Abstract
The poly-γ-d-glutamic acid (PGA) capsule is one of the major virulence factors of Bacillus anthracis, which causes a highly lethal infectious disease. The PGA capsule disguises B. anthracis from immune surveillance and allows its unimpeded growth in the host. The PGA capsule recently was reported to be associated with lethal toxin (LT) in the blood of experimentally infected animals (M. H. Cho, et al., Infect. Immun. 78:387-392, 2010). The effect of PGA, either alone or in combination with LT, on macrophages, which play an important role in the progression of anthrax disease, has not been thoroughly investigated. In this study, we investigated the effect of PGA on LT cytotoxicity using the mouse macrophage cell line J774A.1. PGA produced a concentration-dependent enhancement of the cytotoxicity of LT on J774A.1 cells through an enhancement in the binding and accumulation of protective antigen to its receptors. The increase of LT activity was confirmed using Western blot analysis, which showed that the combination of PGA and LT produced a greater degree of degradation of mitogen-activated protein kinase kinases and an increased level of the activation of the proform of caspase-1 to its processed form compared to the effects of LT alone. In addition, mice that received a tail vein injection of both PGA and LT had a significantly increased rate of death compared to that of mice injected with LT alone. PGA had no effect when added to cultures or administered to mice in the absence of LT. These results emphasize the importance of PGA in the pathogenesis of anthrax infection.
INTRODUCTION
Anthrax is a highly lethal infectious disease caused by the spore-forming bacterium Bacillus anthracis (22). After entering the host, anthrax spores are rapidly recognized and phagocytosed by antigen-presenting cells, such as macrophages and dendritic cells, and are carried to regional lymph nodes (7, 25). Normally, antigen-presenting cells act as the first line of defense against microbial pathogens by engulfing and killing infectious agents. Antigen-presenting cells also produce numerous inflammatory mediators that recruit and activate additional immune cells and stimulate adaptive immune responses (10). Anthrax spores can, however, survive within phagocytes and germinate into vegetative bacilli, multiply, and escape from the control of the innate immune system (24, 25). The vegetative form of B. anthracis then penetrates into the circulatory system by disrupting the phagocytes and secretes high levels of exotoxin, sheds capsule, and multiplies systemically, reaching 109 organisms/ml of blood (24, 25, 31). Because macrophages are used to bypass the host immune system during B. anthracis infection, it is important to define the interactions between macrophages and B. anthracis spores as well as the virulence factors of B. anthracis.
The major virulence factor of B. anthracis, exotoxin, is composed of three distinct proteins, protective antigen (PA), edema factor (EF), and lethal factor (LF), that are secreted individually as nontoxic monomers (22). The binding of LF or EF to PA results in the formation of active lethal toxin (LT) or edema toxin, respectively (22). LF is a metalloprotease that cleaves most isoforms of mitogen-activated protein kinase kinase (MAPKK) (36), and MAPKK plays an intermediate role in the activation of MAPK signaling pathways (35). LF promotes macrophage death by disrupting the MAPK-dependent pathways that regulate prosurvival genes (26, 35) and by activating proteasome- and inflammasome-dependent pathways that cleave NALP1b and activate caspase-1 (5, 12, 35). EF is a calmodulin-dependent adenylate cyclase that causes a prolonged increase in cytosolic cyclic AMP, which then triggers an efflux of fluid from the cell and localized edema (20). The EF-induced rise in cyclic AMP also inhibits phagocytosis, microbicidal activity, neutrophil chemotaxis, and superoxide production, and it thereby contributes to profound immune dysfunction (35).
B. anthracis contains another virulence factor, the capsule, which is composed of PGA (21). The weakly immunogenic and antiphagocytic PGA capsule disguises the bacilli from immune surveillance through a mechanism that is similar to that of the capsular polysaccharides that protect other pathogens, such as pneumococci and meningococci, from phagocytosis (21). The immunogenicity of PGA, like other T-cell-independent polysaccharide antigens, is enhanced when it is conjugated with other proteins, such as PA (9, 18, 29). A recent report showed that the capsule of B. anthracis activates caspase-1 and induces the release of interleukin-1β (IL-1β) from differentiated THP-1 cells and from human monocyte-derived dendritic cells (11). Another recent study showed that the capsule released from B. anthracis is associated with LT in the blood of experimentally infected animals (13). These results indicate that the PGA capsule is important in the pathogenesis of anthrax infection, but the combined effect of PGA and LT has not been studied.
In this study, we investigated the effect of PGA on LT-mediated cytotoxicity in both a mouse macrophage cell line (J774A.1) and BALB/c mice. We used PGA that was purified from the culture supernatant of Bacillus licheniformis strain ATCC 9945a (18, 29). PGA enhanced the cytotoxic effect of LT on J774A.1 cells in a concentration-dependent manner and also augmented the death of mice challenged with LT. Our experimental results indicate that PGA, in combination with LT, is important in anthrax pathogenesis and acts to intensify the toxemia that occurs at the terminal stage of anthrax infection.
MATERIALS AND METHODS
Cell lines and culture conditions.
A mouse macrophage-like cell line, J774A.1, was obtained from the American Type Culture Collection (ATCC; Manassas, VA). Cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco Life Technologies, Eggenstein, Germany) supplemented with 10% fetal bovine serum (FBS; Gibco Life Technologies), 1% penicillin-streptomycin (Biosource International, Camarillo, CA), 4 mM l-glutamine (Gibco Life Technologies), and 10 mM HEPES (Gibco Life Technologies) and were cultured at 37°C in a humidified incubator containing 5% CO2.
