Abstract
Many enteric bacteria use bile as an environmental cue to signal resistance and virulence gene expression. Microarray analysis of enterohemorrhagic Escherichia coliO157:H7 (EHEC) treated with bile salts revealed upregulation of genes for an efflux system (acrAB), a two-component signal transduction system (basRS/pmrAB), and lipid A modification (arnBCADTEFand ugd). Bile salt treatment of EHEC produced a basS- and arnT-dependent resistance to polymyxin.
TEXT
Enterohemorrhagic Escherichia coli(EHEC), including serotype O157:H7, causes a severe food-borne illness associated with diarrhea, hemorrhagic colitis (HC), and hemolytic-uremic syndrome (HUS) (17, 29). Upon ingestion, en route to the colon, the bacteria encounter a variety of antimicrobial stresses, including gastric acids in the stomach (27) and bile in the duodenum and small intestine. Bile is a complex mixture composed mainly of bile salts, as well as phospholipids, cholesterol, proteins, and bilirubin (15). Bile salts are amphipathic molecules that act as detergents aiding in lipid solubilization and digestion but also play a role in host defense, as they have potent antimicrobial properties (26). For this reason, bile resistance is an essential characteristic of enteric bacteria and is achieved primarily via active efflux mechanisms (6, 32, 35, 53, 64) and altered permeability of the outer membrane (64, 70). The RND efflux systems have been well described as playing a significant role in bile resistance among Gram-negative bacteria (45). Additionally, the use of two-component regulatory systems (TCRS) (52, 68) and alterations of the lipopolysaccharide (LPS) layer have been shown to be involved in resistance to bile in several bacteria (8, 42, 43, 49, 71).
Bile has also been demonstrated to be an environmental signal that controls the expression of colonization and virulence factors of several enteric bacteria (13, 27, 28, 31, 36, 50, 51, 54, 55, 65). Much of the work on Gram-negative bacteria's response to bile has been performed with Salmonella(9, 44, 51–54, 57, 59, 68). Since marked differences in gene expression after bile stress have been observed even between Salmonella entericaserovar Typhimurium and S. entericaserovar Typhi, differences may also exist in EHEC (68). Thus, here we investigated the response of E. coliO157:H7 to bile salt stress and the influence bile salts have on bile resistance mechanisms and virulence gene expression.
Transcriptional analysis of bile salt-treated EHEC.
The bile salt stress protocol used here was modified from reference 13. Briefly, bacteria were grown in Luria-Bertani medium (LB) at 37°C with shaking overnight and then subcultured in Dulbecco's modified Eagle's medium (DMEM) at pH 7.4 and statically incubated at 37°C in 5% CO2until an optical density at 600 nm of 0.4 was reached. Bacteria were then gently pelleted by centrifugation, and the medium was replaced with either DMEM (Wisent) at pH 7.4 or a 0.15% bile salt mixture (BSM; Sigma B-3426) in DMEM at pH 7.4. These cultures were statically incubated at 37°C in 5% CO2for 90 min. Bacteria were then harvested for analysis or additional treatments. Initially, we used microarray-based expression profiling of EHEC strain 86-24 (MWG E. coliO157:H7 array [GenBank accession number GPL533] [27]) in both the presence and the absence of BSM. RNA purification and microarray analysis were performed as described by House et al. (27). Computational analysis of four control and four BSM-treated EHEC RNA samples on four microarrays was performed by the University Health Network Microarray Center (Toronto, Ontario, Canada), and significance was determined by significance analysis of microarrays (SAM) analysis and ttests. The complete data set is available under NCBI Gene Expression Omnibus Series accession number GSE22060(14). Our analysis showed that 30 genes were upregulated (Table 1) and 35 genes were downregulated 1.5-fold or more after exposure to BSM relative to the control (Table 2). Semiquantitative reverse transcriptase PCR (as described in reference 66) was used to confirm several upregulated genes of interest (data not shown). Promoters of genes of interest were identified using the RegulonDB online database (16) and cloned into the promoterless β-galactosidase expression vector pMC1403 (5). β-Galactosidase reporter assays (5) were performed under a variety of conditions to further examine the bile responsiveness of promoters of interest (Fig. 1).
Table 1.
