Abstract
Group B streptococcus (GBS) is a common commensal of the gastrointestinal and vaginal mucosa and a leading cause of serious infections in newborns, the elderly, and immunocompromised populations. GBS also causes infections of the urinary tract. However, little is known about host responses to GBS urinary tract infection (UTI) or GBS virulence factors that participate in UTI. Here we describe a novel murine model of GBS UTI that may explain some features of GBS urinary tract association in the human host. We observed high titers and heightened histological signs of inflammation and leukocyte recruitment in the GBS-infected kidney. However, extensive inflammation and leukocyte recruitment were not observed in the bladder, suggesting that GBS may suppress bladder inflammation during cystitis. Acute GBS infection induced the localized expression of proinflammatory cytokines interleukin-1α (IL-1α), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, and IL-9, as well as IL-10, more commonly considered an anti-inflammatory cytokine. Using isogenic GBS strains with different capsule structures, we show that capsular sialic acid residues contribute to GBS urinary tract pathogenesis, while high levels of sialic acid O-acetylation attenuate GBS pathogenesis in the setting of UTI, particularly in direct competition experiments. In vitro studies demonstrated that GBS sialic acids participate in the suppression of murine polymorphonuclear leukocyte (PMN) bactericidal activities, in addition to reducing levels of IL-1α, tumor necrosis factor alpha, IL-1β, MIP-1α, and KC produced by PMNs. These studies define several basic molecular and cellular events characterizing GBS UTI in an animal model, showing that GBS participates simultaneously in the activation and suppression of host immune responses in the urinary tract.
INTRODUCTION
Group B streptococcus (GBS) is the leading cause of bacterial sepsis and meningitis in human newborns and is increasingly associated with invasive infections in adult populations such as pregnant women, diabetics, and the elderly (11, 16, 48, 65). GBS colonizes the gastrointestinal and vaginal mucosa of approximately 30% of individuals tested at a given time and nearly 2/3 of those tested at multiple intervals over a year (35). While GBS colonization occurs asymptomatically, localized infections of the skin, lungs, and urinary tract are associated with progression to serious systemic infections in individuals with a compromised or underdeveloped immune system (4, 16, 48, 65). For example, vaginal colonization in late pregnancy is associated with vertical transmission of GBS in utero due to ascending amniotic infection or by aspiration of contaminated vaginal fluids during delivery. Pneumonia, sepsis, and meningitis are potential complications of GBS transmission to the neonate, reflecting an array of bacterial virulence factors that act to impede phagocytic clearance, resulting in host tissue injury (10). Much of what is known about GBS virulence has been defined in animal models of systemic infection (18, 20, 23, 25, 28, 46, 47). GBS has also been described in animal models of localized colonization and infection, including vaginal (7, 8), pulmonary (15, 19, 22), mammary (52), and orogastric (27) infection models. GBS can also cause cystitis and pyelonephritis (54, 55), but the urinary tract is less well characterized as a site of GBS-host interactions.
Asymptomatic bacteriuria and urinary tract infection (UTI) caused by GBS are common among pregnant, elderly, and otherwise immunocompromised individuals, groups with a higher risk of ascending pyelonephritis that can progress to bacteremia (11, 16, 53, 55). It has been estimated that up to 7% of pregnant women have significant titers of GBS in their urine (36). While typically asymptomatic, GBS bacteriuria in pregnancy is an independent risk factor for maternal pyelonephritis and chorioamnionitis as well as neonatal GBS disease (1, 36, 41, 42). Other studies of elderly populations with UTIs show an involvement of GBS in as many as 39% of nursing home residents over 70 years of age (4). This result stands in striking contrast to the proportion of UTIs associated with GBS in younger populations, estimated at 1 to 2% (14). Despite the abundant molecular epidemiological data regarding GBS, little is known about GBS UTI pathogenesis. This dearth of understanding contrasts dramatically to our extensive knowledge of disease processes involving the more common etiologic agent of UTI, uropathogenic Escherichia coli (UPEC), which successfully utilizes multiple immune evasion strategies to persist within the urinary tract (45, 49, 61, 62).
During systemic infection, GBS employs a number of strategies to avoid recognition and clearance by the host immune system. Capsular polysaccharide is one of the best-characterized GBS virulence factors. GBS capsule is critical for systemic disease progression in animal models (60). More specifically, the outermost sialic acid residues of the capsule polymer, which are identical to host surface moieties, limit complement deposition, prevent phagocytosis, and suppress neutrophil oxidative burst and granule protease release (2, 6, 12, 34, 50). Recent studies demonstrated that GBS surface sialic acids interact with host sialic acid binding immunoglobulin-like lectins (Siglecs), a family of surface receptors that are expressed on immune cells (5). Interactions between GBS sialic acids and human polymorphonuclear leukocyte (PMN) Siglec-9 lead to reduced PMN bactericidal functions and limit GBS killing, whereas modification of GBS sialic acids with high levels of O-acetylation impairs Siglec-9 binding and neutrophil suppression (6, 57, 58).
Another important virulence factor of GBS is the β-hemolysin/cytolysin, a pore-forming toxin responsible for the characteristic beta-hemolytic phenotype of GBS on blood agar plates (13). Expression of β-hemolysin/cytolysin is correlated with disease severity in murine models of intranasal and intravenous administration, consistent with its ability to produce injury in both red blood cells and pulmonary epithelial and endothelial cells (33, 43, 51). Studies also demonstrate a role of β-hemolysin/cytolysin during adherence of lung epithelium and induction of neutrophil chemoattractant cytokine interleukin-8 (IL-8) (9).