For PGA production, B. licheniformis ATCC 9945a, which produces a capsule composed of γ-linked glutamic acid residues (3), was used. The capsule produced by B. licheniformis ATCC 9945a has been used as a surrogate for B. anthracis in several studies (17, 18, 29). However, unlike B. anthracis, which has d-glutamic acid residues exclusively, B. licheniformis 9945a contains both d- and l-enantiomers according to culture conditions (3). In our experiment, E medium, which contains 2 mM MnCl2·4H2O, was used to stimulate the maximal production of PGA in the D isoform (18, 19, 33). Usually under these conditions, approximately 84% of the glutamic acid was d-enantiomer. PGA was purified from the supernatant of B. licheniformis cultures as described previously (18, 29). B. anthracis has been reported to produce a high-molecular-mass capsule of >100 kDa, which first is polymerized on the bacterial cell surface. It then is degraded to a lower-molecular-mass capsule of <14 kDa, resulting in the release of capsule from the bacterial cell surface into the culture supernatant (24). Since the average molecular mass of native PGA from B. licheniformis ATCC 9945a was approximately 500 kDa, PGA degraded to a molecular mass of less than 30 kDa as previously described (11, 18) was used for experiments.
Reagents.
PA, purified as described previously (30), was obtained from Green Cross (South Korea). LF was obtained from List Biological Laboratories (Campbell, CA). Antibodies against α-tubulin and MAPKK4 were purchased from Cell Signaling (Denvers, MA). Antibody against caspase-1 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against MAPKK2 was from BD Transduction Laboratories (San Jose, CA), and antibody against MAPKK6 was from Upstate Biotechnology (Charlottesville, VA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and HRP-conjugated goat anti-mouse IgG were purchased from Invitrogen (Carlsbad, CA) and GE Healthcare (Piscataway, NJ), respectively. Antibody against α1-Na+/K+-ATPase was from Abcam (Cambridge, United Kingdom).
Determination of the effect of PGA on LT-mediated cytotoxicity and on the LT-neutralizing effect of anti-PA antibody.
To evaluate the effect of PGA on LT cytotoxicity, we performed LT-mediated cytotoxicity experiments with J774A.1 cells (18, 30). Monolayers of J774A.1 cells in DMEM containing 10% FBS were cultured at 37°C in 96-well plates (SPL Plastic Labware, South Korea) up to a concentration of 1.5 × 105 cells/well. Prior to addition to the cells, PGA was diluted with DMEM (without or with 5% FBS) and added to a set of 96-well plates at final concentrations of 0 (medium alone), 31.3, 62.5, 125, 250, and 500 μg/ml. PA was added to the PGA dilutions at a final concentration of 0.5 μg/ml, and LF was added to the PGA dilutions at final concentrations of 0.00625, 0.0125, 0.025, 0.05, and 0.1 μg/ml. The mixtures were incubated for 1 h at 37°C before addition to J774A.1 cells. Medium was removed from the J774A.1 cell monolayers, and 100 μl of the mixtures containing PGA, PA, and LF was added to the cells. After a 4-h incubation at 37°C with 5% CO2, 100 μl of 3-[4,5-dimethylthylthiazol-2-yl]-2,5-dimethyl-tetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO) was added to each well at a final concentration of 0.5 mg/ml. After an additional 1-h incubation at 37°C, the J774A.1 cells were lysed by adding 100 μl of extraction buffer (90% isopropyl alcohol containing 25 mM HCl and 0.5% [wt/vol] SDS). Absorbance then was measured at 570 nm with an ELISA reader (Tecan, Männedorf, Switzerland). For each assay, controls consisted of four wells that received only LT and four wells that contained only culture medium. Each sample was tested in duplicate and averaged for analysis.
To determine the effects of PGA on the LT-neutralizing effects of antibodies against PA, serum containing anti-PA was produced in rabbits as described previously (18). The serum was diluted with DMEM without 10% FBS and added to the wells of 96-well plates containing PGA (at final concentrations of 0, 31.3, 62.5, 125, 250, and 500 μg/ml) and LT (which consisted of 0.5 μg/ml PA and 0.1 μg/ml LF). The plates were incubated at 37°C for 1 h before the mixtures were added to J774A.1 cells as described above.
The necrotic and apoptotic death of J774A.1 cells by PGA and LT was measured using an FC500 flow cytometer (Beckman Coulter, Krefeld, Germany) using a propidium iodide (PI) and annexin V staining kit (Invitrogen).
Measurement of binding of labeled PA to J774A.1 cells by flow-cytometric analysis.
Alexa Fluor 488-conjugated PA was produced by an Alexa labeling kit (Invitrogen). To analyze the effect of PGA on the binding of PA to receptors on J774A.1 cells, cultured J774A.1 cells were washed with phosphate-buffered saline (PBS), stained with Alexa Fluor 488-conjugated PA (1 μg/ml in PBS) in the presence of various concentrations of PGA (0, 31.3, 62.5, and 125 μg/ml in PBS), and cultured at 37°C for 30 min. After three washes with PBS, the stained cells were resuspended in PBS, and a flow-cytometric analysis was conducted using the FC500 flow cytometer.
Western blot analysis.
To prepare whole-cell lysates for Western blotting, 2 × 106 J774A.1 cells were inoculated in six-well plates (Nunc, Denmark) and cultured for 24 h at 37°C in a humidified incubator containing 5% CO2. Cells were incubated with LT (0.5 μg/ml PA and 0.1 μg/ml LF for PA-63 heptamer and caspase-1 analyses, 0.5 μg/ml PA and 0.05 μg/ml LF for MAPKK analysis) and PGA (0, 62.5, 125, 250, or 500 μg/ml) for the indicated times at 37°C. After incubation, cells were washed three times with ice-cold PBS. Cells were harvested and resuspended in 100 μl of 1× bacterial protein extraction solution (Intron, Seoul, South Korea) supplemented with 1× protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and incubated on ice for 30 min to produce whole-cell lysates. The cell lysates were clarified by microcentrifugation, and 30 μg of each clarified lysate was electrophoresed on a 12% NuPage Bis-Tris gel (Invitrogen). After electrophoresis, the separated proteins were transferred to 0.45-μm nitrocellulose membranes (Bio-Rad, Hercules, CA). Blots were blocked for 1 h in 100 mM Tris-HCl (pH 7.5), 0.9% NaCl, and 0.05% Tween 20 (TBS-T) containing 5% skim milk. Blots then were rinsed three times in TBS-T for 5 min and subsequently incubated with primary antibodies at dilutions specified by each manufacturer: rabbit anti-caspase-1, 1:1,000; rabbit anti-α-tubulin, 1:1,000; mouse anti-MAPKK2, 1:2,500; rabbit anti-MAPKK4, 1:1,000; rabbit anti-MAPKK6, 1:1,000; and mouse anti-α1-Na+/K+-ATPase, 1:5,000. HRP-conjugated goat anti-rabbit IgG (1:5,000) and HRP-conjugated goat anti-mouse IgG (1:5,000) were used as secondary antibodies. After three washes with TBS-T, the blots were developed with ECL substrate (Pierce, Rockford, IL) and exposed to X-ray film (Kodak XAR5; Eastman Kodak, Rochester, NY).
