Abstract
A gene encoding a 29-kDa protein from Neisseria meningitidis serogroup B strain MC58 with homology to the macrophage infectivity potentiator (MIP) protein of Legionella pneumophila was cloned and expressed in Escherichia coli, and the purified soluble recombinant protein (rMIP) was used for immunization studies. Analysis of the predicted amino acid sequences of MIP from 13 well-characterized meningococcal strains, isolated from carriers or patients and differing in serogroup, serotype, and subtype, showed that the protein was highly conserved (98 to 100%), with only three distinct sequence types (designated I, II, and III) found. Western blotting showed that the MIP protein was expressed at similar levels by all of these strains. Immunization of mice with type I MC58 rMIP in detergent micelles and liposomes containing monophosphoryl lipid A (MPLA) induced high levels of surface-reactive antibodies with serum bactericidal activity (SBA) titers of 1/1,024 against the homologous strain. Bactericidal antibodies were also induced with the protein in saline alone and liposomes alone (titers, 1/128) but not following adsorption to Al(OH)3. Significantly, antisera raised against type I rMIP administered in saline or liposomes killed strains of heterologous sequence types II and III with similar SBA titers (1/128 to 1/256). Taken together, these findings suggest that rMIP can provide cross-strain protection against meningococci and should be considered a potential antigen for inclusion in new vaccines against meningococcal infection.
INTRODUCTION
Infections caused by Neisseria meningitidis (meningococcus) are significant causes of mortality and morbidity worldwide. Despite the success of the meningococcal serogroup C conjugate vaccines introduced into the routine immunization schedules of developed countries (2), no such vaccines exist for serogroup B infection. The conjugate strategy that has been successful for both serogroup C and serogroup A capsular polysaccharides is unlikely to be effective for serogroup B strains due to structural similarities between the B capsule and human fetal brain neural cell adhesion molecules (13). As a result, serogroup B vaccine development has focused on the use of isolated outer membranes (OMs) for epidemic control, but these are complex and protection is largely directed against the PorA protein and therefore serosubtype specific (25, 36, 38). In addition to PorA, many other OM proteins, including porin PorB (59), the opacity proteins Opa (3) and Opc (31), factor H binding protein (fHBP) (15, 30), and other adhesins (17, 19), have been prepared as recombinant proteins and investigated as vaccines in preclinical studies. In addition, human clinical trials with bivalent and multivalent vaccines containing recombinant OM antigens have recently been carried out (12, 19, 45, 46). However, due to immune pressure, many OM antigens are variable and the goal for effective vaccine development is to identify those antigens that are more conserved and capable of inducing cross-protective antibody responses.
Recently, we used nanocapillary liquid chromatography-tandem mass spectrometry to investigate the proteome of the meningococcal OM. These studies identified the presence in relatively high abundance of a protein with an Mr of ∼29,000 and with homology to the macrophage infectivity potentiator (MIP) protein of Legionella pneumophila (Lp-MIP), which we have termed the meningococcal MIP (57). Concurrently, Leuzzi and colleagues reported the presence of a surface-exposed lipoprotein on the closely related organism Neisseria gonorrhoeae with an Mr of ∼30,000, which they termed Ng-MIP (34). The bacterial MIP proteins may represent a new class of bacterial pathogenicity factors. The first MIP protein to be studied in detail was from Legionella pneumophila, and its potential role was identified by inactivating a gene encoding an ∼24-kDa protein which reduced the bacterium's ability to infect monocytes/macrophages (8, 55). The protein is exposed on the cell surface of Legionella (23), and sera from patients with Legionella infection have been shown to react with Lp-MIP (1), demonstrating its expression during infection. MIP homologues have subsequently been found in other bacteria, including Chlamydia trachomatis (35) and C. psittaci (47), Salmonella enterica serovar Typhimurium (26), Trypanosoma cruzi (40, 42), Mycoplasma (53), Coxiella (39), and Rickettsia, Ehrlichia, and Rochalimaea species (9).
The L. pneumophila MIP protein shows some similarity to the immunophilin family of human FK506-binding proteins (FKBPs), which are a family of conserved, widely distributed eukaryotic proteins (10, 49) that are active as peptidyl-prolyl-cis-trans-isomerases (PPIases) (50) and are targets for the macrolide immunosuppressants FK506 (14, 44) and rapamycin (16). Although contradictory evidence has been presented for the requirement of the active enzyme site within the protein for virulence of Legionella (22, 33), many of the studies described above clearly demonstrated that expression of microbial MIP (and homologues) appeared to have direct relevance to the survival of important human pathogens that have intracellular stages in their life cycles (20).
The high abundance of meningococcal MIP in the OM and evidence that a similar protein in gonococci was surface exposed (34, 57) suggested to us that MIP may have potential as a vaccine antigen. In the current study, we therefore cloned and expressed MIP as a recombinant protein and tested its ability to induce functional bactericidal antibodies, the generally accepted laboratory correlate of protection for serogroup B meningococci (52), using a variety of adjuvant formulations suitable for human use.
MATERIALS AND METHODS
Bacterial strains, vectors, and growth conditions.
Neisseria meningitidis strain MC58 (B:15:P1.7,16b: Cap+ Opa+ Opc+ PorA+ PorB+ Pil+ [class I] Rmp+ LOS+) was isolated from an outbreak of meningococcal infection that occurred in Stroud, Gloucestershire, United Kingdom, in the mid-1980s (37). The other strains included in the study are listed in Table 1. Meningococcal strains were grown on supplemented proteose-peptone agar (GC agar) at 37°C for 18 h in an atmosphere containing 5% (vol/vol) CO2. Outer membranes of strain MC58 were prepared by extraction of whole cells by lithium acetate as previously described (57).
Table 1.