Summary of EHEC 86-24 transcripts with a 1.5-fold or greater increase in expression after bile salt treatment relative to that of the untreated controla
| Gene | Product and predicted function | Fold change | Pvalue |
|---|---|---|---|
| ais | Protein induced by aluminum; function unknown | 6.85 | <0.005 |
| arnC | Undecaprenyl phosphate-l-Ara4FN transferase | 6.39 | <0.001 |
| arnD | Undecaprenyl phosphate-alpha-l-Ara4FN deformylase | 4.46 | <0.001 |
| hycF | Formate hydrogen lyase complex iron-sulfur protein | 3.43 | <0.005 |
| hycB | Hydrogenase 3, Fe-S subunit | 3.21 | <0.005 |
| hydN | Putative electron transport protein HydN/iron-sulfur protein required for Hyd-3 activity | 2.90 | 0.022 |
| arnTb | l-Ara4N transferase | 2.65 | 0.005 |
| arnB | UDP-4-amino-4-deoxy-l-arabinose synthase; UDP-4″-ketopentose aminotransferase; l-glutamate is the amine donor | 2.63 | 0.028 |
| hycA | Transcriptional repression of hycand hypoperons | 2.59 | 0.011 |
| arnF | Undecaprenyl phosphate-alpha-l-Ara4N exporter; flippase ArnEF subunit | 2.45 | 0.0066 |
| acrR | acrABoperon repressor | 2.37 | 0.015 |
| fdhF | Formate dehydrogenase | 2.36 | 0.014 |
| ugdb | UDP-glucose 6-dehydrogenase | 2.16 | <0.001 |
| basSb | Sensory histidine kinase in two-component regulatory system with BasR | 2.13 | 0.0057 |
| eptA | Predicted metal-dependent hydrolase/lipid A phosphoethanolamine transferase, associated with polymyxin resistance | 2.12 | 0.031 |
| hycI | Protease involved in processing of the C-terminal end of the large subunit of hydrogenase 3 | 2.05 | <0.001 |
| hycG | Component of hydrogenase 3; formate hydrogen lyase complex | 2.04 | 0.01 |
| arnA | UDP-glucuronate dehydrogenase and UDP-Ara4N formyltransferase | 2.01 | 0.031 |
| basR | DNA-binding response regulator in two-component regulatory system with BasS | 2.00 | <0.005 |
| hycD | Formate hydrogen lyase complex inner membrane protein | 1.91 | 0.026 |
| arnF | Undecaprenyl phosphate-alpha-l-Ara4N exporter; flippase ArnEF subunit | 1.90 | <0.005 |
| acrAb | Membrane fusion protein/component of AcrAB-TolC multidrug efflux system/acridine efflux pump | 1.82 | <0.005 |
| acrB | AcrB RND-type permease/component of AcrAB-TolC multidrug efflux system | 1.75 | <0.005 |
| hycC | Formate hydrogen lyase complex inner membrane protein | 1.72 | 0.044 |
| yeeF | Putative amino acid/amine transport protein; required for swarming phenotype, function unknown | 1.65 | <0.005 |
| gatY | d-Tagatose 1,6-bisphosphate aldolase 2, catalytic subunit | 1.56 | 0.019 |
| hycE | Hydrogenase 3, large subunit | 1.56 | 0.016 |
| prmC | N5-Glutamine methyltransferase, modifies release factors RF-1 and RF-2 | 1.55 | 0.017 |
| yfbQ | Predicted aminotransferase | 1.51 | <0.01 |
| yehDb | Predicted fimbrial adhesin-like protein; FimA homologue | 1.50 | <0.01 |
As determined by SAM analysis. n= 4 independent cultures (4 treatment, 4 control), n= 4 chips, n= 2 replicate spots per chip. Pvalues were determined using a one-way Student ttest. Bolded genes names indicate genes in operons in which increased expression was verified by β-galactosidase reporter assay.
Increased expression verified by semiquantitative reverse transcriptase PCR.
Table 2.
Summary of EHEC 86-24 transcripts with a 1.5-fold or greater decrease in expression after bile salt treatment relative to that of the untreated controla
| Gene | Product and predicted function | Fold change | Pvalue |
|---|---|---|---|
| Z1540 | Hypothetical protein | −3.21 | <0.05 |
| ymfP | Pseudogene, e14 prophage | −2.77 | <0.05 |
| ompF | Outer membrane protein 1a | −2.54 | <0.005 |
| ECS2038 | Similar to putative membrane transport protein B1433 (E. coli) | −1.91 | <0.05 |
| Z0273 | Hypothetical protein | −1.81 | <0.05 |
| yrbL | Hypothetical protein | −1.80 | <0.05 |
| ECS3219 | Similar to B2335 (E. coli), minor fimbrial subunit StfE protein (S. entericaserovar Typhimurium) | −1.75 | 0.016 |
| Z5401 | Hypothetical protein | −1.71 | 0.013 |
| yciO | Hypothetical protein | −1.71 | <0.05 |
| ydfZ | Conserved protein | −1.70 | 0.0011 |
| yaiS | Conserved protein | −1.67 | <0.005 |
| exoP | Putative exodeoxyribonuclease (cryptic prophage CP-933P) | −1.67 | 0.012 |
| Z4067 | Hypothetical protein | −1.64 | <0.05 |