Mouse injection with LT and PGA.
To verify the effect of PGA in vivo, 6-week-old female BALB/c mice (Orient Bio Inc., Seoul, South Korea) received tail vein injections of PGA (200 or 500 μg) only or PGA with LT (which consisted of 48 μg PA and 20 μg LF). Previously, a mixture of PA (60 μg) and LF (25 μg) was determined as five times the 50% lethal dose (LD50) of LT in BALB/c mice by intravenous injections (28). The amount of LT used in our study is the equivalent of approximately four times the LD50 of LT in BALB/c mice (29). For the protection experiment with anti-PA, 0.5 ml of rabbit anti-PA serum was injected into the intraperitoneal cavities of mice before the tail vein injection of LT and 500 μg of PGA. Mice were observed for a period of 14 days. Animals that survived for 14 days after the challenge were considered survivors. The animal procedures were approved by the Institutional Animal Care and Use Committee at the Korea National Institute of Health.
Statistical analysis.
Differences in mean survival times after LT and/or PGA injections were determined by Kaplan-Meier log-rank tests using SAS software (SAS Institute Inc., Cary, NC). For other measures, the means ± standard deviations (SD) of treatment groups were compared to appropriate controls to determine statistical significance using a two-tailed Student's t test. Differences were considered significant if P < 0.05.
RESULTS
PGA treatment enhances the cytotoxic activity of LT on mouse macrophage cell line J774A.1.
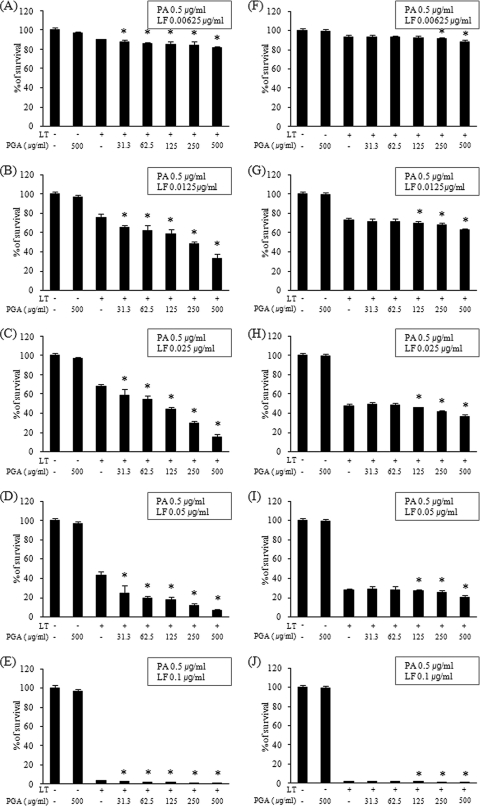
We determined the effect of PGA, which was isolated from the culture supernatant of B. licheniformis ATCC 9945a, on the cytotoxic activity of LT on J774A.1 cells. J774A.1 cells were treated with various concentrations of PGA and/or LT, and cell viability was assessed after 4-h incubations using the MTT assay. These assays were performed both in the absence and presence of 5% FBS, and the LT consisted of 0.5 μg/ml PA and 0.00625, 0.0125, 0.025, 0.05, or 0.1 μg/ml LF. The treatment of J774A.1 cells with LT caused cell death, and the rates of cell death increased with increasing LF concentrations (Fig. 1). The addition of PGA (0, 31.3, 62.5, 125, 250, or 500 μg/ml) enhanced the cytotoxic effects of LT in a concentration-dependent manner (Fig. 1). In assays performed without FBS, the addition of 500 μg/ml of PGA to LT containing 0.00625, 0.0125, 0.025, 0.05, or 0.1 μg/ml of LF significantly decreased cell viability by 9.4 (P = 0.019), 56.3 (P = 6.2 × 10−7), 77.6 (P = 3.4 × 10−10), 85.0 (P = 0.01), and 66.8% (P = 0.00003), respectively, compared to results for samples that did not contain PGA (Fig. 1A to E). The enhancement of LT-mediated cytotoxic activity by PGA also was observed in the presence of 5% FBS, although the enhancement effect was modest in the presence of FBS (5 to 28%) compared to effect in the absence of FBS (9 to 85%). The addition of 500 μg/ml of PGA to LT containing 0.00625, 0.0125, 0.025, 0.05, or 0.1 μg/ml of LF significantly decreased cell viability by 5.37 (P = 0.0004), 14.3 (P = 2.2 × 10−7), 22.7 (P = 0.00001), 27.9 (P = 0.003), and 21.1% (P = 0.01), respectively, compared to results for controls in the presence of 5% FBS (Fig. 1F to J). In all assay conditions, treatment with medium alone (control) or 500 μg/ml PGA alone (without LT) did not produce cytotoxic effects on J774A.1 cells (Fig. 1A to J). LF has been reported to promote macrophage apoptotic death by disrupting the MAPK-dependent pathways that regulate prosurvival genes (26, 35) and macrophage pyroptotic death by activating caspase-1 through inflammasome-dependent pathways (5, 12). Pyroptotic cell death features DNA fragmentation, the secretion of activated inflammatory cytokines, and the lytic release of inflammatory intracellular contents mediated by the formation of membrane pores (15). In our study, the PGA-induced increase in death of J774A.1 cells was confirmed by staining for PI and annexin V and subsequent flow-cytometric analysis (Fig. 2). Apoptotic cells were stained with annexin V only, and pyroptotic cells were stained with PI. Live cells were not stained. The treatment of J774A.1 cells with 250 μg/ml of PGA alone did not increase the proportion of dead cells compared to the proportion of dead cells with medium only (Fig. 2A and B), whereas the treatment of J774A.1 cells with 250 μg/ml PGA in combination with LT (consisting of 0.5 μg/ml PA and 0.1 μg/ml LF) increased the proportion of dead cells due to apoptosis and pyroptosis by 498.8% compared to results the levels for the medium-only control (P = 0.0001) and by 23.3% compared to results for cells treated with LT only (P = 0.004) (Fig. 2A and B). The addition of PGA of increasing concentrations resulted in significantly greater proportions of dead cells (Fig. 2A and B). These results showed that PGA enhances the cytotoxic effect, having both proapoptotic and propyroptotic activities of LT on J774A.1 cells in a concentration-dependent manner.