Meningococcal strains used in the study
| Strain | Phenotype | Reference |
|---|---|---|
| MC58 | B:15:P1.7,16b | 37 |
| MC54 | B:2b:P1.18-1,3 | 27 |
| MC90 | B:9:P1.5,2 | 27 |
| MC168 | B:4:P1.5,2 | 32 |
| MC172 | B:1:P1.22,14 | 32 |
| MC179 | B:1:P1.19-2,13-1 | 58 |
| MC180 | B:1:P1.22,14 | 58 |
| L2470 | B:4:P1.7-2,4 | 27 |
| MC161 | C:2-37, P1.5-1,10-4 | 56 |
| MC162 | C:2-36:P1.5,2 | 56 |
| MC173 | C:2b:P1.5,2 | 32 |
| MENC11 | C:16, P17a,1 | 56 |
| MC174 | 29E:4:P1.5-1,10-8 | 32 |
The pRSETA plasmid (Invitrogen, United Kingdom), maintained in Escherichia coli DH5α (Invitrogen, United Kingdom), was used for cloning genes encoding MIP. E. coli BL21(DE3)/pLysS (Invitrogen, United Kingdom) was transformed by recombinant pRSETA for protein expression. E. coli strains were grown using Luria-Bertani (LB) broth and agar. For protein expression, transformed BL21(DE3)/pLysS bacteria were cultured on super optimal broth (SOB) medium (Invitrogen) supplemented with ampicillin (50 μg/ml; Sigma-Aldrich, Poole, United Kingdom) and chloramphenicol (30 μg/ml; Sigma-Aldrich, United Kingdom).
Cloning and expression of mip gene in E. coli.
Genomic DNA of strain MC58 was extracted by alkaline lysis as described previously (6). A single colony of overnight growth of MC58 was suspended in 10 μl of ultra-high-quality (UHQ) water and was lysed by the addition of 10 μl of 0.25 M potassium hydroxide, followed by boiling for 5 min. The sample was neutralized by the addition of 10 μl of 0.5 M Tris-HCl buffer, pH 7.5, the final volume was adjusted to 130 μl with the addition of UHQ water, and the sample was stored at −20°C until used.
Using the gene sequence encoding MIP (NMB1567), two primers were designed to amplify the MIP protein by PCR, with the forward primer (NMB1567F, 5′-GGCTATCTCGAGATGAACACCATTTTCAAAAT-3′) incorporating the restriction site for XhoI (underlined) and the reverse primer (NMB1567R; 5′-GGCTATAAGCTTCTATTAATTTACTTTTTTGATGT-3′) incorporating the restriction site for HindIII (underlined). Amplification of the target DNA sequence from MC58 genomic DNA was done using the 2× Phusion PCR master mix (Finnzymes, United Kingdom) with the following PCR conditions: initial denaturation (98°C, 30s) and then 30 cycles of denaturation (98°C, 10 s), annealing (60°C, 30s), and extension (72°C, 25s) and a final extension at 72°C for 5 min. The PCR product was subjected to gel purification using a Wizard SV gel and PCR cleanup system (Promega, United Kingdom). The purified PCR product was digested with XhoI and HindIII restriction enzymes (Promega, United Kingdom) at 37°C for 3 h and then cleaned as described above.
The pRSETA plasmid was digested with XhoI and HindIII restriction enzymes at 37°C for 3 h prior to dephosphorylation using calf intestinal alkaline phosphatase (Promega, United Kingdom). The DNA was subsequently purified using the Wizard SV gel and PCR cleanup system. For ligation of insert and vector, a molar ratio of insert/vector of 3:1 was used and a 10-μl ligation reaction mixture contained 10 ng of vector, an appropriate amount of insert, 1 μl (3 U) of T4 DNA ligase (Promega, United Kingdom), and 5 μl of 2× rapid ligation buffer (Promega, United Kingdom). The mixture was incubated at room temperature for 15 min and then used for transformation of competent E. coli strain DH5α (Invitrogen) with selection on LB-ampicillin agar plates. Colonies were selected and DNA templates were prepared for PCR screening for the presence of recombinant plasmid, which was then transformed into competent E. coli BL21(DE3)/pLysS. Transformants were selected on LB agar plates containing ampicillin (50 μg/ml) and chloramphenicol (30 μg/ml).
Expression of recombinant MIP (rMIP) was induced in a 2-liter culture of transformant in SOB medium using 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (final concentration, 1 mM) for 5 h, as determined by pilot experiments. The bacterial cell pellet was collected by centrifugation and suspended in 50 mM NaH2PO4, pH 8.0, lysis buffer containing 300 mM NaCl and 10 mM imidazole (4 to 5 ml/g pellet). The bacterial suspension was subjected to three cycles of freeze (−80°C)-thaw and sonicated (Soniprep 150; MSE, United Kingdom) on ice, using 10-s bursts 10 to 20 times. The lysate was then centrifuged at 10,000 × g for 30 min at 4°C. Recombinant protein was produced as a soluble protein, as judged by its presence in the supernatant following repeated freeze-thaw cycles and analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Purification of rMIP protein.
The soluble rMIP protein was purified using nickel-nitrilotriacetic acid (Ni-NTA) metal-affinity chromatography (Qiagen, Crawley, United Kingdom). Bound protein was eluted with 50 mM NaH2PO4 buffer, pH 8.0, containing 300 mM NaCl and 250 mM imidazole, and eluate fractions were analyzed by SDS-PAGE and then pooled and dialyzed against repeated changes of phosphate-buffered saline (PBS; pH 7.4) containing thimerosal (1/10,000, wt/vol). The protein concentration of the purified rMIP was determined by bicinchoninic acid protein assay (Pierce, United Kingdom), and the protein was stored at −20°C in aliquots until used. The presence of any contaminating lipopolysaccharide (LPS) in the pure rMIP protein preparation was investigated by low-Mr SDS-PAGE using the method of Schagger and von Jagow (48) with silver staining (24). In addition, rMIP was evaluated using the Limulus amebocyte lysate assay (Lonza Biologics, Slough, United Kingdom) according to the manufacturer's instructions, in order to quantify the level of any contaminating LPS.
Sequencing of PCR product amplified from other meningococcal strains.
Genomic DNA was extracted from a selection of meningococcal strains (Table 1) using a QIAamp extraction kit according to the manufacturer's instructions (Qiagen, United Kingdom). Next, the target gene was amplified by PCR with forward primer 5′-GAAACATTCAAACTCGGCTA-3′ and reverse primer 5′-GTTTCAGACGGCATTTGCCG-3′. The PCR mixture and PCR were the same as those for cloning the mip gene from MC58. All sequencing was done by Source BioScience (Oxford, United Kingdom).
Preparation of adjuvant and delivery vehicles for rMIP. (i) Incorporation into liposomes.