| proW | Glycine betaine transporter membrane protein | −1.64 | 0.0057 |
| ECS1528 | Similar to hypothetical protein (bacteriophage 933W) | −1.60 | 0.012 |
| moaA | Molybdenum cofactor biosynthesis protein A | −1.60 | <0.05 |
| ECS1219 | Similar to putative small subunit terminase (bacteriophage 933W) | −1.60 | 0.017 |
| Z5162 | Hypothetical protein | −1.58 | <0.05 |
| terA2 | Putative phage inhibition, colicin resistance and tellurite resistance protein | −1.58 | <0.005 |
| ECS2283 | Hypothetical protein | −1.58 | <0.05 |
| ECS1211 | Similar to hypothetical protein (bacteriophage 933W) | −1.56 | <0.05 |
| Z1466 | Unknown protein (bacteriophage BP-933W) | −1.56 | <0.05 |
| Z2042 | Unknown protein (prophage CP-933O) | −1.56 | <0.05 |
| yaiY | Predicted inner membrane protein | −1.56 | <0.05 |
| yajO | 2-Carboxybenzaldehyde reductase, function unknown | −1.55 | <0.05 |
| modD | Molybdenum transport protein | −1.54 | <0.05 |
| Z1491 | Unknown protein (bacteriophage BP-933W) | −1.53 | <0.05 |
| B2640 | Hypothetical protein | −1.53 | <0.005 |
| ECS0337 | Similar to probable transcription regulator YkgA | −1.52 | <0.05 |
| terD | Putative tellurium resistance protein TerD | −1.50 | 0.012 |
| engA | GTP-binding protein EngA | −1.50 | <0.05 |
| stx2B | Shiga toxin 2 B subunit | −1.50 | <0.05 |
| Z2087 | Unknown protein (prophage CP-933O) | −1.50 | 0.0066 |
| stx2A | Shiga toxin 2 A subunit | −1.50 | 0.017 |
| ECS1329 | Hypothetical protein | −1.50 | <0.05 |
As determined by SAM analysis. n= 4 independent cultures (4 treatment, 4 control), n= 4 chips, n= 2 replicate spots per chip. The Pvalues presented were determined using a one-way Student ttest.
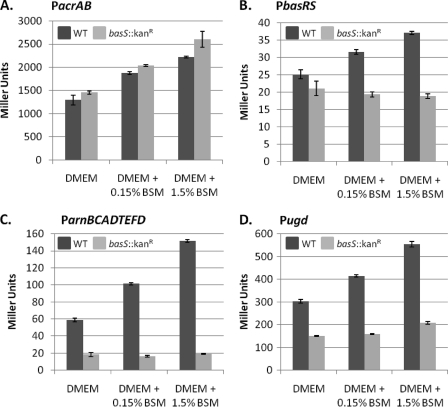
Fig. 1.
β-Galactosidase reporter assays demonstrate EHEC promoters of efflux, and lipid A remodeling operons display concentration-dependent responses to bile salts. The activity of the promoters for acrAB(A), basRS(B), arnBCADTEFD(C), and ugd(D) were examined in β-galactosidase expression assays in both the wild-type (WT) 86-24 (dark gray bars) and basS::Kanr(light gray bars) backgrounds. In the WT background, all of the promoters tested showed statistically significant and reproducible enhanced responses to exposure to increasing concentrations of the bile salt mixture. In the basS::Kanrbackground, PacrAB(A) remained responsive to the presence of bile salts; however, the activity of Pugd(D) was significantly diminished and the responses of PbasRS(B) and ParnBCADTEFD(C) to bile salts were abrogated. The same responses to bile were observed with another base medium (50% LB; with or without 0.15% BSM). Student ttests of the difference between the control (DMEM) and each treatment, as well as between both treatments, were done. A statistically significant difference (P< 0.01) was observed between all compared treatments within the same background strain, with the exception of PbasRSand ParnBCADTEFDin the basS::Kanrbackground. The data shown are for one experiment, but the experiment was repeated four times with similar results (3 independent experiments, 4 replicates within each experiment).
Bile salts alter the expression of genes for efflux systems and porins.
Microarray analysis revealed that genes encoding the AcrA-AcrB RND efflux pump and its regulator (acrA, acrB, and acrR) were upregulated in EHEC by BSM (Table 1). This efflux system has been shown to be a crucial component of bile resistance in E. coliK-12 and S. Typhimurium, as it actively pumps bile out of the cell (32, 44, 45, 53, 64). Using β-galactosidase assays (2), we further demonstrated that the acrABpromoter showed a concentration-dependent response to BSM (Fig. 1A). Bile has previously been demonstrated to pass into the periplasm of E. colivia the OmpF outer membrane porin channel (64). Our microarray results show that BSM treatment downregulates the expression of ompF(Table 2). Combined, these data demonstrate that EHEC employs several bile resistance mechanisms that are similar to those of other Gram-negative bacteria and that our bile salt treatment is effective at eliciting a bona fide physiological response to bile.
Bile salts do not induce Shiga toxin expression or release.
