Abstract
Arthritis is one of the most common complications of human brucellosis, but its pathogenic mechanisms have not been elucidated. Fibroblast-like synoviocytes (FLS) are known to be central mediators of joint damage in inflammatory arthritides through the production of matrix metalloproteinases (MMPs) that degrade collagen and of cytokines and chemokines that mediate the recruitment and activation of leukocytes. In this study we show that Brucella abortus infects and replicates in human FLS (SW982 cell line) in vitro and that infection results in the production of MMP-2 and proinflammatory mediators (interleukin-6 [IL-6], IL-8, monocyte chemotactic protein 1 [MCP-1], and granulocyte-macrophage colony-stimulating factor [GM-CSF]). Culture supernatants from Brucella-infected FLS induced the migration of monocytes and neutrophils in vitro and also induced these cells to secrete MMP-9 in a GM-CSF- and IL-6-dependent fashion, respectively. Reciprocally, culture supernatants from Brucella-infected monocytes and neutrophils induced FLS to produce MMP-2 in a tumor necrosis factor alpha (TNF-α)-dependent fashion. The secretion of proinflammatory mediators and MMP-2 by FLS did not depend on bacterial viability, since it was also induced by heat-killed B. abortus (HKBA) and by a model Brucella lipoprotein (L-Omp19). These responses were mediated by the recognition of B. abortus antigens through Toll-like receptor 2. The intra-articular injection of HKBA or L-Omp19 into the knee joint of mice resulted in the local induction of the proinflammatory mediators MMP-2 and MMP-9 and in the generation of a mixed inflammatory infiltrate. These results suggest that FLS, and phagocytes recruited by them to the infection focus, may be involved in joint damage during brucellar arthritis through the production of MMPs and proinflammatory mediators.
INTRODUCTION
Human brucellosis is a systemic febrile illness with a plethora of somatic complaints resulting from infection with Brucella species. The disease usually spreads to humans by direct contact with infected animals, by the ingestion of unpasteurized milk or milk products, through cuts and abrasions, or by the inhalation of aerosols. Brucellosis is chiefly an inflammatory disease. Inflammation is present in both the acute and chronic phases of the disease and in virtually all of the organs affected. The most common clinical features of human brucellosis are undulant fever, sweats, arthralgias, myalgias, lymphadenopathy, and hepatosplenomegaly (39).
Arthritis is one of the most common complications or focalizations of human brucellosis and may be caused by different Brucella species. Articular involvement may be observed for either acute or chronic cases of human brucellosis (1, 17, 25) and may affect patients of any age (1, 3, 17, 25). Arthritis is frequently polyarticular and migratory, affecting mainly the large joints, but monoarthiritis may be the presenting feature of brucellosis (25, 29). The clinical features include joint pain, which may be severe and of sudden onset or mild and gradual in onset. Joint swelling, tenderness, increased local warmth, and limitation of movement are common, and the arthritis may last while the disease is active and not treated. Image studies have revealed cartilage loss and bone erosion in brucellar arthritis affecting different joints (3, 28). These lesions may eventually lead to permanent joint dysfunction. Brucella sp. is isolated from synovial fluid samples in about 50% of the cases. The synovial membrane of the affected joint may present a lymphomononuclear infiltrate in the chronic phase of the disease but usually presents a polymorphonuclear infiltrate in acute cases (17, 29).
Septic arthritis may be caused by different bacteria, among which Staphylococcus aureus is the leading cause and has been commonly used in animal models to study the pathogenesis of this condition (4). Both clinical and animal studies have shown that septic arthritis may be secondary to the hematogenous seeding of a joint during transient or persistent bacteremia (4, 35). The infiltration and growth of bacteria within the synovium result in inflammation with the infiltration of leukocytes into the joint fluid. There is a rapid influx of polymorphonuclear leukocytes (PMN), later followed by the migration of mononuclear phagocytes (48). An increased expression or secretion of proinflammatory cytokines can be detected in the synovial membrane or the synovial fluid during infection in animal models and clinical cases of septic arthritis (21, 38, 58). Under most circumstances, the inflammatory response contains the invading pathogen and resolves the infection. However, when the infection is not quickly cleared by the host, the potent activation of the immune response with the associated high levels of cytokines leads to joint destruction. High cytokine concentrations increase the release of host matrix metalloproteinases (MMPs) that degrade collagen and thus lead to cartilage loss (15, 44, 54).
MMPs comprise a family of calcium- and zinc-dependent proteinases that degrade collagens and other extracellular matrix proteins through selective and overlapping substrate specificities (5, 36). These enzymes have many physiological functions but may have detrimental effects when their levels are highly increased, as occurs in many inflammatory processes. MMP-2 and MMP-9 (gelatinases A and B, respectively) are important in the pathogenesis of osteoarticular diseases, since they can degrade a variety of collagens, including the basement membrane (type IV collagen), denatured fibrillar type I collagen (gelatin), and type V collagen (34). Notably, in a recent study we found a high level of gelatinase activity in the synovial fluid of a patient with prepatellar bursitis due to Brucella abortus, as revealed by zymography, and also confirmed the presence of high levels of MMP-9 by an enzyme-linked immunosorbent assay (ELISA), which suggests that MMPs may be involved in the osteoarticular damage associated with Brucella infection (52).
While MMPs can be produced by activated neutrophils and monocytes recruited to the inflamed synovium, fibroblast-like synoviocytes (FLS) have been increasingly recognized as a key source of these proteases in inflammatory arthritides (2, 33, 37). FLS respond to, and themselves produce, inflammatory mediators, including interleukin-1 (IL-1), IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, IL-18, IL-21, tumor necrosis factor alpha (TNF-α), transforming growth factor β (TGF-β), and gamma interferon (IFN-γ). In particular, TNF-α and IL-1β have been shown to be the main inducers of MMP production by FLS (33). It is assumed that these cytokines are produced mainly by neutrophils and monocytes recruited to the infected or inflamed synovium. Such a recruitment may be mediated by chemokines produced by FLS in response to infection, thus establishing a cytokine network between FLS and phagocytes. Notably, FLS may increase their MMP production not only in response to cytokines but also in response to bacterial antigens. FLS have been reported to express Toll-like receptors (TLRs), particularly TLR2 (43). Human FLS treated with a cell lysate or filtered culture supernatants of S. aureus had significantly enhanced expressions of several MMPs compared with untreated controls (23). In another study, the expression of MMP mRNAs was upregulated in human FLS treated with staphylococcal peptidoglycan, and the levels of IL-6 and IL-8 in the culture supernatants were also increased (27).
Against this background we decided to investigate the production of MMPs by human FLS in response to Brucella infection and upon interactions with Brucella-infected monocytes or neutrophils.
MATERIALS AND METHODS
Bacterial culture.
Brucella abortus S2308 cells were grown overnight in 10 ml of tryptic soy broth with constant agitation at 37°C. Bacteria were harvested by centrifugation for 15 min at 6,000 × g at 4°C and washed twice in 10 ml of phosphate-buffered saline (PBS). Bacterial numbers in the cultures were estimated by comparing the optical densities at 600 nm with a standard curve obtained in our laboratory. To prepare inocula, cultures were diluted in sterile PBS to the desired bacterial concentration on the basis of the optical density readings, but the precise concentrations of inocula were determined by plating cells onto tryptic soy agar. To obtain heat-killed B. abortus (HKBA), bacteria were washed five times for 10 min each in sterile PBS, heat killed at 70°C for 20 min, aliquoted, and stored at −70°C until they were used. The total absence of B. abortus viability after heat killing was verified by the absence of bacterial growth on tryptose soy agar. All live Brucella manipulations were performed in biosafety level 3 facilities.
Cell culture.
The immortalized human FLS cell line SW982 was obtained from the ATCC (Rockville, MD). The fibroblast phenotype of SW982 cells has been demonstrated by their expression of vimentin, a fibroblast marker (30, 51). This cell line has been successfully used to study the expressions of inflammatory cytokines and MMPs by FLS upon different stimuli (30, 47, 56). The SW982 cell line was cultured as monolayers in a 5% CO2 atmosphere at 37°C in a Dulbecco's modified Eagle medium F-12 nutrient mixture (Gibco, Grand Island, NY) supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum (FBS) (Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin.
Human neutrophils were isolated from venous blood from healthy human volunteers by a Ficoll-Paque (GE Healthcare, Uppsala, Sweden) gradient, followed by the sedimentation of erythrocytes in 6% dextran and hypotonic lysis. Neutrophils were harvested, washed twice with sterile PBS, and resuspended at a concentration of 1 × 106 cells/ml in RPMI 1640 medium supplemented with 5% FBS, 1 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cell viability was >98%, as determined by trypan blue exclusion. The purity of the final neutrophil preparation was >95%, as assessed by morphological examination after Giemsa staining and flow cytometry light scatter patterns. The human monocytic cell line THP-1 was cultured in a 5% CO2 atmosphere at 37°C in RPMI 1640 medium (Gibco) supplemented with 2 mM l-glutamine, 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cell lines were seeded at 5 × 105 cells/well in 24-well plates.
Lipoproteins and LPS.
The lipidated and unlipidated forms of the 19-kDa outer membrane protein from B. abortus (L-Omp19 and U-Omp19, respectively) were obtained in a recombinant form in Escherichia coli cells as described previously (14). Both recombinant proteins contained less than 0.25 endotoxin units per μg of protein as assessed by a Limulus amebocyte lysate test (Associates of Cape Cod, East Falmouth, MA). The protein concentration was determined by the bicinchoninic acid method (Pierce, Rockford, IL) using bovine serum albumin (BSA) as a standard. B. abortus S2308 lipopolysaccharide (LPS) and E. coli O111k58H2 LPS were provided by I. Moriyon (University of Navarra, Pamplona, Spain). The synthetic lipohexapeptide tripalmitoyl-S-glyceryl-Cys-Ser-Lys4-OH (Pam3Cys) was purchased from Boehringer Mannheim (Indianapolis, IN).