Fig. 1.
PGA enhances LT-mediated cytotoxicity in a concentration-dependent manner. The viability of J774A.1 cells was determined after a 4-h incubation with LT, which consisted of 0.5 μg/ml PA as well as 0.00625 (A and F), 0.0125 (B and G), 0.025 (C and H), 0.05 (D and I), and 0.1 (E and J) μg/ml LF in the absence (A, B, C, D, and E) or presence (F, G, H, I, and J) of 5% FBS. PGA was isolated from B. licheniformis ATCC 9945a and added to the LT at concentrations of 0, 31.3, 62.5, 125, 250, and 500 μg/ml before incubation with the J774A.1 cells. After the 4-h incubation, the viability of cells was determined using the MTT assay. The y axis represents the percentage of survival relative to control values, given as the means ± SD derived from three separate experiments. *, P < 0.05 versus LT without PGA control.
Fig. 2.
PGA increases LT-mediated cell death. J774A.1 cells were treated with medium only; 250 μg/ml PGA only; LT (consisting of 0.5 μg/ml PA and 0.1 μg/ml LF); or 62.5, 125, or 250 μg/ml of PGA plus LT (consisting of 0.5 μg/ml PA and 0.1 μg/ml LF). After a 2-h incubation, cells were stained for PI and annexin V and analyzed by flow cytometry. (A) Each panel shows a representative result obtained for each of the six conditions from three separate experiments. (B) The y axis represents the percentage of dead cells relative to control values, given as means ± SD derived from three separate experiments. *, P < 0.05 versus LT without PGA control.
PGA increases the binding and accumulation of LT in J774A.1 cells, which results in enhancement of LT activity.
After PA binds to its receptors, ANTXR1 (tumor endothelial marker-8) and ANTXR2 (capillary morphogenesis protein 2), a furin-like surface protease cleaves PA at the N terminus to release a soluble 20-kDa fragment and membrane-associated 63-kDa PA (PA-63) that forms a ring-shaped heptameric oligomer or prepore. The PA-63 heptamer binds LF, and endocytosis follows via a lipid raft-mediated and clathrin-dependent process (2, 37). To investigate whether the presence of PGA affects the binding of PA to its receptors, Alexa Flour 488-labeled PA was incubated with various amounts of PGA (0, 31.3, 62.5, and 125 μg/ml) for 30 min, and flow cytometry was used to determine the proportion of cells to which PA had bound. The addition of PGA increased the binding of PA to its receptors on J774A.1 cells in a concentration-dependent manner (Fig. 3A). The addition of 125 μg/ml PGA significantly increased the proportion of cells labeled with PA by 36.2% (P = 0.03) compared to levels for cells labeled with PA in the absence of PGA (Fig. 3A).
Fig. 3.
PGA increases, in a concentration-dependent manner, the binding to and internalization of PA in J774A.1 cells. The effect of PGA on the binding of PA to J774A.1 cells was analyzed by flow cytometry after a 30-min incubation with Alexa Fluor 488-labeled PA and increasing concentrations of PGA. Representative histograms under each condition are shown: medium control (upper left); 125 μg/ml PGA (upper middle); 1 μg of Alexa Fluor 488-labeled PA (upper right); and 1 μg of Alexa Fluor 488-labeled PA with 31.3 (lower right), 62.5 (lower middle), or 125 (lower right) μg/ml PGA. (A) The percentage of fluorescence measured in each histogram is indicated by horizontal bars. Western blot analysis (30 μg total protein/lane) was used to quantify the amount of PA-63 heptamer protein in J774A.1 cells after a 1-h incubation at 37°C with LT, consisting of 0.5 μg PA and 0.1 μg LF and 0, 62.5, 125, 250, or 500 μg/ml PGA. Western blots were photographed and quantified, and results are expressed relative to values obtained using α-tubulin as an internal control. (B) α1-Na+/K+-ATPase was used as a membrane marker protein control. Each bar represents the means ± SD derived from three separate experiments. *, P < 0.05 versus LT without PGA control.
The endocytosed PA-63 heptamers then undergo a pH-dependent conformational change and form pores as the carrier endosomes mature into early endosomes, enodosomal carrier vesicles, and late endosomes (1). In acidified endosomes, PA-63 heptamers have been reported to become an SDS-resistant complex (16). The dissociation and release of LF from PA through the PA-63 heptameric pores occurs in the membrane of intralumenal vesicles and results in cytotoxicity to the host cells (1). The amount of PA-63 that had translocated intracellularly was quantified by determining the concentration of SDS-resistant PA-63 heptamer using Western blotting. As expected, the intracellular concentration of PA-63 heptamer increased with increasing concentrations of PGA (Fig. 3B). The addition of 500 μg/ml of PGA significantly increased the intracellular concentration of PA-63 heptamer by 49.1% (P = 0.00001) compared to the level observed in cells treated with LT alone (Fig. 3B). α-Tubulin was used as a control for sample loading and quantifying relative levels of PA-63 heptamers in the Western blotting (Fig. 3B). The experiment with another membrane marker protein control, α1-Na+/K+-ATPase, showed that our cell lysates contained a membrane fraction as well as a cytoplasmic fraction, which explains the existence of residual amounts of PA-83 in Western blotting (Fig. 3B).