Bile has been demonstrated to induce the expression of Vibrio choleraecholera toxin in the small intestine (28). This toxin is responsible for the severe dehydrating diarrhea associated with cholera (48). EHEC produces similar toxins, known as verotoxins or Shiga toxins (Stx1 and Stx2), which are key virulence factors of the pathogen and are associated with the diarrhea, HC, and HUS characteristic of EHEC infection (4, 10, 58, 61). These toxin genes are located on lambdoid prophages integrated into the bacterial genome (41, 60). Our microarray analysis showed that the genes which encode both subunits (stx2A, stx2B) of this multisubunit toxin were slightly downregulated by bile treatment relative to our control (Table 2). Additionally, five other genes associated with the Stx2 bacteriophage BP-933W were similarly downregulated, indicating that bile treatment does not induce the expression of these phage genes in EHEC. This result was supported by an experiment in which we exposed EHEC to various bile salt treatments (glycocholate, deoxycholate, chenodeoxycholate, ursodeoxycholate, and BSM) and evaluated periplasmic and secreted levels of Stx2 using a well-established Vero cell cytotoxicity assay (as in reference 27). We found no increase in periplasmic or secreted Stx2 after treatment of EHEC with individual bile salts (2.5 mM) or the 0.15% BSM relative to the untreated control (see Fig. S1 in the supplemental material).
This microarray also indicated no change in the expression of other known EHEC virulence factors, including those in the locus of enterocyte effacement pathogenicity island, after BSM exposure (Table 1). Thus, although bile acts a signal for virulence gene expression in other bacteria, it does not appear to do so in EHEC under the conditions used in this study.
The BasR-regulated genes for lipid A modification are upregulated by bile salts.
While efflux is a vital means of resisting the deleterious effects of bile, limiting penetration by altering the composition of the outer membrane is an additional strategy used by many bacteria (42, 49, 57). The genes encoding the BasR-BasS (also known as PmrA-PmrB) histidine kinase TCRS were upregulated by BSM treatment on our microarray and by our confirmatory methods (Table 1and Fig. 1B). TCRS sense and respond to environmental signals, producing physiological changes in bacteria (reviewed in reference 30). Regulation of basR-basSexpression has not previously been linked to bile in E. colior Salmonellaspp. but has been associated with other stresses, including metal ion stress (7, 23, 33, 46, 62, 73) and mild acid stress (25, 62). Here, we established that the basRSpromoter follows a concentration-dependent response to BSM treatment (Fig. 1B). BasR (PmrA) is known to control the expression of the arnBCADTEFD(also known as pmrHFIJKLM) operon, members of which along with ugdare responsible for the synthesis and transfer of 4-amino-4-deoxy-l-arabinose (l-Ara4N) to lipid A (56). Our transcriptome analysis showed upregulation of all members of the arnoperon and ugdby treatment with BSM (Table 1). Additionally, a concentration-dependent response was also observed for promoters of arnBand ugdusing a β-galactosidase reporter assay (Fig. 1C and D). Inactivation of basS(12) did not affect the bile response of the acrABpromoter (Fig. 1A) but did abrogate that of the arnBoperon and ugd(Fig. 1C and D), providing further evidence that the BSM is eliciting the expression of these lipid A modification genes. Interestingly, the basRSpromoter lost the ability to respond to BSM in the absence of basS, as BSM-induced expression of the reporter gene was lost in the basS::Kanrmutant (Fig. 1B). This suggests that BasS may function in its self-regulation in response to bile.
Exposure to bile salts confers EHEC resistance to PMB.
The addition of l-Ara4N to lipid A has been shown to confer on Gram-negative bacteria resistance to several cationic antimicrobial peptides (CAMPs), including polymyxin B (PMB), a peptide antibiotic often used to study antimicrobial peptide resistance (19, 20, 37, 38, 40, 63, 67, 74). The lipid A modifications, controlled through the BasRS (PmrAB) TCRS, in both E. coliand Salmonellaspp. are essential for resistance to PMB; however, in neither organism does it appear that these modifications are required for resistance to bile itself (68) (Fig. 2). Therefore, at least in the case of EHEC, bile may be acting as an environmental signal which triggers outer membrane modifications for resistance to CAMPs within the small intestine.
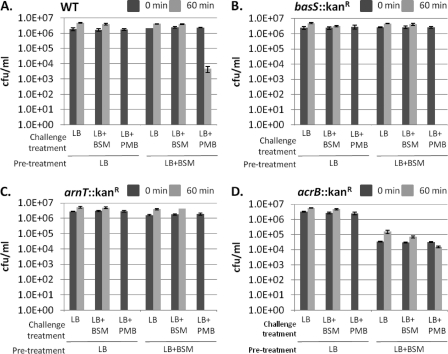
Fig. 2.
Pretreatment of EHEC with bile salts induces a basS- and arnT-dependent resistance to PMB. Bacteria were pretreated with either LB or LB plus BSM (0.15% BSM), and then each was standardized, divided into three samples, and plated for quantification (time, 0 min; dark gray bars). Bacteria were then subjected to one of three challenge treatments (LB, LB plus BSM, or LB plus PMB), incubated for 60 min, and then plated for quantification (light gray bars). Wild-type (WT) 86-24 bacteria (A) pretreated with BSM were able to withstand treatment with PMB, whereas the bacteria pretreated in LB alone were killed by a challenge with PMB. This protection is lost in the basS::Kanr(B) and arnT::Kanr(C) disruption mutants, demonstrating that both basSand arnTare involved in bile salt-induced resistance to PMB. The acrBdisruption (D) was able to resist a challenge with PMB when pretreated with bile salts, although these bacteria were more susceptible to the deleterious effects of bile salts, as demonstrated by reduced levels of growth in the bile salt-treated bacteria relative to those of bacteria grown in LB. Results are from three independent experiments, with three replicates per experiment.