Cellular infection.
SW982 cells were infected with B. abortus S2308 at different multiplicities of infection (MOIs) (100, 250, 500, or 1,000), and neutrophils and THP-1 cells were infected at an MOI of 100.
After the bacterial suspension was dispensed, the plates were centrifuged for 10 min at 1,000 × g and then incubated for 2 h at 37°C under a 5% CO2 atmosphere. Cells were extensively washed with RPMI medium to remove extracellular bacteria and incubated in medium devoid of FBS and supplemented with BSA (0.01%), along with 100 μg/ml gentamicin and 50 μg/ml streptomycin to kill extracellular bacteria (complete medium). Supernatants from infected cultures were harvested at 24 h postinfection (p.i.) to be used as conditioned medium and at 48 h p.i. for measuring MMP concentrations.
Measurement of cytokine concentrations.
Concentrations of human IL-1β, IL-6, IL-8, monocyte chemotactic protein 1 (MCP-1), TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) were measured in culture supernatants by sandwich ELISA, using paired cytokine-specific monoclonal antibodies, according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). Mouse IL-1β, IL-6, MCP-1 (BD Pharmingen, San Diego, CA), and keratinocyte chemoattractant (KC) (CXCL1) secretions were quantified by ELISA (R&D Systems Inc., Minneapolis, MN) of supernatants from tissue homogenates from knee joints injected with Brucella antigens (see below).
Stimulation with conditioned media.
Culture supernatants from Brucella-infected SW982 cells (MOI of 100) were harvested at 24 h p.i., sterilized by filtration through a 0.22-μm nitrocellulose filter, and used to stimulate noninfected neutrophils and THP-1 monocytes. Similarly, culture supernatants from Brucella-infected neutrophils and THP-1 cells were harvested at 24 h p.i. and used to stimulate uninfected SW982 cells. For stimulation, supernatants were used diluted 1/2, 1/5, or 1/10 in complete medium. After 48 h the supernatants from these stimulated cultures were harvested to measure MMP concentrations.
Neutralization experiments were performed with an anti-TNF-α neutralizing antibody (clone MAb1; BD Biosciences, San Diego, CA), an anti-GM-CSF neutralizing antibody (clone BVD2-23B6; BD Biosciences), or an anti-IL-6 neutralizing antibody (clone MQ2-13A5; BD Biosciences). The appropriate isotype control was used in each case. Conditioned media from Brucella-infected cells (FLS, monocytes, or neutrophils) were preincubated with the corresponding antibody (or isotype control) for 1 h at 37°C before being used to stimulate other cell types.
Zymography.
Gelatinase activity was assayed by the method of Hibbs et al. (20). Briefly, a total of 20 μl of cell culture supernatants from infected or stimulated FLS, neutrophils, or monocytes or from untreated controls was mixed with 5 μl of 5× loading buffer (0.25 M Tris [pH 6.8], 50% glycerol, 5% SDS, and bromophenol blue crystals) and loaded onto 10% SDS-PAGE gels containing 1 mg/ml gelatin (Sigma-Aldrich, Argentina SA). Following electrophoresis, gels were washed with a solution containing 50 mM Tris-HCl (pH 7.5) and 2.5% Triton X-100 (buffer A) for 30 min and with buffer A added with 5 mM CaCl2 and 1 μM ZnCl2 for 30 min and were later incubated with buffer A with additional 10 mM CaCl2 and 200 mM NaCl for 48 h at 37°C. This denaturation/renaturation step promotes MMP activity without the proteolytic cleavage of pro-MMP-9. Gelatin activity was visualized by the staining of the gels with 0.5% Coomassie blue. Unstained bands indicated the presence of gelatinase activity, and their positions indicated the molecular weights of the enzymes involved. The identity of the candidate MMP was confirmed by a specific ELISA, as explained below.
Measurement of MMP-9 and MMP-2 levels.
MMP-2 levels present in conditioned medium from SW982 cells and MMP-9 levels present in conditioned medium from neutrophils and THP-1 cells were quantified by sandwich ELISA using paired MMP-specific monoclonal antibodies according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Gelatinase activity under native conditions.
The gelatinase activity in unprocessed culture supernatants (native conditions) was measured by using a gelatinase/collagenase fluorometric assay kit (EnzChek; Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The EnzChek kit contains DQ gelatin, a fluorescein-conjugated gelatin so heavily labeled with fluorescein that fluorescence is quenched. When this substrate is digested by gelatinases or collagenases, it yields highly fluorescent peptides, and the fluorescence increase is proportional to the proteolytic activity. Collagenase purified from Clostridium histolyticum provided in the assay kit served as a control enzyme. Plates were read with a fluorescence plate reader (Victor3; Perkin-Elmer, Waltham, MA).
Migration assay.
Cell migration was evaluated by using 96-well microchemotaxis plates with 5-μm-pore-diameter polycarbonate filters (Corning, Corning, NY). Human neutrophils and THP-1 cells (1 × 106 cells/ml) were placed into the upper well of the chambers, and the indicated stimuli (dilutions of culture supernatants from B. abortus-infected SW982 cells) were placed into the lower wells. Migration was scored by counting the number of monocytes or neutrophils that had reached the bottom well after 2 h. Migration toward N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP) (1 × 10−7 M) (Sigma-Aldrich) served as a positive control. The number of migrating cells was expressed as follows: chemoattractant index = number of cells migrating to the tested medium/number of cells migrating to fresh culture medium.
Stimulation with B. abortus antigens and TLR agonists.
SW982 cells (5 × 105 cells/ml) were incubated with E. coli LPS (100 ng/ml), B. abortus LPS (1,000 ng/ml), Pam3Cys (50 ng/ml), HKBA (1 × 108 bacteria/ml), or L-Omp19 (1,000 ng/ml) in a final volume of 0.4 ml. Cultures were incubated for 24 h, and supernatants were assayed for cytokine production. In some experiments cells were incubated with 20 μg/ml of anti-human TLR2 antibody (clone TL2.1), anti-human TLR4 antibody (clone HTA125), or an IgG2a isotype control (eBioscience) for 30 min at 37°C before incubation with the antigens and agonists mentioned above.
Evaluation of the articular inflammatory reaction in a mouse model.
Six- to eight-week-old female BALB/c mice were anesthetized with ketamine chlorhydrate (150 mg/kg of body weight) and xylazine (15 mg/kg) and then injected intra-articularly into the knee joint with 50 μl of HKBA (1 × 106 CFU), L-Omp19 (500 ng), U-Omp19 (500 ng), E. coli LPS (500 ng) as a positive control, or vehicle (PBS). Mice were sacrificed at 5 days postadministration.
To determine cytokine levels and MMP production in the joints, knees from each mouse were excised by removing the skin and cutting just above and below the joint and were placed immediately into 1 ml of cold PBS. Joint extractions were performed by using a tissue homogenizer. Homogenates were centrifuged at 2,000 × g for 20 min at 4°C, and supernatants were stored at −70°C until cytokine and MMP measurements were performed.
In another group of mice histological examination of joints was carried out at day 5 poststimulation after routine fixation, decalcification, and paraffin embedding. Five-micrometer-thick sections were cut and stained with hematoxylin and eosin.
Statistical analysis.
Statistical analysis was performed with a one-way analysis of variance (ANOVA), followed by a Tukey post hoc test using GraphPad Prism 4.0 software. Data are represented as means ± standard deviations (SD).
RESULTS
Brucella abortus invades and multiplies in human FLS.
Infection experiments showed that Brucella abortus 2308 is internalized by human FLS (SW982 cell line) in vitro. The magnitude of the infection (intracellular CFU) was related directly to the MOI used but was observed even at MOIs as low as 100 (Fig. 1A). Follow-up of infected cultures revealed that B. abortus can replicate inside human FLS. Intracellular CFU counts increased by about 2 to 3 logs (depending on the initial MOI) during the first 48 h p.i. and then increased slightly during the next 24 h.
Fig. 1.
