Abstract
Monophosphoryl lipid A (MPLA) is a Toll-like receptor 4 (TLR4) agonist that is currently used as a vaccine adjuvant in humans. In this study, we evaluated the effect of MPLA treatment on the innate immune response to systemic bacterial infections in mice. Mice treated with MPLA after burn injury showed improved survival and less local and systemic dissemination of bacteria in a model of Pseudomonas aeruginosa burn wound infection. Prophylactic treatment with MPLA significantly enhanced bacterial clearance at the site of infection and reduced systemic dissemination of bacteria despite causing attenuation of proinflammatory cytokine production during acute intra-abdominal infection caused by cecal ligation and puncture. Administration of MPLA at 1 h after CLP also improved bacterial clearance but did not alter cytokine production. MPLA treatment increased the numbers of granulocytes, double-positive myeloid cells, and macrophages at sites of infection and increased the percentage and total numbers of myeloid cells mediating phagocytosis of bacteria. Depletion of Ly6G+ neutrophils, but not macrophages, eliminated the ability of MPLA treatment to improve bacterial clearance. The immunomodulatory effects of MPLA were absent in TLR4-deficient mice. In conclusion, these studies show that MPLA treatment significantly augments the innate immune response to bacterial infection by enhancing bacterial clearance despite the attenuation of proinflammatory cytokine production. The enhanced bacterial clearance is mediated, in part, by increased numbers of myeloid cells with effective phagocytic functions at sites of infection and is TLR4 dependent.
INTRODUCTION
The use of immunomodulatory strategies aimed at improving resistance to bacterial infections could be beneficial in a variety of clinical scenarios in which the host is predisposed to infectious complications. Among those are patients with severe burns or major trauma, patients that have undergone major surgical procedures, or patients that have received immunosuppressive therapies for cancer or organ transplantation. The attractiveness of interventions that can improve the host response to infection is further enhanced by the increasing incidence of antibiotic resistance among bacteria, especially those that commonly cause nosocomial infections.
Bacterial lipopolysaccharide (LPS; endotoxin) is a component of the Gram-negative bacterial cell wall that has known immunomodulatory properties (6). LPS is recognized by Toll-Like receptor 4 (TLR4), which is expressed on a variety of leukocytes and activates both TRIF- and MyD88-dependent signaling pathways (10, 15, 18, 22, 27, 31, 32, 35). Activation of TLR4 signaling induces the production of numerous proinflammatory mediators such as cytokines, chemokines, and nitric oxide that facilitate the cardinal features of inflammation such as increased vascular permeability, edema formation, and leukocyte recruitment. Interestingly, prior exposure to LPS induces a state in which a subsequent challenge with LPS or bacteria results in markedly decreased production of proinflammatory mediators (7). The altered immunological phenotype that is elicited by priming with LPS has historically been referred to as endotoxin tolerance (8). The induction of endotoxin tolerance has been shown to be highly effective in reducing both morbidity and mortality associated with subsequent challenge with a normally lethal dose of LPS (23). Because LPS priming attenuates proinflammatory cytokine production in response to LPS or bacterial challenge, many investigators previously characterized LPS tolerance as a state of immunosuppression. However, few studies have examined the effects of LPS treatment on the host response to live bacterial infections. Recent studies from our laboratory, and others, have demonstrated that mice primed with LPS have improved resistance to bacterial infections (23, 39, 40). However, the clinical applicability of LPS as a therapeutic or prophylactic agent is precluded by toxicity and a narrow therapeutic index in humans. Furthermore, the mechanisms by which LPS exposure leads to improved antimicrobial immunity are currently unknown.
Monophosphoryl Lipid A (MPLA) is an endotoxin derivative that is used as a vaccine adjuvant in humans (33). MPLA is produced by hydrolysis of native diphosphoryl lipid A, the component of LPS that is recognized by TLR4, resulting in the removal of all but a single phosphate group and various degrees of deacylation (26). These structural alterations decrease systemic toxicity by >99% compared to native lipid A, resulting in an immunomodulatory agent with greater potential for clinical use (3, 30). The attenuated toxicity of MPLA is associated with reduced induction of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and gamma interferon (IFN-γ) during initial exposure (13, 29). MPLA retains significant immunomodulatory activity and prior treatment with MPLA increases survival after otherwise lethal exposure to LPS in animal models (2, 16, 39). However, the effect of MPLA on the innate response to clinically relevant models of bacterial infection has not been determined. Furthermore, the cellular and molecular mechanisms underlying the beneficial effects of MPLA treatment on innate antimicrobial immunity have not been characterized. Therefore, further understanding of the immunomodulatory properties of MPLA is required for its development as an immunomodulatory agent. In the present study, we evaluated the effect of MPLA treatment on leukocyte recruitment, cytokine production, and bacterial clearance mechanisms in clinically relevant models of acute bacterial infection.
MATERIALS AND METHODS
Mice.
Male and female C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Female C3H/HeOUJ and C3H/HeJ mice were purchased from the Jackson Laboratory. All mice were 8 to 10 weeks of age. Male mice were used for all intravenous treatment studies, and female mice were used for intraperitoneal treatment studies. Mice were housed and cared for in the animal facility located in the Shriners Hospital for Children (Galveston, TX). All procedures were approved by the University of Texas Medical Branch (UTMB) Institutional Animal Care and Use Committee and meet the criteria established by the Guide for the Care and Use of Experimental Animals (National Institutes of Health).
Antibodies.
Anti-mouse CD16/32 and fluorochrome-conjugated anti-mouse F4/80 (clone BM8) and Gr1 monoclonal antibodies, along with isotype controls, were purchased from eBioscience, Inc., San Diego, CA. Fluorochrome-conjugated and unconjugated anti-mouse Ly6G (clone 1A8) and isotype control monoclonal antibodies were purchased from BD Biosciences, San Jose, CA. Fluorescein isothiocyanate (FITC)-conjugated anti-mouse myeloperoxidase (MPO) specific antibody (clone 8F4) was purchased from HyCult Biotech, Netherlands.
MPLA treatment.
MPLA derived from Salmonella enterica serotype Minnesota Re 595 was purchased from Sigma-Aldrich Corp. (St. Louis, MO). MPLA was dissolved in 0.2% triethylamine (1 mg/ml), heated to 60°C, sonicated for 30 min, and then diluted in phosphate-buffered saline (100 μg/ml) prior to administration. For intraperitoneal or intravenous administration, MPLA was injected (20 μg in 0.2 ml) once daily for two consecutive days (total of 40 μg/mouse). In postinfection treatment experiments, mice received 20 μg of MPLA intravenously in 0.2 ml of vehicle at 1 h after infection. Intravenous injections were performed via the dorsal vein of the penis. Control mice received injections of vehicle in the same volume and by the route as that used in the respective treatment protocols.
CLP.