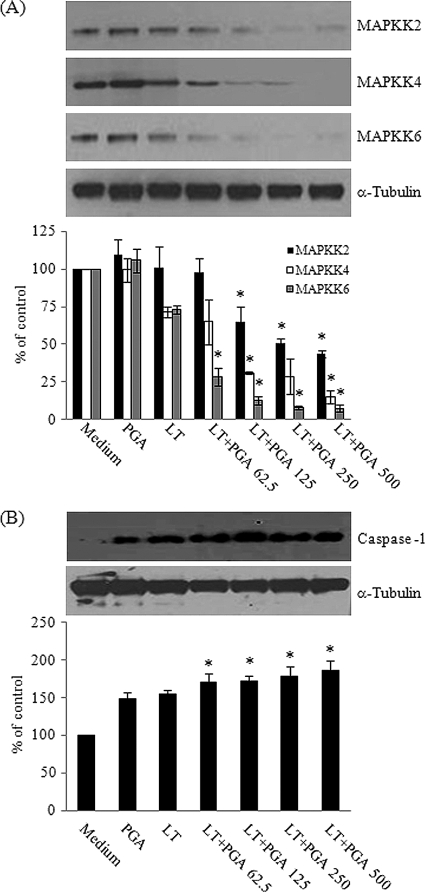
The most prominent effect of LT on macrophages is the induction of cell death (27, 34, 35). The LF present in LT cleaves and inactivates MAPKK1, MAPKK2, MAPKK3, MAPKK4, and MAPKK6, each of which plays an important role in MAPK-dependent immune activation, and the inactivation of the MAPKKs results in impaired host defenses (27, 34, 35). The recent identification of a susceptibility locus for LT in different mouse species highlights the inflammasome component NALP1b as a potential target of LF in macrophage death (5). Inflammasome formation involves the association of NALP1 and NOD2 to activate caspase-1, which then triggers the death of host cells through pyroptosis (15). Caspase-1 is an enzyme that proteolytically cleaves the precursor forms of proinflammatory cytokines, such as IL-1β and IL-18, to form active mature peptides (14). Caspase-1 itself exists as a proform (p45), and upon activation, the proform of caspase-1 ultimately is cleaved into two bioactive forms, p20 and p10 (8). In our study, we hypothesized that the increase in PA binding to J774A.1 cells (Fig. 3) increases the amount of LT inside the cells, which then increases the extent of cell death through the degradation of MAPKK and activation of caspase-1. To test this hypothesis, lysates from LT- and PGA-treated J774A.1 cells were analyzed by Western blotting using antibodies that recognize MAPKK2, MAPKK4, and MAPKK6 to support the shutdown of MAPK-dependent signaling pathways through Erk, p38, and JNK and using an antibody that recognizes the processed form of caspase-1. Levels of MAPKK2, MAPKK4, and MAPKK6 decreased (Fig. 4A), whereas levels of the cleaved p10 subunit of caspase-1 increased (Fig. 4B) after the treatment of J774A.1 cells with LT and increasing concentrations of PGA. The addition of 500 μg/ml PGA significantly decreased the amount of MAPKK2, MAPKK4, and MAPKK6 by 56.3, 78.4, and 89.4%, respectively (P < 0.05), and increased levels of the cleaved p10 subunit of caspase-1 by 21.6% (P = 0.0058) compared to their expression in cells treated with LT only. α-Tubulin was used as a control for sample loading. Our results showed that increased LF due to the presence of PGA leads to more death of J774A.1 cells due to increased intracellular LF activity.
Fig. 4.
PGA enhances LT activity. Cultures of J774A.1 cells were incubated with LT, consisting of 0.5 μg/ml PA and either 0.05 μg/ml LF (for MAPKK analysis) or 0.1 μg/ml LF (for caspase-1 analysis) and 0, 62.5, 125, 250, or 500 μg/ml PGA for 4 h (A) or for 1 h (B). Protein lysates derived from the cultures were analyzed by Western blotting (30 μg total protein/lane) for the presence of MAPKK2 (upper panel), MAPKK4 (middle panel), and MAPKK6 (lower panel) (A) and for the presence of the activated product of caspase-1, p10 (upper panel) (B). Western blots were photographed and quantified, and the results are expressed relative to values obtained using α-tubulin as an internal control. Each bar represents the means ± SD derived from three separate experiments. *, P < 0.05 versus LT without PGA control.
PGA slightly inhibits the LT-neutralizing activity of anti-PA antibody.
Currently licensed anthrax vaccines, as well as those under development, target PA, and PA antibodies are believed to play an important role in the protection against anthrax infection through the neutralization of LT (21, 30). Studies on the effect of PGA on the LT-neutralizing activity of anti-PA antibody are needed, particularly because PGA associates with LT in vivo (13). The LT-neutralizing activity of serum containing anti-PA antibody was determined after incubation with either a fixed concentration of PGA (125 μg/ml) and various dilutions of the serum (1:5 to 1:160) or a fixed concentration of serum (at a dilution of 1:5) and various concentrations of PGA (0, 31.3, 62.5, 125, 250, and 500 μg/ml). The addition of 125 μg/ml of PGA did not inhibit the LT-neutralizing activity of anti-PA antibody at serum dilutions of 1:5 and 1:10, but approximately 50 to 55% inhibition (P < 0.05) was observed with serum dilutions of 1:20 to 1:160 compared to the level for cells that were not treated with PGA (Fig. 5A). With a fixed serum dilution of 1:5, PGA concentrations of 31.3, 62.5, 125, and 250 μg/ml did not inhibit the LT-neutralizing activity of anti-PA antibody, but a PGA concentration of 500 μg/ml did inhibit the LT-neutralizing activity by 27% (P = 0.038) compared to that of cells that were not treated with PGA (Fig. 5B). These results suggest that anti-PA antibody produced by PA vaccination is effective in neutralizing PA in the presence of PGA, but high concentrations of PGA inhibit the toxin-neutralizing activity of anti-PA antibody in vivo.
Fig. 5.