Liposomes were prepared by dialysis-sonication as previously described (27). Briefly, l-α-phosphatidylcholine and cholesterol (Sigma-Aldrich) were combined at a 7:2 molar ratio and dissolved (20 mg total) in 3 ml of chloroform in a glass round-bottom flask, and the solvent was removed under vacuum at 25°C with rotation to achieve an even lipid film. A solution of purified rMIP (1 mg) and 100 mg of octyl β-d-glucopyranoside (Sigma-Aldrich) was prepared in 5 ml of 10 mM HEPES (Sigma-Aldrich) buffer, pH 7.2, and this was then incubated at room temperature for 3 h. The lipid film was then dissolved in this protein solution by incubation at room temperature for 1 h. Subsequently, the detergent-protein solution was extensively dialyzed against PBS containing thimerosal (1/10,000, wt/vol) for 72 h at 4°C and then subjected to sonication (MSE Soniprep 150 sonicator; 15 μm for 20 to 30 bursts of 60 s each) to induce vesicle formation. Any insoluble material was removed by centrifugation at 1,000 × g for 10 min. Liposomes were also prepared by incorporating the adjuvant monophosphoryl lipid A (MPLA; Sigma-Aldrich) at an adjuvant/protein ratio of 1:1. Control liposomes without protein and with or without MPLA were also prepared. All liposome preparations were stored in aliquots at −20°C until used.
(ii) Incorporation of rMIP into Zwittergent micelles (29, 54).
rMIP protein-Zwittergent mixtures were prepared in PBS containing rMIP (0.5 mg ml−1) and Zwittergent 3-14 micelles (8 mg ml−1; Calbiochem, Nottingham, United Kingdom), with or without MPLA (0.5 mg ml−1 dissolved in PBS), and then incubated overnight at room temperature. For immunization, the solutions were diluted with saline to a final concentration of 200 μg of rMIP ml−1, and each mouse was injected with 20 μg of protein. Control micelle preparations without rMIP and/or MPLA were also prepared. All micelle preparations were stored at −20°C in aliquots until used.
(iii) Adsorption to Al(OH)3.
Aluminum hydroxide [Al(OH)3; alum] gel adjuvant (2.0%; Superfos Biosector, Denmark) was used to adsorb rMIP or an MC58 OM preparation. The mixture was composed of 500 μl of alum, 200 μg of rMIP, or OM in saline to make a final volume of 1 ml. Antigen was adsorbed by mixing overnight at 4°C on an angled rotary mixer. These preparations were used immediately for immunization, and the injection dose for each animal was 100 μl containing 20 μg of protein.
(iv) Saline preparations.
Solutions of rMIP (200 μg ml−1) were prepared in saline and used immediately.
Immunization of animals.
BALB/c mice (H-2d haplotype) and New Zealand White rabbits were housed under standard conditions of temperature and humidity with a 12-h lighting cycle and with food and water available ad libitum. Groups of five mice of approximately equal size and weight were immunized intraperitoneally with the following rMIP preparations: rMIP-saline, rMIP-alum, rMIP-liposomes, rMIP-MPLA-liposomes, rMIP–ZW 3-14 micelles, and rMIP–MPLA–ZW 3-14 micelles. The dose of rMIP was 20 μg/mouse, and the immunization schedule was three doses, administered on days 0, 14, and 28. Groups of five mice were also injected with control preparations consisting of saline, saline-MPLA, alum, empty liposomes, and empty ZW 3-14 micelles, with and without MPLA. In addition, one group of mice was injected with OM (20 μg/mouse) adsorbed to alum, and another group was maintained for normal serum. Mice were terminally bled by cardiac puncture under anesthesia on day 42.
Rabbits (n = 2) were immunized subcutaneously with rMIP emulsified in Freund's complete adjuvant for the primary injection and Freund's incomplete adjuvant for a subsequent three injections. Each dose contained 20 μg of protein administered at 14-day intervals. The rabbits were terminally bled from the middle ear vein and by cardiac puncture under anesthesia 14 days after the last dose. To express serum, whole animal blood was allowed to clot at 37°C for an hour, left at 4°C overnight, and then centrifuged at 8,000 × g for 6 min. The resulting sera were removed and stored at −20°C until required. This study complied with the animal experimentation guidelines of the Home Office and the authors' institution, and no animals suffered significant adverse effects.
Characterization of biological and functional properties of antibodies to rMIP. (i) ELISA.
Individual murine antisera were reacted in an enzyme-linked immunosorbent assay (ELISA) with either purified rMIP protein or MC58 OM, as described previously (7). Absorbance was measured at 450 nm after 10 min of incubation with enzyme substrate, and the ELISA titer, extrapolated from the linear portion of the serum titration curve, was taken as the reciprocal dilution which gave an increase in absorbance of 0.1 U after 10 min. A two-sample t test was used to compare differences between mean values for ELISA data sets as previously described (7).
(ii) SDS-PAGE and Western immunoblotting.
SDS-PAGE was done with a 10 to 25% linear gradient gel at 200 V for 18 h at 4°C (21). Samples containing rMIP protein were loaded at 10 to 20 μg per well; OM and whole-cell lysate preparations were loaded at 20 μg per well. The separated proteins were transferred to nitrocellulose by semidry blotting with a current limit of 0.8 mA/cm2 for 1 h, and after incubation with murine or rabbit sera, immunological reactivity was detected by using anti-mouse/rabbit immunoglobulin–alkaline phosphatase conjugate (Bio-Rad, Hemel Hempstead, United Kingdom) as described previously (7).
(iii) IF.
Pooled murine antisera, diluted 1/100, were reacted with fixed whole meningococci as described previously (31). Antibody bound to meningococci was detected by reaction with anti-mouse immunoglobulin–fluorescein isothiocyanate conjugate (Dako; diluted 1/100 in PBS), and the samples were then viewed with a Leica SP2 confocal microscope. Immunofluorescence (IF) reactivity was scored as nonreactivity (−), weak (±), medium (+), and strong (++), determined as previously described (31). For comparison, antiserum raised to OM showed very strong (+++) reactivity with whole cells.
(iv) Complement-mediated killing of meningococci.