Vehicle and MPLA-treated mice were anesthetized with 2% isoflurane in oxygen via facemask and presented to the surgeon in a blinded fashion to minimize experimental bias. A 1- to 2-cm midline incision was made through the abdominal wall. The cecum was identified and ligated 0.7 cm from the tip with a 3-0 silk tie. A double puncture of the cecum was performed using a 20-gauge needle, and cecal contents were expressed from the puncture. Great care was taken to avoid ligation-induced obstruction of flow between the ileum and colon. The cecum was returned to the abdominal cavity, and the incision was closed with surgiclips. All mice received 0.1 mg of buprenorphine/kg subcutaneously immediately after cecal ligation and puncture (CLP) and twice daily thereafter. Control mice did not receive surgical manipulation. For survival studies, mice received a single intraperitoneal injection of Primaxin (imipenem/cilistatin, 25 mg/kg) immediately after the CLP procedure.
Pseudomonas aeruginosa challenge.
P. aeruginosa (strain 15692; American Type Culture Collection, Rockville, MD) was propagated in tryptic soy broth overnight in a shaker incubator (37°C). Bacteria were washed twice in 30 ml of sterile 0.9% normal saline (NS), resuspended in 4 ml of NS, and stored at 4°C until use. The concentration of viable CFU was determined by plating serial dilutions on tryptic soy agar. Plates were incubated overnight at 37°C. Mice received intraperitoneal injection with 108 CFU of Pseudomonas or vehicle (0.2 ml of NS) at 48 h after receiving their last dose of MPLA or vehicle. For intravenous challenge, mice received 108 CFU of Pseudomonas or vehicle (0.2 ml of NS) via the dorsal vein of the penis at 48 h after receiving their last dose of MPLA or vehicle.
Burn wound infection.
A cutaneous burn wound was induced as previously described (14). Briefly, mice were anesthetized with 2 to 3% isoflurane; the backs were shaved and covered with a Zetex cloth containing a rectangular opening corresponding to 15% of the mouse total body surface area. NS was injected subcutaneously in the burn target area to prevent injury to underlying tissues. A full-thickness flame burn was achieved using a Bunsen burner applied to the exposed skin for ∼10 s. Fluid resuscitation was administered immediately by intraperitoneal injection of lactated Ringer's solution (3 ml), followed by an additional injection (1 ml) 24 h later. Buprenorphine (0.1 mg/kg, given subcutaneously) was administered for analgesia prior to initiation of the burn injury and twice daily thereafter. At days 3 and 4 after burn injury, mice received intraperitoneal treatment with MPLA (20 μg) or vehicle. P. aeruginosa (108 CFU) was applied to the caudad portion of the wound on day 5 postburn. Wound samples were harvested at 48 and 72 h after inoculation with bacteria and homogenized for measurement of the bacterial CFU. Lung tissue was also harvested at 72 h after infection for measurement of bacterial burden.
Cytokine analysis.
Cytokine levels in plasma and peritoneal fluid were measured by using a Bio-Plex mouse 23-Plex panel (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. IL-6 concentrations in plasma were measured using an enzyme-linked immunosorbent assay according to the manufacturer's protocol (eBioscience).
Measurement of bacterial counts.
Arterial blood was aseptically collected by laceration of the internal carotid artery as described above. Peritoneal lavage was performed with 7 ml of sterile phosphate-buffered saline using an aseptic technique. Lungs liver, spleen, and burn eschar were dispersed by using a glass tissue homogenizer and resuspended in NS to a standard wet weight/volume ratio under aseptic conditions. Serial dilutions of whole blood, peritoneal lavage fluid, and tissue homogenates were plated onto tryptic soy agar and incubated overnight at 37°C. Isolate colonies were counted to determine bacterial burden.
Flow cytometry.
Leukocytes were harvested and incubated with anti-mouse CD16/32 at a concentration of 1 μg per million cells for 30 min at 4°C to block Fc receptors. Two million leukocytes were transferred into polystyrene tubes containing isotype control or labeling antibodies (0.5 μg/tube) and incubated for 30 min at 4°C. Labeled cells were then washed with 2 ml of cold phosphate-buffered saline and resuspended in 0.5 ml of 1% paraformaldehyde fixative. The resultant samples were analyzed in the UTMB flow cytometry core facility using a FACSCanto flow cytometer (BD Biosciences). The data were analyzed by using FlowJo software (Tree Star, Inc., Ashland, OR).
For multispectral imaging flow cytometric analysis of nuclear morphology, peritoneal leukocytes were harvested and prepared as described above. Cells were surface labeled with fluorochrome-conjugated antibodies against F4/80, Gr1, and Ly6G. The cells were fixed in 1% paraformaldehyde and shipped overnight to Amnis Corp. in Seattle, WA. Upon arrival, the cells were stained with propidium iodide, and images were collected on more than 10,000 cells per sample by using an ImageStream flow cytometer. Single-color controls were collected and used to calculate a spectral cross talk compensation matrix to compensate the imagery. Using algorithms in the IDEAS image analysis software (Amnis, Inc., Seattle, WA), the cells were immunophenotyped, and the nuclei were analyzed for morphological characteristics.
In vivo phagocytosis assay.
P. aeruginosa was propagated as described above and heat killed by incubation in a water bath for 1 h at 56°C. The heat-killed bacteria were labeled with FITC as previously described (20). The labeled bacteria were resuspended in NS and stored at −80°C. Mice received intraperitoneal injection with 108 CFU of heat-killed, FITC-labeled P. aeruginosa or vehicle (0.2 ml of NS). Peritoneal leukocytes were collected 3 h after injection of bacteria, labeled with antibodies against F4/80 and Ly6G, and fixed in 1% paraformaldehyde. The samples were analyzed in the UTMB flow cytometry core facility using a FACSCanto flow cytometer (BD Biosciences). The data were analyzed by using FlowJo software (Tree Star).
Measurement of peripheral blood leukocyte counts.
Mice were challenged with 108 CFU of P. aeruginosa or vehicle (0.2 ml of NS) at 48 h after receiving their last dose of MPLA or vehicle. At 4 h after bacterial challenge, arterial blood was collected, and the cellular profile was analyzed within an hour by using a Hemavet 950FS (Drew Scientific Group, Waterbury, CT).
Monocyte depletion.
To deplete monocytes/macrophages, clodrinate liposomes (200 μl, given intraperitoneally, obtained from Nico van Rooijen, Vrije Universiteit, VUMC, Department of Molecular Cell Biology, Amsterdam, Netherlands) were administered 2 days prior to and 1 day after intraperitoneal MPLA or vehicle treatment. Control mice received liposomes containing vehicle according to this same regimen. The effectiveness of monocyte/macrophage depletion was determined by examination of intraperitoneal leukocytes after infection using surface marker labeling (F4/80, Ly6G) and flow cytometry.
Neutrophil depletion.
At 24 h after their final dose of MPLA or vehicle, mice received intraperitoneal injections with 100 μg of Ly6G specific antibody. At 16 h after CLP, peritoneal cells were harvested and assessed for myeloid surface marker (F4/80, Ly6G) expression by flow cytometry.
MPO analysis.
Peritoneal leukocytes were collected and stained for Ly6G and F4/80 surface marker expression. The cells were then washed and treated with Cytofix/Cytoperm according to the manufacturer's instructions (BD Biosciences, San Diego, CA). After fixation and permeabilization, the cells were labeled with FITC-conjugated myeloperoxidase (MPO) specific antibody (clone 8F4; HyCult Biotech, Netherlands) and analyzed by flow cytometry.
Complement-mediated erythrocyte lysis assay.
