Abstract
Virulent mycobacterial infections progress slowly, with a latent period that leads to clinical disease in a proportion of cases. Mycobacterium avium subsp. paratuberculosis is an intracellular pathogen that causes paratuberculosis or Johne's disease (JD), a chronic intestinal disease of ruminants. Indoleamine 2,3-dioxygenase (IDO), an enzyme that regulates tryptophan metabolism, was originally reported to have a role in intracellular pathogen killing and has since been shown to have an important immunoregulatory role in chronic immune diseases. Here we demonstrate an association between increased IDO levels and progression to clinical mycobacterial disease in a natural host, characterizing gene expression, protein localization, and functional effects. IDO mRNA levels were significantly increased in M. avium subsp. paratuberculosis-infected monocytic cells. Levels of both IDO gene and protein expression were significantly upregulated within the affected tissues of sheep with JD, particularly at the site of primary infection, the ileum, of animals with severe multibacillary disease. Lesion severity was correlated with the level of IDO gene expression. IDO gene expression was also increased in the peripheral blood cells of M. avium subsp. paratuberculosis-exposed sheep and cattle. IDO breaks down tryptophan, and systemic increases were functional, as shown by decreased plasma tryptophan levels, which correlated with the onset of clinical signs, a stage well known to be associated with Th1 immunosuppression. IDO may be involved in downregulating immune responses to M. avium subsp. paratuberculosis and other virulent mycobacteria, which may be an example of the pathogen harnessing host immunoregulatory pathways to aid survival. These findings raise new questions about the host-mycobacterium interactions in the progression from latent to clinical disease.
INTRODUCTION
Many similarities exist between the pathogeneses of diseases due to virulent mycobacteria in humans and animals. The differences relate mainly to host preference and tissue trophism, such that studies in one natural model may inform others (51). Slow progression of the disease and latent infection are hallmarks of pathogenesis and have been related to both host and microbe factors (22, 51). Paratuberculosis, or Johne's disease, is a chronic infection of ruminants such as cattle, sheep, and goats. It is caused by Mycobacterium avium subsp. paratuberculosis, an obligate intracellular pathogen that resides within macrophages and inhibits certain macrophage phagosomal and activation processes in order to survive (25, 50). M. avium subsp. paratuberculosis resides mainly in the gut mucosa and associated lymph nodes (LN), causing severe granulomatous enteritis and lymphadenitis, though dissemination in blood, milk, and peripheral tissues occurs at certain times during infection (7, 14, 23). Exposure, generally thought to occur at a young age, is followed by a long (>1-year) subclinical or latent phase that in a proportion of animals progresses to clinical disease, characterized by wasting, diarrhea, and death (4, 13). The later stages of disease are associated with shedding of M. avium subsp. paratuberculosis organisms in the feces, which contaminates the environment and results in infection of other animals (23, 29). Paratuberculosis has spread to every continent, and disease control is limited by the difficulties associated with the diagnosis of subclinically infected animals and a lack of therapeutic options (51). Aside from animal welfare aspects, the disease has a major economic impact on the livestock industries due to stock and production losses (10, 23). Public health concerns have also arisen, with an association found between M. avium subsp. paratuberculosis and Crohn's disease, a debilitating inflammatory bowel disease of humans (39, 44).
The mechanisms leading to the progression from subclinical to clinical disease are poorly understood in paratuberculosis, as they are in other mycobacterial diseases. Immune regulation during chronic infection is required to eradicate the pathogen while minimizing the destruction of host tissues. Indoleamine 2,3-dioxygenase (IDO) is a rate-limiting enzyme in the breakdown of the essential amino acid tryptophan within a highly conserved metabolic pathway (37). It was originally described for its antimicrobial role, depleting tryptophan essential for the growth of some microbial pathogens (53), but has also been found to be a potent immunoregulatory molecule in pregnancy and long-term immune responses, including chronic infections and tumors (28, 37, 53). IDO is expressed by antigen (Ag)-presenting cells, such as macrophages (11) and dendritic cells (38), as well as neutrophils (8) and epithelial cells (19). The key cytokine that induces IDO expression is gamma interferon (IFN-γ).
The immunoregulatory effects of IDO depend on T cells, which are very susceptible to tryptophan depletion and to its breakdown products (the kynurenines), leading to inhibition of T cell proliferation, anergy, increased T cell apoptosis, and also alteration of the Th1/Th2 balance (37). Tolerogenic dendritic cells (DC) expressing IDO have immunosuppressive functions and are involved in the generation of regulatory T cells (12, 38). Previous studies have indicated that IDO may be induced in association with mycobacterial infections (1, 17); however, nothing is known about the role of IDO in a natural host during the course of a chronic mycobacterial infection from exposure through latency to clinical disease.
The aim of this study was to examine immune-regulatory pathways in M. avium subsp. paratuberculosis infection, particularly the tryptophan metabolic pathway and IDO, as a model for mycobacterial infection in a natural host. We hypothesized that IDO may be an important immune-regulatory molecule in the progression of disease because macrophages are the target cells of chronic M. avium subsp. paratuberculosis infection and because the main site of infection is the gut, which is a highly regulated environment. In the first comprehensive study examining IDO gene expression, protein localization, and functional analysis, we show that high IDO expression at the site of infection and by peripheral blood cells is associated with clinical but not subclinical disease. IDO induction is associated with decreased plasma tryptophan levels; this precedes clinical signs and is not found in subclinical cases. These findings may have broader applicability to mycobacterial pathogenesis and inform studies of the processes involved in the transition from latent to clinical disease.
MATERIALS AND METHODS
Bacterial cultures.
M. avium subsp. paratuberculosis sheep strain Telford 9.2 (passage level 5) was reconstituted from lyophilized stock and inoculated into a radiometric Bactec culture vial (Becton Dickinson) containing egg yolk and mycobactin J, cultured at 37°C for 2 to 3 weeks, and then subcultured on modified Middlebrook 7H10 agar slopes supplemented with mycobactin J for 6 weeks, as previously described (4, 16). An M. avium subsp. paratuberculosis cattle strain field isolate (CM00/416/C4) at passage level 6 (including its primary isolation from cattle feces) was cultured as described above. Total and viable M. avium subsp. paratuberculosis cells were enumerated by visual counting (Thoma ruled counting chamber) using a standard three-tube most-probable-number (MPN) method Bactec culture (42).
Experimental animals and tissue sampling.
All animal procedures were approved by the Animal Ethics Committee, University of Sydney. Merino sheep were obtained from properties in New South Wales that were either classified as free from JD (unexposed controls) or known to have endemic disease (naturally infected). JD-free farms were located in a region of Australia with a negligible prevalence of JD, which was confirmed by a negative serum antibody enzyme-linked immunosorbent assay (serum Ab ELISA) and whole-flock fecal culture. The disease status of naturally infected sheep was confirmed by serum Ab ELISA, pooled fecal culture and/or a specific IFN-γ assay, histopathology and mycobacterial tissue culture of culled sheep, and direct fecal quantitative PCR (qPCR), as previously described (29, 48). Full details of individual animals, infection status, and samples collected from naturally infected sheep have been previously published (48).
In addition, Merino lambs obtained from JD-free farms were used in two separate experimental-infection trials (4). Lambs were obtained from the same farm, and all tested negative for M. avium subsp. paratuberculosis infection using fecal culture, serum Ab ELISA, and IFN-γ ELISA prior to the study. Trial A involved a comparison of three cohorts of sheep: 20 unexposed control sheep, 20 sheep inoculated at 3 to 4 months of age with an M. avium subsp. paratuberculosis sheep strain (Telford 9.2), and 20 sheep inoculated at 3 to 4 months with gut homogenate from a clinically infected sheep (4, 16). The animals were managed under conventional Australian sheep farming conditions by allowing them to graze in paddocks, with age-matched controls housed separately in paddocks where no M. avium subsp. paratuberculosis-infected sheep had been kept previously. Trial B involved a similar design, comprising 20 lambs inoculated with an M. avium subsp. paratuberculosis sheep strain (Telford 9.2) in three doses (4.3 × 107, 2.3 × 107, and 9.3 × 107 cells), with a total infectious dose of 1.6 × 108 viable cells of M. avium subsp. paratuberculosis, and 10 control unexposed sheep. An experimental-infection trial in cattle (trial C) was performed using a protocol similar to the validated sheep infection model (4) but using an M. avium subsp. paratuberculosis cattle strain. Twenty calves at 3 to 6 months of age were inoculated with a low-passage-number laboratory seed stock culture of an M. avium subsp. paratuberculosis cattle strain (CM00/416/C4) in three doses over a 1-month period (2.3 × 108, 9.3 × 107, and 1.5 × 108 cells), with a total infectious dose of 4.8 × 108 viable M. avium subsp. paratuberculosis organisms (unpublished data). Ten age-matched calves were used as controls.