In this study, we sought to develop a robust murine model to more fully characterize infection dynamics and host response to GBS in the urinary tract. Here we elucidate several fundamental aspects of GBS-induced soluble and cellular inflammation in the murine bladder and kidney and define GBS capsule sialic acid (but not β-hemolysin/cytolysin) to be an important determinant for host association in the urinary tract.
MATERIALS AND METHODS
Ethics statement.
All animal studies were performed in accordance with approvals from the Committee for Animal Studies at Washington University School of Medicine.
Bacterial strains and growth conditions.
Uropathogenic E. coli strain UTI89 (37) or UTI89::HKGFPkanr (63) was inoculated from single colonies grown on LB agar into Luria broth containing kanamycin at 25 μg/ml where appropriate and grown statically overnight (18 to 24 h) at 37°C. Similarly, the well-characterized Streptococcus agalactiae strain COH1 or clinical strains provided by Walt Stamm (University of Washington) and Thomas Hooten (University of Florida) were inoculated from single colonies grown on Todd-Hewitt (TH) agar into TH broth (Difco) with antibiotics where appropriate and grown statically overnight (18 to 24 h) at 37°C. For GBS, bacteria were diluted and grown to log phase (optical density at 600 nm, 0.4) just prior to infection. Evaluation of GBS virulence factors important for urinary tract pathogenesis was carried out using previously published strains. Genetic manipulation of neuA, a bifunctional O-acetylesterase/sialic acid synthetase, was utilized for isogenic variation of capsular structure as previously described (29, 30). Specifically, GBS strains lacking a chromosomal copy of neuA (ΔneuA/perm) accumulate intracellular O-acetylated sialic acids and do not express capsule sialic acids (asialo). GBS strains that overexpress wild-type NeuA (ΔneuA/pneuA or COH1 wild type/pneuA) express indistinguishable levels of sialic acids with minimal O-acetylation (GBS-loOAc), whereas a point mutation in NeuA leads to hyper-O-acetylation of sialic acids (ΔneuA/pneuA301; GBS-hiOAc). These strains have been extensively characterized (29, 30, 57, 58). The acapsular GBS strain was previously described and compared in our experiments to the parent wild-type strain (64). The cylE mutant was constructed in the highly hemolytic NCTC 10/84 strain background and was kindly provided by Victor Nizet (9).
Murine infections.
Bacterial cultures, grown as described above, were collected by centrifugation and resuspended in phosphate-buffered saline (PBS). Female wild-type mice 7 to 8 weeks of age were obtained from Harlan (C3H/HeN). Mice were anesthetized by inhalation of 3% isoflurane and inoculated transurethrally with ∼1 × 107 CFU in 50 μl (16, 29). At indicated time points, mice were euthanized and bladders and kidneys were aseptically removed. The number of bacteria present in the tissues was determined by homogenization of bladders or kidney pairs in PBS and plating of serial dilutions on TH agar (for GBS) or LB agar (for UPEC) supplemented with antibiotics when appropriate. For competition experiments, GBS mutants were coinoculated at equal doses of 107 CFU per strain, followed by organ CFU enumeration on TH agar supplemented with appropriate antibiotics. Statistical analyses were performed using the Mann-Whitney U test with Prism software (version 5.00 for Windows; GraphPad Software). Recovered titers of zero were set to the limit of detection of the assay.
Histological and microscopic analyses.
At 24 h after inoculation with either GBS or PBS (mock infection), animals were euthanized and bladders and kidneys were fixed in 10% neutral buffered formalin, embedded in paraffin, cut in 5-μm-thick sections, and stained with hematoxylin and eosin (H&E) for histological analysis.
Cytokine measurement.
GBS or PBS was inoculated into mouse bladders, and organs were homogenized in 1 ml PBS at the indicated time points postinfection. Homogenates were spun at 14,000 rpm and 4°C for 5 min, and supernatants were frozen at −80°C until the time of the assay. Serum was obtained by submandibular bleeding, and blood was placed into BD Microtainer serum separator tubes. Following centrifugation at 14,000 rpm for 5 min, serum was frozen at −20°C until the time of cytokine assay. Cytokine expression in serum and organ supernatants was measured using a Bio-Plex multiplex cytokine bead kit (Bio-Rad), which measures protein levels of 23 different proinflammatory cytokines and chemokines. Statistical analyses were performed using the 1-sample t test (GraphPad Prism).
Peritoneal PMN elicitation and killing assays.
Murine peritoneal neutrophils (PMNs) were elicited by intraperitoneal injection of 2 ml of thioglycolate solution (BBL), followed by cervical dislocation and extraction of the peritoneal exudates with Hank's balanced salt solution without Ca2+, Mg2+, or phenol red (HBSS; Sigma) 4 to 6 h later for oxidative burst assessment (below) or RPMI (Gibco)-10% heat-inactivated fetal calf serum for killing assays. PMNs (5 × 105) were plated in sterile 24-well tissue culture-treated plates, GBS was added to each well at a multiplicity of infection (MOI) of 10 to 20 in at least 3 replicate wells per experiment, and the plate was centrifuged at 400 × g for 5 min at 10°C. Cells were then incubated at 37°C for 30 min and lysed in 0.05% Triton X-100 in PBS, prior to serial dilution and bacterial enumeration. The number of viable CFU was determined as the percentage of the input number of CFU, with subsequent normalization to the percent killing of wild-type bacteria to correct for biological variability in the degree of killing between experiments. For cytokine analysis following bacterial stimulation of PMNs (at an MOI of approximately 10:1 bacteria/PMNs), cell-free culture supernatants were collected after 60 min of incubation with live GBS or 180 min of incubation with heat-killed GBS and assessed for PMN cytokine expression relative to that of PBS-treated PMNs using the Bio-Plex assay described above.