The bactericidal activities of pooled antisera were determined with 5 to 10% (vol/vol) baby rabbit serum (AbD Serotec; Kidlington, United Kingdom) as a source of exogenous complement, as previously described (7). Murine antisera raised to purified outer membranes were used as a positive control. Complement-dependent bactericidal activity was determined from the numbers of bacteria surviving in the presence of serum and complement compared to the numbers surviving with complement but without test serum. Sera that showed bactericidal activity in two or more dilutions were considered positive, and statistical significance was analyzed using F and t tests (7). P values of <0.05 were considered to be significant.
RESULTS
Cloning of mip gene in E. coli using pRSETA system: expression and purification of rMIP.
In order to obtain MIP free from other meningococcal components, the pRSETA vector system was used, in which the gene of interest is expressed as a fusion protein with an N-terminal 39-amino-acid polypeptide that contains the 6 histidine residues which function as a nickel-binding domain to facilitate purification by affinity chromatography (5, 31, 59). The plasmid constructed contained the entire coding sequence for MIP protein under the control of the T7 promoter. Pilot experiments demonstrated that following induction with IPTG, optimal expression of rMIP occurred by 5 h and that following freeze-thawing and centrifugation, the protein was present in the supernatant fraction.
In large-scale experiments conducted to prepare sufficient rMIP for immunization studies, the protein was readily purified from the supernatant fraction under native conditions by affinity chromatography on a Ni2+ column. Following elution with imidazole, the fractions containing the highest levels of rMIP protein were pooled and dialyzed exhaustively against PBS. SDS-PAGE showed a single homogeneous band with an apparent Mr of 33,000 (Fig. 1A), which corresponds to the expected Mr of the whole MIP protein plus the additional N-terminal 39-amino-acid fusion polypeptide. From a typical 2-liter culture volume, the yield of rMIP was estimated to be approximately 74 mg per liter of culture. PAGE and silver staining also showed that no contaminating LPS was visible in the rMIP preparation (Fig. 1B), and the Limulus amebocyte lysate assay confirmed the presence of a very low level of LPS (≤0.7%, wt/wt).
Fig. 1.
Purification of recombinant MIP. (A) The protein was purified to homogeneity using nickel affinity chromatography under native conditions. Lane 1, molecular mass markers; lane 2, rMIP (10 μg) as a single band with a molecular mass of ∼33 kDa. (B) Silver staining shows that no contaminating LPS is visible in the rMIP preparation. Lane 1, molecular mass markers; lane 2, rMIP (10 μg); lane 3, loading buffer only; lanes 4 and 5, E. coli LPS (10 μg and 1 μg, respectively).
Humoral immune response to rMIP protein.
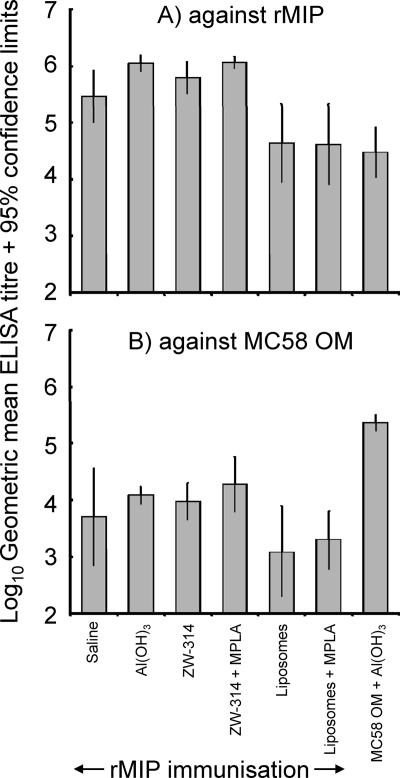
The purified rMIP was used for immunization studies in adjuvant formulations that are suitable for human immunization, including adsorption to aluminum hydroxide gel and incorporation into liposomes with and without the nontoxic adjuvant MPLA. In addition, mice were immunized with protein in ZW 3-14 micelles with and without MPLA and in saline alone. The murine humoral immune response was initially studied by ELISA against purified rMIP (Fig. 2A). High titers of antibodies that reacted with purified rMIP were raised using all the different adjuvant and delivery systems. rMIP in ZW 3-14 micelles or adsorbed to Al(OH)3 induced statistically higher titers than protein delivered in liposomes only (P < 0.05). The addition of MPLA did not significantly increase the induction of anti-rMIP antibody in either ZW 3-14 micelles or liposomes (P > 0.05). Significantly, antibodies to rMIP could be induced by immunization with protein in saline alone. In addition, immunization with MC58 OM also induced antibodies that reacted with the recombinant MIP protein (Fig. 2A). Animals that were sham immunized did not produce antibodies that reacted with rMIP in ELISA.
Fig. 2.
ELISA reactivity of antisera raised against different rMIP formulations. Antisera from individual animals raised against rMIP in various formulations were reacted against homologous rMIP protein (A) and OM from MC58 (B). The columns represent the geometric mean reciprocal ELISA titers (n = 5 animals per group), and the error bars represent the 95% confidence limits. No reactivity was observed with sera from sham-immunized animals.
Humoral immune response to OM.
Antisera raised against rMIP were also tested in ELISA against OM from the homologous strain MC58 (Fig. 2B). Significant reactivity to MIP present in OM was observed with antisera raised with all the different adjuvants and delivery systems, and there were no statistically significant differences between the mean antibody levels observed for any of the groups (P > 0.05). As expected, significantly higher (P < 0.05) levels of anti-OM antibodies were induced by immunization with OM than rMIP preparations. No significant reactivity against OM was observed with antisera from sham-immunized animals.
The specificity of the immune response against rMIP was also investigated by Western blotting with whole-cell lysate and an OM preparation of the homologous strain MC58. All antisera raised to rMIP showed strong reactivity with a band with an apparent Mr of 29,000, and sera from the corresponding sham-immunized animals were nonreactive (data not shown).
Antibody binding to MIP on meningococcal cells.
IF was used to investigate the ability of sera to recognize and bind to MIP on meningococcal cells. Antisera raised against rMIP in ZW 3-14 micelles and liposomes alone showed weak (±) IF binding reactivity with meningococcal cells, but with the addition of MPLA, raised antibodies showed stronger (++) reactivity. Similarly, strong (++) IF signals were observed when sera raised to rMIP adsorbed to Al(OH)3 or in saline were reacted with bacteria. All sera from the corresponding sham-immunized animals were nonreactive (−).
Bactericidal activity of antisera raised to rMIP for homologous strain MC58.