Blood and fecal samples were collected from naturally infected animals and age-matched controls prior to necropsy. Animals in experimental-infection trials were sampled prior to inoculation and then every 1 to 4 months. Blood samples were used for proliferation assays, RNA isolation, whole-blood IFN-γ ELISA, serum Ab ELISA (Pourquier ELISA; IDEXX), as previously described (3–4, 16, 48), and plasma tryptophan analysis. Methods for peripheral blood cell isolation, whole-blood IFN-γ ELISA, and fecal culture for cattle were validated based on published methods used for sheep (unpublished data). Necropsies were performed on all naturally infected sheep and sheep from experimental-infection trial A, as well as a subset of clinically diseased sheep from trial B, as previously described (4). Briefly, multiple samples of intestinal tissue and the associated draining LN were collected, with tissues for RNA extraction and M. avium subsp. paratuberculosis culture immediately frozen and stored at −80°C. Tissue samples for histopathology and immunohistochemistry (IHC) were placed in 10% buffered formalin prior to being paraffin embedded.
Animals were classified by exposure history, histopathology results, and tissue culture for M. avium subsp. paratuberculosis and divided into three groups: unexposed, uninfected (exposed but uninfected at the time of necropsy), and infected (showing histopathological and tissue culture evidence of disease). Infected sheep were further categorized, based on histopathological findings in the terminal ileum and according to the criteria of Pérez et al. (41), into low-grade lesion (grades 1 and 2), early paucibacillary lesion (grade 3a), or multibacillary lesion (grade 3b) cases (3, 41).
Cell infection.
THP-1 cells, a human monocytic cell line, were obtained from the European collection of cell cultures and grown at 37°C with 5% CO2 in culture medium: RPMI 1640 medium (Gibco) supplemented with 10% fetal calf serum (FCS) (Invitrogen), 0.2 mM l-glutamine, penicillin at 100 U/ml, and streptomycin at 100 μg/ml (Gibco). Cells were plated 24 h prior to infection at 2 × 105 cells/well in a 48-well plate tissue culture plate (BD Falcon). Cultures were either uninfected (control) or infected with M. avium subsp. paratuberculosis (Telford 9.2 sheep strain) at a multiplicity of infection of 10:1. Four replicate infected cultures and two to four replicate control cultures were harvested at 0, 4, 8, 24, and 48 h postinfection, resuspended in culture medium, and centrifuged at 3,000 × g for 3 min prior to extraction of RNA for qPCR studies.
Proliferation assay.
In vitro studies on the proliferation of peripheral blood cells from sheep and cattle experimentally infected with M. avium subsp. paratuberculosis were performed using a fluorescent dye (carboxyfluorescein succinimidyl ester [CFSE]) flow cytometric assay (16). Buffy coat cells were prepared by centrifugation of lithium heparin blood tubes at 1,455 × g for 20 min. The cells at the interface were aspirated and red blood cells (RBC) lysed, and then the remaining cells were washed with phosphate-buffered saline (PBS), centrifuged at 233 × g for 10 min, and resuspended in culture medium (as for THP-1 cells with 2-mercaptoethanol). These were cultured at 2.5 × 106 cells/well in triplicate in 96-well plates and stimulated with 10 μg/ml M. avium subsp. paratuberculosis Ag (cattle strain 316v whole-cell Ag; Elizabeth Macarthur Agricultural Institute, New South Wales, Australia), 5 μg/ml pokeweed mitogen (PWM), or culture medium alone as a control. Replicate cultures were performed in the presence of the tryptophan analogue 1-methyl-l-tryptophan (1-MT; Sigma-Aldrich), an inhibitor of IDO function. At the end of the culture period (6 days), samples were acquired on a flow cytometer (FACScan; BD) and gated for lymphocytes, and the percent proliferation was determined from the change in CFSE fluorescence.
RNA extraction and cDNA synthesis.
Methods for peripheral blood mononuclear cell (PBMC) isolation from sheep blood, RNA extraction, DNase treatment of RNA, and cDNA synthesis were as previously described (48). For RNA extraction from cattle blood, buffy coat cells were prepared as described above for proliferation assays from EDTA blood tubes, and the cell pellet was extracted using the illustra RNAspin minikit (GE Healthcare). RNA was extracted from THP-1 cell cultures using the illustra RNAspin minikit. RNA quality was assessed using a spectrophotometer (NanoDrop; Thermo Scientific). One to five micrograms of RNA was DNase treated to remove genomic DNA, using 10 U RQ1 DNase (Promega) and 1 μl RNasin Plus RNase inhibitor (Promega), followed by synthesis of cDNA using AffinityScript reverse transcriptase (Agilent) according to the manufacturer's instructions. cDNA was diluted 1/10 or 1/100 with nuclease-free water prior to qPCR analysis.
qPCR.
Primers were designed using Beacon Designer 4.0 (Premier Biosoft International, Palo Alto, CA) or Primer3 software, and all sequences are given in Table 1. As the ovine IDO sequence is not available, bovine/ovine-specific IDO primers were designed based on the bovine IDO sequence (National Center for Biotechnology Information (NCBI) reference sequence accession no. NM_001101866), and primers that displayed strong homology (90%) between the bovine and equine IDOs (XM_001490681.1), spanning a 396-bp intron (Table 1), were selected. Primers were optimized for use on both sheep and cattle cDNA. Reference genes for sheep tissues and PBMCs were identified and validated in previous studies (48, 54). A reference gene for cattle quantitative-PCR (qPCR) analyses was identified from microarray studies as a stable, unregulated gene in peripheral blood cells at 9, 13, and 21 weeks after experimental infection (unpublished data). For studies on THP-1 cells, human primers were designed and optimized for IDO, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-glucuronidase, histone H3, interleukin-12B (IL-12B), and IL-10 (Table 1). The reference gene (β-glucuronidase) for these studies was selected based on the method developed for M. avium subsp. paratuberculosis infection of RAW 264.7 cells (47).
Table 1.
Primer sequences and references
| Primer name | Gene product | Sequence | Product length (bp) | NCBI reference sequence no.a | % PCR efficiencyb |
|---|---|---|---|---|---|
| Bov IDO | IDO | 5′-CGAATATACTTGTCTGGTTGG (forward) | 139 | NM_001101866 | 99.3 |
| 5′-GGAGAACATCAAAGCACTG (reverse) | XM_001490681.1 | ||||
| Bov RGc | ZC3H13 | 5′-ATGCTTCAGACGGGATTCTG (forward) | 204 | XM_002691780 | 98.2 |
| 5′-TGTTGGATACCGGACTGTGA (reverse) | |||||
| Hu βglucd | β-Glucuronidase | 5′-TGTTTGAACAGCTACTACTCTTG (forward) | 131 | NM_000181.3 | 101.8 |
| 5′-GCTCCATACTCGCTCTGAAT (reverse) | |||||
| Hu GAPDH | GAPDH | 5′-CATCAAGAAGGTGGTGAAGCAG (forward) | 119 | NM_002046 | 92.4 |
| 5′-CGTCAAAGGTGGAGGAGTTGG (reverse) | |||||
| Hu Hist | Histone H3 | 5′-GTAAAGCACCCAGGAAGCAA (forward) | 137 | NM_002107.3 | 96.1 |
| 5′-AGTGGACTTCTGATAACGTCTAAT (reverse) | |||||
| Hu IDO | IDO | 5′-AGTCCGTGAGTTTGTCCTTTC (forward) | 145 | NM_002164 | 95.9 |
| 5′-CTTTGGCTGCTGGCTTGC (reverse) | |||||
| Hu IL-12B | IL-12B (p40) | 5′-GCAAGAGCAAGAGAGAAAAGAAAG (forward) | 135 | NM_002187.2 | 105.4 |
| 5′-ATGCCCATTCGCTCCAAGAT (reverse) | |||||
| Hu IL-10 | IL-10 | 5′-CAGCAGAGTGAAGACTTTCTTTC (forward) | 143 | NM_000572.2 | 103.8 |
| 5′-GCATCACCTCCTCCAGGTAA (reverse) |
NCBI nucleotide reference sequence accession number.
Efficiency of the primer set in qPCR, determined from a cDNA standard curve.
Bov RG, reference gene for bovine PBMC (identified by microarray studies) encoding predicted Bos taurus zinc finger CCCH-type domain-containing protein 13 (ZC3H13; Affymetrix identifier Bt.28167.1.S1).