PGA affects the LT-neutralizing activity of anti-PA antibody. The LT-neutralizing activity of anti-PA antibody was determined in J774A.1 cells either at a fixed concentration of 125 μg/ml of PGA with 1:5 to 1:160 dilutions of serum containing anti-PA antibody (A) or at a fixed 1:5 dilution of serum containing anti-PA antibody with 0, 31.3, 62.5, 125, 250, or 500 μg/ml PGA (B). LT consisted of 0.5 μg/ml PA and 0.1 μg/ml LF. The y axis represents the percentage of surviving cells relative to that obtained with the medium-only control. Each bar represents means ± SD derived from three separate experiments. *, P < 0.05 versus LT without PGA control.
PGA significantly increases the rate of death of mice challenged with LT.
To determine the effect of PGA on LT in vivo, 6-week-old female BALB/c mice (n = 8 to 12) received a tail vein injection of 200 or 500 μg of PGA alone or with approximately four times the LD50 of LT (29). The mice then were observed for a period of 14 days. Mice that received 200 or 500 μg of PGA alone survived, but only 50% of the mice that received four times the LD50 of LT survived (Table 1). The percentage of mice that died rose significantly to 91.7 (P = 0.0325) and 100% (P = 0.0138) among mice that received 200 or 500 μg/ml of PGA, respectively, with addition to four times the LD50 of LT. The tail vein injection of PBS (control) did not alter survival rates (Table 1). The intraperitoneal injection of 0.5 ml of serum containing anti-PA antibody 4 h before the tail vein injection of 500 μg of PGA with four times the LD50 of LT rescued the mice completely, confirming the effectiveness of the PA-based vaccine (Table 1).
Table 1.
PGA increases mortality of BALB/c mice challenged with LT
| Groupa | No. (%) of dead miceb |
|---|---|
| PBS control | 0/8 (0) |
| 200 μg PGA | 0/8 (0) |
| 500 μg PGA | 0/8 (0) |
| LT | 6/12 (50) |
| 200 μg PGA with LT | 11/12 (91.7) |
| 500 μg PGA with LT | 12/12 (100) |
| 500 μg PGA with LT + anti-PA antibodyc | 0/10 (0) |
Mice were challenged with 200 or 500 μg of PGA only or with four times the LD50 of LT (48 μg PA, 20 μg LF) through their tail veins.
Mice were observed for a period of 14 days for survival.
Mice were injected intraperitoneally with 0.5 ml rabbit serum containing anti-PA antibody 4 h before LT with 500 μg of PGA challenge.
DISCUSSION
The PGA capsule is produced primarily by Bacillus strains; however, the biological function of PGA in B. anthracis remains largely unclear, except for its role in sheltering bacteria from phagocytosis (22). The PGA capsule in B. anthracis first is polymerized on the bacterial cell surface to produce a structure with a high molecular mass (24). The capsule then is degraded to a lower molecular mass that is released from the bacterial cell surface and acts as a decoy to protect bacteria from complement (24). Although the production of PGA by B. anthracis at each stage of anthrax infection has not been thoroughly studied, serum levels of PGA have been reported to become high in later stages of mice and rhesus macaque infection, during which the actively produced virulence factors from B. anthracis stimulate cytokine production (6, 31). Recently, the PGA capsule has been reported to activate caspase-1 and to induce the release of IL-1β from THP-1 cells that had differentiated into macrophages and from human monocyte-derived dendritic cells (11). This finding raises the possibility that the high serum concentrations of PGA observed in the later stage of infection with B. anthracis could result in septic shock secondary to an increase in IL-1β (11). Our results further suggest that high concentrations of PGA during later stages of anthrax infection augment the cytotoxic activity of LT and thereby accelerate toxemia and the death of the host.
The binding of PA to its receptors on macrophage cells is the first step in the creation of the cytotoxic effects of LT (37). The endocytosed LT dissociates and releases LF from PA through PA-63 pores in the membranes of intralumenal vesicles (1). Cytosolic LF then cleaves MAPKKs, which blocks the activation of the p38 MAPK and NF-κB signaling pathways, and it activates various caspases, including caspase-1, resulting in the death of activated macrophages (12, 26, 27, 35). The death of macrophages by LT prevents their secretion of cytokines and chemokines that otherwise would alert other immune cells (26). Thus, anthrax infection can proceed undetected to the terminal stage. Our results suggest that the association of PGA with LT enhances the binding of PA to its receptors, resulting in a greater accumulation of LT inside macrophages and an increase in LT cytotoxicity that leads to an increase in macrophage cell death. Further study is required to elucidate whether PGA forms a complex with LT at physiological pH in vitro as well as in vivo and whether PGA enhances the binding of PA to its receptors in vivo. In contrast to the effects observed in combination with LT, PGA itself did not show any effects on MAPKKs or caspase-1 in J774A.1 cells in our experimental conditions. It remains to be determined whether PGA affects other signaling pathways or contributes to the pathogenesis of B. anthracis in vivo, perhaps through the regulation of the expression of IL-1β and/or other cytokines, as previously suggested (11).