The complement activity of serum collected from MPLA-treated and control mice, both before and after infection, was assessed as previously described (11, 21). Briefly, rabbit erythrocytes were incubated with goat anti-rabbit serum and then with serial dilutions of mouse serum samples. The absorbance of the resultant supernatant was measured at 540 nm. Various concentrations of sensitized erythrocytes were incubated in red cell lysis buffer (Sigma-Aldrich Corp., St. Louis, MO), and the resultant supernatant was serially diluted to generate a standard curve.
Statistics.
Each experiment was repeated at least twice, except for the multispectral imaging flow cytometry trial, which was conducted to validate traditional flow cytometry findings. The data were analyzed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). Cytokine measurements, bacterial counts, and flow cytometry data were analyzed using one-way analysis of variance and Tukey's multiple-comparison test or the Mann-Whitney U test. Peripheral blood leukocyte counts were analyzed by using an unpaired t test. Experimental groups in survival studies were compared by log-rank analysis. A P value of <0.05 was considered statistically significant.
RESULTS
MPLA treatment prevents hypothermia, improves survival, and improves bacterial clearance in mice with systemic infection.
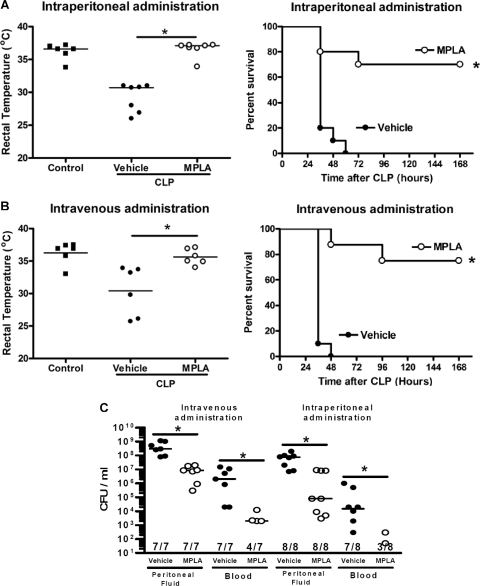
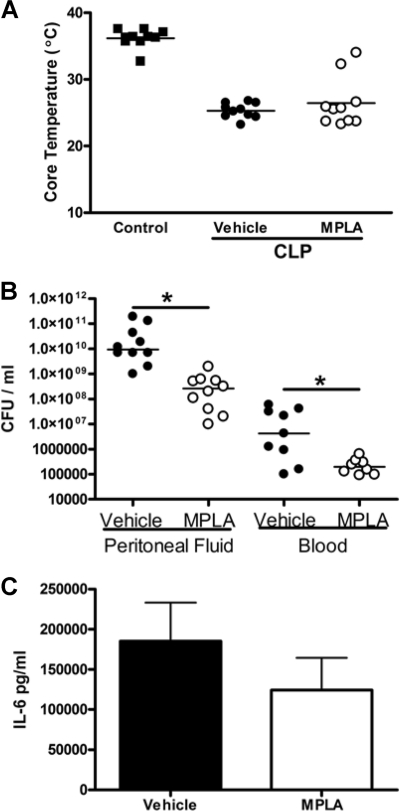
In rodents, sepsis is characterized by a decline in core body temperature, and sepsis-induced hypothermia is a predictor of mortality (5). Vehicle-treated mice showed a significant decrease in core temperature after CLP that paralleled a mortality rate of 100% (Fig. 1 A and B). Mice that were treated with MPLA, either by the intraperitoneal or the intravenous route, did not show a significant decrease in core temperature compared to control mice, and their core temperatures were significantly (P < 0.05) higher than those of vehicle-treated mice after CLP (Fig. 1A and B). Survival rates of 70 to 75% were observed in mice receiving MPLA treatment, which was significantly higher than that observed in vehicle-treated mice. Administration of MPLA by either the intraperitoneal or the intravenous route also resulted in significantly (P < 0.05) lower bacterial counts in peritoneal fluid and blood after CLP compared to vehicle-treated mice (Fig. 1C).
Fig. 1.
MPLA treatment improves the host response to systemic infection caused by CLP. Mice were treated with MPLA by either intraperitoneal (A) or intravenous (B) routes prior to CLP. The rectal temperature was measured at 24 h, and survival was monitored out to 2 weeks after CLP. (C) Bacterial counts in peritoneal lavage fluid and blood were measured at 16 h after CLP. *, P < 0.05 compared to vehicle (n = 6 to 10 mice per group).
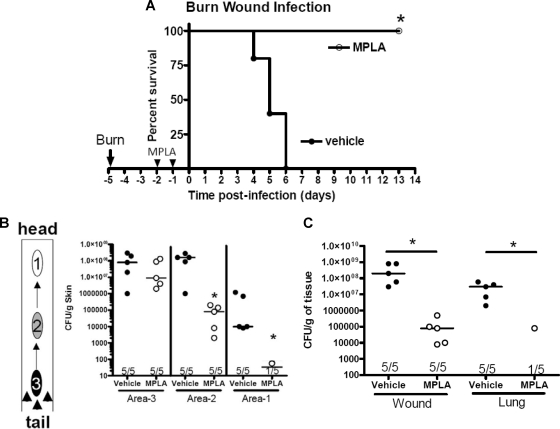
MPLA treatment also improved survival in a model of Pseudomonas burn wound infection (Fig. 2 A). Mice with a 15% total body surface area burn were treated with MPLA on days 3 and 4 postburn, followed by inoculation of the wound with P. aeruginosa on day 5. Mice receiving MPLA treatment showed 100% survival, whereas 100% mortality was observed in vehicle-treated mice at 6 days after wound inoculation (Fig. 2A). MPLA treatment also decreased the local and systemic dissemination of bacteria in mice with Pseudomonas burn wound infection (Fig. 2B). On day 5 postburn, the caudal portion of the burn wound (area 3) was inoculated with P. aeruginosa, and bacterial counts at the inoculation site and at distant sites (areas 1 and 2) were measured. At the inoculation site (area 3), the median number of organisms was not significantly decreased in MPLA-treated mice compared to vehicle-treated controls (Fig. 2B). However, the numbers of Pseudomonas in sections of the wound distant from the inoculation site (areas 2 and 1) were significantly lower (P < 0.05) in MPLA-treated mice compared to vehicle-treated controls (Fig. 2B). In further studies, the entire burn wound was excised and lungs were harvested at 72 h after inoculation of the burn wound with Pseudomonas (Fig. 2C). Total counts of Pseudomonas in the burn wound were significantly decreased in MPLA-treated mice compared to vehicle-treated controls (Fig. 2C). All mice in the vehicle-treated group grew Pseudomonas from the lungs, with a median bacterial count of 4 × 107 CFU/g. Only one of five mice in the MPLA-treated group grew Pseudomonas from the lungs (Fig. 2C).
Fig. 2.