The murine antisera were tested for their ability to promote complement-mediated killing of the homologous meningococcal strain MC58 (Table 2). No bactericidal activity was shown by antisera raised to rMIP adsorbed to alum or incorporated into ZW 3-14 micelles. rMIP administered in liposomes alone showed bactericidal activity, with a median titer of 1/128. However, the incorporation of MPLA into both ZW 3-14 micelles and liposomes significantly increased median bactericidal titers to 1/1,024. Interestingly, rMIP injected in saline alone was also able to induce bactericidal antibodies, with a median titer of 1/128 (Table 2). In addition, colonies of bacteria that survived in the presence of rMIP antisera were selected from agar plates and were run on SDS-polyacrylamide gels, and the expression of MIP was detected by Western blotting. All selected surviving colonies expressed MIP protein, demonstrating that MIP-negative strains were not induced (data not shown). No significant bactericidal activity was observed for antisera from sham-immunized animals.
Table 2.
Bactericidal activity of pooled antisera raised against rMIP protein for the homologous strain MC58
| Formulation | Serum bactericidal titera against MC58 |
|
|---|---|---|
| With rMIP | Without rMIP | |
| Saline | 128 | <8 |
| Al(OH)3 | <4 | <4 |
| ZW 3-14 micelle | <4 | 8 |
| ZW 3-14 micelle + MPLA | 1,024 | <4 |
| Liposome | 128 | <4 |
| Liposome + MPLA | 1,024 (512, 2,048) | <4 |
Titers are expressed as the reciprocal of the highest dilution at which 50% killing was observed. Titers for normal mouse serum and sera from mice immunized with MC58 OM were <8 and 20,000, respectively. Data are the median values, with the range of values in parentheses, for serum bactericidal activity from three or more independent measurements of bactericidal activity of all pooled serum samples. Single values denote that the serum bactericidal activity titers were identical from the independent experiments.
Conservation and expression of MIP in Neisseria meningitidis.
In order to investigate the conservation of MIP in meningococci, the mip gene encoding the protein from a collection of carriage isolates and strains isolated from patients was amplified and sequenced (Fig. 3). The inferred amino acid sequences showed that MIP had a high degree of conservation across the strains, with only three sequence types being identified in a total of 13 strains of different serogroups, serotypes, and serosubtypes. The MIP proteins of five strains (MC168, MC172, MC174, MC180, and L2470) showed 100% homology with the MIP of MC58 (sequence type I), the MIP proteins of five strains (MENC11, MC90, MC161, MC162, and MC179) shared a sequence that showed 97.8% homology with the MIP protein of MC58 (sequence type II), and the MIP proteins of two strains (MC54 and MC173) had a sequence with 98.9% homology with the MIP protein of MC58 (sequence type III).
Fig. 3.
Sequencing of MIP across Neisseria strains. The target mip gene for the meningococcal strains listed in Table 1 was amplified by PCR and sequenced, and the amino acid sequences were annotated. Amino acid sequences for MIP from strains MC168, MC172, MC174, MC180, and L2470 were identical to those of MIP from MC58 (I); the amino acid sequences for MIP from strains MC90, MC161, MC162, MC179, and MENC11 were similar (II); and the amino acid sequences for MIP from strains MC54 and MC173 showed a third amino acid sequence type (III). Shading denotes amino acid deletions or changes.
The levels of expression of MIP were also investigated across the same 13 strains. Western blotting with rabbit antisera to rMIP showed that the protein was expressed to high and similar levels in all the strains tested (Fig. 4). The bands also showed a small difference in apparent Mr between the different sequence types, with the protein from strains expressing sequence type II MIP migrating at a marginally lower molecular weight than the protein from strains expressing type I or III MIP, corresponding to the deletion of 4 amino acids observed in the type II proteins (Fig. 4).
Fig. 4.
Western blot reactivity of antisera raised to rMIP against lysates of sequenced meningococcal strains. Rabbit antiserum (1/100) to rMIP from strain MC58 was reacted with lysates of meningococcal strains of different sequence types (I, II, and III). In order from the left, unnumbered lanes are as follows: lane 1, molecular mass markers; lanes 2 and 3, recombinant MIP (0.1 μg/well); lanes 4 to 11, MC58, L2470, MC168, MC172, MC174, MC180, MC173, and MC54 (type I and III MIP proteins; 15 μg of each lysate/well); lanes 11 to 15, MC90, MC161, MC162, MC179, and MENC11 (type II MIP; 15 μg of each lysate/well).
Antisera to rMIP shows cross-strain bactericidal activity.
The antisera raised against rMIP from MC58 (type I) were tested for their ability to promote complement-mediated killing of other strains from sequence types II and III (Table 3). Antisera from animals immunized with rMIP in saline, liposomes only, and liposomes and ZW 3-14 micelles with MPLA, which showed the highest levels of reactivity against the homologous strain MC58 (Table 2), were tested against heterologous strains of types II and III. For type II strain MC90, the median levels of bactericidal activity were similar to those for strain MC58. In addition, for type III strain MC54, antisera raised to rMIP in saline or liposomes also showed similar bactericidal activity. In contrast, in formulations that contained MPLA, lower levels of bactericidal activity were observed with strain MC54 (Table 3).
Table 3.
Cross-strain bactericidal activity of rMIP antiseraa
| Sequence type/ representative strain | Serum bactericidal titer of antisera raised to rMIP in: |
|||
|---|---|---|---|---|
| Saline | Liposomes | Liposomes + MPLA | ZW 3-14 micelles + MPLA | |
| I/MC58 | 128 | 128 | 1,024 (512, 2,048) | 1,024 |
| II/MC90 | 256 (256, 512) | 256 (128, 256) | 512 (512, 1,024) | 2,048 (1,024, 4,096) |
| III/MC54 | 256 | 256 | <4 | 64 |
Pooled antisera raised to rMIP from MC58 were tested against heterologous strains with variations in MIP sequence. The titers are expressed as the reciprocal of the highest dilution at which 50% killing was observed. Titers for normal mouse sera and sera from mice immunized with controls without rMIP were <4. Data are the median values, with the range of values in parentheses, for serum bactericidal activity from three or more independent measurements of bactericidal activity of all pooled serum samples. Single values denote that the serum bactericidal activity titers were identical from the independent experiments.