Oxidative burst assessment.
PMN suspensions from five mice were pooled, and cell numbers were adjusted to 500,000 cells/ml. The cells were centrifuged at 1,000 × g and 4°C for 5 min, the supernatant was aspirated, and the cells were gently resuspended in cold burst assay buffer: HBSS supplemented with 2 mM CaCl2 and 1 mM luminol sodium salt from a freshly prepared 100 mM stock. PMNs were kept on ice until initiation of the assay. In each well of a sterile 96-well plate (Costar 3903), 50 μl of GBS suspended in Dulbecco's PBS buffer was combined with 100 μl of PMNs (50,000 cells) in burst assay buffer at multiplicities of infection ranging from 0.2 to 200. The plate was then sealed with optically clear film, and luminescence was measured every 3 min with incubation at 37°C and gentle shaking prior to each reading.
RESULTS
The murine model of GBS UTI.
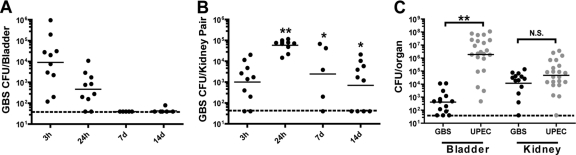
GBS serotypes Ia, III, and V are most prevalent among human UTIs caused by GBS (55). Among these, serotype III appears to have a unique predilection for causing symptomatic compared to asymptomatic UTI (55). Here we establish a urinary tract infection model using the well-studied serotype III strain COH1. After transurethral inoculation of young (7- to 8-week-old) female C3H/HeN mice with a single instilment of GBS strain COH1, we observed low-level bladder GBS colonization in organ homogenates that peaked at 3 h postinfection (hpi) and gradually diminished thereafter (Fig. 1A). The numbers of CFU recovered from kidney homogenates were highest at 24 hpi and were significantly higher than the numbers of CFU recovered from the bladders at 1, 3, and 7 days postinfection (dpi) (Fig. 1B). As a comparison, inoculation of mice with UPEC strain UTI89 resulted in significantly higher bladder titers than inoculation of mice with GBS, while GBS and UPEC colonized the kidney equally at 24 hpi (Fig. 1C). A minimally passaged clinical isolate of GBS, isolated from a human UTI, also demonstrated significant kidney tropism (data not shown), similar to other major Gram-positive uropathogens, Staphylococcus saprophyticus and Enterococcus faecalis (24, 26).
Fig. 1.
GBS preferentially colonizes the murine kidney. Young C3H/HeN female mice (7 to 8 weeks old) were transurethrally inoculated with 107 CFU GBS or UPEC in 50 μl, and the CFU in the bladder (A) or kidney (B) was enumerated at the indicated time points. The numbers of GBS or UPEC CFU were directly compared at 24 hpi (C). Results represent a compilation of 1 to 2 experiments (A and B) and a compilation of 4 experiments (C), using at least 5 mice per time point. The limit of detection of the assay is indicated by a dashed line. Statistical significance was determined by a two-tailed Mann-Whitney U test: *, P < 0.05; **, P < 0.01; n.s., not significant. Statistical significance shown in panel B denotes comparison to the numbers of bladder CFU at the same time point.
Innate immune response to GBS UTI.
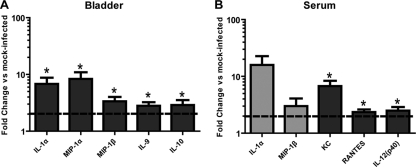
To assess the host factors that contribute to GBS UTI, the profiles of 23 different cytokines and chemokines were examined in bladder homogenates of mice at 24 hpi. GBS infection resulted in induction of IL-1α, macrophage inflammatory protein-1α (MIP-1α), MIP-1β, IL-9, and IL-10 compared to PBS mock-infected bladders (Fig. 2 A). At 24 hpi, the serum cytokines induced in the same animals during GBS infection included KC, RANTES, and IL-12p40 (Fig. 2B). In addition, IL-1α and MIP-1β were induced 15- and 3-fold (P < 0.10), respectively. These observations together represent a host response to GBS UTI consisting of cytokines and chemokines that are primarily secreted by monocytes and/or epithelial cells and that serve to attract and activate PMNs.
Fig. 2.
Cytokine/chemokine induction in the bladder and serum of GBS-infected mice. At 24 hpi, the bladders from C3H/HeN mice infected transurethrally with GBS or mock infected with PBS were removed and homogenized (A) or the serum was collected (B), and cytokine expression was assessed. The data are a composite of 2 separate experiments consisting of 4 to 5 mice each and are calculated as fold change compared to the results for PBS mock-infected animals. Dark gray bars and asterisks indicate values significantly different from those for mice mock infected with PBS (P < 0.05), as determined by a 1-sample t test; light gray bars, P < 0.10; dotted lines, 2-fold induction cutoff.