Human β-glucuronidase was selected as the reference gene for THP-1 studies, as it had the lowest standard deviations (SD) (0.18) across treatment groups (control and M. avium subsp. paratuberculosis infected) and time points (0, 4, 24, and 48 h) at levels closest to those of the genes of interest (48).
qPCR was performed as previously described, using an Mx3000P real-time PCR system (Stratagene, Agilent) and QuantiTect SYBR green PCR kit (Qiagen) (48). Reaction mixtures (25 μl) contained 10 ng template cDNA, or 5 μl of a 1/10 to 1/100 dilution of cDNA, and an optimized concentration of forward and reverse primers (300 to 600 nM). qPCR experiments were run using the following program: 95°C for 15 min, and then 40 cycles of 95°C for 20 s, 52 to 60°C for 30 s, and 72°C for 30 s, with fluorescence acquisition at the end of the annealing step. Reaction specificity was confirmed postamplification using a melt curve analysis. Standard curves were performed for all primer sets once they were optimized using a dilution series (0.1 to 100 ng) of cDNA (Table 1). Fold changes in gene expression were calculated using the 2−ΔΔCT comparative quantitation method (33), following normalization against the appropriate reference gene. Data are presented as fold changes in gene expression using predicted means from the linear mixed-model statistical analysis (see below). This was calculated by comparison of the mean change in threshold cycle (ΔCT) for each treatment group to that for the unexposed group, which was given an arbitrary expression level of 1. Significant differences were also confirmed using the Relative Expression Software Tool (REST 2008; Corbett Research and M. W. Pfaffl).
Immunohistochemistry.
IHC was performed on sections of paraffin-embedded ileal tissues from control and clinically infected sheep, necropsied 14 to 16 months after M. avium subsp. paratuberculosis experimental infection. Sections were deparaffinized in xylene and then rehydrated through graded-ethanol baths. Ag retrieval was performed using Target retrieval solution, pH 6.1 (Dako), and boiled for 10 min. Sections were washed with Tris buffer, and endogenous peroxidase activity was blocked using 3% hydrogen peroxide solution for 10 min in a humidified chamber. Anti-human/mouse IDO monoclonal Ab (clone 10.1; Millipore), which cross-reacts with bovine and ovine IDO, was used at 2 μg/ml in Ab diluent (Dako). Sections were stained with IDO Ab or a mouse IgG isotype control for 1 h in a humidified chamber, washed with Tris buffer, and then incubated for 1 h with secondary Ab from the EnVision system horseradish peroxidase (HRP) kit (Dako), followed by 3,3′ diaminobenzidine (DAB) substrate solution for 5 min. Sections were counterstained with hematoxylin and bluing solution and dehydrated through ethanol and xylene baths; coverslips were placed over them using DPX mounting medium.
Plasma tryptophan determination.
Blood was collected in lithium heparin vacuum tubes (Vacuette) from experimentally infected and control sheep and centrifuged at 1,455 × g for 20 min. Plasma was collected and stored at −80°C. Tryptophan concentrations were determined using reverse-phase high-pressure liquid chromatography (HPLC) as described previously (34).
Statistics.
Gene expression differences in monocytic cultures and within tissues and PBMCs were examined using restricted maximum-likelihood (REML) analysis in a linear mixed model (GenStat release 12.1 2009; VSN International Ltd.), as previously described (48, 54). For tissues, the analysis considered property of origin as a random factor, with fixed effects of exposure (unexposed, exposed), infection status (uninfected, infected), and histopathological lesion group (no lesion, early paucibacillary lesions, multibacillary lesions). Similarly, a REML linear mixed-model analysis was performed on PBMC gene expression data that considered exposure, infection status, and lesion group as fixed effects, with type of animal and time as the random terms to account for potential correlation across sampling time points and for individual animals. Significant differences in serum tryptophan levels were assessed using Student's t test.
RESULTS
IDO is expressed by monocytes infected with M. avium subsp. paratuberculosis.
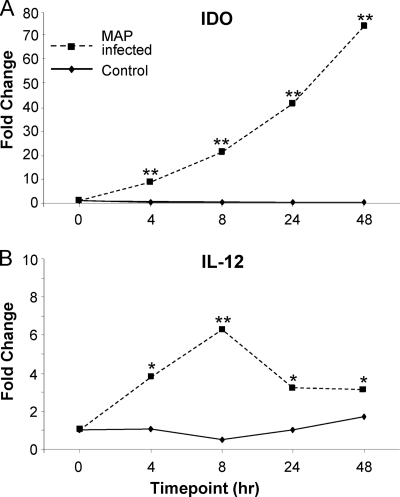
To determine if IDO was induced in paratuberculosis infections, we first examined IDO gene expression by monocytes infected with M. avium subsp. paratuberculosis in vitro. IDO was expressed at low levels by uninfected THP-1 monocytes. Infection with M. avium subsp. paratuberculosis led to a rapid and highly significant (P < 0.0001) induction of IDO gene expression (Fig. 1 A). This was evident within 4 h postinfection and reached over 75-fold of that of the control within 48 h. These results were confirmed in a second experiment, where IDO gene expression was induced >200-fold (data not shown). By comparison, IL-12 gene expression was induced about 6-fold and peaked at 8 h postinfection (Fig. 1B).
Fig. 1.
IDO gene expression is increased in M. avium subsp. paratuberculosis (MAP)-infected monocytes. Expression of IDO (A) and IL-12 (B) by the human monocytic cell line THP-1, infected at a 10:1 multiplicity of infection with M. avium subsp. paratuberculosis. Gene expression was assessed by qPCR at early time points postinfection. Replicates were carried out for control uninfected (n = 2 to 4) and M. avium subsp. paratuberculosis-infected (n = 4) cultures, harvested at 0, 4, 8, 24, and 48 h postinfection. *, P < 0.05; **, P < 0.0001 (compared to control cultures at the respective time point).
To confirm the origin of the IDO expression, the M. avium subsp. paratuberculosis K10 complete genome (NCBI reference sequence accession no. NC_002944) was searched, but it did not contain a sequence similar to that of IDO, nor were the IDO primers used able to recognize sequences in the K10 genome, based on BLAST searches. Thus, the increased expression of IDO was derived from the monocytic cells and not the intracellular M. avium subsp. paratuberculosis organisms.
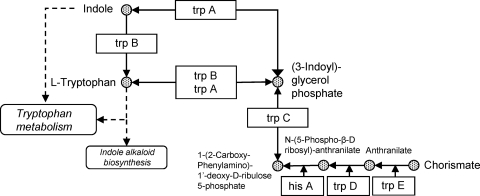
The tryptophan biosynthesis pathway is present in M. avium subsp. paratuberculosis.
Interestingly, the tryptophan biosynthesis (indole) pathway is present in M. avium subsp. paratuberculosis organisms, as shown in Fig. 2. The K10 genome contains trpA (tryptophan synthase subunit alpha, MAP1307), trpB (tryptophan synthase subunit beta, MAP1306), trpC (indole-3-glycerol-phosphate synthase, MAP1305), hisA (phosphoribosyl isomerase A, MAP1297), trpD (anthranilate phosphoribosyltransferase, MAP1931c), and trpE (anthranilate synthase component I, MAP1303), which are involved in l-tryptophan biosynthesis. Thus, M. avium subsp. paratuberculosis organisms may be protected from the effects of local tryptophan depletion due to IDO, as they can synthesize this essential amino acid.
Fig. 2.
Tryptophan biosynthesis pathway in M. avium subsp. paratuberculosis, based on KEGG pathway mpa00400 (phenylalanine, tyrosine, and tryptophan biosynthesis in Mycobacterium avium subsp. paratuberculosis strain K10).
IDO is increased in the affected tissues of M. avium subsp. paratuberculosis-infected animals.