MPLA treatment improves the host response to P. aeruginosa burn wound infection. Mice underwent a cutaneous burn followed by intraperitoneal treatment with MPLA or vehicle on days 3 and 4 postburn. The wounds were then infected with P. aeruginosa on day 5 postburn. (A) Survival was determined out to 2 weeks after burn wound infection, but no mice died beyond 7 days after wound infection. (B) Bacterial counts at the site of wound inoculation (area 3) and at locations distant from the inoculation site (areas 1 and 2) were determined 3 days after wound infection. (C) Total bacterial counts within the entire burn wound and in the lungs were determined 3 days after burn wound infection. *, P < 0.05 compared to vehicle (n = 6 to 10 mice per group).
MPLA treatment attenuates sepsis-induced proinflammatory cytokine production.
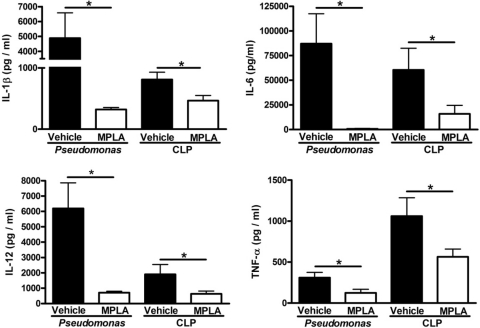
Production of proinflammatory cytokines has been linked to many of the pathophysiologic alterations associated with sepsis and treatment with TLR4 agonists has been shown to attenuate infection-induced cytokine production (28). Studies were undertaken to determine whether the improved survival and enhanced bacterial clearance seen in MPLA-treated mice was associated with a change in systemic cytokine production. Examination of plasma cytokine concentrations showed that MPLA treatment significantly attenuated infection-induced production of several proinflammatory cytokines after systemic Pseudomonas challenge or CLP. After infection with P. aeruginosa or CLP, vehicle-treated mice showed high plasma concentrations of IL-1β, IL-6, IL-12, and TNF-α (Fig. 3). MPLA-treated mice had significantly lower (P < 0.05) concentrations of the measured cytokines compared to vehicle-treated mice (Fig. 3).
Fig. 3.
MPLA pretreatment attenuates the production of proinflammatory cytokines in response to infection or CLP. Mice were treated with MPLA for 2 days prior to infection. Plasma cytokine levels were measured in plasma at 16 h after CLP or intraperitoneal P. aeruginosa infection. *, P < 0.05 compared to vehicle-treated and infected mice (n = 7 [CLP] to 9 [P. aeruginosa] mice per group).
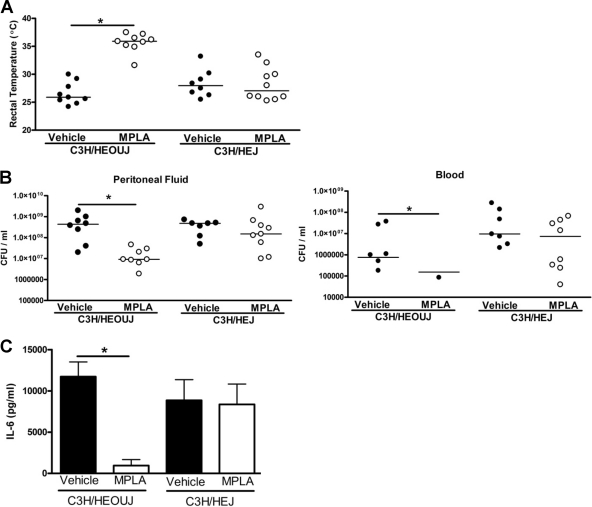
The protective effects of MPLA are mediated through TLR4.
Mice with intact (C3H/HeOUJ) and nonfunctional (C3H/HeJ) TLR4 were treated with MPLA prior to CLP (Fig. 4). Rectal temperature at 24 h after CLP was significantly higher in C3H/HeOUJ mice treated with MPLA compared to vehicle-treated mice (Fig. 4A). However, rectal temperatures were not significantly different between C3H/HeJ mice treated with MPLA or vehicle and were comparable to the core temperatures observed in vehicle-treated C3H/HeOUJ mice (Fig. 4A). The numbers of viable bacteria in MPLA-treated C3H/HeOUJ mice were significantly lower than in vehicle-treated C3H/HeOUJ mice, whereas no significant differences in bacterial counts were observed among vehicle- and MPLA-treated C3H/HeJ mice (Fig. 4B). Analysis of plasma IL-6 concentrations showed that MPLA treatment caused significant attenuation of CLP-induced IL-6 production in C3H/HeOUJ mice but not in C3H/HeJ mice (Fig. 4C).
Fig. 4.
The immunomodulatory effects of MPLA are dependent on TLR4. Mice with functional (C3H/HeOUJ) and nonfunctional (C3H/HeJ) TLR4 were pretreated with MPLA or vehicle prior to CLP. Rectal temperature (A), bacterial counts in plasma and peritoneal lavage fluid (B), and plasma IL-6 production concentrations (C) were measured at 16 h after CLP. *, P < 0.05 compared to vehicle-treated mice (n = 6 to 8 mice per group).
Postinfection treatment with MPLA enhances bacterial clearance but does not attenuate cytokine production or prevent hypothermia.
The studies reported above show that prophylactic treatment with MPLA is beneficial in murine models of sepsis. Further studies were undertaken to determine whether the administration of MPLA after the septic insult would improve outcome. The administration of MPLA after CLP did not prevent decline in core temperature (Fig. 5 A). However, bacterial counts in the peritoneal cavity and blood were significantly lower in mice treated with MPLA compared to vehicle-treated mice (Fig. 5B). Yet, plasma IL-6 concentrations were not significantly different among mice treated with MPLA or vehicle (Fig. 5C). Higher doses of MPLA, a 100-μg bolus instead of 20 μg, did not improve the therapeutic effect of MPLA (data not shown).
Fig. 5.
Treatment with MPLA after CLP enhances bacterial clearance but does not alter IL-6 production or prevent infection-associated hypothermia. At 1 h after CLP, mice received an intraperitoneal injection with MPLA or vehicle. Core temperature (A), bacterial counts within the peritoneal cavity and peripheral blood (B), and IL-6 concentrations in plasma (C) were determined at 16 h after CLP. *, P < 0.05 (n = 10 mice per group, data from three independent trials).
Intraperitoneal MPLA treatment increases the numbers of phagocytic myeloid cells at sites of infection.