DISCUSSION
Our studies show that the MIP protein of Neisseria meningitidis meets several important criteria for a potential meningococcal vaccine antigen. We identified only three different MIP protein sequences in 13 meningococcal strains isolated from carriers or patients of different serogroups, serotypes, and serosubtypes (32, 56, 58). Type I MIP was expressed by MC58 and is ∼99% homologous to the surface-exposed lipoprotein Ng-MIP, which is found on the closely related organism Neisseria gonorrhoeae (34). Type II MIP showed ∼98% homology to type I MIP (strain MC58), containing a deletion of four sequential amino acids and the substitution of two amino acids only. Type III MIP showed ∼99% homology to type I MIP, with only three closely spaced amino acid substitutions. Unlike variable OM proteins such as PorA, PorB, and Opa, these limited variations did not appear to be clustered in discrete regions that might indicate immunogenic regions most susceptible to immune attack. In addition, MIP showed similar levels of expression across this collection of meningococcal strains, as judged by reactivity on a Western blot with rabbit anti-rMIP serum.
The key findings from our study were that MIP induced antibodies that activated complement-mediated killing of meningococci, which is the generally accepted correlate of protection against meningococcal infection (18, 52). For OM proteins, the generation of serum bactericidal antibodies is dependent on refolding to a native-like conformation, and we therefore incorporated rMIP protein into liposomes and ZW 3-14 micelles, which we and others have used successfully with other meningococcal OM proteins, e.g., PorA (27, 28), PorB (59), and Opc (31), as well as the gonococcal PorB (54). MPLA was also included in several of these formulations as a potent and nontoxic immunomodulator. Moreover, MPLA is acceptable for human vaccines and is included in the human papillomavirus vaccine Cervarix and in the hepatitis B vaccine Fendrix (43) and functions by acting through Toll-like receptor 4 to increase antibody production (4), induce antigen-specific CD8+ T-cell responses (41), and enhance the intrinsic adjuvant effect of liposomes (51).
The addition of MPLA into both rMIP-liposomes and rMIP-ZW 3-14 micelles resulted in a significant increase in antibodies that were bactericidal for the homologous strain MC58 compared with the equivalent formulation delivered without the adjuvant. These findings are similar to previous observations on the adjuvant effect of MPLA on the PorB (59) and Opc (31) OM proteins. However, a significant property of rMIP was its ability to induce bactericidal antibodies, when it was administered in saline alone, which were not observed with the major OM proteins PorA, PorB, and Opc. This functional immunogenicity could be due to the fact that rMIP is able to refold to a native-like conformation without the need for incorporation into liposomes or detergent micelles. Thus, removing the need to both use liposomal technology and include extrinsic adjuvants would be useful properties for commercial vaccine manufacture in eliminating processes that could introduce batch-to-batch variation.
Most importantly, antiserum raised to rMIP from the type I MIP strain MC58 was capable of promoting complement-mediated killing of heterologous strains expressing type II and III MIP proteins. Cross-reactive killing was observed against the type II strain (MC90) at similar levels to the type I strain. In addition, antisera raised to protein in saline or liposomes alone killed the type III strain (MC54) as effectively as the type I and II strains. In contrast, with the type III strain, the level of cross-reactive bactericidal activity was significantly lower with antisera raised against both preparations that contained MPLA. Clearly, the presence and type of adjuvant can influence immunogenicity, and it is possible that MPLA can bind to the protein and subtly alter the folding and native-like conformation of the protein. The consequence of this may be that the antibodies generated do not recognize the functional epitope(s) on the type III MIP protein. A similar observation was reported for recombinant PorA porin incorporated into liposomes, where the addition of MPLA resulted in a complete loss of porin-induced bactericidal activity, which was attributed to interference with the native conformation of the porin in the lipid bilayer (5). In contrast, although lower levels of antibody are induced by rMIP protein delivered in saline or liposomes alone, these antibodies appear to recognize bactericidal epitopes on the different MIP proteins equally. In addition, care needs to be taken in the choice of exogenous adjuvant used: indeed, this is exemplified by the use of alum, which, although it is a potent adjuvant in stimulating high levels of antibodies reactive with MIP on meningococci, as judged by IF labeling, failed to induce a bactericidal response. The likely explanation for this finding is that physical adsorption to alum alters the native-like conformation of the rMIP bactericidal epitope(s). This explanation is supported by the study from Wetzler and colleagues (54) with gonococcal PorB protein, where adsorption to alum resulted in the induction of antiporin antibodies with diminished functional activity compared to antibodies induced by PorB in liposomes. In our study, IF labeling also demonstrated that antibodies raised to rMIP with other preparations could bind to meningococcal cells. Thus, IF labeling appeared to be useful for demonstrating the surface accessibility of the MIP antigen, but it was not wholly predictive of serum bactericidal activity. Similar observations have also been reported for the major surface-exposed OM antigens PorA, PorB, and Opc (27, 31, 59).
In our study, a recombinant meningococcal OM protein, rMIP, was shown to induce antibodies with high levels of cross-strain bactericidal activity against meningococci. MIP is also highly conserved and expressed by all the meningococcal strains tested thus far. In addition, since MIP shares 99% homology to surface-located gonococcal MIP, which has been shown to play a role in bacterial persistence within macrophages (34), it is possible that MIP is also important during interactions of meningococci with immune effector cells and potentially at other intracellular sites of infection, e.g., during invasion of epithelial and endothelial cells. Indeed, a role for MIP in virulence has recently been suggested by the observation that expression of the mip gene was upregulated in an ex vivo human whole-blood model of wild-type MC58 infection; moreover, a mutant strain lacking MIP protein expression was found to be sensitive to killing by human blood compared to the wild-type strain (11). In light of all these observations, MIP may be a significant candidate for inclusion in novel meningococcal vaccines, either singly or in combination with other protective antigens.
ACKNOWLEDGMENTS
M.-C. Hung is a Ph.D. student sponsored by the government of Taiwan. The study was supported by Wessex Medical Trust, and M.C. and J.E.H. now receive grant funding from GlaxoSmithKline.
Footnotes
Published ahead of print on 27 June 2011.