In our study, serum containing anti-PA antibody, produced by PA vaccination, was effective in inhibiting the PGA-induced increase in the cytotoxic activity of LT both in vitro and in vivo, which supports the use of current PA-based anthrax vaccines (21, 30). The effect of PGA did, however, depend on both the concentration of PGA and the concentration of anti-PA antibody (Fig. 5). Thus, information about serum concentrations of PGA as well as PA at each stage of B. anthracis infection would be necessary to find the optimal anti-PA antibody levels required for protection against anthrax infection. Recently, levels of PGA in rhesus macaques infected with B. anthracis Ames spores by inhalation were reported (6). During the course of disease progression, PGA in the blood exhibited a triphasic kinetic profile. PGA was not detected at 24 h, increased at 48 h (2,037 ± 2 ng/ml; range, 48 to 10,394 ng/ml), declined at 72 h (14 ± 0.2 ng/ml; range, 7 to 20 ng/ml), and then increased at 96 h (3,401 ± 8 ng/ml; range, 3 to 1,965,663 ng/ml) and 120 h (6,004 ± 187 ng/ml; range, 32 to 1,126,388 ng/ml). LF also showed a triphasic pattern, while PA was not detected until later stages of infection, which might be due to the saturation of host receptors, decreased cellular uptake, and thus the accumulation of PA in serum (6). At 96 and 120 h, three of five rhesus macaques showed PA levels ranging from 147 to 19,434 ng/ml (6). The PGA level of each animal at the terminal stage is much higher than that of PA (the PA/PGA ratio [ng/ml] for each animal was 147:32,000, 19,434:1,965,663, 3,153:1,126,388, undetected:3, 541, and undetected:32) (6). Bacteremia also was triphasic: positive at 48 h, negative at 72 h, and positive at euthanasia (6). These data indicate the initial rapid and extended clearance of PGA-producing bacilli (72 h) after infection, followed by a resurgence of bacterial load in the blood of rhesus macaques. In the case of mice, the production of PGA by B. anthracis at each stage of anthrax infection has not been thoroughly studied. However, serum levels of PGA and PA range from 500 to 1,000 μg/ml and 0 to 408 ng/ml, respectively, in the later stages of murine infection, during which the actively produced virulence factors from B. anthracis stimulate cytokine production (31, 32). Levels of PA but not PGA also were determined in the blood of rabbit and guinea pigs after anthrax spore infection (23). At the terminal stage, the levels of PA in rabbits and guinea pigs were 80 to 100 μg/ml and 0.1 to 1.7 μg/ml, respectively (23). In the case of rhesus macaques, the level of PGA is approximately 100- to 350-fold higher than that of PA at the later stage of infection, and it is possible that a high concentration of PGA at this stage inhibits the toxin-neutralizing activity of anti-PA antibody. However, further studies are necessary to clarify the relationship between the toxin-neutralizing activity of anti-PA antibody and PGA and to find the optimal level of anti-PA antibody for protection against anthrax pathogenesis. This observation supports the utilization of both anti-PA antibody and antibiotics as a clinical treatment in the later stage of infection in addition to prior prevention with PA-based anthrax vaccines. In addition to PA-based vaccines, efforts have been made to include the PGA capsule as a component of anthrax vaccines to enhance protection through the inactivation of the vegetative forms of B. anthracis (9, 18, 29). Because of the association of PGA with LT, the effectiveness of an antibody against PGA was questioned previously (13). The effect of anti-PGA antibody on the LT-PGA complex was not tested in this study. Anti-PGA antibody produced by capsule vaccination might prove to be effective, because the protection provided by anti-PGA antibody appears to be due primarily to the opsonophagocytic activity of the PGA antibodies in combination with the activities of complement and neutrophils (9, 29).
To determine the effect of PGA on LT-mediated cytotoxic activity on J774A.1 cells in more physiologically relevant conditions (i.e., resembling the blood of anthrax-infected animals), cytotoxic activity assays were performed in the presence of 5% FBS as well as in the absence of FBS. In both conditions, PGA showed the enhancement of the cytotoxic effects of LT in a concentration-dependent manner; however, PGA showed a relatively modest enhancement of LT activity in the presence of 5% FBS compared to that of LT without FBS. It is not clear at this point how the effect of PGA is inhibited by the presence of FBS. As PGA capsule associates with LT during experimental infection (13), PGA might bind other serum proteins, which results in the partial sequestration of serum PGA and a lower level of PGA association with LT, thereby resulting in the moderate enhancement of LT cytotoxicity. Actually, it has been reported that the proinflammatory activity of bacterial lipoproteins such as Mip of Chlamydia trachomatis in THP-1 cells differentiated into macrophage was significantly higher in the absence than in the presence of serum, possibly because of interactions of acyl chains of the lipid part of bacterial lipoproteins with serum lipoprotein (4). It is expected that the recognition and responses would be much more sensitive for Chlamydia strains that are not primarily bloodstream infectious reagents but rather are infecting lungs, the urogenital system, or eyes, i.e., serum-free compartments (4). Although the effect of PGA on LT-mediated cytotoxicity is relatively modest in the presence of serum compared to that in the absence of serum, our in vivo study using BALB/c mice injected with LT and PGA, and the fact that a relatively high concentration of PGA is produced at the terminal stage of infection in animal models (6, 31), strongly support the conclusion that PGA enhances the cytotoxic activity of LT in vivo.
To the best of our knowledge, this is the first study to track the effects of PGA on LT activity. Here, we demonstrate that PGA increases, in a concentration-dependent manner, the cytotoxicity of LT in the mouse macrophage cell line J774A.1 by enhancing the binding of PA to its receptors, which results in an increase in intracellular LF, which then results in the death of macrophages through the enhanced degradation of MAPKKs and the increased activation of caspase-1. The effect of PGA on LT was confirmed in vivo using BALB/c mice. Anti-PA antibody is effective in inhibiting LT cytotoxicity in the presence of PGA, which supports the use of current PA-based anthrax vaccines. These results yield important insights that increase our understanding of the role of PGA in anthrax pathogenesis. Our study also provides evidence related to PGA that is relevant to the development of anthrax diagnostics, therapeutics, and clinical treatment.
ACKNOWLEDGMENTS
We thank S. Han for helpful discussions.
This work was supported by a GRRC grant of Gyeonggi province (HUFS2010-B03 to J.P.) and by a Korea National Institute of Health grant (to G.R.).
Footnotes
Published ahead of print on 20 June 2011.