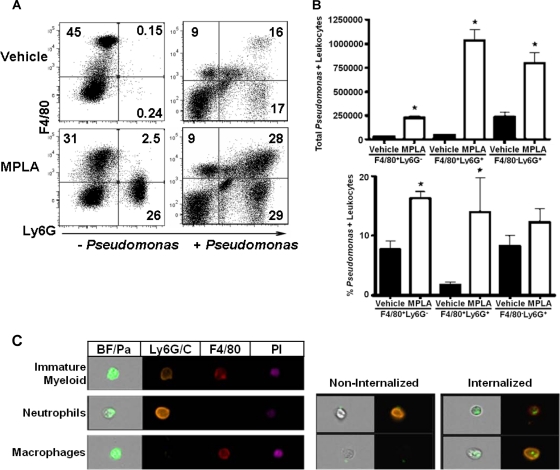
Myeloid cells are responsible for the majority of cell-mediated bacterial clearance after infection and are key participants in the acute inflammatory response. Studies were undertaken to assess the effect of MPLA treatment on the recruitment of myeloid cells to the primary site of infection. In one study, mice received intraperitoneal treatment with MPLA, followed 2 days later by intraperitoneal challenge with P. aeruginosa. Peritoneal leukocytes were harvested from mice and labeled with fluorochrome-conjugated antibodies against the myeloid cell biomarkers Ly6G (neutrophils) and F4/80 (macrophages) at 6 h after Pseudomonas challenge (Fig. 6 A). Approximately 45% of peritoneal leukocytes isolated from control (vehicle-treated, noninfected) mice were macrophages (F4/80+ Ly6G−) (Fig. 6A). The remainders of the peritoneal cells from control mice (vehicle-treated, noninfected) were predominantly negative for Ly6G and F4/80 (Fig. 6A). Analysis of the non-myeloid-cell populations showed that they are composed primarily of B and T lymphocytes (data not shown). Treatment with MPLA increased the numbers of leukocytes in the peritoneal cavity compared to vehicle-treated mice (1.9 × 107 ± 4.4 × 106 versus 4.8 × 106 ± 1.2 × 106). Peritoneal myeloid cells from MPLA-treated mice were composed primarily of macrophages (F4/80+ Ly6G−) and neutrophils (F4/80− Ly6G+) cells (Fig. 6A). Intraperitoneal infection of vehicle-treated mice with Pseudomonas caused an increase in the percentage of neutrophils and double-positive myeloid cells that coexpress both F4/80 and Ly6G, as well as a decrease in the numbers of resident macrophages (Fig. 6A). MPLA-treated mice that were challenged with Pseudomonas had significantly (P < 0.05) increased percentages and total numbers of neutrophils (6.4 ± 0.8 × 106 versus 2.8 ± 0.7 × 106) and F4/80+ Ly6G+ double-positive myeloid cells (7.9 ± 1.1 × 106 versus 1.6 ± 0.5 × 106) in peritoneal lavage samples compared to vehicle-treated and infected mice (Fig. 6A).
Fig. 6.
Characterization of peritoneal myeloid cell composition and phagocytic function from MPLA-treated mice. Mice were treated with MPLA for 2 days prior to challenge with FITC-labeled and heat-killed P. aeruginosa. Cells were harvested for analysis at 6 h after bacterial challenge. (A) Flow cytometric analysis of peritoneal leukocytes after labeling with anti-F4/80 and anti-Ly6G. (B) Total numbers and percentages of myeloid cells that were positive for FITC-labeled P. aeruginosa. (C) Representative figures from multispectral imaging flow cytometry analysis, which was used to verify the results of our phagocytosis and myeloid marker experiments. *, P < 0.05 compared to vehicle-treated mice (n = 4 mice per group).
The phagocytic function of recruited myeloid cells from MPLA- and vehicle-treated mice was evaluated after intraperitoneal challenge with FITC-labeled P. aeruginosa (Fig. 6B). MPLA pretreatment significantly (P < 0.05) increased the percentages and total numbers of macrophages (F4/80+ Ly6G−), double-positive myeloid cells (F4/80+Ly6G+) and neutrophils (F4/80− Ly6C+) that phagocytosed FITC-labeled Pseudomonas (Fig. 6B). The FITC mean fluorescence intensity, an indicator of the total number of Pseudomonas engulfed per cell, was not significantly different compared to controls (data not shown). Analysis by multispectral flow cytometry (ImageStream flow cytometer; Amnis Corp., Seattle, WA) confirmed the presence of macrophages (F4/80+ Ly6G−), double-positive myeloid cells (F4/80+ Ly6G+) and neutrophils (F4/80− Ly6C+) in the peritoneal cavities of MPLA-treated mice and was able to differentiate between surface associated and internalized bacteria. The analysis showed that >77% of Pseudomonas organisms were intracellular and not bound to the cell surface of leukocytes (Fig. 6C).
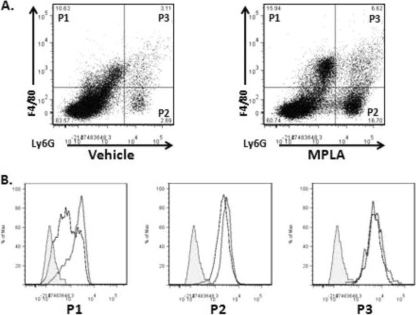
Myeloperoxidase (MPO) is an important antimicrobial enzyme produced in neutrophils and macrophages that is responsible for the generation of reactive oxygen and nitrogen compounds capable of killing pathogens (1). To assess the effect of MPLA on MPO production, mice received intraperitoneal priming with MPLA or vehicle and were infected with 108 CFU of Pseudomonas. At 1 h after infection, peritoneal cells were harvested and MPO production was determined by antibody labeling and flow cytometry. MPLA treatment facilitated infection-induced recruitment of F4/80+ and Gr-1+ myeloid cells to the peritoneal cavity compared to vehicle-treated mice (Fig. 7 A). Furthermore, infection caused an increase in leukocyte MPO production in both vehicle- and MPLA-treated mice (Fig. 7B). MPO expression was greater in F4/80+ Ly6G− monocytes from MPLA-treated mice than in vehicle-treated mice after infection (Fig. 7B). Pseudomonas infection induced the production of MPO among Ly6G+ F4/80− neutrophils or Ly6G+ F4/80+ double-positive myeloid cells, but no difference in MPO levels in those populations was observed among vehicle- and MPLA-treated mice (Fig. 7B).
Fig. 7.
MPLA treatment augmented sepsis-induced myeloperoxidase (MPO) production in macrophages but not neutrophils or double-positive myeloid cells. Mice were treated with MPLA or vehicle, followed by an intraperitoneal challenge with P. aeruginosa. (A) Representative dot plots from intraperitoneal cells that were recovered and assayed for F4/80 and Ly6G expression. (B) Histogram demonstrates intracellular MPO staining from the three myeloid populations in panel A (P1, F4/80+ monocytes; P2, Ly6G+ polymorphonuclear leukocytes; P3, F4/80+ and Ly6G+ immature myeloid cells). Isotype control, shaded curve; vehicle-treated mice, solid line; MPLA-treated mice, dotted line. The data are representative of three independent trials.
Intravenous MPLA treatment increases the numbers of myeloid cells at the site of infection and attenuates the decline of leukocytes in peripheral blood during systemic infection.
In the intraperitoneal priming model described above, both the route of MPLA administration and the site of insult were the same, possibly resulting in the prerecruitment of myeloid cells to the site of infection. Therefore, further studies were undertaken to assess the effect of intravenous MPLA treatment on the recruitment of leukocytes to the primary site of infection. This was determined by harvesting intraperitoneal leukocytes at 6 h after intraperitoneal challenge with P. aeruginosa in mice that received intravenous treatment with MPLA (Fig. 8). Compared to vehicle-treated controls, intravenous MPLA treatment did not significantly affect the numbers or percentages of myeloid cell populations within the peritoneal cavity in the absence of Pseudomonas infection (Fig. 8). In both groups, the predominant myeloid cell population in noninfected mice consisted of F4/80+ Ly6G− resident macrophages. After challenge with P. aeruginosa, MPLA-primed mice had a larger percentage and total number of intraperitoneal macrophages (F4/80+ Ly6G−) and double-positive myeloid cells (F4/80+ Ly6G+) compared to vehicle-treated mice (Fig. 8). The numbers of neutrophils in MPLA-treated mice were not significantly different from those in vehicle-treated controls (Fig. 8).