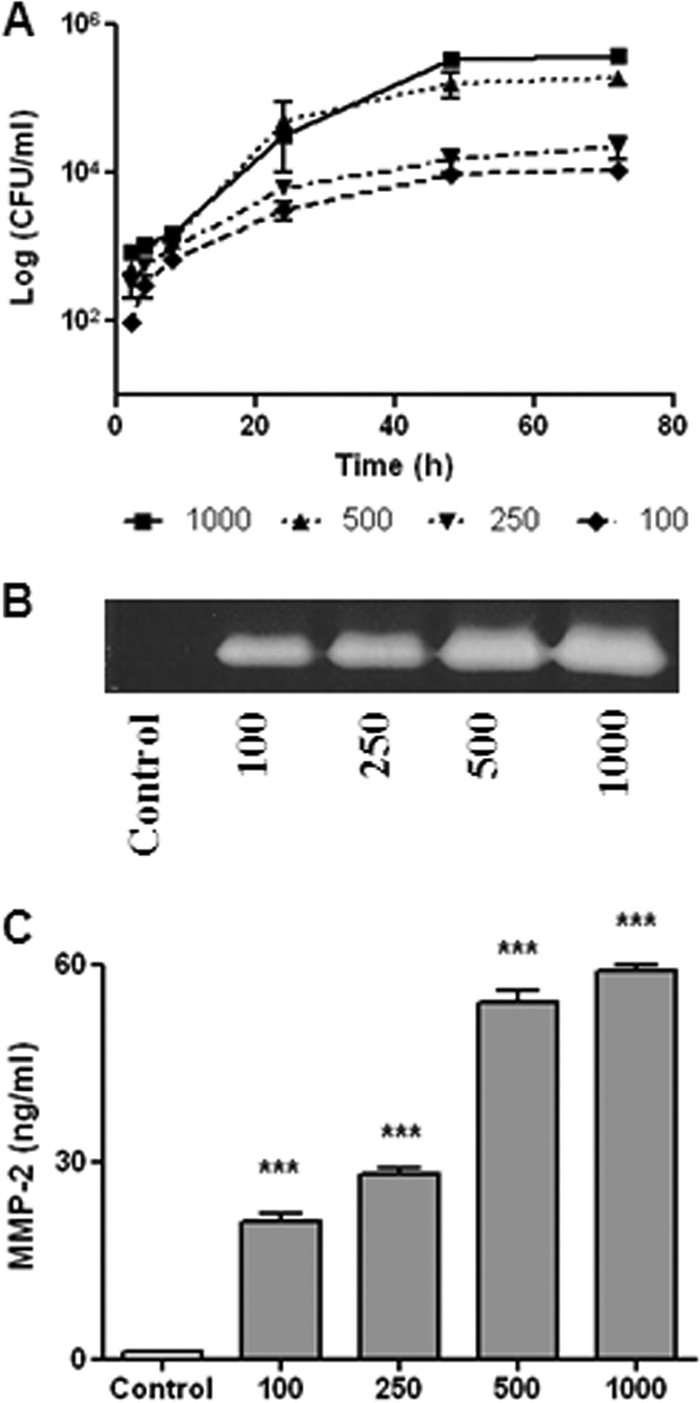
Infection and replication of B. abortus within human FLS (SW982 cell line). After infection at different MOIs (from 100 to 1,000), cells were incubated with antibiotics to kill extracellular bacteria. (A) Cell lysates obtained at different times p.i. were plated onto agar to determine intracellular CFU. (B and C) MMP-2 production at 48 h p.i. by B. abortus-infected FLS was determined by zymography (B) and ELISA (C). Data shown are from a representative experiment of five performed. ***, P < 0.001 versus the control.
Brucella abortus infection induces MMP-2 by human FLS.
As mentioned above, human FLS can respond to bacterial antigens with an enhanced secretion of MMPs. Therefore, we tested whether B. abortus infection induces MMP expression in SW982 cells. As shown in Fig. 1B, an increased gelatinase activity was detected by zymography in the supernatants of infected cells at 48 h p.i., which, according to the molecular weight of the band, corresponded to MMP-2. This was confirmed by ELISA, which revealed significantly increased levels of MMP-2 in supernatants of infected cells compared to uninfected cells (Fig. 1C). By both methods the magnitude of MMP-2 release was related directly to the MOI used. These results indicate that B. abortus can infect and replicate in human FLS in which it induces the secretion of MMP-2.
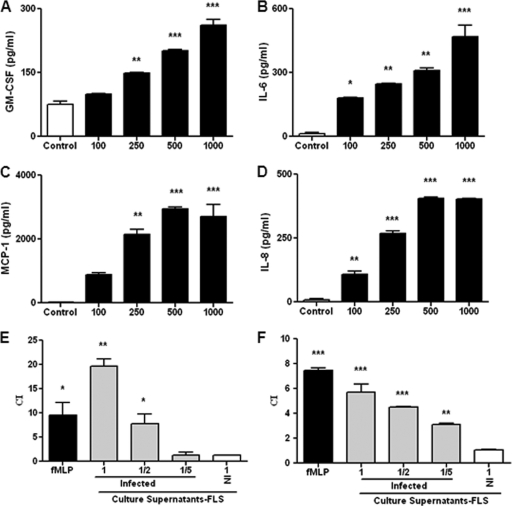
Brucella abortus infection induces GM-CSF, IL-6, MCP-1, and IL-8 but not TNF-α and IL-1β production by FLS.
As mentioned above, FLS can produce several cytokines and chemokines in response to bacterial antigens (27, 40). As shown in Fig. 2A to D, B. abortus infection of the synoviocyte cell line SW982 elicited the secretion of GM-CSF, IL-6, MCP-1, and IL-8 in an MOI-dependent fashion. The maximum stimulus-specific levels of these factors were detected 48 h after infection. No further increases in levels of these cytokines and chemokines were detected at 72 h p.i. (not shown). In addition, B. abortus infection did not induce a significant increase of TNF-α or IL-1β secretion (not shown).
Fig. 2.
Cytokine and chemokine production by FLS infected with Brucella abortus at an MOI of 100 to 1,000. (A to D) Levels of GM-CSF (A), IL-6 (B), MCP-1 (C), and IL-8 (D) measured at 48 h p.i. (E and F) Migration of human monocytes (E) and neutrophils (F) induced by culture supernatants from Brucella-infected or noninfected (NI) FLS, as measured in a microchemotaxis plate. Supernatants were added pure or diluted 1/2 or 1/5 in fresh culture medium. Migrated cells were counted at 2 h, and results are expressed as a chemoattractant index (CI) (the number of cells that migrated to conditioned medium divided by the number of cells that migrated to fresh culture medium). Migration toward N-formyl-l-methionyl-l-leucyl-phenylalanine (fMLP) served as a positive control. Data shown are from a representative experiment of five performed. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (versus the control).
Supernatants from B. abortus-infected FLS induce monocyte and neutrophil migration.
Since we found that Brucella abortus-infected FLS secrete the chemokines IL-8 and MCP-1, experiments were conducted to evaluate if supernatants from Brucella-infected FLS were able to induce the migration of neutrophils and monocytes. For this purpose, human peripheral blood neutrophils or monocytic THP-1 cells were placed into the top wells of microchemotaxis chambers, and different dilutions of supernatants from infected or noninfected FLS were placed into the bottom wells of the chambers. Migration was scored by counting the number of cells that reached the lower chamber after a 2-h incubation period. The background migration of both cell types in chambers containing fresh culture medium instead of culture supernatants was computed. As shown in Fig. 2E and F, the migrations of both monocytes and neutrophils in chambers containing supernatants from infected FLS were significantly greater than background migration, and the same was true for migration in the fMLP controls. In contrast, the migration in chambers containing supernatants from noninfected FLS remained at background levels. These results suggest that IL-8 and MCP-1 secreted by Brucella-infected FLS may play an important local regulatory role in inflammation due to its chemoattractant function, recruiting inflammatory cells to the site of infection. These recruited cells may respond to bacterial antigens with the secretion of proinflammatory cytokines, which in turn may induce FLS to secrete MMPs. Therefore, we decided to study the effects of cytokines secreted by Brucella-infected monocytes and neutrophils on the production of MMPs by FLS.
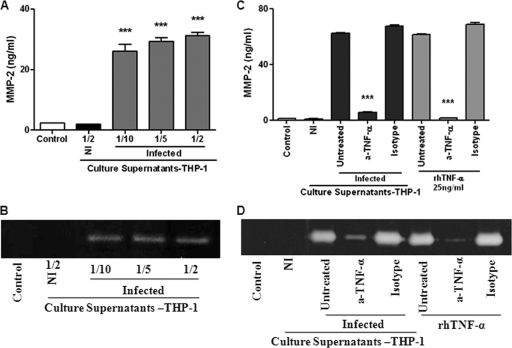
Culture supernatants from B. abortus-infected monocytes induce MMP-2 production by FLS through TNF-α.
The addition of supernatants from B. abortus-infected monocytes at different proportions (1/2 to 1/10) to uninfected FLS induced a significant secretion of MMP-2 by the latter cells compared to that in unstimulated cultures (Fig. 3 A and B). In contrast, MMP-2 secretion was not induced when FLS were stimulated with supernatants from noninfected monocytes.
Fig. 3.
(A and B) MMP-2 production by FLS upon stimulation with culture supernatants from B. abortus-infected monocytes. FLS were stimulated with culture supernatants from infected THP-1 monocytes (added at a 1/2, 1/5, or 1/10 proportion) or from noninfected (NI) monocytes (at a 1/2 proportion), and MMP-2 production was determined by ELISA (A) and zymography (B). Data shown are from a representative experiment of five performed. ***, P < 0.001 versus the control. (C and D) Inhibition of the stimulating effect of culture supernatants from infected monocytes by preincubation with a neutralizing antibody against TNF-α. FLS were incubated with culture supernatants preincubated or not (untreated) with the neutralizing antibody. As a control, other FLS cultures were incubated with fresh culture medium supplemented with recombinant human TNF-α (rhTNF-α), pretreated or not with the neutralizing antibody. MMP-2 levels were determined by ELISA (C) or zymography (D). Data shown are from a representative experiment of five performed. ***, P < 0.001 versus untreated supernatants.
In agreement with data from previous reports (10), significantly elevated levels of TNF-α, IL-1β, and IL-6 expression were detected in culture supernatants of THP-1 cells at 24 h after exposure to live B. abortus S2308 cells (MOI of 25 to 100) (not shown). Since TNF-α is known to be a strong inducer of MMP-2 production by different cell types, including FLS (32, 53), we decided to determine the contribution of this cytokine to the induction of MMP-2 in FLS by supernatants from B. abortus-infected monocytes. As shown in Fig. 3C and D, the pretreatment of supernatants with an anti-TNF-α neutralizing antibody reduced markedly their ability to stimulate MMP-2 secretion by FLS, while the isotype control had no effect. Compared to untreated supernatants, pretreatment reduced the MMP-2 expression level induced by stimulation with supernatants from Brucella-infected THP-1 cells by 91.1% (Fig. 3C). The ability of the anti-TNF-α antibody to neutralize the stimulating effect of TNF-α on MMP-2 secretion was demonstrated by a parallel experiment in which FLS were stimulated with recombinant TNF-α. These results indicated that the inducing effect of supernatants from Brucella-infected monocytes on MMP-2 expression by FLS was mediated almost exclusively by TNF-α.
Culture supernatants from B. abortus-infected neutrophils induce MMP-2 production by FLS through TNF-α.