REFERENCES
- 1. Bangsborg J. M., Shand G., Pearlman E., Hoiby N. 1991. Cross-reactive Legionella antigens and the antibody response during infection. APMIS 99:854–865 [PubMed] [Google Scholar]
- 2. Borrow R., Miller E. 2006. Long-term protection in children with meningococcal C conjugate vaccination: lessons learned. Expert Rev. Vaccines 5:851–857 [DOI] [PubMed] [Google Scholar]
- 3. Callaghan M. J., et al. 2011. Potential of recombinant Opa proteins as vaccine candidates against hyperinvasive meningococci. Infect. Immun. 79:2810–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casella C. R., Mitchell T. C. 2008. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell. Mol. Life Sci. 65:3231–3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christodoulides M., Brooks J. L., Rattue E., Heckels J. E. 1998. Immunisation with recombinant class 1 outer membrane protein from Neisseria meningitidis: influence of liposomes and adjuvants on antibody avidity, recognition of native protein and the induction of a bactericidal immune response against meningococci. Microbiology 144:3027–3037 [DOI] [PubMed] [Google Scholar]
- 6. Christodoulides M., Jolley K., Heckels J. E. 2001. Recombinant proteins in vaccine development, p. 167–180 In Pollard A. J., Maiden M. C. J. (ed.), Meningococcal vaccines. Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 7. Christodoulides M., McGuinness B. T., Heckels J. E. 1993. Immunisation with synthetic peptides containing epitopes of the class 1 outer membrane protein of Neisseria meningitidis: production of bactericidal antibodies on immunisation with a cyclic peptide. J. Gen. Microbiol. 139:1729–1738 [DOI] [PubMed] [Google Scholar]
- 8. Cianciotto N. P., Eisenstein B. I., Mody C. H., Toews G. B., Engleberg N.C. 1989. A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect. Immun. 57:1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cianciotto N. P., Oconnell W., Dasch G. A., Mallavia L. P. 1995. Detection of MIP-like sequences and MIP-related proteins within the family Rickettsiaceae. Curr. Microbiol. 30:149–153 [DOI] [PubMed] [Google Scholar]
- 10. Dornan J., Taylor P., Walkinshaw M. D. 2003. Structures of immunophilins and their ligand complexes. Curr. Top. Med. Chem. 3:1392–1409 [DOI] [PubMed] [Google Scholar]
- 11. Echenique-Rivera H., et al. 2011. Transcriptome analysis of Neisseria meningitidis in human whole blood and mutagenesis studies identify virulence factors involved in blood survival. PLoS Pathog. 7:e10002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Findlow J., et al. 2010. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin. Infect. Dis. 51:1127–1137 [DOI] [PubMed] [Google Scholar]
- 13. Finne J., Leinonen M., Makela P. H. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355–357 [DOI] [PubMed] [Google Scholar]
- 14. Fischer G., Bang H., Ludwig B., Mann K., Hacker J. 1992. MIP protein of Legionella pneumophila exhibits peptidyl-prolyl-cis trans isomerase (PPIase) activity. Mol. Microbiol. 6:1375–1383 [DOI] [PubMed] [Google Scholar]
- 15. Fletcher L. D., et al. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 72:2088–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fretz H., et al. 1991. Rapamycin and Fk506 binding-proteins (immunophilins). J. Am. Chem. Soc. 113:1409–1411 [Google Scholar]
- 17. Giuliani M. M., et al. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. U. S. A. 103:10834–10839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldschneider I., Gotschlich E. C., Artenstein M. S. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Granoff D. M. 2010. Review of meningococcal group B vaccines. Clin. Infect. Dis 50:S54–S65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hacker J., Fischer G. 1993. Immunophilins-structure-function relationship and possible role in microbial pathogenicity. Mol. Microbiol. 10:445–456 [DOI] [PubMed] [Google Scholar]
- 21. Heckels J. E. 1981. Structural comparison of Neisseria gonorrhoeae outer membrane proteins. J. Bacteriol. 145:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Helbig J. H., et al. 2003. The PPIase active site of Legionella pneumophila MIP protein is involved in the infection of eukaryotic host cells. Biol. Chem. 384:125–137 [DOI] [PubMed] [Google Scholar]
- 23. Helbig J. H., Luck P. C., Steinert M., Jacobs E., Witt M. 2001. Immunolocalization of the MIP protein of intracellularly and extracellularly grown Legionella pneumophila. Lett. Appl. Microbiol. 32:83–88 [DOI] [PubMed] [Google Scholar]
- 24. Hitchcock P. J., Brown T. M. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holst J., et al. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27:B3–B12 [DOI] [PubMed] [Google Scholar]
- 26. Horne S. M., Kottom T. J., Nolan L. K., Young K. D. 1997. Decreased intracellular survival of an fkpA mutant of Salmonella typhimurium Copenhagen. Infect. Immun. 65:806–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Humphries H., et al. 2006. Multivalent liposome-based vaccines containing different serosubtypes of PorA protein induce cross-protective bactericidal immune responses against Neisseria meningitidis. Vaccine 24:35–44 [DOI] [PubMed] [Google Scholar]
- 28. Idanpaan-Heikkila I., et al. 1995. The antibody response to a prototype liposome vaccine containing Neisseria meningitidis outer membrane protein P1 produced in Bacillus subtilis. Vaccine 13:1501–1508 [DOI] [PubMed] [Google Scholar]
- 29. Idanpaan-Heikkila I., et al. 1996. Immunization with meningococcal class 1 outer membrane protein produced in Bacillus subtilis and reconstituted in the presence of Zwittergent or Triton X-100. Vaccine 14:886–891 [DOI] [PubMed] [Google Scholar]
- 30. Jiang H. Q., et al. 2010. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 28:6086–6093 [DOI] [PubMed] [Google Scholar]
- 31. Jolley K., Appleby L., Wright J. C., Christodoulides M., Heckels J. E. 2001. Immunisation with recombinant Opc outer membrane protein from Neisseria meningitidis: influence of sequence variation and levels of expression on the bactericidal immune response against meningococci. Infect. Immun. 69:3809–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jordens J. Z., Williams J. N., Jones G. R., Christodoulides M., Heckels J. E. 2004. Development of immunity to serogroup B meningococci during carriage of Neisseria meningitidis in a cohort of university students. Infect. Immun. 72:6503–6510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kohler R., et al. 2003. Biochemical and functional analyses of the MIP protein: influence of the N-terminal half and of peptidylprolyl isomerase activity on the virulence of Legionella pneumophila. Infect. Immun. 71:4389–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leuzzi R., et al. 2005. Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol. Microbiol. 58:669–681 [DOI] [PubMed] [Google Scholar]