Fig. 8.
Intravenous MPLA enhanced recruitment of phagocytic granulocytes to the site of infection. Mice were treated intravenously with MLPA or vehicle, followed by an intraperitoneal challenge with P. aeruginosa. Peritoneal leukocytes recovered after infection were assayed for myeloid marker expression. (A) Representative dot plots from vehicle- and MPLA-treated mice. Quadrant percentages are displayed. (B) The total number of cells from the respective myeloid cell populations from MPLA-treated and vehicle-treated mice that were recruited to the peritoneum. *, P < 0.05 compared to vehicle-treated mice (n = 4 mice per group, data from two independent trials).
Neutrophil, but not monocyte/macrophage, depletion eliminates the antimicrobial effect of MPLA treatment.
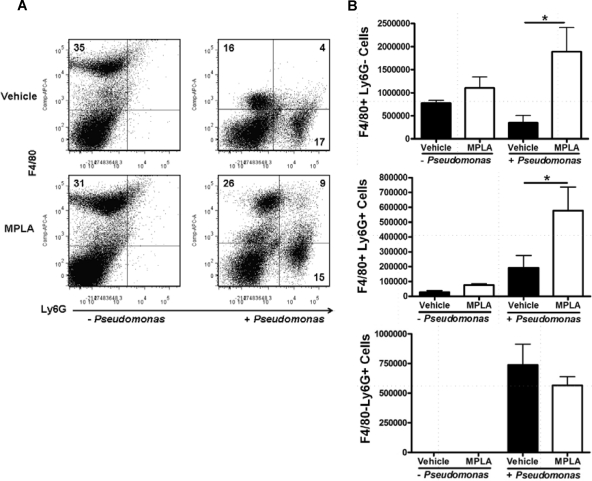
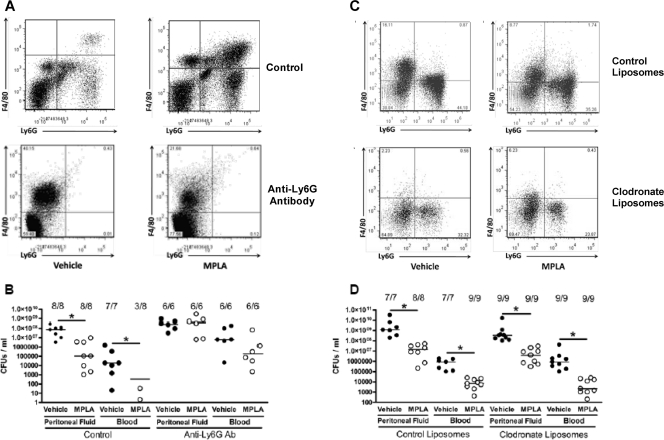
As shown above, MPLA priming causes a significant influx of phagocytic Ly6G+ neutrophils and double-positive (Ly6G+ F4/80+) myeloid cells into the peritoneal cavity, and this population is increased further after infection. To determine the functional importance of these Ly6G+ populations in MPLA-mediated immunomodulation, mice received treatment with anti-Ly6G specific antibody (clone 1A8) after being primed with vehicle or MPLA. Treatment with anti-Ly6G antibody eliminated the Ly6G+ F4/80− neutrophil population, as well as the Ly6G+ F4/80+ double-positive myeloid population (Fig. 9 A). Depletion of Ly6G+ cells also eliminated the enhanced bacterial clearance seen in MPLA-primed mice (Fig. 9B).
Fig. 9.
Effect of myeloid cell depletion on MPLA-induced alterations in bacterial clearance. Mice received treatment with MPLA or vehicle. After treatment, Ly6G+ cells were depleted by intraperitoneal treatment with anti-Ly6G antibody (clone 1A8), followed by infection with P. aeruginosa. (A) Peritoneal myeloid cells from mice treated with control or anti-Ly6G antibodies. (B) Antibody-mediated Ly6G+ cell depletion eliminated the enhanced bacterial clearance normally conferred by MPLA pretreatment. (C) MPLA- or vehicle-treated prime mice underwent treatment with control or clodronate liposomes to deplete monocytes, and mice were then challenged with P. aeruginosa. (C) Peritoneal myeloid populations after clodronate liposome treatment in vehicle- and MPLA-treated mice. (D) Clodronate liposome-mediated monocyte depletion did not eliminate the enhanced bacterial clearance seen in MPLA-treated mice. *, P < 0.05 compared to vehicle (n = 6 to 9 mice per group).
To determine whether monocytes/macrophages participate in the antimicrobial effect elicited by MPLA, this population was depleted by treatment with clodronate-containing liposomes. Treatment with clodronate liposomes decreased the numbers of F4/80+ monocytes and F4/80+ Ly6G+ immature myeloid cells by >98% (Fig. 9C). However, monocyte/macrophage depletion did not alter the MPLA-induced augmentation of bacterial clearance (Fig. 9D).
DISCUSSION
The results of this study demonstrate that mice treated with the TLR4 agonist MPLA show increased resistance to clinically relevant models of systemic bacterial infection. MPLA pretreatment improved survival, prevented hypothermia, attenuated proinflammatory cytokine production, and enhanced the clearance of bacteria in the CLP model of sepsis and in mice with Pseudomonas burn wound infection. The improved resistance to infection occurred independently of the route of MPLA administration, since both intraperitoneal and intravenous routes of administration were protective. The full benefit of MPLA, as utilized in the present study, required prophylactic administration. Nevertheless, a prophylactic strategy has potential utility in clinical scenarios in which patients are at increased risk of developing infectious complications. Among these are patients that have suffered major trauma or large burns or have undergone high-risk surgery. Patients with severe cutaneous burns are highly susceptible to burn wound and systemic infections. In the present study, treatment of burned mice with MPLA markedly decreased local spread and systemic dissemination of P. aeruginosa in a clinically relevant model of wound infection. Patients undergoing major intra-abdominal surgery are also at high risk for infection, especially bacterial peritonitis. Prophylactic treatment with MPLA markedly improved resistance to peritonitis caused by CLP in our study by improving bacterial clearance and attenuating systemic cytokine production. This approach has potential clinical relevance since immunomodulators such as MPLA could be given preoperatively to decrease the incidence or severity of postoperative infections and inflammation-induced morbidity in patients undergoing high-risk surgical procedures.