REFERENCES
- 1. Abrami L., Lindsay M., Parton R. G., Leppla S. H., G. van der Goot F. 2004. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J. Cell Biol. 166:645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abrami L., Liu S., Cosson P., Leppla S. H., G. van der Goot F. 2003. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160:321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashiuchi M., Misono H. 2002. Biochemistry and molecular genetics of poly-γ-glutamate synthesis. Appl. Microbiol. Biotechnol. 59:9–14 [DOI] [PubMed] [Google Scholar]
- 4. Bas S., James R. W., Gabay C. 2010. Serum lipoproteins attenuate macrophage activation and Toll-Like Receptor stimulation by bacterial lipoproteins. BMC Immunol. 11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyden E. D., Dietrich W. F. 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38:240–244 [DOI] [PubMed] [Google Scholar]
- 6. Boyer A. E., et al. 2009. Kinetics of lethal factor and poly-D-glutamic acid antigenemia during inhalation anthrax in rhesus macaques. Infect. Immun. 77:3432–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brittingham K. C., et al. 2005. Dendritic cells endocytose Bacillus anthracis spores: implications for anthrax pathogenesis. J. Immunol. 174:5545–5552 [DOI] [PubMed] [Google Scholar]
- 8. Cerretti D. P., et al. 1992. Molecular cloning of the interleukin-1 beta converting enzyme. Science 256:97–100 [DOI] [PubMed] [Google Scholar]
- 9. Chabot D. J., et al. 2004. Anthrax capsule vaccine protects against experimental infection. Vaccine 23:43–47 [DOI] [PubMed] [Google Scholar]
- 10. Chakrabarty K., et al. 2006. Bacillus anthracis spores stimulate cytokine and chemokine innate immune responses in human alveolar macrophages through multiple mitogen-activated protein kinase pathways. Infect. Immun. 74:4430–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cho M. H., et al. 2010. Bacillus anthracis capsule activates caspase-1 and induces interleukin-1β release from differentiated THP-1 and human monocyte-derived dendritic cell. Infect. Immun. 78:387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cordoba-Rodriguez R., Fang H., Lankford C. S., Frucht D. M. 2004. Anthrax lethal toxin rapidly activates caspase-1/ICE and induces extracellular release of Interleukin (IL)-1β and IL-18. J. Biol. Chem. 279:20563–20566 [DOI] [PubMed] [Google Scholar]
- 13. Ezzell J. W., et al. 2009. Association of Bacillus anthracis capsule with lethal toxin during experimental infection. Infect. Immun. 77:749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fantuzzi G., Dinarello C. A. 1999. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J. Clin. Immunol. 19:1–11 [DOI] [PubMed] [Google Scholar]
- 15. Fink S. L., Bergsbaken T., Cookson B. T. 2008. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. U. S. A. 105:4312–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ha S. D., et al. 2010. Cathepsin B-mediated autophagy flux facilitates the anthrax toxin receptor 2-mediated delivery of anthrax lethal factor into the cytoplasm. J. Biol. Chem. 285:2120–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kozel T. R., et al. 2004. mAbs to Bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc. Natl. Acad. Sci. U. S. A. 101:5042–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee D. Y., et al. 2009. Poly-γ-D-glutamic acid and protective antigen conjugate vaccines induce functional antibodies against the protective antigen and capsule of Bacillus anthracis in guinea-pigs and rabbits. FEMS Immunol. Med. Microbiol. 57:165–172 [DOI] [PubMed] [Google Scholar]
- 19. Leonard C. G., Housewright R. D., Thorne C. B. 1958. Effects of some metallic ions on glutamyl polypeptide synthesis by Bacillus subtilis. J. Bacteriol. 76:499–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leppla S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. U. S. A. 79:3162–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leppla S. H., Robbins J. B., Schneerson R., Shiloach J. 2002. Development of an improved vaccine for anthrax. J. Clin. Invest. 110:141–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Little S. F., Ivins B. E. 1999. Molecular pathogenesis of Bacillus anthracis infection. Microbes Infect. 1:131–139 [DOI] [PubMed] [Google Scholar]
- 23. Mabry R., et al. 2006. Detection of anthrax toxin in the serum of animals infected with Bacillus anthracis by using engineered immunoassays. Clin. Vaccine Immunol. 13:671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Makino S., Watarai M., Cheun H. I., Shirahata T., Uchida I. 2002. Effect of the lower molecular capsule released from the cell surface of Bacillus anthracis on the pathogenesis of anthrax. J. Infect. Dis. 186:227–233 [DOI] [PubMed] [Google Scholar]
- 25. Mock M., Fouet A. 2001. Anthrax. Annu. Rev. Microbiol. 55:647–671 [DOI] [PubMed] [Google Scholar]
- 26. Park J. M., Greten F. R., Li Z. W., Karin M. 2002. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297:2048–2051 [DOI] [PubMed] [Google Scholar]
- 27. Popov S. G., et al. 2002. Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem. Biophys. Res. Commun. 293:349–355 [DOI] [PubMed] [Google Scholar]
- 28. Price B. M., et al. 2001. Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect. Immun. 69:4509–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rhie G. E., et al. 2003. A dually active anthrax vaccine that confers protection against both bacilli and toxins. Proc. Natl. Acad. Sci. U. S. A. 100:10925–10930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rhie G. E., et al. 2005. Expression and secretion of the protective antigen of Bacillus anthracis in Bacillus brevis. FEMS Immunol. Med. Microbiol. 45:331–339 [DOI] [PubMed] [Google Scholar]
- 31. Sutherland M. D., Thorkildson P., Parks S. D., Kozel T. R. 2008. In vivo fate and distribution of poly-γ-D-glutamic acid, the capsular antigen from Bacillus anthracis. Infect. Immun. 76:899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang S., et al. 2009. Detection of anthrax toxin by an ultrasensitive immunoassay using europium nanoparticle. Clin. Vaccine Immunol. 16:408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thorne C. B., Leonard C. G. 1958. Isolation of D- and L-glutamyl polypeptides from culture filtrates of Bacillus subtilis. J. Biol. Chem. 233:1109–1112 [PubMed] [Google Scholar]
- 34. Tournier J. N., Paccani S. R., Quesnel-Hellmann A., Baldari C. T. 2009. Anthrax toxin: A weapon to systematically dismantle the host immune defenses. Mol. Aspects Med. 30:456–466 [DOI] [PubMed] [Google Scholar]
- 35. Turk B. E. 2007. Manipulation of host signaling pathways by anthrax toxins. Biochem. J. 402:405–417 [DOI] [PubMed] [Google Scholar]
- 36. Vitale G., Bernardi L., Napolitani G., Mock M., Montecucco C. 2000. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 352:739–745 [PMC free article] [PubMed] [Google Scholar]
- 37. Young J. A., Collier R. J. 2007. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 76:243–265 [DOI] [PubMed] [Google Scholar]