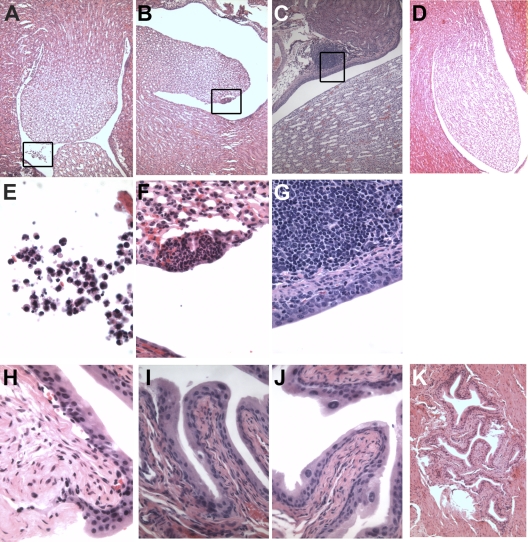
Next, organ histology was examined at 3 h and 24 h after infection. H&E staining of GBS-infected kidneys, which have higher numbers of bacterial CFU than the bladder (Fig. 1), displays large collections of immune cells in the renal pelvis and at focal sites at 3 and 24 hpi (Fig. 3 A to C), with both organs containing bacteria and immune cells with morphology indicative of PMNs, monocytes, and lymphocytes (Fig. 3E to G). No inflammation was observed in PBS-treated kidneys (Fig. 3D). In contrast, GBS-infected bladders revealed minimal visible inflammatory infiltrate at the time points examined (Fig. 3H to J), similar to PBS-treated bladders (Fig. 3K). Given the composition of bladder cytokines, it is likely that PMNs are also present in the bladder but are not detectable by gross histology. Similarly, low and dispersed bacterial titers in the bladder at this time point were difficult to detect by H&E staining, even though they were quantifiable by CFU analysis.
Fig. 3.
Robust inflammation in the kidney of GBS-infected animals. Hematoxylin-eosin staining of GBS-infected C3H/HeN murine kidneys (A to G) or bladders (H to K) at 3 hpi (A B, E, F, and H) or 24 hpi (C, G, I, and J). PBS mock-infected kidney (D) and bladder (K) at 24 hpi are shown. Representative images are shown at magnifications of ×10 (A to D, K), ×40 (I and J), ×60 (F to H), and ×100 (E). (E to G) Boxed areas from panels A to C, respectively.
GBS glycan mimicry limits murine PMN oxidative burst and bactericidal activity.
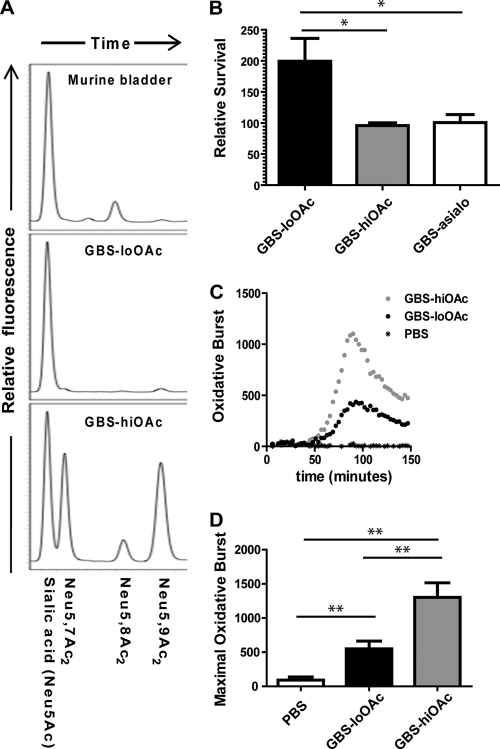
We have previously characterized a naturally occurring GBS capsule modification (sialic acid O-acetylation), which is expressed at low to moderate levels in all tested serotypes (30, 58). Site-specific mutation of a GBS O-acetylesterase has facilitated genetic control of O-acetylation levels (here referred to as GBS-hiOAc [80% of total surface sialic acids modified] and GBS-loOAc [∼2% of total surface sialic acids modified]) (29). Previous studies have shown that O-acetylation does not impact GBS complement evasion but does impede sialic acid-Siglec-9 engagement and thus attenuates bacterial evasion of human neutrophils (57, 58). Murine neutrophils express a homolog of human Siglec-9 called Siglec-E. However, it is not known whether GBS can evade murine neutrophils by sialic acid-dependent processes.
Therefore, we investigated the hypothesis that the GBS sialic acid capsule facilitates the evasion of murine neutrophil killing in early stages of UTI as part of a mechanism to persist in the murine urinary tract. We hypothesized that GBS-loOAc would better mimic the host sialic acid structure (Fig. 4A) and promote PMN suppression and bacterial survival than GBS-hiOAc. To test this hypothesis in vitro, GBS-loOAc and GBS-hiOAc were incubated with murine peritoneal PMNs for 30 min, and the surviving bacteria were enumerated. GBS-loOAc survived significantly better than GBS-hiOAc in four independent experiments (Fig. 4B). As a control, we show that bacteria lacking capsular sialic acids are also killed significantly better by murine PMNs than GBS-loOAc. In contrast, GBS-hiOAc and GBS-asialo were killed similarly by murine PMNs. Consistent with previous studies with peripheral human leukocytes (59), we show that GBS lacking capsule is killed significantly more readily by murine PMNs than its isogenic wild-type parent strain (data not shown). In addition, we measured the oxidative burst of isolated murine PMNs using kinetic luminol detection of the reactive oxygen species released following pathogen exposure. This analysis revealed that PMNs were significantly more activated in the presence of GBS-hiOAc than in the presence of GBS-loOAc (Fig. 4C and D).
Fig. 4.
Unmodified capsular sialic acids of GBS suppress oxidative burst and bactericidal activity of murine neutrophils. (A) Biochemical similarity of the surfaces of the bladder and GBS is shown by high-pressure liquid chromatography analysis of sialic acids from uninfected murine bladder and GBS strains used in this study. The latter have been previously published and are shown here for comparison. The most common sialic acid in mammals is N-acetylneuraminic acid (Neu5Ac). O-acetylation of Neu5Ac occurs at the carbon 7 and 9 positions of the sialic acid molecule (Neu5,7Ac2 and Neu5,9Ac2, respectively). (B) GBS was incubated with murine peritoneal PMNs at an MOI of 10 to 20:1 bacteria/PMNs, and bacterial survival was assessed after 30 min. Data shown are the compilation of 4 separate experiments. Kinetic measurement (C) and maximal luminescence measurement (D) of in vitro oxidative burst of murine peritoneal PMNs were assessed after incubation with GBS-hiOAc or GBS-loOAc. The MOI was 20:1 bacteria/PMNs. Similar results were obtained at an MOI of 200:1. Significant differences were determined by the paired t test: *, P < 0.05; **, P < 0.01.