- 35. Lundemose A. G., Birkelund S., Fey S. J., Larsen P. M., Christiansen G. 1991. Chlamydia trachomatis contains a protein similar to the Legionella pneumophila MIP gene-product. Mol. Microbiol. 5:109–115 [DOI] [PubMed] [Google Scholar]
- 36. Martin D. R., Ruijne N., McCallum L., O'Hallahan J., Oster P. 2006. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin. Vaccine Immunol. 13:486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McGuinness B. T., et al. 1991. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet 337:514–517 [DOI] [PubMed] [Google Scholar]
- 38. Milagres L. G., Gorla M. C., Sacchi C. T., Rodrigues M. A. 1998. Specificity of bactericidal antibody response to serogroup B meningococcal strains in Brazilian children after immunisation with an outer membrane vaccine. Infect. Immun. 66:4755–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mo Y. Y., Seshu J., Wang D., Mallavia L. P. 1998. Synthesis in Escherichia coli of two smaller enzymically active analogues of Coxiella burnetii macrophage infectivity potentiator (CbMIP) protein utilizing a single open reading frame from the cbmip gene. Biochem. J. 335:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moro A., Ruizcabello F., Fernandezcano A., Stock R. P., Gonzalez A. 1995. Secretion by Trypanosoma cruzi of a peptidyl-prolyl cis-trans isomerase involved in cell infection. EMBO J. 14:2483–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nordly P., Agger E. M., Andersen P., Nielsen H. M., Foged C. 2011. Incorporation of the TLR4 agonist monophosphoryl lipid A into the bilayer of DDA/TDB liposomes: physico-chemical characterization and induction of CD8(+) T-cell responses in vivo. Pharm. Res. 28:553–562 [DOI] [PubMed] [Google Scholar]
- 42. Pereira P. J. B., et al. 2002. Trypanosoma cruzi macrophage infectivity potentiator has a rotamase core and a highly exposed alpha-helix. EMBO Rep. 3:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Perrie Y., Mohammed A. R., Kirby D. J., Mcneil S. E., Bramwell V. W. 2008. Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int. J. Pharm. 364:272–280 [DOI] [PubMed] [Google Scholar]
- 44. Riboldi-Tunnicliffe A., et al. 2001. Crystal structure of MIP, a prolylisomerase from Legionella pneumophila. Nat. Struct. Biol. 8:779–783 [DOI] [PubMed] [Google Scholar]
- 45. Richmond P., et al. 2010. Safety and immunogenicity of serogroup B Neisseria meningitidis (MnB) rLP2086 vaccine in adults and adolescent subjects: overview of 3 clinical trials, Abstr. VWO4. In Abstr. 17th Int. Pathog. Neisseria Conf Banff, Alberta, Canada [Google Scholar]
- 46. Rinaudo C. D., Telford J. L., Rappuoli R., Seib K. L. 2009. Vaccinology in the genome era. J. Clin. Invest. 119:2515–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rockey D. D., Chesebro B. B., Heinzen R. A., Hackstadt T. 1996. A 28 kDa major immunogen of Chlamydia psittaci shares identity with MIP proteins of Legionella spp and Chlamydia trachomatis—cloning and characterization of the C-psittaci MIP-like gene. Microbiology 142:945–953 [DOI] [PubMed] [Google Scholar]
- 48. Schagger H., von Jagow G. 1987. Tricine-sodium dodecyl sulphate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368–379 [DOI] [PubMed] [Google Scholar]
- 49. Schreiber S. L. 1991. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science 251:283–287 [DOI] [PubMed] [Google Scholar]
- 50. Siekierka J. J., et al. 1990. The cytosolic-binding protein for the immunosuppressant Fk-506 is both a ubiquitous and highly conserved peptidyl-prolyl cis-trans isomerase. J. Biol. Chem. 265:21011–21015 [PubMed] [Google Scholar]
- 51. Verma J. N., et al. 1992. Adjuvant effects of liposomes containing lipid A-enhancement of liposomal antigen presentation and recruitment of macrophages. Infect. Immun. 60:2438–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vermont C., van den Dobbelsteen G. 2002. Neisseria meningitidis serogroup B: laboratory correlates of protection. FEMS Immunol. Med. Microbiol. 34:89–96 [DOI] [PubMed] [Google Scholar]
- 53. Vogtherr M., et al. 2002. NMR solution structure and dynamics of the peptidylprolyl cis-trans isomerase domain of the trigger factor from Mycoplasma genitalium compared to FK506-binding protein. J. Mol. Biol. 318:1097–1115 [DOI] [PubMed] [Google Scholar]
- 54. Wetzler L. M., Blake M. S., Barry K., Gotschlich E. C. 1992. Gonococcal porin vaccine evaluation—comparison of por proteosomes, liposomes, and blebs isolated from rmp deletion mutants. J. Infect. Dis. 166:551–555 [DOI] [PubMed] [Google Scholar]
- 55. Wieland H., Faigle M., Lang F., Northoff H., Neumeister B. 2002. Regulation of the Legionella MIP promotor during infection of human monocytes. FEMS Microbiol. Lett. 212:127–132 [DOI] [PubMed] [Google Scholar]
- 56. Williams J. N., Jones G. R., Christodoulides M., Heckels J. E. 2003. Serological correlates of protection against meningococci in a cohort of university students before and during an outbreak of serogroup C infection. J. Infect. Dis. 187:1433–1441 [DOI] [PubMed] [Google Scholar]
- 57. Williams J. N., et al. 2007. Proteomic analysis of outer membranes and vesicles from wild-type serogroup B Neisseria meningitidis and a lipopolysaccharide-deficient mutant. Infect. Immun. 75:1364–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Williams J. N., Skipp P. J., O'Connor C. D., Christodoulides M., Heckels J. E. 2009. Immunoproteomic analysis of the development of natural immunity in subjects colonized by Neisseria meningitidis reveals potential vaccine candidates. Infect. Immun. 77:5080–5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wright J. C., Williams J. N., Christodoulides M., Heckels J. E. 2002. Immunisation with recombinant PorB outer membrane protein induces a bactericidal immune response against Neisseria meningitidis. Infect. Immun. 70:4028–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]