MPLA improved bacterial clearance, at least in part, by promoting the recruitment of phagocytic myeloid cells to sites of infection. Irrespective of the route of administration, MPLA-treated mice showed increased numbers of phagocytic macrophages, neutrophils, and double-positive myeloid cells at sites of infection, although the phenotypic characteristics of the recruited leukocytes differed somewhat between mice receiving intraperitoneal or intravenous treatment with MPLA. In general, the overall numbers of phagocytic myeloid cells at sites of infection were increased in mice receiving MPLA treatment. However, phagocytic function and MPO activity on a per-cell basis were not altered by MPLA treatment, implying that the effect of MPLA may not be mediated through an increase in the antimicrobial functions of individual cells but by the recruitment and activation of larger numbers of phagocytes, particularly neutrophils. Depletion of Ly6G+ cells proved to abrogate the ability of MPLA treatment to improve bacterial clearance. Anti-Ly6G treatment caused depletion of neutrophils, as well as myeloid cells that coexpress Ly6G and F4/80. We have previously reported that the double-positive myeloid cell population has characteristics consistent with the monocyte phenotype, including a kidney-shaped nucleus and coexpression of Ly6C. In the present study, we show that the double-positive population is also eliminated by treatment with clodronate-containing liposomes, which further supports the conclusion that the cells are of the monocyte/macrophage lineage. Clodronate liposome treatment did not significantly alter MPLA-induced enhancement of bacterial clearance, which implies that the double-positive myeloid cells and true macrophages are not essential for MPLA-enhanced bacterial clearance. Thus, it appears that the innate immunomodulating effect associated with MPLA pretreatment is mediated predominantly through the neutrophil myeloid lineage.
The large influx of myeloid cells into sites of infection was not associated with increased proinflammatory cytokine production. Paradoxically, the production of proinflammatory cytokines was markedly attenuated in MPLA-treated mice. Treatment with MPLA, like other endotoxin derivatives, has been shown to attenuate subsequent cytokine production normally triggered by inflammatory stimuli, such as infection or LPS exposure (4, 9). Numerous reports have shown that exposure of leukocytes to endotoxin analogs causes intrinsic alterations in intracellular signaling mechanisms that result in decreased production of proinflammatory gene products, a phenomenon known as endotoxin tolerance (25, 38, 41). The alterations are most evident in the monocyte/macrophage population and include decreased mitogen-activated protein kinase phosphorylation and alterations in NF-κB translocation. Based on extensive prior research, it is likely that the attenuation of proinflammatory cytokine production observed in the present study is due to effects on macrophages and monocytes. However, MPLA-induced enhancement of bacterial clearance appears to be independent of those cell populations and is mediated by neutrophils. The recruitment of neutrophils during infection is dependent on complex interactions with macrophages and endothelial cells (17). One could conclude, based on our findings, that MPLA treatment induces a state of priming that facilitates neutrophil recruitment in response to a subsequent infection. Whether this state is due to a direct effect of MPLA on neutrophils and/or endothelial cells remains to be determined. Little is known about the effects of exposure to TLR4 agonists on neutrophil antibacterial functions, and our study clearly shows that the enhanced bacterial clearance seen after MPLA treatment is dependent on TLR4. Some prior studies have reported that endotoxin exposure causes a state of tolerance among endothelial cells characterized by decreased cytokine production and adhesion molecule expression (19, 34). Recent work indicates that endothelial LPS tolerance induction may not impede proinflammatory cytokine production; however, like most previous studies, this work was performed in vitro (37). Our studies were performed in vivo using live infections, thus creating a better model of true immune function. Future studies are needed to determine the impact of MPLA treatment on neutrophil and endothelial cell functions and interactions during in vivo infection.
A novel finding in the present study is that while MPLA administration after the onset of infection does not induce a preservation of core temperature or a dramatic attenuation in proinflammatory cytokine production, it does confer a heightened state of bacterial clearance. This indicates that the anti-inflammatory and antibacterial effects of MPLA may be elicited by different mechanisms and temporally separate, as noted above. It appears that the antimicrobial effects induced by MPLA treatment are activated rapidly, whereas the effects on cytokine production require prior exposure and cellular reprogramming, which is consistent with the development of endotoxin tolerance. It is possible that systemic treatment with MPLA accelerates the induction of antimicrobial mechanisms and facilitates a more rapid antimicrobial response. As noted above, neutrophil and endothelial cell activation may be among those responses. Future studies will be required to determine the impact of MPLA on those cell populations, as noted above.
Previous studies from our laboratory, and others, have demonstrated that treatment with LPS is capable of conferring resistance to infection by mechanisms that have not been fully elucidated (23, 24, 36). However, the systemic administration of LPS in humans is not an attractive option due to inflammation-associated toxicity and a narrow therapeutic index. MPLA provides a more plausible alternative because of its decreased toxicity and side effects. MPLA is relatively low in toxicity and is currently approved for clinical use as a vaccine adjuvant. As shown here, MPLA retains potent immunomodulatory properties and improves the innate host response to infection by increasing bacterial clearance while attenuating the production of proinflammatory cytokines. These characteristics are attractive and make MPLA a strong candidate immunomodulator for application in the clinical setting.
In conclusion, the present study shows that treatment with the TLR4 agonist MPLA causes improved resistance to systemic infection that is characterized by attenuation of systemic proinflammatory cytokine production but improved clearance of bacteria. The improvement in bacterial clearance is mediated, in part, by enhanced recruitment of phagocytic myeloid cells to sites of infection. This effect is lost with depletion of neutrophils, but not macrophages. Although the overall response to a bacterial challenge is improved by MPLA treatment, per-cell functions do not appear to be enhanced, and the improvement in bacterial clearance appears to be mediated through increased recruitment of neutrophils to sites of infection. Postinfection administration of MPLA is capable of improving bacterial clearance but does not significantly attenuate proinflammatory cytokine production. The ability of MPLA to improve survival, reduce inflammation, and enhance bacterial clearance makes it an attractive agent for potential application in patients that are at high risk of developing infectious complications.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health grants R01 GM66885 and F31 AI080159.
We thank the Amnis Corp. for analysis of our samples with the ImageStream cytometer, and we also acknowledge the technical support of Geping Fang.
Footnotes
Published ahead of print on 6 June 2011.