Paneth cells within the small intestine produce CAMPs known as defensins as part of the innate immune system (1, 3, 11, 47). CAMPs are attracted to negative charges of the outer membrane; in Gram-negative bacteria, they function by penetrating this membrane and disrupting the inner membrane (1, 34, 69, 72). Lipid A is an anionic molecule that contributes to the negative charge of the outer membrane. Modification of the outer portion of lipid A with l-Ara4N reduces the negative charge, resulting in resistance to several CAMPs. Gunn et al. demonstrated that in S. Typhimurium, these lipid A modifications, regulated by PmrA-PmrB, were required for resistance to PMB (18, 20). Pseudomonas aeruginosamutants which constitutively expressed pmrB(basS) were observed to be not only resistant to PMB but also cross-resistant to α-defensins, β-defensins-1 and -2, α-helical peptides, and protegrin-1 (40). Enteric bacteria encounter defensins within the small intestine. Therefore, since we observed that the genes associated with l-Ara4N modification of lipid A are upregulated by BSM treatment in EHEC, we asked whether BSM treatment could induce resistance to PMB. Using a broth microdilution method, we first determined the MIC of PMB (Sigma, P0972) for EHEC 86-24 in our system to be 0.15 μg/ml. Bacteria were then cultured in LB in the presence or absence of 0.15% BSM overnight, subcultured in the same treatment (“pretreatment”), incubated under static conditions at 37°C in 5% CO2for 3 to 4 h, and then washed with PBS. Bacteria (1 × 106CFU/ml) were resuspended in a “challenge” medium, i.e., LB, LB plus 0.15% BSM, or LB plus 0.15 μg/ml PMB, for 1 h at 37°C with shaking and then quantified by serial dilutions and plating (Fig. 2). Although these growth conditions varied slightly from those of the initial microarray experiment, β-galactosidase expression assays demonstrated that the promoters of our genes of interest displayed similar trends of upregulation (data not shown). Notably, pretreatment with BSM significantly improved the ability of EHEC 86-24 to survive a lethal concentration of PMB (Fig. 2A). Conversely, when the same experiment was performed with an EHEC basS::Kanrmutant, BSM pretreatment failed to induce resistance to PMB (Fig. 2B). This is further evidence that BasS is a sensor for bile salts and suggests that, in its absence, EHEC cannot respond with the lipid A modifications that protect it from PMB. As arnTencodes the enzyme that transfers l-Ara4N to lipid A (67), the same experiment was performed with an EHEC arnTdisruption mutant in order to determine if this is the modification that results in BSM-induced PMB resistance and not another downstream BasS target. Significantly, bile-induced resistance to PMB was abrogated by inactivation of arnT(12) (Fig. 2C) and restored when the arnTmutation was complemented (24) (see Fig. S2 in the supplemental material), providing physiological evidence that this biochemical pathway is induced by BSM and that it results in resistance to PMB, likely due to l-Ara4N modification of lipid A.
To establish that the bile-induced PMB resistance seen is not a consequence of increased efflux by AcrA-AcrB, we performed the same experiment with an acrBdisruption mutant (12). We observed that BSM-induced resistance to PMB was not affected (Fig. 2D); however, the BSM pretreatment was observed to affect overall bacterial viability, pointing to the significant role this efflux system has in bile resistance. Interestingly, acrABmutants of S. Typhimurium are killed by even low concentrations of bile (53); however, here we see that this is not the case in EHEC. Thanassi et al. also observed that while an E. coliK-12 acrAmutant was hypersensitive to bile, this mutant and an acrA-emrBdouble mutant were still able to survive under bile stress (64). The authors remarked that an additional, unknown, efflux system(s) for managing bile must be in place in E. coli.
We have demonstrated increased transcription of BasRS (PmrAB) and their downstream targets, the l-Ara4N lipid A modification genes, in response to bile in EHEC. In contrast, in Salmonella, neither PmrAB nor its regulator PhoPQ has been shown to be upregulated in response to bile, although, interestingly, both TCRS appear to be important for bile and antimicrobial peptide resistance (18, 18, 20–22, 68). Merighi et al. demonstrated in an in vivoexpression system that both the phoPQand pmrABoperons of S. enterica serovar Typhimurium were upregulated within the mouse intestinal lumen and spleen in response to an unidentified signal (39). Since the authors controlled for known inducers of these operons, it is possible that bile is a signal to which at least one of these TCRS is responding.
Our data are consistent with a model where bile salts in the small intestine serve as an environmental signal for EHEC, one that triggers changes in gene expression which result in protective alterations of the outer membrane, thereby permitting successful transit through the small intestine. We report, for the first time, that bile causes upregulation of the BasR-BasS TCRS, the l-Ara4N LPS alteration pathway, and concomitant antimicrobial resistance in EHEC. These findings offer insights into potential strategies used by EHEC to resist the antimicrobial effects of bile and CAMPs of the small intestine.