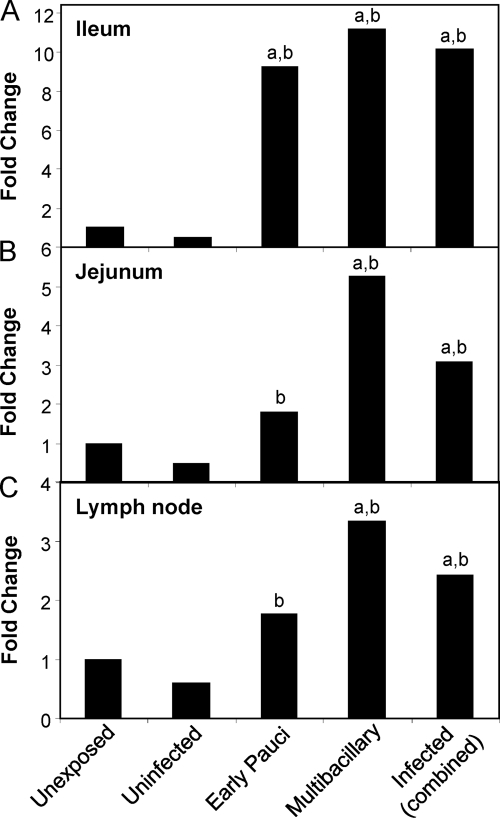
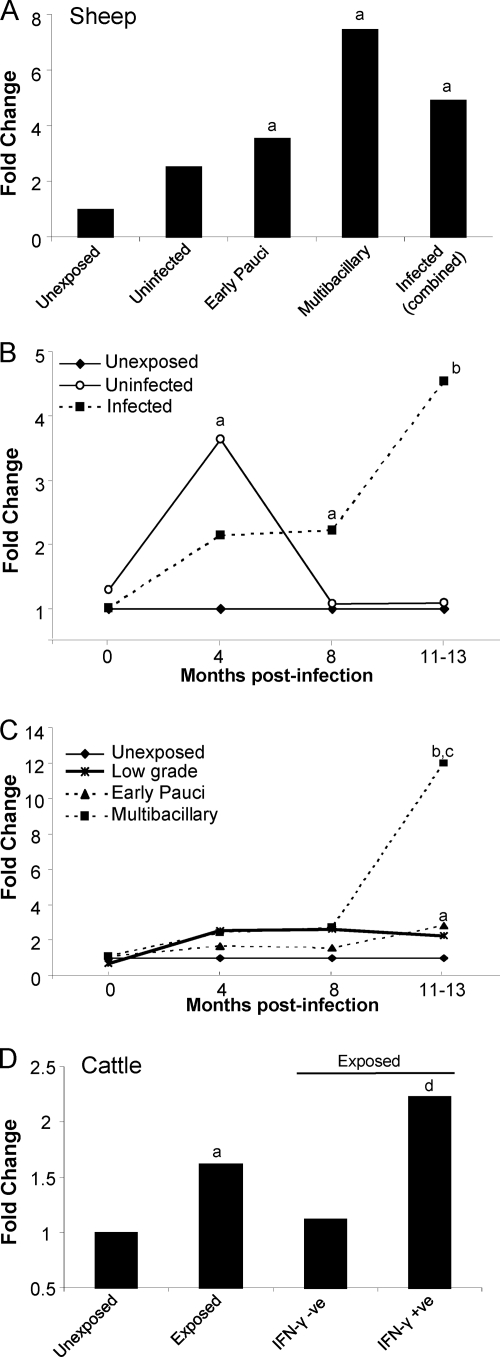
IDO gene expression was assessed in the tissues of the gut and draining LN, the sites known to be pathologically affected in animals with JD. Unexposed control sheep were compared to sheep exposed to M. avium subsp. paratuberculosis. Exposed sheep were classified at the time of necropsy as either uninfected or infected based on M. avium subsp. paratuberculosis tissue culture results, and the infected animals were further categorized into low-grade, early paucibacillary, or multibacillary lesion types. Multibacillary lesions are the most severe, characterized by granulomas with high numbers of M. avium subsp. paratuberculosis organisms, and are generally found in the later stages of disease associated with clinical manifestations.
IDO mRNA was significantly increased in the gut tissue of M. avium subsp. paratuberculosis-infected sheep compared to in control unexposed animals (Fig. 3A and B). Significant effects were identified using a linear mixed model that took into consideration the property of origin, with data (means ± standard errors of the means [SEM]) shown in Fig. S1 in the supplemental material, and also by REST analysis software (see Table S1 in the supplemental material). IDO expression in the ilea and jejuna of infected animals (combined paucibacillary and multibacillary cases) was significantly higher than that in unexposed controls and exposed uninfected sheep (P < 0.005 and P < 0.05, respectively). Multibacillary cases were associated with the highest IDO expression levels. The fold change in IDO expression tended to be higher in the ileum (9- to 11-fold), the site in the gut where the disease is most expressed, than in the jejunum, though this difference did not reach significance. As animals were classified based on the most severe lesion type detected along the length of the gut (in the ileum), jejunum data could also be analyzed based on the local lesion severity within these sections. When this was done, the IDO fold changes within this tissue were much higher (multibacillary, 8.24-fold, and paucibacillary, 3.76-fold), indicating correlation of the IDO expression level with the local lesion severity.
Fig. 3.
IDO gene expression is increased in the affected tissues of infected sheep with JD. qPCR results of IDO gene expression, normalized against the reference gene, in the ileum (A), jejunum (B), and LN draining the ileum (C). Fold change was relative to gene expression in an unexposed (control) group. Unexposed sheep, n = 12; uninfected sheep (sheep exposed to M. avium subsp. paratuberculosis but uninfected at necropsy), n = 6; sheep with early paucibacillary lesions, n = 6; sheep with multibacillary lesions, n = 6; and infected sheep (combined early paucibacillary and multibacillary), n = 12. For significant differences, “a” indicates a P of <0.005 in comparisons with the same tissue in unexposed sheep and “b” indicates a P of <0.05 in comparisons with the same tissue in exposed uninfected sheep.
Similar results were found in the draining LN, both ileal (Fig. 3C) and midjejunal (data not shown), where infected sheep had significantly higher IDO gene expression than unexposed or exposed uninfected sheep (P < 0.005 and P < 0.05, respectively). As for the gut tissue, the multibacillary cases had the highest levels of IDO expression in their LN.
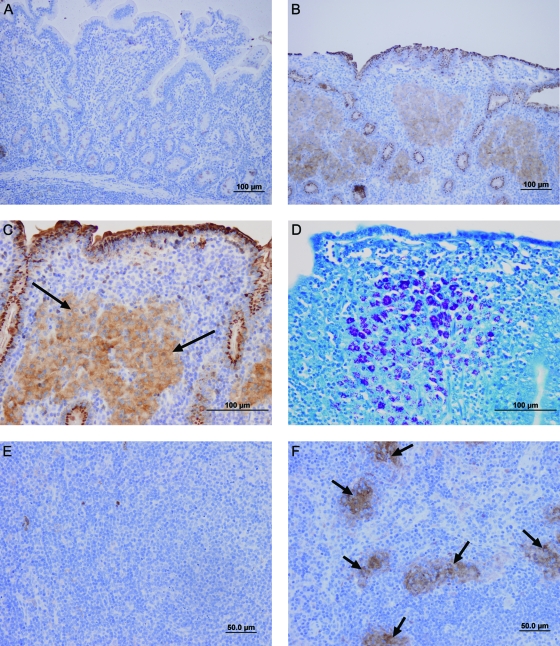
The increased IDO mRNA expression was confirmed to lead to increased IDO protein using immunohistology (Fig. 4). There was strong staining of gut and LN tissues from sheep with granulomatous paratuberculosis lesions, both paucibacillary and multibacillary. IDO protein levels were low in the normal gut tissue of sheep, with light staining confined to crypt cells (Fig. 4A). However, in the infected ileum, IDO was strongly expressed by epithelial cells and also by macrophages, which were seen as “islands” of staining in granulomas (Fig. 4B and C). These same macrophage “islands” in the multibacillary lesions were the regions with the highest mycobacterial numbers, confirmed by Ziehl-Neelsen staining for acid-fast organisms (Fig. 4D). There was also strong staining for IDO by cells within the crypts of infected gut tissues that may be Paneth cells or macrophages entering the luminal space. Within the LN, IDO staining was associated with granulomatous foci comprised of infiltrating macrophages, typical of late-stage disease (Fig. 4F). These occurred within the parafollicular zones.
Fig. 4.
IDO protein is associated with granulomatous lesions containing acid-fast bacilli. Immunohistochemical staining for IDO protein levels or acid-fast bacilli within gut and LN tissues of sheep. (A) Ileum of an unexposed control sheep stained for IDO; (B) ileum from an infected sheep with multibacillary JD lesions stained for IDO (brown); (C) infected-sheep ileum, showing a region of diffuse IDO staining (brown) associated with granulomas (arrows); (D) same infected ileum section as in panel C stained using a Ziehl-Neelsen technique to detect acid-fast bacilli, visible as dark-pink staining within the granuloma; (E) unexposed control sheep ileal LN stained for IDO; (F) infected-sheep ileal LN stained for IDO, showing IDO staining (brown) associated with granulomatous lesions (arrows). Isotype control Ab staining was negative in all sections (not shown).
IDO gene expression is increased in peripheral blood cells of M. avium subsp. paratuberculosis-exposed and -infected animals.
IDO mRNA expression was measured in peripheral blood cells of sheep and cattle with JD (Fig. 5; see also Fig. S2 in the supplemental material). When PBMCs were examined from a group of naturally and experimentally infected sheep (1.5 to 4 years), late in the disease process, IDO expression was significantly increased (P < 0.05) compared to that in unexposed controls (Fig. 5A). Further studies were conducted to examine the expression of IDO throughout the course of the disease.
Fig. 5.