Similar to the results obtained with infected monocytes, supernatants from B. abortus-infected neutrophils induced MMP-2 secretion by uninfected FLS in a dose-dependent fashion (1/2 to 1/10) (Fig. 4A and B). In agreement with previous reports (9), significantly elevated levels of TNF-α and IL-1β were detected in culture supernatants from human neutrophils at 24 h after exposure to live B. abortus S2308 cells (MOI of 25 to 100) (not shown). Therefore, to test the participation of TNF-α in the stimulating effect of such supernatants on MMP-2 production by FLS, we used the same experimental approach as that described in the previous section for supernatants from infected monocytes.
Fig. 4.
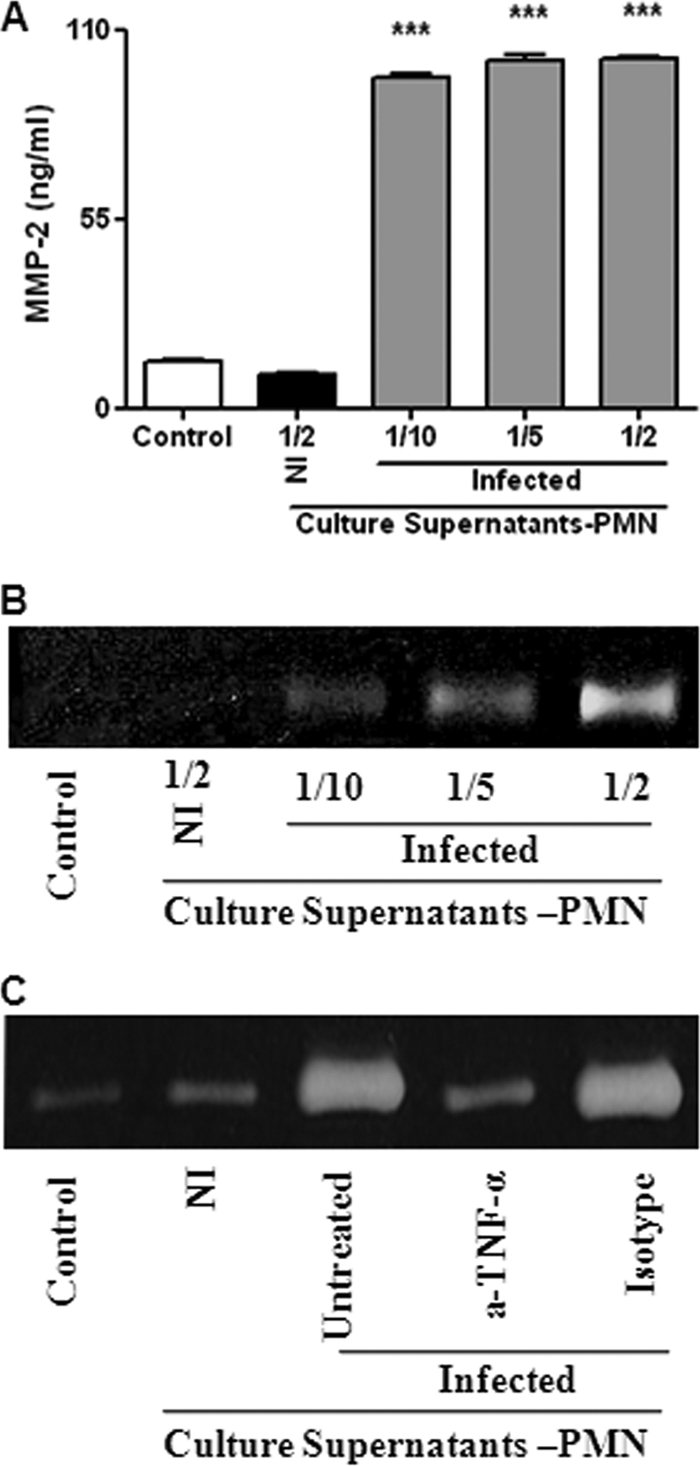
MMP-2 production by FLS upon stimulation with culture supernatants from B. abortus-infected neutrophils. (A and B) FLS were stimulated with supernatants from infected neutrophils (added at a 1/2, 1/5, or 1/10 proportion) or from noninfected (NI) neutrophils (at a 1/2 proportion), and MMP-2 production was determined by ELISA (A) and zymography (B). Data shown are from a representative experiment of five performed. ***, P < 0.001 versus control. (C) This stimulating effect was inhibited by the pretreatment of supernatants with a neutralizing antibody to TNF-α for 1 h before addition to FLS.
As shown in Fig. 4C, pretreatment with an anti-TNF-α neutralizing antibody reduced markedly the ability of supernatants from Brucella-infected neutrophils to stimulate MMP-2 secretion by FLS, while the isotype control had no effect. These results suggest that TNF-α is the main mediator of the inducing effect of supernatants from Brucella-infected neutrophils on MMP-2 expression by FLS.
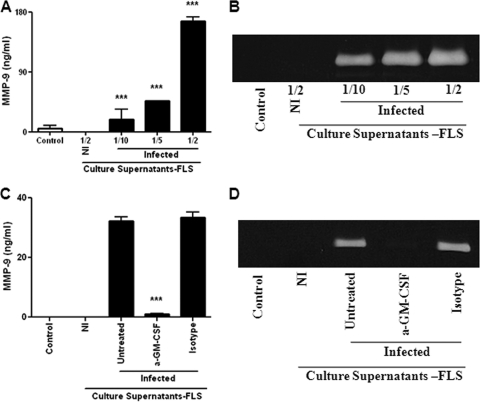
Culture supernatants from Brucella-infected FLS induce MMP-9 production by monocytes through GM-CSF.
As mentioned above, monocytes and neutrophils could be attracted to the site of infection in response to chemokines produced by Brucella-infected FLS. Upon such recruitment, cytokines produced by the latter cells may have several effects on the attracted phagocytes, including the induction of MMP secretion. Therefore, we evaluated the production of MMP-9 by noninfected monocytes upon stimulation with culture supernatants from Brucella-infected FLS. Supernatants added at different proportions induced a significant production of MMP-9 by THP-1 monocytes compared to unstimulated cultures (Fig. 5A and B). In contrast, MMP-9 secretion was not stimulated by supernatants from noninfected FLS. Several cytokines and growth factors have been shown to stimulate MMP-9 production by monocytes, including IL-1β, TNF-α, and GM-CSF, and were candidates for mediators of the stimulating effect of synoviocyte culture supernatants (19, 26, 57). However, we have mentioned above that Brucella-infected FLS did not secrete IL-1β or TNF-α. In contrast, as shown in Fig. 2A, B. abortus-infected FLS secrete GM-CSF, which is known to stimulate MMP-9 production by monocytes (57). To evaluate whether GM-CSF may be involved in the ability of conditioned media from Brucella-infected FLS to stimulate MMP-9 in monocytes, these conditioned media were preincubated or not with an anti-GM-CSF monoclonal neutralizing antibody (or an isotype control) before being used to stimulate THP-1 cells. As shown in Fig. 5C and D, the neutralization of GM-CSF almost abolished the ability of FLS supernatants to stimulate MMP-9 secretion by monocytes, while the isotype control had no effect. These results indicated that the inducing effect of supernatants from Brucella-infected FLS on MMP-9 expression by monocytes was mediated almost exclusively by GM-CSF.
Fig. 5.
MMP-9 production by monocytes upon stimulation with supernatants from B. abortus-infected FLS. (A and B) Monocytes were stimulated with supernatants from infected FLS (added at a 1/2, 1/5, or 1/10 proportion) or from noninfected (NI) FLS (added at a 1/2 proportion), and MMP-9 production was determined by ELISA (A) and zymography (B). Data shown are from a representative experiment of five performed. ***, P < 0.001 versus the control. (C and D) Inhibition of the stimulating effect of culture supernatants by preincubation with a neutralizing antibody to GM-CSF. Culture supernatants from infected FLS were incubated with the neutralizing antibody or with an isotype control for 1 h before addition to monocytes. MMP-9 production by the latter cells was determined by ELISA (C) and zymography (D). Data shown are from a representative experiment of five performed. ***, P < 0.001 versus untreated supernatants.
Culture supernatants from infected FLS induce MMP-9 production by neutrophils through IL-6.
Experiments were conducted to assess whether supernatants from B. abortus-infected FLS were able to induce MMP-9 secretion by neutrophils. The addition of supernatants from infected FLS at different proportions (1/2 to 1/10) to uninfected neutrophils induced a significant secretion of MMP-9 by the latter cells compared to that in unstimulated cultures. In contrast, MMP-9 secretion was not induced when neutrophils were stimulated with supernatants from noninfected FLS (Fig. 6A and B).
Fig. 6.
(A and B) MMP-9 production by neutrophils stimulated with supernatants from B. abortus-infected FLS (added at a 1/2, 1/5, or 1/10 proportion) or from noninfected (NI) FLS, as determined by ELISA (A) and zymography (B). Data shown are from a representative experiment of five performed. ***, P < 0.001 versus the control. (C) The stimulating effect was not inhibited by the pretreatment of supernatants with different doses of a neutralizing antibody to GM-CSF or with an isotype control for 1 h before addition to monocytes, as determined by zymography. (D) MMP-9 production by neutrophils was inhibited by the pretreatment of FLS supernatants with a neutralizing antibody to IL-6 for 1 h before addition to neutrophils, while an isotype control had no effect.