GBS capsule sialic acids suppress PMN proinflammatory cytokine induction.
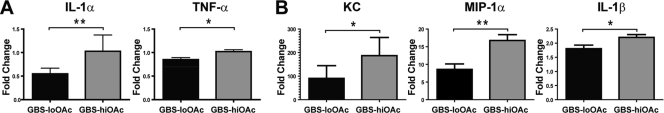
We hypothesized that the observed suppression of bactericidal activity by GBS sialic acids may also be accompanied by a suppression of PMN cytokine responses. Consistent with the in vitro data showing suppression of PMNs in the presence of GBS-loOAc compared to the level of PMN induction in the presence of GBS-hiOAc (Fig. 4B and D), we observed reduced PMN production of the proinflammatory cytokines IL-1α, tumor necrosis factor alpha (TNF-α), IL-1β, MIP-1α, and KC upon exposure to GBS-loOAc compared to that upon exposure to GBS-hiOAc (Fig. 5). After short-term exposures of PMNs to live bacteria (60 min), GBS-loOAc induced significantly lower levels of IL-1α and TNF-α than GBS-hiOAc (Fig. 5A). To better define the impact of GBS sialic acid structure on cytokine levels at later time points, heat-killed GBS was used to stimulate PMNs for 180 min, followed by cytokine measurement (Fig. 5B). Heat-killed bacteria were used to eliminate the confounding effects of differential bacterial survival in the presence of PMNs over time. At the later time point, there were significantly reduced levels of IL-1β, MIP-1α, and KC induced by GBS-loOAc compared to those induced by GBS-hiOAc. Thus, in vitro experiments demonstrate that GBS is able to suppress murine PMN cytokine responses in a sialic acid-dependent fashion that is impaired by sialic acid O-acetylation.
Fig. 5.
GBS capsular sialic acid modification alters PMN cytokine production. Murine PMN culture supernatants were collected after 60 min of incubation with live GBS or PBS (A) or 180 min of incubation with heat-killed GBS or PBS (B) and assayed for cytokine production. Data are represented as fold change compared to the results for PBS-treated PMN at the same time point (n = 3). Significant differences between GBS-hiOAc or GBS-loOAc infection were measured by the unpaired t test: *, P < 0.05; **, P < 0.01. Each panel and statistical evaluation includes data from at least 3 independent experiments.
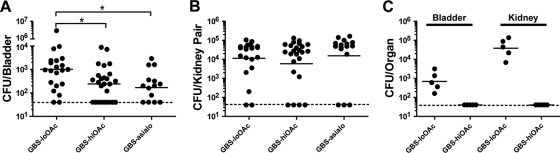
GBS capsule sialic acids impair bladder virulence and are disarmed by O-acetylation.
To directly test whether GBS capsular sialic acids contribute to pathogenesis in the urinary tract, we infected mice with GBS-loOAc and GBS-hiOAc and compared the disease outcome to that in mice infected with GBS expressing no sialic acid residues. Consistent with the in vitro data, strains expressing high levels of O-acetylation or lacking sialic acid residues displayed significantly reduced numbers of CFU in the murine bladder at 24 hpi compared to GBS-loOAc (Fig. 6 A). In fact, GBS with high levels of sialic acid O-acetylation did no better in the bladder than GBS lacking sialic acids. Interestingly, very few (2/21) of the animals infected with GBS-loOAc had completely cleared the infection by 24 h, whereas over one-third (9/26) of the animals infected with GBS-hiOAc had sterile bladders at this time point (Fig. 6A). The GBS sialic acid structure did not appear to alter the outcome of infection in the kidney at the 24-h time point (Fig. 6B). This was somewhat surprising, since both PMNs and monocytic cellular infiltrates were seen in the kidney in response to GBS infection (Fig. 3). Thus, further evaluation of GBS titers at 72 hpi were performed and showed a moderate but statistically significant reduction in GBS-hiOAc titers in the kidney compared to the GBS-loOAc strain titers (data not shown). More strikingly, competition studies showed a rapid and complete exclusion of GBS-hiOAc in the presence of GBS-loOAc in both the bladder and kidney in all animals coinfected with both strains (Fig. 6C). In contrast to the apparent role of sialic acid structure in GBS UTI pathogenesis, investigation of the GBS β-hemolysin/cytolysin encoded by cylE, which is important for virulence in virtually all other animal infection models (9, 19, 31, 44), showed no significant differences in acute bladder or kidney UTI compared to the isogenic wild-type strain (data not shown).
Fig. 6.
GBS capsular sialic acid O-acetylation limits virulence in the bladder. C3H/HeN mice were infected with isogenic GBS bearing surface structural alterations of the capsular polysaccharide. The numbers of CFU in bladder (A) and kidney (B) were enumerated at 24 hpi (n = 3 to 5 experiments with at least 5 mice per group). The limit of detection of the assay is indicated by a dashed line, and statistical analysis was performed as described in the legend to Fig. 1. (C) Competition experiments were performed by coinoculation of 107 each of GBS-hiOAC and GBS-loOAc containing different antibiotic resistance markers, followed by bacterial enumeration at 24 hpi.