Nucleotide sequence accession number.
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (14) and are accessible under GEO Series accession number GSE22060(http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE22060).
Supplementary Material
Acknowledgments
This work was supported by funding to J.V.K. through an NSERC postdoctoral fellowshipand a Ryerson University postdoctoral fellowship, to V.D. through NSERC-USRA, and to D.B.F. through NSERCdiscovery grant 238684.
We thank Victor Gannon (Public Health Agency of Canada) for the microarray chips, Jorge Giron (University of Florida) for EHEC strain 86-24, Brett Finlay (University of British Columbia) for pMC1403, Lori Burrows (McMaster University) for pBADGr, and Jeffery Fillingham for helpful discussions and suggestions for the manuscript.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 1 July 2011.
REFERENCES
- 1. Ayabe T., Ashida T., Kohgo Y., Kono T. 2004. The role of Paneth cells and their antimicrobial peptides in innate host defense. Trends Microbiol. 12:394–398 [DOI] [PubMed] [Google Scholar]
- 2. Baker S. J., Daniels C., Morona R. 1997. PhoP/Q regulated genes in Salmonella typhi: identification of melittin sensitive mutants. Microb. Pathog. 22:165–179 [DOI] [PubMed] [Google Scholar]
- 3. Bevins C. L. 2006. Paneth cell defensins: key effector molecules of innate immunity. Biochem. Soc. Trans. 34:263–266 [DOI] [PubMed] [Google Scholar]
- 4. Boerlin P., et al. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coliand disease in humans. J. Clin. Microbiol. 37:497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casadaban M. J., Chou J., Cohen S. N. 1980. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coliplasmid vectors for the detection and cloning of translational initiation signals. J. Bacteriol. 143:971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cerda-Maira F. A., Ringelberg C. S., Taylor R. K. 2008. The bile response repressor BreR regulates expression of the Vibrio choleraebreABefflux system operon. J. Bacteriol. 190:7441–7452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chamnongpol S., Dodson W., Cromie M. J., Harris Z. L., Groisman E. A. 2002. Fe(III)-mediated cellular toxicity. Mol. Microbiol. 45:711–719 [DOI] [PubMed] [Google Scholar]
- 8. Clements A., et al. 2007. Secondary acylation of Klebsiella pneumoniaelipopolysaccharide contributes to sensitivity to antibacterial peptides. J. Biol. Chem. 282:15569–15577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crawford R. W., Gibson D. L., Kay W. W., Gunn J. S. 2008. Identification of a bile-induced exopolysaccharide required for Salmonellabiofilm formation on gallstone surfaces. Infect. Immun. 76:5341–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Creydt V. P., Silberstein C., Zotta E., Ibarra C. 2006. Cytotoxic effect of Shiga toxin-2 holotoxin and its B subunit on human renal tubular epithelial cells. Microbes Infect. 8:410–419 [DOI] [PubMed] [Google Scholar]
- 11. Cunliffe R. N. 2003. Alpha-defensins in the gastrointestinal tract. Mol. Immunol. 40:463–467 [DOI] [PubMed] [Google Scholar]
- 12. Datsenko K. A., Wanner B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coliK-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Jesus M. C., Urban A. A., Marasigan M. E., Barnett Foster D. E. 2005. Acid and bile-salt stress of enteropathogenic Escherichia colienhances adhesion to epithelial cells and alters glycolipid receptor binding specificity. J. Infect. Dis. 192:1430–1440 [DOI] [PubMed] [Google Scholar]
- 14. Edgar R., Domrachev M., Lash A. E. 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30:207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esteller A. 2008. Physiology of bile secretion. World J. Gastroenterol. 14:5641–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gama-Castro S., et al. 2011. RegulonDB version 7.0: transcriptional regulation of Escherichia coliK-12 integrated within genetic sensory response units (Gensor Units). Nucleic Acids Res. 39:D98–D105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffin P. M., et al. 1988. Illnesses associated with Escherichia coliO157:H7 infections. A broad clinical spectrum. Ann. Intern. Med. 109:705–712 [DOI] [PubMed] [Google Scholar]
- 18. Gunn J. S., Miller S. I. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimuriumantimicrobial peptide resistance. J. Bacteriol. 178:6857–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gunn J. S., Ryan S. S., Van Velkinburgh J. C., Ernst R. K., Miller S. I. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella entericaserovar Typhimurium. Infect. Immun. 68:6139–6146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gunn J. S., et al. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171–1182 [DOI] [PubMed] [Google Scholar]
- 21. Guo L., et al. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189–198 [DOI] [PubMed] [Google Scholar]
- 22. Guo L., et al. 1997. Regulation of lipid A modifications by Salmonella typhimuriumvirulence genes phoP-phoQ. Science 276:250–253 [DOI] [PubMed] [Google Scholar]
- 23. Hagiwara D., Yamashino T., Mizuno T. 2004. A genome-wide view of the Escherichia coliBasS-BasR two-component system implicated in iron-responses. Biosci. Biotechnol. Biochem. 68:1758–1767 [DOI] [PubMed] [Google Scholar]
