ, illustrating the potential importance of this pathway (21). Because CD23 is expressed by BCRμ+ cells, it stands to reason that antigen capture in the bloodstream by the low-affinity BCR also occurs in intravascular schistosomiasis (21). Our results demonstrate that BCR cross-linking reduces CXCR5 surface levels. Thus, the dominance of CD23-IgE activation may be significant where the transportation of specific antigens (those that bind antigen-specific IgE) is critical for the development of immunity. Interestingly, our previous report indicates that schistosome antigens inherently suppress human B cell function (21). Here we show that exposure to schistosome egg antigens reduces CXCR5 levels on B cells through undefined mechanisms. Resistant individuals likely have the ability to prevail over the immunoevasive tactics by maintaining high levels of both surface CXCR5 and CD23 (3, 34). Our data indicate that there may be multiple mechanisms to generate the uniquely activated CD23b+ CXCR5high B cells in schistosomiasis. For example, CD40 stimulation upregulates both CD23b and CXCR5 and can overcome the effects of SEA on CXCR5 expression. In addition, TLR ligands may also promote an increase in CXCR5high CD23high B cells, as schistosomiasis is associated with elevated microbial and endogenous TLR ligands and TLR2+ B cells (38). Nevertheless, we also observed a modest increase in CD23b expression by B cells in schistosome-uninfected Kenyans (n = 5), suggesting that CD23b expression may rise through multiple mechanisms. Overall, as we begin to better understand the role of IgE in protective immunity in human helminthiasis, we can develop improved vaccines and adjuvants for controlling disease (6). Further characterization of the functional significance of CD23 expression by B cells may shed light on human immunological mechanisms critical for understanding multiple diseases.
ACKNOWLEDGMENTS
This work was supported by funding from the NIAID (grant A1074843) and a 2007 BD Research Grant to L.G.-L. and a Wellcome Trust grant (08360) to P.M.
We thank Barbara Nikolajczyk (BU, Boston, MA) for technical support, V. O. Ofulla for helpful comments, and especially the study participants.
Footnotes
Published ahead of print on 27 June 2011.
REFERENCES
- 1. Ansel K. M., et al. 2000. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406:309–314 [DOI] [PubMed] [Google Scholar]
- 2. Aubry J., Pochon S., Graber P., Jansen K., Bonnefoy J. 1992. CD21 is a ligand for CD23 and regulates IgE production. Nature 358:505–507 [DOI] [PubMed] [Google Scholar]
- 3. Black C. L., et al. 2010. Increases in levels of schistosome-specific immunoglobulin E and CD23(+) B cells in a cohort of Kenyan children undergoing repeated treatment and reinfection with Schistosoma mansoni. J. Infect. Dis. 202:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bohnhorst J. O., Bjorgan M. B., Thoen J. E., Natvig J. B., Thompson K. M. 2001. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjogren's syndrome. J. Immunol. 167:3610–3618 [DOI] [PubMed] [Google Scholar]
- 5. Brandes M., Legler D. F., Spoerri B., Schaerli P., Moser B. 2000. Activation-dependent modulation of B lymphocyte migration to chemokines. Int. Immunol. 12:1285–1292 [DOI] [PubMed] [Google Scholar]
- 6. Burke J. M., Ganley-Leal L. M., Khatri A., Wetzler L. M. 2007. Neisseria meningitidis PorB, a TLR2 ligand, induces an antigen-specific eosinophil recall response: potential adjuvant for helminth vaccines? J. Immunol. 179:3222–3230 [DOI] [PubMed] [Google Scholar]
- 7. Carrasco Y. R., Batista F. D. 2007. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27:160–171 [DOI] [PubMed] [Google Scholar]
- 8. Coutinho H. M., et al. 2005. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J. Infect. Dis. 192:528–536 [DOI] [PubMed] [Google Scholar]
- 9. Dugas B., Mossalayi M. D., Damais C., Kolb J. P. 1995. Nitric oxide production by human monocytes: evidence for a role of CD23. Immunol. Today 16:574–580 [DOI] [PubMed] [Google Scholar]
- 10. Dunne D. W., Butterworth A. E., Fulford A. J., Ouma J. H., Sturrock R. F. 1992. Human IgE responses to Schistosoma mansoni and resistance to reinfection. Mem. Inst. Oswaldo Cruz 87(Suppl. 4):99–103 [DOI] [PubMed] [Google Scholar]
- 11. Ewart M. A., Ozanne B. W., Cushley W. 2002. The CD23a and CD23b proximal promoters display different sensitivities to exogenous stimuli in B lymphocytes. Genes Immun. 3:158–164 [DOI] [PubMed] [Google Scholar]
- 12. Ferguson A. R., Youd M. E., Corley R. B. 2004. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int. Immunol. 16:1411–1422 [DOI] [PubMed] [Google Scholar]
- 13. Fooksman D. R., et al. 2010. Functional anatomy of T cell activation and synapse formation. Annu. Rev. Immunol. 28:79–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fournier S., et al. 1995. The two CD23 isoforms display differential regulation in chronic lymphocytic leukaemia. Br. J. Haematol. 89:373–379 [DOI] [PubMed] [Google Scholar]