DISCUSSION
Urinary tract infections are among the most common bacterial infections in humans. UTI ranges from asymptomatic bacteria in the urine to a painful infection of the bladder (cystitis) or kidney (pyelonephritis) and can also progress to infection of the blood in susceptible individuals. UPEC is the most common cause of UTIs. However, both the incidence of UTI and the profile of etiologic agents responsible for UTIs differ across the age spectrum. For example, whereas GBS causes only ∼1% of UTIs in young healthy individuals, it causes nearly 40% of infections in elderly populations, where it is also strongly associated with both pyelonephritis and invasive infections (38, 53, 55). GBS cystitis and asymptomatic bacteriuria are also more prevalent during pregnancy and are independent risk factors for pyelonephritis, chorioamnionitis, and preterm labor (56). The basis for such a diverse spectrum of disease states is not well understood.
A robust animal model is required to elucidate host and bacterial factors that contribute to GBS asymptomatic urinary tract colonization and UTI pathogenesis. It is known that different mouse backgrounds are differentially susceptible to UTIs with UPEC (21). For example, the C57BL/6 mouse strain displays rapid uroepithelial exfoliation and is susceptible to quiescent intracellular reservoir formation in response to UPEC infection (39, 40). Alternatively, the C3H/HeN mouse strain is susceptible to both acute and chronic active cystitis in response to UPEC inoculation (17). A recent study described a murine model of GBS UTI using an inoculum of 109 CFU instilled transurethrally into C57BL/6 mice (54). In the current study, we use a much lower dose of 107 CFU GBS in the mouse strain C3H/HeN.
Using this model, we have found that GBS is significantly better able to cause disease in the kidney than in the bladder, a feature shared with the Gram-positive uropathogens E. faecalis and S. saprophyticus (24, 26). The cytokine profile observed in GBS UTIs is unique compared to that observed in UTIs caused by other uropathogens. However, not surprisingly, the overall host cytokine response to GBS is more similar to responses seen in catheter-associated Enterococcus faecalis UTI than UPEC UTI (17, 19). GBS infection of the murine kidney results in an immune response characterized by innate cellular recruitment. The proinflammatory response in the urinary bladder included IL-1α, which is produced by epithelial cells as well as macrophages and other leukocytes, and is consistent with an earlier study showing strong induction of this cytokine upon GBS urinary tract infection (54). The soluble response to GBS infection in the bladder also included the monocyte/macrophage chemokines MIP-1α and MIP-1β. The significant induction of macrophage-associated cytokines and chemokines produced in response to GBS infection in the absence of a significant macrophage infiltrate into the infected bladder suggests that resident macrophages may be activated during infection. The presence of PMN migration and maturation signals such as IL-1α and MIP1-α/β, along with KC, in the serum is also consistent with a role for a PMN response to the GBS-infected bladder.
Taken together, the data suggest that during UTI, GBS triggers proinflammatory signals while simultaneously suppressing immune responses. Past in vitro studies have shown that serotype III GBS can directly dampen the host bactericidal response by engaging Siglecs on human PMNs via capsular sialic acids (6). The experiments presented here show that the sialic acid-containing capsular polysaccharide of serotype III GBS can also modulate the function of murine PMNs to dampen bactericidal capacity and cytokine responses and enhance bacterial virulence during a localized murine urinary tract infection. Our finding of increased IL-10 levels in a GBS-infected bladder is consistent with earlier findings showing GBS-specific upregulation of IL-10 in other infection models (6, 32). This is potentially relevant to GBS-dependent immune suppression since IL-10 has been described to be a cytokine able to control the degree and duration of an inflammatory response. It is able to simultaneously dampen inflammation by blocking the expression of proinflammatory cytokines while also promoting the expression of anti-inflammatory molecules (3). Interestingly, GBS is the first example of a uropathogen that stimulates production of IL-10 in the urinary tract. Taken together, these observations suggest that GBS acts through multiple mechanisms to suppress immune processes during urinary tract infection.
While GBS causes both cystitis and pyelonephritis in humans, it is difficult to know the nidus of infection on the basis of clinical symptoms alone. For example, it cannot be ruled out that the clinical presentation of GBS infection in the kidney might present as cystitis or that the kidney itself could be a reservoir of asymptomatic GBS colonization. Here we demonstrate a tropism of GBS for the murine kidney, a finding that may be relevant to human UTI. Taken together, the apparently mild inflammatory response initiated by GBS in the bladder, tropism of GBS for the kidney, and sialic acid-mediated GBS immune evasion in the bladder show that GBS has multiple overlapping strategies for persistence in the urinary tract.
ACKNOWLEDGMENTS
We thank Patrick Olsen, Justin Perry, and Will Planer for additional technical assistance and Karen Dodson for critical reading of the manuscript.
This work was supported by NIH grant R01DK51406 (to S.J.H.) and startup funds (to A.L.L.).
Footnotes
Published ahead of print on 20 June 2011.