IL-1β and TNF-α are well-known inducers of MMPs in neutrophils, but as mentioned above, these cytokines are not produced by Brucella-infected FLS and were thus ruled out as mediators of the inducing effect described above. Since GM-CSF and IL-6 are also known to induce MMP production by different cell types (8, 26, 46) and both factors are produced by Brucella-infected FLS (Fig. 2A and B), we decided to test their potential role as mediators of the MMP-9 induction by culture supernatants from infected FLS. Neutrophils were incubated in the presence of supernatants from Brucella-infected FLS preincubated or not for 1 h with an anti-GM-CSF monoclonal neutralizing antibody, an anti-IL-6 neutralizing antibody, or the corresponding isotype controls. As shown in Fig. 6C, the neutralization of GM-CSF did not inhibit the induction of MMP-9 in neutrophils by supernatants from infected FLS, even when we used an anti-GM-CSF concentration higher than that suggested to neutralize completely the GM-CSF concentration detected by ELISA in supernatants from B. abortus-infected FLS. In contrast, the neutralization of IL-6 in culture supernatants from Brucella-infected FLS partially inhibited the inducing effect of these supernatants on MMP-9 secretion by neutrophils (Fig. 6D), suggesting that IL-6 is involved in such an induction.
Brucella abortus-induced secretion of MMP-2 and cytokines in FLS is mediated by L-Omp19 recognition through TLR-2.
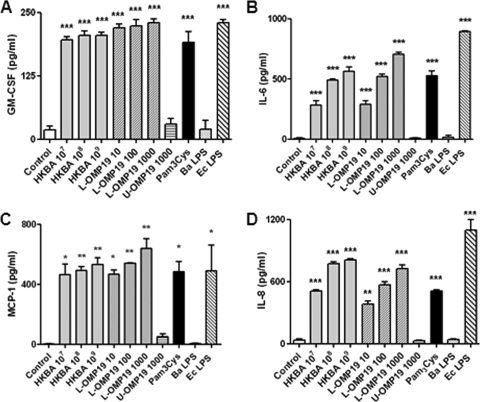
Next, we assessed whether viable B. abortus cells are necessary to induce GM-CSF, IL-6, IL-8, MCP-1, and MMP-2 secretion by FLS or, alternatively, whether secretion can be induced by structural components of the bacterium. To accomplish this goal, we examined the role of specific bacterial components and heat-killed B. abortus S2308 (HKBA).
As shown in Fig. 7, FLS stimulated with HKBA produced elevated levels of GM-CSF, IL-6, IL-8, and MCP-1, in general in a dose-dependent fashion, indicating that the secretion of these factors can be stimulated by a structural component of B. abortus. Since we previously found that B. abortus lipoproteins induce cytokine, chemokine, and MMP secretion in different cell types (13, 14, 41, 59), we hypothesized that lipoproteins could also mediate such effects in FLS.
Fig. 7.
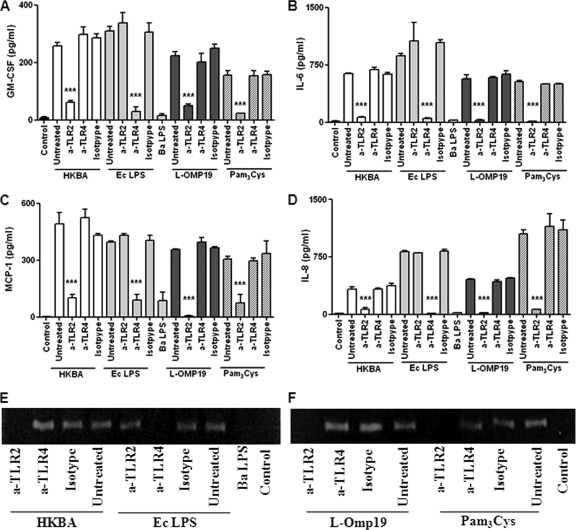
Cytokine and chemokine production by FLS stimulated with B. abortus antigens and TLR agonists. Cells were stimulated with heat-killed B. abortus (HKBA) (1 × 107 to 1 × 109 bacteria/ml), E. coli LPS (Ec LPS) (100 ng/ml), B. abortus LPS (Ba LPS) (1,000 ng/ml), L-Omp19 (10 ng/ml, 100 ng/ml, or 1,000 ng/ml), U-Omp19 (1,000 ng/ml), or Pam3Cys (50 ng/ml) or were not stimulated (control), and the levels of GM-CSF (A), IL-6 (B), MCP-1 (C), and IL-8 (D) were determined by ELISA of culture supernatants. Data shown are from a representative experiment of five performed. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (versus the control).
To test this hypothesis, we used recombinant L-Omp19 as a Brucella lipoprotein model. FLS were incubated with L-Omp19, and culture supernatants were harvested 48 h later to measure the secretion of cytokines and chemokines by ELISA. As shown in Fig. 7, L-Omp19 induced the secretion of GM-CSF, IL-6, IL-8, and MCP-1, in general in a dose-dependent fashion, and a significant production of all these factors was seen with as little as 10 ng/ml of L-Omp19. In all cases induction was dependent on the lipidation of L-Omp19, as U-Omp19 failed to upregulate the expression of the factors evaluated, even when it was used at a concentration of 1,000 ng/ml. To ascertain whether the effects elicited by L-Omp19 could be extended to all B. abortus lipoproteins, the production of cytokines was also evaluated in FLS incubated with a synthetic lipohexapeptide (Pam3Cys) that mimics the structure of the lipoprotein lipid moiety. As shown in Fig. 7, Pam3Cys also stimulated cytokine and chemokine expression by FLS. These results indicate that the Pam3-modified cysteine is the molecular structure of L-Omp19 that induces GM-CSF, IL-6, IL-8, and MCP-1 upregulation. At variance with the results obtained with L-Omp19, B. abortus LPS did not induce cytokine or chemokine production, even when used at high doses (1,000 ng/ml), while LPS from E. coli used at 100 ng/ml elicited the secretion of high levels of all these factors (Fig. 7).
We have previously demonstrated that TLR2 mediates responses to HKBA and B. abortus lipoproteins in different cell types (14, 41, 59). Consequently, we analyzed the role of TLR2 in the induction of cytokine and chemokine secretion from FLS in response to HKBA and L-Omp19. In addition, we evaluated whether TLR2-mediated stimulation with HKBA and lipoproteins can also induce MMP-2 secretion by these cells. FLS were preincubated with anti-TLR2 or anti-TLR4 antibodies or the corresponding isotype controls and then cultured with HKBA or L-Omp19. E. coli LPS and Pam3Cys were used as control agonists for TLR4 and TLR2 evaluations, respectively. The production of cytokines (Fig. 8A to D) and MMP-2 (Fig. 8E and F) in culture supernatants was evaluated. The preincubation of FLS with anti-TLR4 had no effect on the production of GM-CSF, IL-6, IL-8, MCP-1, and MMP-2 in response to HKBA or L-Omp19 but, as expected, significantly blocked the production of these factors in response to E. coli LPS. In contrast, preincubation with the anti-TLR2 antibody inhibited significantly the production of all evaluated factors induced by Pam3Cys, L-Omp19, and HKBA. Isotype controls had no effect on any of the responses investigated. These results indicate that, in FLS, the secretion of cytokines, chemokines, and MMP-2 induced by B. abortus depends on ligand recognition by TLR2. In addition, our results strongly suggest that the TLR2 ligands on HKBA are lipoproteins. In contrast, B. abortus LPS, which signals through TLR4, is not involved in the induction of cytokines, chemokines, or MMP-2 secretion by FLS in response to HKBA.
Fig. 8.
TLR2 dependency of cytokine, chemokine, and MMP-2 expressions induced in FLS by HKBA and L-Omp19. Cells were incubated with anti-TLR2 or anti-TLR4 blocking antibodies or an isotype control for 30 min before the addition of heat-killed B. abortus (HKBA) (1 × 107 bacteria/ml), E. coli LPS (Ec LPS) (100 ng/ml), L-Omp19 (100 ng/ml), or Pam3Cys (50 ng/ml). Culture supernatants were harvested 48 h later to assess the levels of GM-CSF (A), IL-6 (B), MCP-1 (C), and IL-8 (D) by ELISA, and MMP-2 expression was assessed by zymography (E and F). Data shown are from a representative experiment of five performed. ***, P < 0.001 versus anti-TLR2 or anti-TLR4 treatment, whichever is applicable.
Supernatants from Brucella-infected FLS and neutrophils cleave gelatin in the fluid phase.
In vivo, the activity of MMPs is counterbalanced by the activity of inhibitors, including those belonging to the tissue inhibitor of metalloproteinage (TIMP) family (5). Therefore, the net gelatinase or collagenase activity in a complex sample, such as culture supernatants, depends on the balance between MMP and TIMP activities. This net activity is not revealed by zymographic methods, since MMP-TIMP complexes may dissociate during gel electrophoresis. To assess whether an increased net gelatinase activity is generated by Brucella-infected FLS or neutrophils, culture supernatants from these cells were incubated with a nonfluorescent gelatin-fluorescein conjugate, and the fluorescence unmasked as a consequence of gelatin degradation was measured with a fluorometer. As shown in Fig. 9, the enzymatic activity (measured as fluorescence) increased significantly in supernatants of Brucella-infected FLS or neutrophils compared to uninfected cells, and the increase was MOI dependent. These results showed that Brucella infection leads to a net increase of gelatinase activity in the vicinity of infected FLS or neutrophils.
Fig. 9.