IDO gene expression in peripheral blood cells is increased with M. avium subsp. paratuberculosis infection and modulated throughout the disease course. qPCR results of IDO gene expression, normalized against the appropriate reference gene, in peripheral blood cells from sheep and cattle. Fold change is relative to gene expression in an unexposed (control) group. (A) Expression of IDO in PBMCs from naturally and experimentally infected sheep at later stages of disease (1.5 to 4 years of age). Unexposed sheep (control), n = 11; uninfected sheep (sheep exposed to M. avium subsp. paratuberculosis but uninfected at necropsy), n = 5; sheep with early paucibacillary lesions, n = 7; sheep with multibacillary lesions, n = 7; infected sheep (combined early pauci- and multibacillary), n = 14. (B and C) Time course of IDO gene expression in sheep from experimental-infection trial A. Unexposed sheep (control), n = 20; uninfected sheep, n = 10; infected sheep (combined early pauci- and multibacillary), n = 18; sheep with low-grade lesions (infected sheep with a lesion grade of 1 or 2), n = 5; sheep with early paucibacillary lesions, n = 8; sheep with multibacillary lesions, n = 10. (D) Expression of IDO in PBMCs from experimentally infected cattle (n = 20) and unexposed controls (n = 10). Exposed cattle were further subdivided into those that responded in an M. avium subsp. paratuberculosis-specific IFN-γ assay (IFN-γ + ve [where “+ ve” indicates “positive”]; n = 6) compared to those that did not respond (IFN-γ − ve; n = 14), performed at 5 months postexposure. For significant differences, “a” indicates a P of <0.05 in comparisons with unexposed controls, “b” indicates a P of <0.001 in comparisons with unexposed controls and an uninfected group, “c” indicates a P of <0.01 in comparisons with low-grade and early paucibacillary lesion groups, and “d” indicates a P of <0.001 in comparisons with unexposed and IFN-γ-negative groups.
Sheep were experimentally infected with a known dose of viable M. avium subsp. paratuberculosis organisms, and blood was collected throughout the disease course for RNA extraction. The infection model has been validated for sheep and leads to a disease progression and proportions of clinical cases similar to those found with natural infections (4). Only a proportion of exposed animals subsequently develop clinical disease. Some animals that were exposed did not show signs of infection (termed “uninfected”), as they were negative in tests to detect M. avium subsp. paratuberculosis shedding in feces and in tissue culture at necropsy. When unexposed, uninfected, and infected cases were compared, sheep that were infected had significantly increased IDO expression (P < 0.001) detectable in their peripheral blood late in the disease course (Fig. 5B). Interestingly, the exposed-but-uninfected animals had an early and significant spike in IDO expression (P < 0.05) that later returned to baseline. The infected group was subdivided into low-grade-lesion, paucibacillary, and multibacillary cases, showing that the increased IDO gene expression was clearly associated with the multibacillary cases (Fig. 5C).
A similar experimental infection was conducted in cattle. Blood cells from exposed cattle (5 months postinoculation) had significantly increased IDO expression (P < 0.05) compared to unexposed controls (Fig. 5D). The fold change in expression was highest in exposed cattle that responded early in an M. avium subsp. paratuberculosis-specific IFN-γ assay compared to those that did not respond (P < 0.001).
Effect of IDO on responses of lymphocytes from experimentally infected animals.
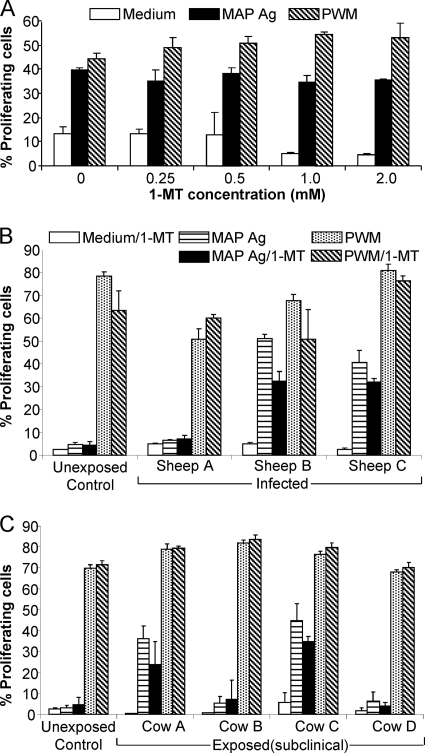
We examined proliferative responses of peripheral blood lymphocytes (PBL) to M. avium subsp. paratuberculosis Ag alone and in the presence of the tryptophan analogue 1-methyl-l-tryptophan (1-MT), an inhibitor of IDO. Studies were performed on both sheep and cattle comparing control unexposed animals and animals experimentally exposed to M. avium subsp. paratuberculosis. An initial optimization of 1-MT concentration based on the reported effective dose range (34) was performed using blood cells from exposed cattle stimulated with M. avium subsp. paratuberculosis Ag or a positive control (PWM) (Fig. 6A). This showed that the best concentration was 0.5 mM 1-MT, used in all subsequent studies.
Fig. 6.
Proliferation of PBL from M. avium subsp. paratuberculosis (MAP)-exposed and -infected sheep and cattle. (A) Titration of the concentration of the IDO-blocking agent 1-MT (0 to 2 mM) in CFSE proliferation assays of cattle peripheral blood cells. Cultures were stimulated with M. avium subsp. paratuberculosis Ag, PWM, or medium alone as a control. Values are means ± SD of percentages of proliferation, determined by decreased CFSE fluorescence compared to that in nonproliferating controls. (B and C) Representative results of PBL from experimentally exposed animals are shown, specifically, proliferation of PBL from sheep (B) and cattle (C). Cultures were stimulated with M. avium subsp. paratuberculosis Ag, PWM, or medium with or without 1-MT. Values are means ± SD of percentages of proliferation in a CFSE assay. A representative unexposed control animal (sheep or cow) was compared to individual infected animals. Results for medium controls with and without 1-MT were not significantly different for all animals; therefore, only the medium-1-MT results are shown.
Three clinically diseased sheep >12 months postinfection (experimental-infection trial B) were analyzed, all with acute weight loss, fecal shedding, and severe granulomatous lesions associated with M. avium subsp. paratuberculosis, confirmed at the time of necropsy (Fig. 6B). PBL from unexposed control sheep (n = 3) proliferated to the positive control (PWM) but not to specific M. avium subsp. paratuberculosis Ag, as expected from their exposure history (results for one representative animal are shown). Of the three clinically infected sheep, two responded to M. avium subsp. paratuberculosis Ag in the proliferation assay (sheep B and C), while the third was unresponsive (sheep A). All responded appropriately to PWM, indicating that the cells were viable. The addition of 1-MT did not enhance the proliferative response of cells from control or diseased sheep to M. avium subsp. paratuberculosis Ag or PWM. Thus, blocking IDO by adding 1-MT did not restore M. avium subsp. paratuberculosis-specific proliferative responses of the nonresponsive sheep.
Proliferative studies were also performed on four experimentally inoculated cattle at 17 months postinfection and three age-matched, unexposed control cattle (results for one representative control are shown) (Fig. 6C). Two of the cattle shed M. avium subsp. paratuberculosis in their feces, detectable by fecal culture, but their PBL were unresponsive to M. avium subsp. paratuberculosis Ag (cows B and D). The other two did not shed M. avium subsp. paratuberculosis, and PBL proliferated in response to M. avium subsp. paratuberculosis Ag. As with the sheep, the addition of 1-MT did not enhance the cellular proliferative response of any of the cattle, including the unresponsive infected cattle, to M. avium subsp. paratuberculosis Ag or PWM (Fig. 6C). Thus, blocking the effect of IDO in vitro did not restore or enhance proliferative responses to M. avium subsp. paratuberculosis Ag of sheep with JD or experimentally inoculated cattle.
Decreased plasma tryptophan levels precede the onset of clinical disease.
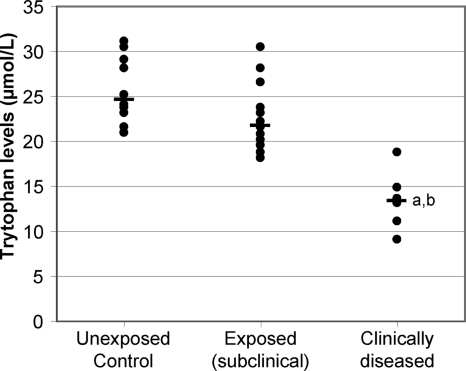
Plasma was collected at 14 months postinoculation from control and exposed sheep from experimental-infection trial B. Within 2 months of this sampling, six of the sheep displayed clinical signs consistent with JD, including a >15% loss of body mass and shedding of M. avium subsp. paratuberculosis in the feces, detected by fecal culture. These sheep were sacrificed, and necropsy confirmed clinical disease associated with severe granulomatous lesions in the gut and dissemination of the infection to the draining LN and liver.