REFERENCES
- 1. Arnhold J., Flemmig J. Human myeloperoxidase in innate and acquired immunity. Arch. Biochem. Biophys. 500:92–106 [DOI] [PubMed] [Google Scholar]
- 2. Astiz M., Galera A., Saha D., Carpati C., Rackow E. 1994. Monophosphoryl lipid A protects against gram-positive sepsis and tumor necrosis factor. Shock 2:271–274 [DOI] [PubMed] [Google Scholar]
- 3. Astiz M. E., Saha D. C., Brooks K., Carpati C. M., Rackow E. C. 1993. Comparison of the induction of endotoxin tolerance in endotoxemia and peritonitis by monophosphoryl lipid A and lipopolysaccharide. Circ, Shock 39:194–198 [PubMed] [Google Scholar]
- 4. Aybay C., Imir T. 1998. Comparison of the effects of Salmonella minnesota Re595 lipopolysaccharide, lipid A, and monophosphoryl lipid A on nitric oxide, TNF-α, and IL-6 induction from RAW 264.7 macrophages. FEMS Immunol. Med. Microbiol. 22:263–273 [DOI] [PubMed] [Google Scholar]
- 5. Blanque R., Meakin C., Millet S., Gardner C. R. 1996. Hypothermia as an indicator of the acute effects of lipopolysaccharides: comparison with serum levels of IL-1β, IL-6, and TNF α. Gen. Pharmacol. 27:973–977 [DOI] [PubMed] [Google Scholar]
- 6. Broad A., Jones D., Kirby J. 2006. Toll-like receptor (TLR) response tolerance: a key physiological “damage limitation” effect and an important potential opportunity for therapy. Curr. Med. Chem. 13:2487–2502 [DOI] [PubMed] [Google Scholar]
- 7. Cavaillon J., Adrie C., Fitting C., Adib-Conquy M. 2003. Endotoxin tolerance: is there a clinical relevance? J. Endotoxin Res. 9:101–107 [DOI] [PubMed] [Google Scholar]
- 8. Cross A. S. 2002. Endotoxin tolerance-current concepts in historical perspective. J. Endotoxin Res. 8:83–98 [DOI] [PubMed] [Google Scholar]
- 9. De Becker G., et al. 2000. The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int. Immunol. 12:807–815 [DOI] [PubMed] [Google Scholar]
- 10. Elner S. G., et al. 2005. TLR4 mediates human retinal pigment epithelial endotoxin binding and cytokine expression. Trans. Am. Ophthalmol. Soc. 103:126–135 [PMC free article] [PubMed] [Google Scholar]
- 11. Flierl M. A., et al. 2008. Functions of the complement components C3 and C5 during sepsis. FASEB J. 22:3483–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gutierrez-Fernandez J., Maroto M. C., Piedrola G., Zamora E. 1987. Complement and sepsis. Allergol. Immunopathol. (Madr.) 15:145–149 [PubMed] [Google Scholar]
- 13. Henricson B., Benjamin W., Vogel S. 1990. Differential cytokine induction by doses of lipopolysaccharide and monophosphoryl lipid A that result in equivalent early endotoxin tolerance. Infect. Immun. 58:2429–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang Z., et al. 2006. Effect of transforming growth factor-beta neutralization on survival and bacterial clearance in a murine model of Pseudomonas aeruginosa burn wound infection. J. Burn Care Res. 27:682–687 [DOI] [PubMed] [Google Scholar]
- 15. Jiang H. W., Zhang W., Ren B. P., Zeng J. F., Ling J. Q. 2006. Expression of Toll-like receptor 4 in normal human odontoblasts and dental pulp tissue. J. Endod. 32:747–751 [DOI] [PubMed] [Google Scholar]
- 16. Johnson A. G., Tomai M., Solem L., Beck L., Ribi E. 1987. Characterization of a nontoxic monophosphoryl lipid A. Infect. Dis. 9:512. [DOI] [PubMed] [Google Scholar]
- 17. Kadl A., Leitinger N. 2005. The role of endothelial cells in the resolution of acute inflammation. Antioxid. Redox Signal. 7:1744–1754 [DOI] [PubMed] [Google Scholar]
- 18. Lebre M. C., et al. 2007. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Invest. Dermatol. 127:331–341 [DOI] [PubMed] [Google Scholar]
- 19. Lush C. W., Cepinskas G., Kvietys P. R. 2000. LPS tolerance in human endothelial cells: reduced PMN adhesion, E-selectin expression, and NF-κB mobilization. Am. J. Physiol. Heart Circ. Physiol. 278:H853–H861 [DOI] [PubMed] [Google Scholar]
- 20. Mariencheck W. I., Savov J., Dong Q., Tino M. J., Wright J. R. 1999. Surfactant protein A enhances alveolar macrophage phagocytosis of a live, mucoid strain of Pseudomonas aeruginosa. Am. J. Physiol. 277:L777–L786 [DOI] [PubMed] [Google Scholar]
- 21. Morgan B. P. 2000. Measurement of complement hemolytic activity, generation of complement-depleted sera, and production of hemolytic intermediates. Methods Mol. Biol. 150:61–71 [DOI] [PubMed] [Google Scholar]
- 22. Muir A., et al. 2004. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 30:777–783 [DOI] [PubMed] [Google Scholar]
- 23. Murphey E., Fang G., Sherwood E. 2008. Endotoxin pretreatment improves bacterial clearance and decreases mortality in mice challenged with Staphylococcus aureus. Shock 29:512–518 [DOI] [PubMed] [Google Scholar]
- 24. Murphey E. D., Sherwood E. R. 2006. Bacterial clearance and mortality are not improved by a combination of IL-10 neutralization and IFN-gamma administration in a murine model of post-CLP immunosuppression. Shock 26:417–424 [DOI] [PubMed] [Google Scholar]
- 25. Ogawa H., et al. 2003. Mechanisms of endotoxin tolerance in human intestinal microvascular endothelial cells. J. Immunol. 170:5956–5964 [DOI] [PubMed] [Google Scholar]
- 26. Qureshi N., Takayama K., Ribi E. 1982. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J. Biol. Chem. 257:11808–11815 [PubMed] [Google Scholar]
- 27. Raetz C. R., Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Redl H., Schlag G. 1999. Cytokines in severe sepsis and septic shock. Birkhauser Verlag, Basel, Switzerland [Google Scholar]
- 29. Salkowski C., Detore G., Vogel S. 1997. Lipopolysaccharide and monophosphoryl lipid A differentially regulate interleukin-12, gamma interferon, and interleukin-10 mRNA production in murine macrophages. Infect. Immun. 65:3239–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takayama K., Qureshi N., Ribi E., Cantrell J. L. 1984. Separation and characterization of toxic and nontoxic forms of lipid A. Rev. Infect. Dis. 6:439–443 [DOI] [PubMed] [Google Scholar]
- 31. Takeda K., Akira S. 2004. TLR signaling pathways. Semin. Immunol. 16:3–9 [DOI] [PubMed] [Google Scholar]
- 32. Tang S. C., et al. 2008. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp. Neurol. 213:114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thoelen S., et al. 1998. Safety and immunogenicity of a hepatitis B vaccine formulated with a novel adjuvant system. Vaccine 16:708–714 [DOI] [PubMed] [Google Scholar]
- 34. Uhrig A., et al. 2005. Development and functional consequences of LPS tolerance in sinusoidal endothelial cells of the liver. J. Leukoc. Biol. 77:626–633 [DOI] [PubMed] [Google Scholar]
- 35. Ungaro R., Abreu M. T., Fukata M. 2009. Practical techniques for detection of Toll-like receptor-4 in the human intestine. Methods Mol. Biol. 517:345–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Varma T., et al. 2005. Endotoxin priming improves clearance of Pseudomonas aeruginosa in wild-type and interleukin-10 knockout mice. Infect. Immun. 73:7340–7347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang W., et al. 28 September 2010. TLR4 activation induces nontolerant inflammatory response in endothelial cells. Inflammation [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 38. West M. A., Koons A. 2008. Endotoxin tolerance in sepsis: concentration-dependent augmentation or inhibition of LPS-stimulated macrophage TNF secretion by LPS pretreatment. J. Trauma 65:893–900 [DOI] [PubMed] [Google Scholar]
- 39. Wy C., Goto M., Young R., Myers T., Muraskas J. 2000. Prophylactic treatment of endotoxic shock with monophosphoryl lipid A in newborn rats. Biol. Neonate 77:191–195 [DOI] [PubMed] [Google Scholar]
- 40. Wynn J. L., et al. 2008. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 112:1750–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ziegler-Heitbrock L. 2001. The p50-homodimer mechanism in tolerance to LPS. J. Endotoxin Res. 7:219–222 [PubMed] [Google Scholar]