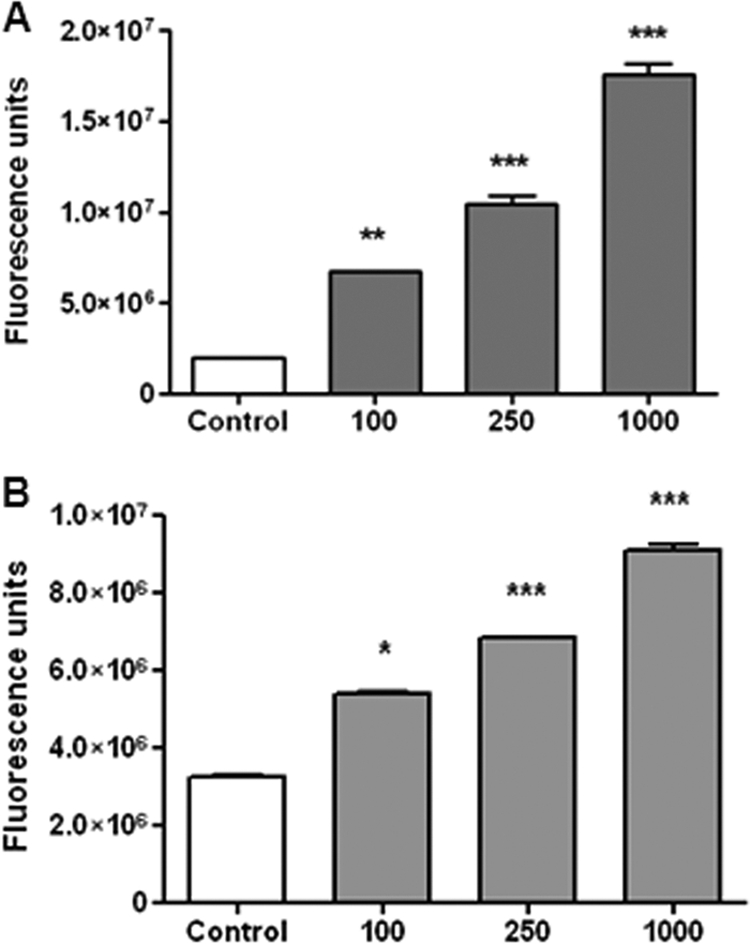
MMP-2 and MMP-9 activities in culture supernatants from B. abortus-infected FLS (A) and neutrophils (B) under native conditions. Supernatants were incubated with a fluorescein-conjugated gelatin substrate (DQ; Invitrogen) that produces highly fluorescent peptides when gelatin is digested. Data are expressed in fluorescence units informed by the fluorometer. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (versus uninfected cells [control]).
B. abortus and L-Omp19 induce inflammation in the knee joints of BALB/c mice.
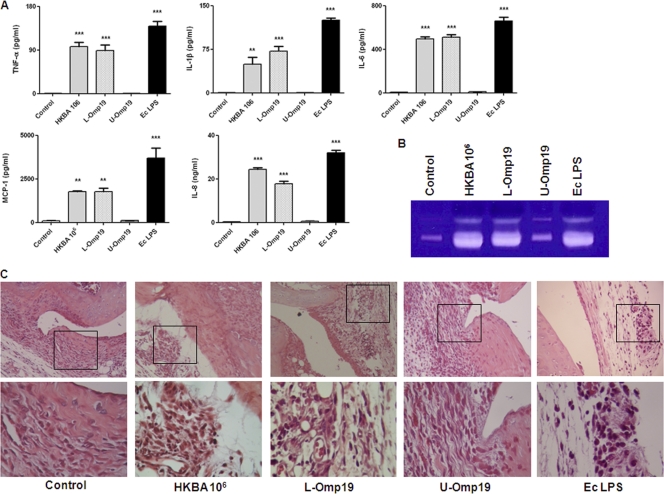
Our hypothesis is that B. abortus organisms that have access to the joint can cause inflammation and that this inflammatory response may lead to articular damage. To corroborate our hypothesis, HKBA, L-Omp19, and U-Omp19 were injected into the knee joints of mice, and animals were sacrificed 5 days later. Joints were cut, homogenized, and extracted with PBS for cytokine and MMP determinations. As shown in Fig. 10A, levels of TNF-α, IL-1β, IL-6, MCP-1, and IL-8 were significantly increased in the joints of mice injected with HKBA, L-Omp19, or E. coli LPS (positive control) compared to joints injected with PBS (negative control). In contrast, the levels of these cytokines were not increased in mice injected with U-Omp19. As revealed by zymography (Fig. 10B), the expression levels of MMP-2 and MMP-9 were increased in the joints of mice injected with HKBA or L-Omp19 compared to PBS controls but remained unchanged in mice injected with U-Omp19.
Fig. 10.
Inflammatory phenomena in knee joints from mice intra-articularly injected with HKBA (1 × 106 bacteria/ml), L-Omp19 (100 ng/ml), U-Omp19 (1,000 ng/ml), E. coli LPS (100 ng/ml), or PBS (control). Mice were killed at 5 days poststimulation, and knee joints were separated to prepare tissue homogenates or for histological evaluation. (A and B) Extracts from tissue homogenates were used to measure cytokine and chemokine levels by ELISA (A), and MMP expression was determined by zymography (B). (C) Histopathological examination of representative joints. The top panels show images taken at the original magnification (×100), and the bottom panels show a detail of perisynovial areas selected to document the presence or absence of leukocytic infiltrates (magnification, ×400).
Articular sections were analyzed after eosin-hematoxylin staining. As shown in Fig. 10C, joints inoculated with HKBA, L-Omp19, or E. coli LPS exhibited a mixed inflammatory infiltrate (neutrophils and monocytes), vasodilation, stasis, edema, and synovial hyperplasia, whereas those inoculated with U-Omp19 or PBS showed only an extravasation of red cells due to the injection procedure. No recruitment of any type of leukocyte was observed for the contralateral joints of animals injected with the antigens (not shown) or for any joint of animals injected with PBS (Fig. 10C). These results indicate that the presence of B. abortus within the joint can induce an inflammatory response that leads to a neutrophilic and monocytic infiltrate.
DISCUSSION
Fibroblast-like synoviocytes (FLS) have been recognized as central mediators of joint damage in inflammatory arthritides of either infectious or noninfectious origins. Besides their direct pathogenic role due to the production of MMPs that degrade collagen, FLS may produce several cytokines, chemokines, and growth factors that may mediate the recruitment of leukocytes to the synovium and their subsequent activation. In agreement with this proposed role of FLS in arthritides, in the present study we show that FLS respond to B. abortus infection with the production of MMP-2 and proinflammatory mediators and that the latter can promote the transmigration of monocytes and neutrophils. Moreover, we show that reciprocal interactions may be established between FLS and phagocytic cells, such that factors produced by each cell type in response to B. abortus infection induce the production of MMPs in the other cell type.
Although joint damage has been widely described for arthritis due to Brucella species, the pathogenic mechanisms underlying such lesions have not been investigated. Given the central role of FLS in destructive lesions of septic and nonseptic inflammatory arthritides, we hypothesized that these cells may also have an important role in lesions associated with brucellar arthritis. We found that B. abortus infects and replicates in human FLS and that such an infection induces these cells to produce MMP-2 and proinflammatory mediators. The ability of B. abortus to invade, survive, and replicate within FLS is in line with its capacity to replicate in other nonphagocytic cells, including epithelial cells, hepatocytes, and osteoblasts (9–11). To our knowledge, there are no reports about the ability of Brucella to infect human FLS in vivo. This may be explained in part by ethical restrictions, since a biopsy of the affected joint may be justified only in exceptional situations. Nevertheless, in the few published studies in which a culture of synovial membrane samples was performed, Brucella was isolated from such samples (6, 24). Importantly, cultures of synovial fluid were negative in these cases, suggesting that the bacterium was located intracellularly in the synovial membrane. In support of this hypothesis, foci of intracytoplasmic granular reactions were detected by immunofluorescence with anti-Brucella antibodies in the synovial surface of joints obtained from cattle experimentally infected with B. abortus S19 (22, 55). Interestingly, FLS are located in the intimal layer of the synovium, which agrees with the “synovial surface” location described in that study.
In spite of the proposed role of FLS in the articular damage of septic arthritis, the ability of bacteria to infect FLS and elicit an inflammatory response or the secretion of MMPs in these cells has been scarcely evaluated (18, 31, 45). The present study demonstrates that FLS respond to B. abortus infection with the production of MMP-2 and proinflammatory mediators, which agrees with the findings of those previous studies. As mentioned above, a proposed pathogenic role of FLS in inflammatory arthritides is the degradation of articular cartilage through the secretion of MMPs. It is well known that in vivo, the activity of MMPs is regulated by natural inhibitors called TIMPs. Since such an inhibition may not be evidenced by zymography or capture ELISA, we assessed the gelatinase activity of culture supernatants from Brucella-infected FLS under native conditions by using a fluorometric method. This assay demonstrated the presence of a net gelatinase activity in these supernatants, suggesting that the MMP-2 induction detected in Brucella-infected FLS may contribute to collagen degradation.