REFERENCES
- 1. Anderson B. L., Simhan H. N., Simons K. M., Wiesenfeld H. C. 2007. Untreated asymptomatic group B streptococcal bacteriuria early in pregnancy and chorioamnionitis at delivery. Am. J. Obstet. Gynecol. 196:524.e1–e5 [DOI] [PubMed] [Google Scholar]
- 2. Aoyagi Y., et al. 2005. Role of l-ficolin/mannose-binding lectin-associated serine protease complexes in the opsonophagocytosis of type III group B streptococci. J. Immunol. 174:418–425 [DOI] [PubMed] [Google Scholar]
- 3. Bazzoni F., Tamassia N., Rossato M., Cassatella M. A. Understanding the molecular mechanisms of the multifaceted IL-10-mediated anti-inflammatory response: lessons from neutrophils. Eur. J. Immunol. 40:2360–2368 [DOI] [PubMed] [Google Scholar]
- 4. Beyer I., Mergam A., Benoit F., Theunissen C., Pepersack T. 2001. Management of urinary tract infections in the elderly. Z. Gerontol. Geriatr. 34:153–157 [DOI] [PubMed] [Google Scholar]
- 5. Carlin A. F., Lewis A. L., Varki A., Nizet V. 2007. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J. Bacteriol. 189:1231–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carlin A. F., et al. 2009. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113:3333–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Q., Fischetti V. A. 2007. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl. Microbiol. Biotechnol. 74:1284–1291 [DOI] [PubMed] [Google Scholar]
- 8. Cheng Q., Nelson D., Zhu S., Fischetti V. A. 2005. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob. Agents Chemother. 49:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doran K. S., Chang J. C., Benoit V. M., Eckmann L., Nizet V. 2002. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J. Infect. Dis. 185:196–203 [DOI] [PubMed] [Google Scholar]
- 10. Doran K. S., Nizet V. 2004. Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol. Microbiol. 54:23–31 [DOI] [PubMed] [Google Scholar]
- 11. Edwards M. S., Baker C. J. 2005. Group B streptococcal infections in elderly adults. Clin. Infect. Dis. 41:839–847 [DOI] [PubMed] [Google Scholar]
- 12. Edwards M. S., Kasper D. L., Jennings H. J., Baker C. J., Nicholson-Weller A. 1982. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J. Immunol. 128:1278–1283 [PubMed] [Google Scholar]
- 13. Facklam R. R., Padula J. F., Thacker L. G., Wortham E. C., Sconyers B. J. 1974. Presumptive identification of group A, B, and D streptococci. Appl. Microbiol. 27:107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foxman B. 2003. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis. Mon. 49:53–70 [DOI] [PubMed] [Google Scholar]
- 15. Gauthier T. W., et al. 2009. In utero ethanol exposure impairs defenses against experimental group B Streptococcus in the term Guinea pig lung. Alcohol Clin. Exp. Res. 33:300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haft R. F., Kasper D. L. 1991. Group B streptococcus infection in mother and child. Hosp. Pract. (Off. Ed.) 26:111–122, 125-128, 133-134 [DOI] [PubMed] [Google Scholar]
- 17. Hannan T. J., Mysorekar I. U., Hung C. S., Isaacson-Schmid M. L., Hultgren S. J. 2010. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 6:e1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henneke P., et al. 2008. Lipoproteins are critical TLR2 activating toxins in group B streptococcal sepsis. J. Immunol. 180:6149–6158 [DOI] [PubMed] [Google Scholar]
- 19. Hensler M. E., et al. 2005. Virulence role of group B Streptococcus beta-hemolysin/cytolysin in a neonatal rabbit model of early-onset pulmonary infection. J. Infect. Dis. 191:1287–1291 [DOI] [PubMed] [Google Scholar]
- 20. Hensler M. E., Quach D., Hsieh C. J., Doran K. S., Nizet V. 2008. CAMP factor is not essential for systemic virulence of Group B Streptococcus. Microb. Pathog. 44:84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hopkins W. J., Gendron-Fitzpatrick A., Balish E., Uehling D. T. 1998. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect. Immun. 66:2798–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones A. L., Mertz R. H., Carl D. J., Rubens C. E. 2007. A streptococcal penicillin-binding protein is critical for resisting innate airway defenses in the neonatal lung. J. Immunol. 179:3196–3202 [DOI] [PubMed] [Google Scholar]
- 23. Jones A. L., Needham R. H., Rubens C. E. 2003. The delta subunit of RNA polymerase is required for virulence of Streptococcus agalactiae. Infect. Immun. 71:4011–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kau A. L., et al. 2005. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 73:2461–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kenzel S., et al. 2006. c-Jun kinase is a critical signaling molecule in a neonatal model of group B streptococcal sepsis. J. Immunol. 176:3181–3188 [DOI] [PubMed] [Google Scholar]
- 26. Kline K. A., et al. 2010. Characterization of a novel murine model of Staphylococcus saprophyticus urinary tract infection reveals roles for Ssp and SdrI in virulence. Infect. Immun. 78:1943–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lalioui L., et al. 2005. The SrtA sortase of Streptococcus agalactiae is required for cell wall anchoring of proteins containing the LPXTG motif, for adhesion to epithelial cells, and for colonization of the mouse intestine. Infect. Immun. 73:3342–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lamy M. C., et al. 2004. CovS/CovR of group B Streptococcus: a two-component global regulatory system involved in virulence. Mol. Microbiol. 54:1250–1268 [DOI] [PubMed] [Google Scholar]
- 29. Lewis A. L., et al. 2007. NeuA sialic acid O-acetylesterase activity modulates O-acetylation of capsular polysaccharide in group B Streptococcus. J. Biol. Chem. 282:27562–27571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lewis A. L., Nizet V., Varki A. 2004. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc. Natl. Acad. Sci. U. S. A. 101:11123–11128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu G. Y., et al. 2004. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc. Natl. Acad. Sci. U. S. A. 101:14491–14496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Madureira P., et al. 2007. Streptococcus agalactiae GAPDH is a virulence-associated immunomodulatory protein. J. Immunol. 178:1379–1387 [DOI] [PubMed] [Google Scholar]