The exposed sheep were categorized into those that developed clinical disease (at 14 to 16 months postinfection) and those that were exposed but did not show clinical signs. The clinically diseased sheep had all shown a specific IFN-γ response during the course of the infection; however, two of these animals (both multibacillary cases) had a decline in their IFN-γ responses concurrent with clinical disease onset. Of the exposed (subclinical) group, all except one had shown a specific IFN-γ response over the course of the infection and 9/13 had evidence of intermittent shedding of M. avium subsp. paratuberculosis, detected by fecal culture.
The plasma tryptophan levels are shown in Fig. 7. The range of tryptophan levels in the unexposed age-matched control sheep was 21.0 to 31.1 μmol/liter. Sheep that subsequently developed clinical disease showed a highly significant (P < 0.00001) reduction in their plasma tryptophan levels that preceded clinical signs, with a range of 9.1 to 18.8 μmol/liter. This clinical group also had significantly reduced plasma tryptophan levels compared to those of the remainder of the exposed cohort that did not develop clinical signs (P < 0.0005). This indicated that tryptophan levels in the blood decreased, coincident with increased IDO expression in the gut and periphery.
Fig. 7.
Plasma tryptophan levels are decreased in clinically diseased sheep compared to in control animals and M. avium subsp. paratuberculosis-exposed subclinical cases. Results for individual animals are shown, with the median of each group indicated by a horizontal bar. Plasma was collected from experimentally exposed sheep at 15 months postexposure. Clinically diseased sheep were identified in the 2-month period following sampling and necropsies performed to confirm disease status. Unexposed controls, n = 10; exposed (subclinical) cases, n = 13; clinically diseased cases, n = 6. For significant differences, “a” indicates a P of <0.00001 in comparisons with the unexposed control group and “b” indicates a P of <0.0005 in comparisons with the exposed (subclinical) group.
DISCUSSION
This study examined the association of IDO with progression of paratuberculosis in sheep and cattle as a model for disease pathogenesis of virulent mycobacteria. We hypothesized that IFN-γ release at the site of immune activation may be associated with induction of IDO, a key immunoregulatory molecule. This could inhibit the local adaptive immune response to the infection, allowing the M. avium subsp. paratuberculosis organism to survive and contributing to the chronicity of the infection.
We found that IDO gene and protein expression was increased in infected tissues of clinically diseased sheep. Systemically, increased IDO gene expression could be detected in the peripheral blood cells of exposed sheep and cattle, particularly in the later stages and associated with severe disease. The IDO gene expression levels in the gut, the main site of infection, showed the highest fold change. This was particularly noted within the ileum, thought to be the primary site of infection (23) and reported to be the site of the gut more likely to have demonstrable evidence of disease and the most severe lesions (4, 14). IDO expression was also increased in other affected regions of the gut and associated LN, with the greatest change in expression found in animals with multibacillary lesions. These constitute a severe disease stage, with high numbers of acid-fast bacilli and macrophages and reduced lymphocyte numbers, recently shown to represent an irreversible end-stage in the progression of the disease (15). Interestingly, increased IDO expression has also been found in intestinal lesions of human Crohn's disease patients (2). In a study of cells in the sputa of patients diagnosed with active tuberculosis, IDO was one of the genes that was significantly upregulated compared to levels of expression in healthy controls or patients with other lung conditions, suggesting a correlation with human tuberculosis (1).
Within the gut and LN of JD-affected sheep, IDO protein was associated with granulomas, strongly suggesting expression by macrophages in these lesions. An interesting finding was the strong IDO staining of epithelial cells and possibly Paneth cells associated with JD pathology. IDO is expressed constitutively at low levels in the lower gastrointestinal tract, perhaps to limit the response to commensal bacteria (19). In inflammatory bowel conditions, IDO has been shown to be strongly expressed by epithelial cells, particularly those on the margins of ulcers or abscesses (19), consistent with our findings. Epithelial IDO expression is thought to play a dual role, acting as an antimicrobial barrier as well as limiting potentially damaging inflammatory responses. In JD, the finding of macrophage-associated staining, colocalized with high acid-fast bacillus numbers, suggests that IDO expression does not limit the growth of M. avium subsp. paratuberculosis organisms. As will be discussed below, M. avium subsp. paratuberculosis may circumvent IDO by endogenous synthesis of tryptophan.
The evidence of systemic changes in IDO levels in peripheral blood cells associated with paratuberculosis is a novel and interesting finding. In sheep, modulated IDO expression was observed throughout the disease course, with greatest expression late in disease. Multibacillary lesion cases had the highest peripheral expression of IDO, similar to findings in tissues. In early stages of disease in cattle, significant differences in IDO gene expression were also detected. The cell type producing IDO is likely CD14+ monocytic cells, as was found in previous studies of human PBMCs (11, 46). The profile of IDO gene expression in peripheral blood cells of uninfected animals that were exposed but had no evidence of infection at the time of necropsy was markedly different from that of the infected group, with an early peak in IDO expression that returned to baseline. It is interesting to hypothesize that these animals may have cleared the infection, as their IDO expression profile reflects an appropriate response, whereas the infected sheep did not have this early response.
Despite evidence of IDO expression by peripheral blood cells, blocking IDO in vitro did not enhance the proliferation of lymphocytes from infected sheep or cattle. Some exposed animals in this study did not show proliferation to M. avium subsp. paratuberculosis Ag, perhaps because it had not yet developed or the response had waned. The unresponsive sheep was a multibacillary case, a stage previously reported to show generalized loss of immune responsiveness in IFN-γ and lymphocyte proliferation assays (3, 16). Loss of responsiveness may be due to mechanisms such as anergy and/or immunosuppression. Blocking with 1-MT may not have been effective for various reasons: (i) changes in the cell environment meant that the cells no longer produced IDO, (ii) available l-tryptophan levels were sufficient to overcome the effect of IDO-induced tryptophan starvation, or (iii) the unresponsiveness was not due to IDO-induced suppression. Other forms of immunosuppression may be involved, such as regulatory T cells. IDO may act indirectly via induction of regulatory T cells (12), identified in chronic disease states, including tuberculosis (5). Alternatively, other cell types not present in the assay may produce IDO, as was shown in mice where IDO was expressed by nonhematopoietic cells (17). The effect of blocking IDO locally at the site of infection by in vivo treatment with 1-MT cannot be assessed in large animals such as cattle and sheep, and reagents to further characterize the immune response in sheep and cattle are less developed than those for use in mice and humans. Studies in mice have shown that in vivo treatment with 1-MT can reverse T cell inhibitory effects of IDO activation (43, 45).
Virulent mycobacteria, such as Mycobacterium tuberculosis and M. avium subsp. paratuberculosis, inhibit macrophage processes, including phagosome maturation (25), apoptosis (27), and Ag presentation (50), to survive intracellularly. IFN-γ, a potent activator of microbicidal functions of macrophages, is induced in mycobacterial infections and is essential for the control of Mycobacterium tuberculosis (21) as well as being a hallmark of M. avium subsp. paratuberculosis exposure (4, 26). Within tuberculosis lesions, IFN-γ is expressed by cells in the granuloma, yet despite this, infection persists (18). This paradox has been partially explained by the finding that intracellular infection of macrophages with virulent mycobacteria inhibits macrophage responsiveness to exogenous IFN-γ, with selective inhibition of genes that are normally induced by IFN-γ, including major histocompatibility complex (MHC) class II and CD64 genes (31).
The most potent known activator of IDO expression is IFN-γ, and the IDO promoter has sequence elements that confer responsiveness to this cytokine (38). In subclinically affected cattle, the expression of IDO in peripheral blood cells correlated with M. avium subsp. paratuberculosis-specific IFN-γ responses. However, in the later stages of disease, there was a lack of correlation between IDO levels and IFN-γ responses in the periphery, as the highest IDO expression was associated with multibacillary cases that have impaired IFN-γ responses (3). IDO was also highly expressed by monocytes infected with M. avium subsp. paratuberculosis. These were pure monocytic cultures with no source of IFN-γ. Thus, it appears that M. avium subsp. paratuberculosis infection itself leads to a rapid and marked expression of IDO. Interestingly, IDO is one of the IFN-γ-inducible genes that is not inhibited by mycobacterial infection and for which expression was actually increased (31). We found that IDO protein expression was associated with lesions that contained high numbers of M. avium subsp. paratuberculosis. Thus, the mechanism of induction of IDO in paratuberculosis may involve not only IFN-γ but also M. avium subsp. paratuberculosis infection of cells per se, inducing high levels of expression. These findings led us to pose the following questions. If M. avium subsp. paratuberculosis is known to affect numerous processes of macrophages to promote its survival, why is IDO spared and indeed selectively induced by this organism? Does it give M. avium subsp. paratuberculosis a survival advantage?