The chemokines MCP-1 and IL-8 were among the inflammatory mediators detected in supernatants from Brucella-infected FLS, which is especially interesting because one of the proposed roles of FLS in inflammatory arthritides is the recruitment of leukocytes through the secretion of chemokines. Notably, in line with this hypothesis, we found that culture supernatants from Brucella-infected FLS induce the migration of monocytes and neutrophils. Both phagocytic types have been implicated in the inflammatory reaction of brucellar arthritis. According to Madkour (29) the synovial membrane may show noncaseating granulomata, but the most frequent finding is a nonspecific inflammatory change. Khateeb et al. described diffuse nonspecific inflammation with scarce granulomatous fibrous deposits (25). In a case affecting the carpus, Seal and Morris described the presence of a mixed infiltrate with a predominance of lymphocytes, plasma cells, and histiocytes and smaller numbers of polymorphonuclear leukocytes (42). The mixed nature of the infiltrate may be the result of a dynamic inflammatory reaction during brucellar arthritis. In cattle experimentally infected with B. abortus S19, a marked inflammation dominated by neutrophils was observed along the joint surface during the first 3 days postinfection, but this infiltrate evolved to a lymphocytic and histiocytic one by 14 days p.i. (22). In vivo, recruited neutrophils and monocytes may contribute to joint damage in at least two ways. First, these cells may phagocytize bacteria present in the infection focus and be activated to produce proinflammatory cytokines that in turn may induce further MMP secretion by FLS. In line with this hypothesis we found that culture supernatants from Brucella-infected monocytes or neutrophils induce MMP-2 production in FLS. As demonstrated by the use of neutralizing antibodies, the main inducer of MMP-2 secretion present in these supernatants was TNF-α. The preponderant role of TNF-α as an MMP inducer in FLS agrees with the findings of several previous studies (12, 32, 33, 53). It has been shown that TNF-α is upregulated in the joints of mice with S. aureus arthritis and that the local secretion of TNF-α in the joint cavity gives rise to an increased severity of arthritis (58). The second potential contribution of phagocytes to joint damage is through their secretion of MMPs upon activation by microbial molecules during phagocytosis or in response to stimulation with proinflammatory factors. We have previously shown that human monocytes and neutrophils produce MMP-9 in response to B. abortus infection (9, 41). In the present study we show that monocytes and neutrophils can also be stimulated to produce MMP-9 by soluble factors produced by Brucella-infected FLS. As shown by neutralization experiments with specific antibodies, these stimulating effects are mediated almost exclusively by GM-CSF in the case of monocytes and mediated at least partially by IL-6 in the case of neutrophils. Overall, these results suggest that a cytokine network may operate during articular infection by B. abortus in which infected FLS secrete chemokines that attract monocytes and neutrophils to the infection focus. Upon contact with the bacterium these phagocytes may secrete TNF-α, which activates MMP-2 secretion by FLS. Reciprocally, MMP-9 secretion may be activated in phagocytes by cytokines (GM-CSF and IL-6) produced by Brucella-infected FLS. In addition, both FLS and phagocytes may produce MMPs (MMP-2 and MMP-9, respectively) directly in response to B. abortus infection.
FLS express Toll-like receptors (TLR), particularly TLR2 (43), and therefore can produce cytokines and MMPs not only in response to infection but also in response to bacterial antigens (23, 27). In line with this last property we found that heat-killed B. abortus (HKBA) can stimulate cytokine and MMP-2 production by human FLS, clearly indicating that these FLS responses may be elicited by one or more structural components of B. abortus. Since Brucella lipoproteins induce the production of proinflammatory cytokines in several cell types (13, 14, 59) and can also induce MMP-9 production by human monocytes (41), we hypothesized that such lipoproteins could also stimulate the production of cytokines and MMP-2 in FLS. Using Omp19 as a model Brucella lipoprotein, we found that its lipidated version (L-Omp19), but not its unlipidated form, induced the production of proinflammatory cytokines and MMP-2 by FLS. The same effect was observed for Pam3Cys, a synthetic lipohexapeptide that mimics the core structure of bacterial lipoproteins. In contrast, high doses of B. abortus LPS did not induce these responses. The actual fatty acid composition of the Omp19 lipoprotein has not been determined. However, it has been shown that the repertoire of fatty acids in a native Brucella lipoprotein analogous to Braun's lipoprotein is similar to that of its E. coli counterpart, including mainly palmitic acid and lower proportions of stearic, oleic, and myristic acids (16). This similarity in lipidation between both bacterial species may extend to other lipoproteins, since the molecular machinery used for the lipidation of Braun's lipoprotein is the same as that used for the lipidation of other lipoproteins. Therefore, also in the case of Omp19, the repertoire of fatty acids of the recombinant lipoprotein obtained from E. coli would probably be similar to that of the native Brucella lipoprotein, including palmitic acid as its main component. The ability of Omp19 to incorporate palmitic acid was demonstrated previously by Tibor et al. (49).
As mentioned above, FLS are known to express TLR, particularly TLR2. In agreement with previous studies that showed that TLR2 mediates responses to HKBA and B. abortus lipoproteins in different cell types (14, 41, 59), we found that the secretion of cytokines, chemokines, and MMP-2 by FLS in response to HKBA and L-Omp19 depends on ligand recognition by TLR2 but not by TLR4. In addition, our results strongly suggest that the TLR2 ligands on HKBA are lipoproteins.
Based on the results mentioned above, our hypothesis is that when B. abortus organisms reach the joint, the bacterial lipoproteins can stimulate FLS to produce inflammatory mediators and MMP-2. In addition, chemokines produced by infected FLS may attract phagocytes to the infection focus, which can also produce MMP-9 in response to bacterial antigens or to soluble factors produced by infected FLS. A mouse model of intra-articular injection was used to determine whether the upregulation of proinflammatory mediators and MMPs in response to Brucella antigens observed in vitro also takes place in vivo. Notably, levels of TNF-α, IL-1β, IL-6, MCP-1, IL-8, MMP-2, and MMP-9 were significantly increased in the knee joints of mice injected with HKBA or L-Omp19 (but not in mice injected with U-Omp19), thus demonstrating in vivo that the presence of Brucella antigens in articular tissues induces the local production of proinflammatory mediators and MMPs. Moreover, the upregulation of proinflammatory mediators in response to HKBA or L-Omp19 was accompanied by the appearance of a mixed inflammatory infiltrate in the synovium. The local upregulation of proinflammatory cytokines and MMPs agrees with previous findings in mouse models of septic arthritis by staphylococci, streptococci, and Borrelia burgdorferi (7, 50, 58) and also agrees with our own findings for a human case of brucellar septic bursitis (52).
In summary, this study demonstrates that B. abortus can infect FLS and induce these cells to produce MMP-2 and proinflammatory cytokines and chemokines, that these effects can be also elicited by the stimulation of FLS with B. abortus antigens, and that FLS-derived chemokines can mediate the migration of monocytes and neutrophils. The study also shows that a network of cytokine-mediated reciprocal stimulations can be established between FLS and phagocytes in response to B. abortus infection, leading to an enhanced secretion of MMP-2 and MMP-9 by these cell types. An increased production of these MMPs in articular tissues in response to B. abortus antigens was demonstrated in mice in vivo. Overall, these results suggest that FLS may have an important role in the pathogenesis of brucellar arthritis by producing MMPs and also by attracting phagocytes and inducing them to produce MMPs in the joint.
ACKNOWLEDGMENTS
We thank the staff of the Centro Nacional de Referencia del Sida, University of Buenos Aires, for their assistance with biosafety level 3 laboratory use.
This work was supported by grants PICT2006-0517, PICT2006-1335, PICT2007-0139, and PICT2008-0764 from the Agencia Nacional of Promoción Científica y Tecnológica (ANPCYT, Argentina) and by grant PIP112-200801-02706 from the Consejo Nacional de Investigación Científica y Tecnológica (CONICET). R.S. is recipient of a fellowship from ANPCYT. M.V.D., P.B., G.H.G., C.A.F., and P.C.B. are members of the Research Career of CONICET. C.A.F. is also a member of the Facultad de Ciencias Exactas, Universidad Nacional de La Plata.
Footnotes
Published ahead of print on 5 July 2011.