- 33. Marchlewicz B. A., Duncan J. L. 1981. Lysis of erythrocytes by a hemolysin produced by a group B Streptococcus sp. Infect. Immun. 34:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marques M. B., Kasper D. L., Pangburn M. K., Wessels M. R. 1992. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect. Immun. 60:3986–3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meyn L. A., Krohn M. A., Hillier S. L. 2009. Rectal colonization by group B Streptococcus as a predictor of vaginal colonization. Am. J. Obstet. Gynecol. 201:76.e71–e77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muller A. E., Oostvogel P. M., Steegers E. A., Dorr P. J. 2006. Morbidity related to maternal group B streptococcal infections. Acta Obstet. Gynecol. Scand. 85:1027–1037 [DOI] [PubMed] [Google Scholar]
- 37. Mulvey M. A., et al. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497 [DOI] [PubMed] [Google Scholar]
- 38. Munoz P., et al. 1992. Group B Streptococcus: a cause of urinary tract infection in nonpregnant adults. Clin. Infect. Dis. 14:492–496 [DOI] [PubMed] [Google Scholar]
- 39. Mysorekar I. U., Hultgren S. J. 2006. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. U. S. A. 103:14170–14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mysorekar I. U., Mulvey M. A., Hultgren S. J., Gordon J. I. 2002. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J. Biol. Chem. 277:7412–7419 [DOI] [PubMed] [Google Scholar]
- 41. Nicolle L. E. 2007. Complicated pyelonephritis: unresolved issues. Curr. Infect. Dis. Rep. 9:501–507 [DOI] [PubMed] [Google Scholar]
- 42. Nicolle L. E. 2008. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol. Clin. North Am. 35:1–12, v [DOI] [PubMed] [Google Scholar]
- 43. Nizet V., et al. 1996. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect. Immun. 64:3818–3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Puliti M., et al. 2000. Severity of group B streptococcal arthritis is correlated with beta-hemolysin expression. J. Infect. Dis. 182:824–832 [DOI] [PubMed] [Google Scholar]
- 45. Seed P. C., Hultgren S. J. 2005. Blueprinting the regulatory response of Escherichia coli to the urinary tract. Trends Microbiol. 13:246–248 [DOI] [PubMed] [Google Scholar]
- 46. Sendi P., et al. 2009. Bacterial phenotype variants in group B streptococcal toxic shock syndrome. Emerg. Infect. Dis. 15:223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shelver D., Rajagopal L., Harris T. O., Rubens C. E. 2003. MtaR, a regulator of methionine transport, is critical for survival of group B Streptococcus in vivo. J. Bacteriol. 185:6592–6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Skoff T. H., et al. 2009. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990-2007. Clin. Infect. Dis. 49:85–92 [DOI] [PubMed] [Google Scholar]
- 49. Svanborg C., et al. 2006. Uropathogenic Escherichia coli as a model of host-parasite interaction. Curr. Opin. Microbiol. 9:33–39 [DOI] [PubMed] [Google Scholar]
- 50. Takahashi S., Aoyagi Y., Adderson E. E., Okuwaki Y., Bohnsack J. F. 1999. Capsular sialic acid limits C5a production on type III group B streptococci. Infect. Immun. 67:1866–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tapsall J. W., Phillips E. A. 1991. The hemolytic and cytolytic activity of group B streptococcal hemolysin and its possible role in early onset group B streptococcal disease. Pathology 23:139–144 [DOI] [PubMed] [Google Scholar]
- 52. Trigo G., et al. 2009. Leukocyte populations and cytokine expression in the mammary gland in a mouse model of Streptococcus agalactiae mastitis. J. Med. Microbiol. 58:951–958 [DOI] [PubMed] [Google Scholar]
- 53. Trivalle C., et al. 1998. Group B streptococcal bacteraemia in the elderly. J. Med. Microbiol. 47:649–652 [DOI] [PubMed] [Google Scholar]
- 54. Ulett G. C., et al. 2010. Group B Streptococcus (GBS) urinary tract infection involves binding of GBS to bladder uroepithelium and potent but GBS-specific induction of interleukin 1alpha. J. Infect. Dis. 201:866–870 [DOI] [PubMed] [Google Scholar]
- 55. Ulett K. B., et al. 2009. Diversity of group B Streptococcus serotypes causing urinary tract infection in adults. J. Clin. Microbiol. 47:2055–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Walker M. J., et al. 2007. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 13:981–985 [DOI] [PubMed] [Google Scholar]
- 57. Weiman S., et al. 2009. Genetic and biochemical modulation of sialic acid O-acetylation on group B Streptococcus: phenotypic and functional impact. Glycobiology 19:1204–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weiman S., et al. O-Acetylation of sialic acid on group B Streptococcus inhibits neutrophil suppression and virulence. Biochem. J. 428:163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wessels M. R., Haft R. F., Heggen L. M., Rubens C. E. 1992. Identification of a genetic locus essential for capsule sialylation in type III group B streptococci. Infect. Immun. 60:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wessels M. R., Rubens C. E., Benedi V. J., Kasper D. L. 1989. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc. Natl. Acad. Sci. U. S. A. 86:8983–8987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wiles T. J., Kulesus R. R., Mulvey M. A. 2008. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 85:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wright K. J., Hultgren S. J. 2006. Sticky fibers and uropathogenesis: bacterial adhesins in the urinary tract. Future Microbiol. 1:75–87 [DOI] [PubMed] [Google Scholar]
- 63. Wright K. J., Seed P. C., Hultgren S. J. 2005. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 73:7657–7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yim H. H., Nittayarin A., Rubens C. E. 1997. Analysis of the capsule synthesis locus, a virulence factor in group B streptococci. Adv. Exp. Med. Biol. 418:995–997 [DOI] [PubMed] [Google Scholar]
- 65. Zaleznik D. F., et al. 2000. Invasive disease due to group B Streptococcus in pregnant women and neonates from diverse population groups. Clin. Infect. Dis. 30:276–281 [DOI] [PubMed] [Google Scholar]