- 15. Ganley-Leal L. M., Liu X., Wetzler L. M. 2006. Toll-like receptor 2-mediated human B cell differentiation. Clin. Immunol. 120:272–284 [DOI] [PubMed] [Google Scholar]
- 16. Ganley-Leal L. M., et al. 2006. Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infect. Immun. 74:2169–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Getahun A., Hjelm F., Heyman B. 2005. IgE enhances antibody and T cell responses in vivo via CD23+ B cells. J. Immunol. 175:1473–1482 [DOI] [PubMed] [Google Scholar]
- 18. Goller M. E., et al. 2002. Regulation of CD23 isoforms on B-chronic lymphocytic leukemia. Leuk. Res. 26:795–802 [DOI] [PubMed] [Google Scholar]
- 19. Gould H. J., et al. 2003. The biology of IgE and the basis of allergic disease. Annu. Rev. Immunol. 21:579–628 [DOI] [PubMed] [Google Scholar]
- 20. Gounni A. S., et al. 1994. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature 367:183–186 [DOI] [PubMed] [Google Scholar]
- 21. Griffith Q. K., Liang Y., Onguru D. O., Mwinzi P. N., Ganley-Leal L. M. 2011. CD23-bound IgE augments and dominates recall responses through human naive B cells. J. Immunol. 186:1060–1067 [DOI] [PubMed] [Google Scholar]
- 22. Hibbert R. G., et al. 2005. The structure of human CD23 and its interactions with IgE and CD21. J. Exp. Med. 202:751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hjelm F., Karlsson M. C., Heyman B. 2008. A novel B cell-mediated transport of IgE-immune complexes to the follicle of the spleen. J. Immunol. 180:6604–6610 [DOI] [PubMed] [Google Scholar]
- 24. Johansson-Lindbom B., Ingvarsson S., Borrebaeck C. A. 2003. Germinal centers regulate human Th2 development. J. Immunol. 171:1657–1666 [DOI] [PubMed] [Google Scholar]
- 25. Karagiannis S. N., et al. 2001. Endocytosis and recycling of the complex between CD23 and HLA-DR in human B cells. Immunology 103:319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karanja D. M., et al. 2002. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet 360:592–596 [DOI] [PubMed] [Google Scholar]
- 27. Kijimoto-Ochiai S. 2002. CD23 (the low-affinity IgE receptor) as a C-type lectin: a multidomain and multifunctional molecule. Cell. Mol. Life Sci. 59:648–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kilmon M., et al. 2001. Regulation of IgE production requires oligomerization of CD23. J. Immunol. 167:3139–3145 [DOI] [PubMed] [Google Scholar]
- 29. Kneitz C., et al. 2002. The CD23b promoter is a target for NF-AT transcription factors in B-CLL cells. Biochim. Biophys. Acta 1588:41–47 [DOI] [PubMed] [Google Scholar]
- 30. Kolb J. P., Abadie A., Proschnicka-Chalufour A., de Gramont A., Poggioli J. 1994. CD23-mediated cell signalling. J. Lipid Mediat. Cell Signal. 9:27–35 [PubMed] [Google Scholar]
- 31. Kuppers R., Zhao M., Hansmann M. L., Rajewsky K. 1993. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 12:4955–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leenstra T., et al. 2006. T-Helper-2 cytokine responses to Sj97 predict resistance to reinfection with Schistosoma japonicum. Infect. Immun. 74:370–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H., et al. 2006. Transcytosis of IgE-antigen complexes by CD23a in human intestinal epithelial cells and its role in food allergy. Gastroenterology 131:47–58 [DOI] [PubMed] [Google Scholar]
- 34. Mwinzi P. N., et al. 2009. Circulating CD23(+) B cell subset correlates with the development of resistance to Schistosoma mansoni reinfection in occupationally exposed adults who have undergone multiple treatments. J. Infect. Dis. 199:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Noronha A. M., et al. 2009. Hyperactivated B cells in human inflammatory bowel disease. J. Leukoc. Biol. 86:1007–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okada T., Cyster J. G. 2006. B cell migration and interactions in the early phase of antibody responses. Curr. Opin. Immunol. 18:278–285 [DOI] [PubMed] [Google Scholar]
- 37. Okada T., et al. 2002. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 196:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Onguru D., et al. 2011. Human schistosomiasis is associated with endotoxemia and Toll-like receptor 2- and 4-bearing B cells. Am. J. Trop. Med. Hyg. 84:321–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paul-Eugene N., et al. 1992. Ligation of CD23 triggers cAMP generation and release of inflammatory mediators in human monocytes. J. Immunol. 149:3066–3071 [PubMed] [Google Scholar]
- 40. Phan T. G., Grigorova I., Okada T., Cyster J. G. 2007. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 8:992–1000 [DOI] [PubMed] [Google Scholar]
- 41. Tu Y., et al. 2005. CD23-mediated IgE transport across human intestinal epithelium: inhibition by blocking sites of translation or binding. Gastroenterology 129:928–940 [DOI] [PubMed] [Google Scholar]
- 42. Yokota A., et al. 1988. Two species of human Fc epsilon receptor II (Fc epsilon RII/CD23): tissue-specific and IL-4-specific regulation of gene expression. Cell 55:611–618 [DOI] [PubMed] [Google Scholar]