REFERENCES
- 1. Aydin M., et al. 2005. Scintigraphic findings in osteoarticular brucellosis. Nucl. Med. Commun. 26:639–647 [DOI] [PubMed] [Google Scholar]
- 2. Bartok B., Firestein G. S. 2010. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233:233–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bosilkovski M., Krteva L., Caparoska S., Dimzova M. 2004. Osteoarticular involvement in brucellosis: study of 196 cases in the Republic of Macedonia. Croat. Med. J. 45:727–733 [PubMed] [Google Scholar]
- 4. Bremell T., Abdelnour A., Tarkowski A. 1992. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect. Immun. 60:2976–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brinckerhoff C. E., Matrisian L. M. 2002. Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 3:207–214 [DOI] [PubMed] [Google Scholar]
- 6. Coventry M. B., et al. 1949. Infection of the hip by Brucella suis. JAMA 141:320–325 [DOI] [PubMed] [Google Scholar]
- 7. Crandall H., et al. 2006. Gene expression profiling reveals unique pathways associated with differential severity of Lyme arthritis. J. Immunol. 177:7930–7942 [DOI] [PubMed] [Google Scholar]
- 8. Dasu M. R., Barrow R. E., Spies M., Herndon D. N. 2003. Matrix metalloproteinase expression in cytokine stimulated human dermal fibroblasts. Burns 29:527–531 [DOI] [PubMed] [Google Scholar]
- 9. Delpino M. V., Barrionuevo P., Scian R., Fossati C. A., Baldi P. C. 2010. Brucella-infected hepatocytes mediate potentially tissue-damaging immune responses. J. Hepatol. 53:145–154 [DOI] [PubMed] [Google Scholar]
- 10. Delpino M. V., Fossati C. A., Baldi P. C. 2009. Proinflammatory response of human osteoblastic cell lines and osteoblast-monocyte interaction upon infection with Brucella spp. Infect. Immun. 77:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrero M. C., Fossati C. A., Baldi P. C. 2009. Smooth Brucella strains invade and replicate in human lung epithelial cells without inducing cell death. Microbes Infect. 11:476–483 [DOI] [PubMed] [Google Scholar]
- 12. Fuchs S., Skwara A., Bloch M., Dankbar B. 2004. Differential induction and regulation of matrix metalloproteinases in osteoarthritic tissue and fluid synovial fibroblasts. Osteoarthritis Cartilage 12:409–418 [DOI] [PubMed] [Google Scholar]
- 13. Garcia Samartino C., et al. 2010. Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis. Am. J. Pathol. 176:1323–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giambartolomei G. H., et al. 2004. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J. Immunol. 173:4635–4642 [DOI] [PubMed] [Google Scholar]
- 15. Gjertsson I., Innocenti M., Matrisian L. M., Tarkowski A. 2005. Metalloproteinase-7 contributes to joint destruction in Staphylococcus aureus induced arthritis. Microb. Pathog. 38:97–105 [DOI] [PubMed] [Google Scholar]
- 16. Gomez-Miguel M. J., Moriyon I. 1986. Demonstration of a peptidoglycan-linked lipoprotein and characterization of its trypsin fragment in the outer membrane of Brucella spp. Infect. Immun. 53:678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gotuzzo E., et al. 1982. Articular involvement in human brucellosis: a retrospective analysis of 304 cases. Semin. Arthritis Rheum. 12:245–255 [DOI] [PubMed] [Google Scholar]
- 18. Hanada H., Ikeda-Dantsuji Y., Naito M., Nagayama A. 2003. Infection of human fibroblast-like synovial cells with Chlamydia trachomatis results in persistent infection and interleukin-6 production. Microb. Pathog. 34:57–63 [DOI] [PubMed] [Google Scholar]
- 19. Heidinger M., Kolb H., Krell H. W., Jochum M., Ries C. 2006. Modulation of autocrine TNF-alpha-stimulated matrix metalloproteinase 9 (MMP-9) expression by mitogen-activated protein kinases in THP-1 monocytic cells. Biol. Chem. 387:69–78 [DOI] [PubMed] [Google Scholar]
- 20. Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. 1985. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J. Biol. Chem. 260:2493–2500 [PubMed] [Google Scholar]
- 21. Jeng G. W., et al. 1997. Measurement of synovial tumor necrosis factor-alpha in diagnosing emergency patients with bacterial arthritis. Am. J. Emerg. Med. 15:626–629 [DOI] [PubMed] [Google Scholar]
- 22. Johnson B., Mosier D. A., Morton R. J., Confer A. W. 1994. Experimental Brucella abortus strain 19 arthritis in young cattle. J. Vet. Diagn. Invest. 6:56–61 [DOI] [PubMed] [Google Scholar]
- 23. Kanangat S., et al. 2006. Induction of multiple matrix metalloproteinases in human dermal and synovial fibroblasts by Staphylococcus aureus: implications in the pathogenesis of septic arthritis and other soft tissue infections. Arthritis Res. Ther. 8:R176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kelly P. J., Martin W. J., Schirger A., Weed L. A. 1960. Brucellosis of the bones and joints. Experience with thirty-six patients. JAMA 174:347–353 [DOI] [PubMed] [Google Scholar]
- 25. Khateeb M. I., Araj G. F., Majeed S. A., Lulu A. R. 1990. Brucella arthritis: a study of 96 cases in Kuwait. Ann. Rheum. Dis. 49:994–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kohno Y., et al. 2004. GM-CSF activates RhoA, integrin and MMP expression in human monocytic cells. Pathol. Int. 54:693–702 [DOI] [PubMed] [Google Scholar]
- 27. Kyburz D., et al. 2003. Bacterial peptidoglycans but not CpG oligodeoxynucleotides activate synovial fibroblasts by Toll-like receptor signaling. Arthritis Rheum. 48:642–650 [DOI] [PubMed] [Google Scholar]
- 28. Madkour M. M., Sharif H. 2001. Bone and joint imaging, p. 90–132In Madkour M. M.(ed.), Madkour's brucellosis, 2nd ed. Springer-Verlag, Berlin, Germany [Google Scholar]
- 29. Madkour M. M. 2001. Osteoarticular brucellosis, p. 74–84In Madkour M. M.(ed.), Madkour's brucellosis, 2nd ed. Springer-Verlag, Berlin, Germany [Google Scholar]
- 30. McNearney T. A., et al. 2010. A peripheral neuroimmune link: glutamate agonists upregulate NMDA NR1 receptor mRNA and protein, vimentin, TNF-alpha, and RANTES in cultured human synoviocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298:R584–R598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyer-Bahlburg A., et al. 2004. Yersinia enterocolitica leads to transient induction of TNF-alpha and activates NF-kappaB in synovial fibroblasts. Clin. Exp. Rheumatol. 22:278–284 [PubMed] [Google Scholar]
- 32. Migita K., et al. 1996. TNF-alpha-mediated expression of membrane-type matrix metalloproteinase in rheumatoid synovial fibroblasts. Immunology 89:553–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mor A., Abramson S. B., Pillinger M. H. 2005. The fibroblast-like synovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction. Clin. Immunol. 115:118–128 [DOI] [PubMed] [Google Scholar]
- 34. Murphy G., Nagase H. 2008. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat. Clin. Pract. Rheumatol. 4:128–135 [DOI] [PubMed] [Google Scholar]
- 35. Nade S. 2003. Septic arthritis. Best Pract. Res. Clin. Rheumatol. 17:183–200 [DOI] [PubMed] [Google Scholar]
- 36. Nagase H., Visse R., Murphy G. 2006. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 69:562–573 [DOI] [PubMed] [Google Scholar]
- 37. Noss E. H., Brenner M. B. 2008. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol. Rev. 223:252–270 [DOI] [PubMed] [Google Scholar]
- 38. Osiri M., Ruxrungtham K., Nookhai S., Ohmoto Y., Deesomchok U. 1998. IL-1beta, IL-6 and TNF-alpha in synovial fluid of patients with non-gonococcal septic arthritis. Asian Pac. J. Allergy Immunol. 16:155–160 [PubMed] [Google Scholar]
- 39. Pappas G., Akritidis N., Bosilkovski M., Tsianos E. 2005. Brucellosis. N. Engl. J. Med. 352:2325–2336 [DOI] [PubMed] [Google Scholar]
- 40. Pierer M., et al. 2004. Chemokine secretion of rheumatoid arthritis synovial fibroblasts stimulated by Toll-like receptor 2 ligands. J. Immunol. 172:1256–1265 [DOI] [PubMed] [Google Scholar]
- 41. Scian R., et al. 2011. Granulocyte-macrophage colony-stimulating factor- and tumor necrosis factor alpha-mediated matrix metalloproteinase production by human osteoblasts and monocytes after infection with Brucella abortus. Infect. Immun. 79:192–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seal P. V., Morris C. A. 1974. Brucellosis of the carpus. Report of a case. J. Bone Joint Surg. Br. 56:327–330 [PubMed] [Google Scholar]
- 43. Seibl R., et al. 2003. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am. J. Pathol. 162:1221–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shirtliff M. E., Mader J. T. 2002. Acute septic arthritis. Clin. Microbiol. Rev. 15:527–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singh S. K., Morbach H., Nanki T., Girschick H. J. 2005. Differential expression of chemokines in synovial cells exposed to different Borrelia burgdorferi isolates. Clin. Exp. Rheumatol. 23:311–322 [PubMed] [Google Scholar]
- 46. Sundararaj K. P., et al. 2009. Interleukin-6 released from fibroblasts is essential for up-regulation of matrix metalloproteinase-1 expression by U937 macrophages in coculture: cross-talking between fibroblasts and U937 macrophages exposed to high glucose. J. Biol. Chem. 284:13714–13724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suzuki M., Hashizume M., Yoshida H., Shiina M., Mihara M. IL-6 and IL-1 synergistically enhanced the production of MMPs from synovial cells by up-regulating IL-6 production and IL-1 receptor I expression. Cytokine 51:178–183 [DOI] [PubMed] [Google Scholar]
- 48. Tarkowski A., et al. 2002. Current status of pathogenetic mechanisms in staphylococcal arthritis. FEMS Microbiol. Lett. 217:125–132 [DOI] [PubMed] [Google Scholar]
- 49. Tibor A., Decelle B., Letesson J. J. 1999. Outer membrane proteins Omp10, Omp16, and Omp19 of Brucella spp. are lipoproteins. Infect. Immun. 67:4960–4962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tissi L., et al. 1999. Role of tumor necrosis factor alpha, interleukin-1beta, and interleukin-6 in a mouse model of group B streptococcal arthritis. Infect. Immun. 67:4545–4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Varani K., et al. P2X(1) and P2X(3) purinergic receptors differentially modulate the inflammatory response in human osteoarthritic synovial fibroblasts. Cell. Physiol. Biochem. 25:325–336 [DOI] [PubMed] [Google Scholar]
- 52. Wallach J. C., et al. 2010. Prepatellar bursitis due to Brucella abortus: case report and analysis of the local immune response. J. Med. Microbiol. 59:1514–1518 [DOI] [PubMed] [Google Scholar]
- 53. Wang Y., et al. 2010. Differential MMP-2 activity induced by mechanical compression and inflammatory factors in human synoviocytes. Mol. Cell. Biomech. 7:105–114 [PubMed] [Google Scholar]
- 54. Wright J. A., Nair S. P. Interaction of staphylococci with bone. Int. J. Med. Microbiol. 300:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wyn-Jones G., Baker J. R., Johnson P. M. 1980. A clinical and immunopathological study of Brucella abortus strain 19-induced arthritis in cattle. Vet. Rec. 107:5–9 [DOI] [PubMed] [Google Scholar]
- 56. Yamazaki T., Yokoyama T., Akatsu H., Tukiyama T., Tokiwa T. 2003. Phenotypic characterization of a human synovial sarcoma cell line, SW982, and its response to dexamethasone. In Vitro Cell. Dev. Biol. Anim. 39:337–339 [DOI] [PubMed] [Google Scholar]
- 57. Zhang Y., McCluskey K., Fujii K., Wahl L. M. 1998. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J. Immunol. 161:3071–3076 [PubMed] [Google Scholar]
- 58. Zhao Y. X., Ljungdahl A., Olsson T., Tarkowski A. 1996. In situ hybridization analysis of synovial and systemic cytokine mRNA expression in superantigen-mediated Staphylococcus aureus arthritis. Arthritis Rheum. 39:959–967 [DOI] [PubMed] [Google Scholar]
- 59. Zwerdling A., et al. 2008. Brucella lipoproteins mimic dendritic cell maturation induced by Brucella abortus. Microbes Infect. 10:1346–1354 [DOI] [PubMed] [Google Scholar]