- 24. Harvey H., Habash M., Aidoo F., Burrows L. L. 2009. Single-residue changes in the C-terminal disulfide-bonded loop of the Pseudomonas aeruginosatype IV pilin influence pilus assembly and twitching motility. J. Bacteriol. 191:6513–6524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herrera C. M., Hankins J. V., Trent M. S. 2010. Activation of PmrA inhibits LpxT-dependent phosphorylation of lipid A promoting resistance to antimicrobial peptides. Mol. Microbiol. 76:1444–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hofmann A. F., Hagey L. R. 2008. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 65:2461–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. House B., et al. 2009. Acid-stress-induced changes in enterohaemorrhagic Escherichia coliO157: H7 virulence. Microbiology 155:2907–2918 [DOI] [PubMed] [Google Scholar]
- 28. Hung D. T., Mekalanos J. J. 2005. Bile acids induce cholera toxin expression in Vibrio choleraein a ToxT-independent manner. Proc. Natl. Acad. Sci. U. S. A. 102:3028–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karmali M. A. 2004. Infection by Shiga toxin-producing Escherichia coli: an overview. Mol. Biotechnol. 26:117–122 [DOI] [PubMed] [Google Scholar]
- 30. Krell T., et al. 2010. Bacterial sensor kinases: diversity in the recognition of environmental signals. Annu. Rev. Microbiol. 64:539–559 [DOI] [PubMed] [Google Scholar]
- 31. Kristoffersen S. M., et al. 2007. Low concentrations of bile salts induce stress responses and reduce motility in Bacillus cereusATCC 14579. J. Bacteriol. 189:5302–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lacroix F. J., et al. 1996. Salmonella typhimuriumacrB-like gene: identification and role in resistance to biliary salts and detergents and in murine infection. FEMS Microbiol. Lett. 135:161–167 [DOI] [PubMed] [Google Scholar]
- 33. Lee L. J., Barrett J. A., Poole R. K. 2005. Genome-wide transcriptional response of chemostat-cultured Escherichia colito zinc. J. Bacteriol. 187:1124–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lehrer R. I., et al. 1989. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Invest. 84:553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin J., Sahin O., Michel L. O., Zhang Q. 2003. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect. Immun. 71:4250–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malik-Kale P., Parker C. T., Konkel M. E. 2008. Culture of Campylobacter jejuniwith sodium deoxycholate induces virulence gene expression. J. Bacteriol. 190:2286–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCoy A. J., Liu H., Falla T. J., Gunn J. S. 2001. Identification of Proteus mirabilismutants with increased sensitivity to antimicrobial peptides. Antimicrob. Agents Chemother. 45:2030–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McPhee J. B., Lewenza S., Hancock R. E. 2003. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol. Microbiol. 50:205–217 [DOI] [PubMed] [Google Scholar]
- 39. Merighi M., Ellermeier C. D., Slauch J. M., Gunn J. S. 2005. Resolvase-in vivoexpression technology analysis of the Salmonella entericaserovar Typhimurium PhoP and PmrA regulons in BALB/c mice. J. Bacteriol. 187:7407–7416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moskowitz S. M., Ernst R. K., Miller S. I. 2004. PmrAB, a two-component regulatory system of Pseudomonas aeruginosathat modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J. Bacteriol. 186:575–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mühldorfer I., et al. 1996. Regulation of the Shiga-like toxin II operon in Escherichia coli. Infect. Immun. 64:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nesper J., et al. 2002. Role of Vibrio choleraeO139 surface polysaccharides in intestinal colonization. Infect. Immun. 70:5990–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nesper J., et al. 2001. Characterization of Vibrio choleraeO1 El tor galUand galEmutants: influence on lipopolysaccharide structure, colonization, and biofilm formation. Infect. Immun. 69:435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nikaido E., Yamaguchi A., Nishino K. 2008. AcrAB multidrug efflux pump regulation in Salmonella entericaserovar Typhimurium by RamA in response to environmental signals. J. Biol. Chem. 283:24245–24253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nikaido H., Takatsuka Y. 2009. Mechanisms of RND multidrug efflux pumps. Biochim. Biophys. Acta 1794:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nishino K., et al. 2006. Identification of the lipopolysaccharide modifications controlled by the SalmonellaPmrA/PmrB system mediating resistance to Fe(III) and Al(III). Mol. Microbiol. 61:645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ouellette A. J. 2005. Paneth cell alpha-defensins: peptide mediators of innate immunity in the small intestine. Springer Semin. Immunopathol. 27:133–146 [DOI] [PubMed] [Google Scholar]
- 48. Petritsch W., et al. 1992. Effect of cholera toxin on the human jejunum. Gut 33:1174–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Picken R. N., Beacham I. R. 1977. Bacteriophage-resistant mutants of Escherichia coliK12. Location of receptors within the lipopolysaccharide. J. Gen. Microbiol. 102:305–318 [DOI] [PubMed] [Google Scholar]