The increased expression of IDO associated with granulomatous lesions in JD may reflect an attempt by the host to control pathogen growth as well as to limit tissue damage caused by inflammatory immune processes. A study in gene knockout mice, in which the nonhematopoietic cells were unable to respond to IFN-γ, suggested that modest expression of IDO may be important in preventing uncontrolled immune activation (17). Loss of IDO expression in this model was associated with an overwhelming immunopathology associated with neutrophils and Th17 activation, and these mice succumbed to M. tuberculosis infection. However, in a mouse model of Leishmania major infection, IDO-mediated suppression of T cell responses in the draining LN was proposed to contribute to the persistence of the pathogen (36). Similarly, we found high expression of IDO in multibacillary lesions, which are characterized by reduced T cell numbers and increased mycobacterial replication (13).
Depletion of local tryptophan stores by IDO may not be detrimental to M. avium subsp. paratuberculosis survival. A number of pathogens have been reported to be susceptible to IDO-mediated tryptophan starvation and restriction of growth (9, 24, 35, 49). This study suggests that M. avium subsp. paratuberculosis is not one of these, as it can synthesize its own l-tryptophan, and IDO protein within tissues was found in regions of uncontrolled M. avium subsp. paratuberculosis proliferation. The tryptophan biosynthesis pathway is conserved in a range of pathogenic and environmental mycobacteria, including M. tuberculosis, Mycobacterium leprae, and Mycobacterium smegmatis (KEGG pathway; phenylalanine, tyrosine, and tryptophan biosynthesis). Thus, the implications for the survival of pathogenic mycobacteria of increasing IDO at the site of infection, based on a lack of sensitivity to its effects, may be minimal. IDO is beneficial to the pathogen and not the host in this context, due to its immunosuppressive effects on host immune responses. This may represent the pathogen harnessing immune-regulatory mechanisms of the host to obtain a survival advantage.
The granuloma has been considered to be a host defense mechanism that attempts to physically contain persistent pathogens by entrapment within an organized cellular “prison.” Alternatively, the granuloma can be considered a stalemate between the host and the pathogen, such that there is control but not eradication of the infectious agent (6). Moving the balance toward excessive immune regulation could lead to local immunosuppression and favor the replication of mycobacteria, potentially breaking this stalemate. As the highest IDO expression is associated with later stages of disease, the associated local and systemic immunosuppression may contribute to the progression from subclinical to clinical disease. Immunosuppression and local enhanced proliferation of M. avium subsp. paratuberculosis may also facilitate dissemination, which is associated with severe disease in cattle and sheep (7, 14).
The fact that tryptophan levels in the plasma were reduced is indicative of a major, systemic, functional effect of the increased IDO levels, rather than just an effect on local inflammatory processes. Most circulating tryptophan is bound to albumin in the blood, which acts as a reservoir (40). The considerable reduction in total plasma tryptophan indicates prolonged IDO elevation, which may deplete body tryptophan reserves. A localized infection with more limited IDO elevation might be expected to affect localized tryptophan levels but not have such an impact upon total plasma tryptophan levels. The combination of chronicity, the large tracts of gut tissue that are pathologically affected, and the peripheral expression of IDO appears to have led to a measurable effect on circulating tryptophan, which combined with malabsorption may contribute to the wasting seen with clinical cases of JD. It may also contribute to the peripheral immune dysfunction in severe clinical disease. Serum levels of tryptophan are reduced upon activation of IDO under conditions of chronic immunity (52), and altered kynurenine/tryptophan ratios have been found in human inflammatory bowel disease patients (20). In pigs with induced lung inflammation, decreased plasma tryptophan was proposed to relate to increased IDO activity in the tissues as well as to tryptophan usage to synthesize acute-phase proteins (32). In a model of colitis in pigs, administration of l-tryptophan led to improvement of clinical and histological signs, increased daily weight gain, and restoration of the integrity of the gut barrier (30). Dietary l-tryptophan may be an option as a potential nutritional supplement in cases of paratuberculosis, acting to reduce immunosuppressive effects and weight loss associated with disease.
These studies identify a role for IDO in the pathogenesis of paratuberculosis. Tryptophan depletion by IDO may not have the desired antimicrobial effect, as M. avium subsp. paratuberculosis can synthesize its own tryptophan and is therefore not dependent on environmental sources. IDO and other immunoregulatory mechanisms that are activated to limit immune-mediated tissue damage may be beneficial to the pathogen, enabling M. avium subsp. paratuberculosis persistence. The induction of IDO by M. avium subsp. paratuberculosis may be another way in which the pathogen modulates macrophage responses to promote its survival. Importantly, an increase in IDO expression and corresponding tryptophan depletion systemically may signal a transition from subclinical to clinical disease, although it is not yet clear whether this is a causal relationship. Studies conducted in model systems, such as experimental infections using an IDO gene knockout host or mycobacterial deletion mutant strains unable to synthesize tryptophan, may further elucidate this. These findings may have important implications for other mycobacterial diseases, including tuberculosis in humans, giving insight into potential mechanisms involved in the transition from latent to active disease.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Meat and Livestock Australia and by the Cattle Council of Australia, the Sheepmeat Council of Australia, and WoolProducers Australia through Animal Health Australia.
We thank the Farm Animal and Veterinary Public Health Laboratory Manager Anna Waldron; Adelyn Bolithon, Sophie Hoft, Ankit Srivastava, and Nicole Carter for laboratory support; Deborah Taylor and Reena Mehta for sample preparation; Craig Kristo and Nobel Toribio for animal husbandry support; Elaine Chew, VPDS Histopathology Laboratory, Faculty of Veterinary Science, for assistance with IHC; and Peter Thomson for advice and assistance with statistical analyses.
We declare that we have no conflict of interest pertaining to this work.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 5 July 2011.
REFERENCES
- 1. Almeida A. S., et al. 2009. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J. Immunol. 183:718–731 [DOI] [PubMed] [Google Scholar]
- 2. Barceló-Batllori S., et al. 2002. Proteomic analysis of cytokine induced proteins in human intestinal epithelial cells: implications for inflammatory bowel diseases. Proteomics 2:551–560 [DOI] [PubMed] [Google Scholar]
- 3. Begg D. J., et al. 2011. Does a Th1 over Th2 dominancy really exist in the early stages of Mycobacterium avium subspecies paratuberculosis infections? Immunobiology 216:840–846 [DOI] [PubMed] [Google Scholar]
- 4. Begg D. J., et al. 2010. Experimental infection model for Johne's disease using a lyophilised, pure culture, seedstock of Mycobacterium avium subspecies paratuberculosis. Vet. Microbiol. 141:301–311 [DOI] [PubMed] [Google Scholar]
- 5. Belkaid Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7:875–888 [DOI] [PubMed] [Google Scholar]
- 6. Bold T. D., Ernst J. D. 2009. Who benefits from granulomas, mycobacteria or host? Cell 136:17–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bower K. L., Begg D. J., Whittington R. J. 2011. Culture of Mycobacterium avium subspecies paratuberculosis (MAP) from blood and extra-intestinal tissues in experimentally infected sheep. Vet. Microbiol. 147:127–132 [DOI] [PubMed] [Google Scholar]
- 8. Bozza S., et al. 2005. A crucial role for tryptophan catabolism at the host/Candida albicans interface. J. Immunol. 174:2910–2918 [DOI] [PubMed] [Google Scholar]
- 9. Brown J., Howie S. E., Entrican G. 2001. A role for tryptophan in immune control of chlamydial abortion in sheep. Vet. Immunol. Immunopathol. 82:107–119 [DOI] [PubMed] [Google Scholar]
- 10. Bush R. D., Windsor P. A., Toribio J. A. 2006. Losses of adult sheep due to ovine Johne's disease in 12 infected flocks over a 3-year period. Aust. Vet. J. 84:246–253 [DOI] [PubMed] [Google Scholar]
- 11. Carlin J. M., Borden E. C., Sondel P. M., Byrne G. I. 1989. Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J. Leukoc. Biol. 45:29–34 [DOI] [PubMed] [Google Scholar]
- 12. Chung D. J., et al. 2009. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood 114:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clarke C. J. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116:217–261 [DOI] [PubMed] [Google Scholar]