- 50. Pope L. M., Reed K. E., Payne S. M. 1995. Increased protein secretion and adherence to HeLa cells by Shigellaspp. following growth in the presence of bile salts. Infect. Immun. 63:3642–3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prouty A. M., Gunn J. S. 2000. Salmonella entericaserovar Typhimurium invasion is repressed in the presence of bile. Infect. Immun. 68:6763–6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prouty A. M., Van Velkinburgh J. C., Gunn J. S. 2002. Salmonella entericaserovar Typhimurium resistance to bile: identification and characterization of the tolQRAcluster. J. Bacteriol. 184:1270–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prouty A. M., Brodsky I. E., Falkow S., Gunn J. S. 2004. Bile-salt-mediated induction of antimicrobial and bile resistance in Salmonella typhimurium. Microbiology 150:775–783 [DOI] [PubMed] [Google Scholar]
- 54. Prouty A. M., et al. 2004. Transcriptional regulation of Salmonella entericaserovar Typhimurium genes by bile. FEMS Immunol. Med. Microbiol. 41:177–185 [DOI] [PubMed] [Google Scholar]
- 55. Pumbwe L., et al. 2007. Bile salts enhance bacterial co-aggregation, bacterial-intestinal epithelial cell adhesion, biofilm formation and antimicrobial resistance of Bacteroides fragilis. Microb. Pathog. 43:78–87 [DOI] [PubMed] [Google Scholar]
- 56. Raetz C. R., Reynolds C. M., Trent M. S., Bishop R. E. 2007. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 76:295–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ramos-Morales F., Prieto A. I., Beuzon C. R., Holden D. W., Casadesus J. 2003. Role for Salmonella entericaenterobacterial common antigen in bile resistance and virulence. J. Bacteriol. 185:5328–5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ritchie J. M., Thorpe C. M., Rogers A. B., Waldor M. K. 2003. Critical roles for stx2, eae, and tirin enterohemorrhagic Escherichia coli-induced diarrhea and intestinal inflammation in infant rabbits. Infect. Immun. 71:7129–7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rychlik I., Barrow P. A. 2005. Salmonellastress management and its relevance to behaviour during intestinal colonisation and infection. FEMS Microbiol. Rev. 29:1021–1040 [DOI] [PubMed] [Google Scholar]
- 60. Schmidt H. 2001. Shiga-toxin-converting bacteriophages. Res. Microbiol. 152:687–695 [DOI] [PubMed] [Google Scholar]
- 61. Sheoran A. S., et al. 2005. Human antibody against Shiga toxin 2 administered to piglets after the onset of diarrhea due to Escherichia coliO157:H7 prevents fatal systemic complications. Infect. Immun. 73:4607–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Soncini F. C., Groisman E. A. 1996. Two-component regulatory systems can interact to process multiple environmental signals. J. Bacteriol. 178:6796–6801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tamayo R., Ryan S. S., McCoy A. J., Gunn J. S. 2002. Identification and genetic characterization of PmrA-regulated genes and genes involved in polymyxin B resistance in Salmonella entericaserovar Typhimurium. Infect. Immun. 70:6770–6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thanassi D. G., Cheng L. W., Nikaido H. 1997. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179:2512–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Torres A. G., et al. 2007. Bile salts induce expression of the afimbrial LDA adhesin of atypical enteropathogenic Escherichia coli. Cell. Microbiol. 9:1039–1049 [DOI] [PubMed] [Google Scholar]
- 66. Torres A. G., et al. 2007. Ler and H-NS, regulators controlling expression of the long polar fimbriae of Escherichia coliO157:H7. J. Bacteriol. 189:5916–5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Trent M. S., Ribeiro A. A., Lin S., Cotter R. J., Raetz C. R. 2001. An inner membrane enzyme in Salmonellaand Escherichia colithat transfers 4-amino-4-deoxy-l-arabinose to lipid A: induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J. Biol. Chem. 276:43122–43131 [DOI] [PubMed] [Google Scholar]
- 68. van Velkinburgh J. C., Gunn J. S. 1999. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonellaspp. Infect. Immun. 67:1614–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. White S. H., Wimley W. C., Selsted M. E. 1995. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 5:521–527 [DOI] [PubMed] [Google Scholar]
- 70. Wibbenmeyer J. A., Provenzano D., Landry C. F., Klose K. E., Delcour A. H. 2002. Vibrio choleraeOmpU and OmpT porins are differentially affected by bile. Infect. Immun. 70:121–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wilkinson R. G., Gemski P., Jr., Stocker B. A. 1972. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J. Gen. Microbiol. 70:527–554 [DOI] [PubMed] [Google Scholar]
- 72. Wimley W. C., Selsted M. E., White S. H. 1994. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci. 3:1362–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wösten M. M., Kox L. F., Chamnongpol S., Soncini F. C., Groisman E. A. 2000. A signal transduction system that responds to extracellular iron. Cell 103:113–125 [DOI] [PubMed] [Google Scholar]
- 74. Yan A., Guan Z., Raetz C. R. 2007. An undecaprenyl phosphate-aminoarabinose flippase required for polymyxin resistance in Escherichia coli. J. Biol. Chem. 282:36077–36089 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.