- 14. Dennis M. M., et al. 2008. Association of severity of enteric granulomatous inflammation with disseminated Mycobacterium avium subspecies paratuberculosis infection and antemortem test results for paratuberculosis in dairy cows. Vet. Microbiol. 131:154–163 [DOI] [PubMed] [Google Scholar]
- 15. Dennis M. M., Reddacliff L. A., Whittington R. J. 2010. Longitudinal study of clinicopathological features of Johne's disease in sheep naturally exposed to Mycobacterium avium subspecies paratuberculosis. Vet. Pathol. 48:565–575 [DOI] [PubMed] [Google Scholar]
- 16. de Silva K., et al. 2010. The early lymphocyte proliferation response in sheep exposed to Mycobacterium avium subsp. paratuberculosis compared to infection status. Immunobiology 215:12–25 [DOI] [PubMed] [Google Scholar]
- 17. Desvignes L., Ernst J. D. 2009. Gamma interferon-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity 31:974–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fenhalls G., et al. 2002. Distribution of IFN-gamma, IL-4 and TNF-alpha protein and CD8 T cells producing IL-12p40 mRNA in human lung tuberculous granulomas. Immunology 105:325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferdinande L., et al. 2008. Inflamed intestinal mucosa features a specific epithelial expression pattern of indoleamine 2,3-dioxygenase. Int. J. Immunopathol. Pharmacol. 21:289–295 [DOI] [PubMed] [Google Scholar]
- 20. Fitzgerald P., et al. 2008. Tryptophan catabolism in females with irritable bowel syndrome: relationship to gamma interferon, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol. Motil. 20:1291–1297 [DOI] [PubMed] [Google Scholar]
- 21. Flynn J. L., et al. 1993. An essential role for gamma interferon in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gumber S., Taylor D. L., Marsh I. B., Whittington R. J. 2009. Growth pattern and partial proteome of Mycobacterium avium subsp. paratuberculosis during the stress response to hypoxia and nutrient starvation. Vet. Microbiol. 133:344–357 [DOI] [PubMed] [Google Scholar]
- 23. Harris N. B., Barletta R. G. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayashi T., et al. 2001. Enhancement of innate immunity against Mycobacterium avium infection by immunostimulatory DNA is mediated by indoleamine 2,3-dioxygenase. Infect. Immun. 69:6156–6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hostetter J., Steadham E., Haynes J., Bailey T., Cheville N. 2003. Phagosomal maturation and intracellular survival of Mycobacterium avium subspecies paratuberculosis in J774 cells. Comp. Immunol. Microbiol. Infect. Dis. 26:269–283 [DOI] [PubMed] [Google Scholar]
- 26. Jungersen G., Huda A., Hansen J. J., Lind P. 2002. Interpretation of the gamma interferon test for diagnosis of subclinical paratuberculosis in cattle. Clin. Diagn. Lab. Immunol. 9:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kabara E., et al. 2010. A large-scale study of differential gene expression in monocyte-derived macrophages infected with several strains of Mycobacterium avium subspecies paratuberculosis. Brief Funct. Genomics 9:220–237 [DOI] [PubMed] [Google Scholar]
- 28. Katz J. B., Muller A. J., Prendergast G. C. 2008. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol. Rev. 222:206–221 [DOI] [PubMed] [Google Scholar]
- 29. Kawaji S., Taylor D. L., Mori Y., Whittington R. J. 2007. Detection of Mycobacterium avium subsp. paratuberculosis in ovine faeces by direct quantitative PCR has similar or greater sensitivity compared to radiometric culture. Vet. Microbiol. 125:36–48 [DOI] [PubMed] [Google Scholar]
- 30. Kim C. J., et al. 2010. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Nutr. Biochem. 21:468–475 [DOI] [PubMed] [Google Scholar]
- 31. Kincaid E. Z., Ernst J. D. 2003. Mycobacterium tuberculosis exerts gene-selective inhibition of transcriptional responses to IFN-gamma without inhibiting STAT1 function. J. Immunol. 171:2042–2049 [DOI] [PubMed] [Google Scholar]
- 32. Le Floc'h N., Melchior D., Sève B. 2008. Dietary tryptophan helps to preserve tryptophan homeostasis in pigs suffering from lung inflammation. J. Anim. Sci. 86:3473–3479 [DOI] [PubMed] [Google Scholar]
- 33. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 34. Logan G. J., et al. 2002. HeLa cells cocultured with peripheral blood lymphocytes acquire an immuno-inhibitory phenotype through up-regulation of indoleamine 2,3-dioxygenase activity. Immunology 105:478–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MacKenzie C. R., Hadding U., Daubener W. 1998. Gamma interferon-induced activation of indoleamine 2,3-dioxygenase in cord blood monocyte-derived macrophages inhibits the growth of group B streptococci. J. Infect. Dis. 178:875–878 [DOI] [PubMed] [Google Scholar]
- 36. Makala L. H., et al. 2011. Leishmania major attenuates host immunity by stimulating local indoleamine 2,3-dioxygenase expression. J. Infect. Dis. 203:715–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mellor A. 2005. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem. Biophys. Res. Commun. 338:20–24 [DOI] [PubMed] [Google Scholar]
- 38. Mellor A. L., Munn D. H. 2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4:762–774 [DOI] [PubMed] [Google Scholar]
- 39. Nacy C., Buckley M. 2008. Mycobacterium avium paratuberculosis: infrequent human pathogen or public health threat? American Academy of Microbiology, Washington, DC: http://academy.asm.org/images/stories/documents/mycobacteriumaviumparatuberculosis.pdf [PubMed] [Google Scholar]
- 40. Pardridge W. M., Fierer G. 1990. Transport of tryptophan into brain from the circulating, albumin-bound pool in rats and in rabbits. J. Neurochem. 54:971–976 [DOI] [PubMed] [Google Scholar]
- 41. Pérez V., Garcia Marin J. F., Badiola J. J. 1996. Description and classification of different types of lesion associated with natural paratuberculosis infection in sheep. J. Comp. Pathol. 114:107–122 [DOI] [PubMed] [Google Scholar]
- 42. Reddacliff L. A., Nicholls P. J., Vadali A., Whittington R. J. 2003. Use of growth indices from radiometric culture for quantification of sheep strains of Mycobacterium avium subsp. paratuberculosis. Appl. Environ. Microbiol. 69:3510–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sakurai K., Zou J. P., Tschetter J. R., Ward J. M., Shearer G. M. 2002. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 129:186–196 [DOI] [PubMed] [Google Scholar]
- 44. Sewell G. W., Marks D. J., Segal A. W. 2009. The immunopathogenesis of Crohn's disease: a three-stage model. Curr. Opin. Immunol. 21:506–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sźantó S., et al. 2007. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis Res. Ther. 9:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tattevin P., et al. 2010. Enhanced indoleamine 2,3-dioxygenase activity in patients with severe sepsis and septic shock. J. Infect. Dis. 201:956–966 [DOI] [PubMed] [Google Scholar]
- 47. Taylor D. L., Thomson P. C., de Silva K., Whittington R. J. 2007. Validation of endogenous reference genes for expression profiling of RAW264.7 cells infected with Mycobacterium avium subsp. paratuberculosis by quantitative PCR. Vet. Immunol. Immunopathol. 115:43–55 [DOI] [PubMed] [Google Scholar]
- 48. Taylor D. L., Zhong L., Begg D. J., de Silva K., Whittington R. J. 2008. Toll-like receptor genes are differentially expressed at the sites of infection during the progression of Johne's disease in outbred sheep. Vet. Immunol. Immunopathol. 124:132–151 [DOI] [PubMed] [Google Scholar]
- 49. Thomas S. M., et al. 1993. IFN-gamma-mediated antimicrobial response. Indoleamine 2,3-dioxygenase-deficient mutant host cells no longer inhibit intracellular Chlamydia spp. or Toxoplasma growth. J. Immunol. 150:5529–5534 [PubMed] [Google Scholar]
- 50. Weiss D. J., Evanson O. A., McClenahan D. J., Abrahamsen M. S., Walcheck B. K. 2001. Regulation of expression of major histocompatibility antigens by bovine macrophages infected with Mycobacterium avium subsp. paratuberculosis or Mycobacterium avium subsp. avium. Infect. Immun. 69:1002–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Whittington R. J., Begg D. J., de Silva K., Plain K. M., Purdie A. C. 2011. Comparative immunological and microbiological aspects of paratuberculosis as a model mycobacterial infection. Vet. Immunol. Immunopathol. doi:10.1016/j.vetimm.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 52. Wirleitner B., Neurauter G., Schrocksnadel K., Frick B., Fuchs D. 2003. Gamma interferon-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr. Med. Chem. 10:1581–1591 [DOI] [PubMed] [Google Scholar]
- 53. Zelante T., Fallarino F., Bistoni F., Puccetti P., Romani L. 2009. Indoleamine 2,3-dioxygenase in infection: the paradox of an evasive strategy that benefits the host. Microbes Infect. 11:133–141 [DOI] [PubMed] [Google Scholar]
- 54. Zhong L., et al. 2009. Identification of differentially expressed genes in ileum, intestinal lymph node and peripheral blood mononuclear cells of sheep infected with Mycobacterium avium subsp. paratuberculosis using differential display PCR. Vet. Immunol. Immunopathol. 131:177–189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.