Abstract
Integrin cell surface receptors are ideally suited to coordinate cellular differentiation and tissue assembly during embryogenesis, as they can mediate both signaling and adhesion. We show that integrins regulate gene expression in the intact developing embryo by identifying two genes that require integrin function for their normal expression in Drosophila midgut endodermal cells. We determined the relative roles of integrin adhesion versus signaling in the regulation of these integrin target genes. We find that integrin-mediated adhesion is not required between the endodermal cells and the surrounding visceral mesoderm for integrin target gene expression. In addition, a chimeric protein that lacks integrin-adhesive function, but maintains the ability to signal, can substitute for the endogenous integrin and regulate integrin target genes. This chimera consists of an oligomeric extracellular domain fused to the integrin βPS subunit cytoplasmic domain; a control monomeric extracellular domain fusion does not alter integrin target gene expression. Therefore, oligomerization of the 47-amino-acid βPS intracellular domain is sufficient to initiate a signaling pathway that regulates gene expression in the developing embryo.
Keywords: Integrin, Drosophila, extracellular matrix, signal transduction, adhesion
Interactions between cells during embryogenesis are vital for the processes of morphogenesis and differentiation. One category of these interactions uses secreted ligands, or morphogens, to specify pattern and cell fate decisions over several cell diameters (Lawrence and Struhl 1996). A second category uses transmembrane ligands, such as Delta and Serrate, to signal to adjacent cells (Artavanis-Tsakonas et al. 1995). A third category of interactions has features of both the other categories: Secreted ligands are used, but because they become incorporated into the insoluble meshwork of secreted proteins between cells, the extracellular matrix (ECM), they signal to those cells in direct contact with the matrix (Adams and Watt 1993). Integrin cell surface receptors, which mediate adhesion to the extracellular matrix and transduce signals, are likely to play important roles in ECM signaling (Juliano and Haskill 1993; Clark and Brugge 1995; Roskelley et al. 1995; Sastry and Horwitz 1996). The simplest way that integrins could contribute to ECM signaling is by mediating adhesion to the ECM so that cells are kept close to the source of signals. Alternatively, ECM signals could be largely transmitted by integrin signaling pathways. The goal of this work is to test the relative importance of these two integrin functions, adhesion and signaling, in the transmission of ECM signals within the intact embryo.
The ECM is a complex mixture of proteins that has important structural functions as well as a role in signaling (for review, see Adams and Watt 1993; Juliano and Haskill 1993; Kreis and Vale 1993; Roskelley et al. 1995). Examples of essential structures formed by the ECM include the tendons that link muscles to the bone, the comparable tendon matrix in insects that links the muscles to the epidermis, and the basement membrane, a thin electron-dense layer that separates cell layers from each other and is important in maintaining their integrity. The major ECM components, such as collagen, fibronectin, and laminin, provide structure to the matrix and contribute to signaling in at least two ways. One, they provide binding sites for other small growth factor peptides, such as members of the Wnt and TGF-β families, which, by binding to the ECM, may be presented to the cell in a higher concentration or in an especially active form (for review, see Adams and Watt 1993; Taipale and Keskioja 1997). Two, these structural components of the ECM also serve as signaling ligands by binding to integrins (Clark and Brugge 1995; Sastry and Horwitz 1996), and in one case, receptor tyrosine kinases (Shrivastava et al. 1997; Vogel et al. 1997).
Each integrin is composed of two type I transmembrane proteins, an α subunit and a β subunit (for review, see Hynes 1992). Both subunits take part in binding to extracellular ligands, which in most cases are ECM proteins, but also include transmembrane proteins. The cytoplasmic tails of almost all of the integrin subunits are very short, <50 amino acids, and do not appear to have any enzymatic activity. Therefore, integrin intracellular function is thought to be mediated through interactions with other proteins, including cytoskeletal molecules required for adhesion and components of signaling pathways. The association of cytoskeletal molecules with integrins requires that the integrins are bound to an extracellular ligand, whereas the initiation of signaling pathways appears to require just aggregation of integrins (Miyamoto et al. 1995a). Integrin aggregation, or clustering, most likely occurs as cells bind to the multivalent ECM, so that integrin adhesion and signaling are normally simultaneous events.
Perhaps the best characterized ECM signaling event occurs when cells in culture are transferred from suspension to an ECM substrate. Within a few minutes, a number of intracellular proteins are transiently activated by phosphorylation (for review, see Clark and Brugge 1995; Juliano 1996; Sastry and Horwitz 1996; Schlaepfer and Hunter 1998). This initial rapid response to cells binding to the ECM results in the activation of at least two signal transduction molecules that transmit signals to the nucleus, mitogen-activated protein kinase (MAPK) and Jun amino (N)-terminal kinase (JNK) (Chen et al. 1994; Schlaepfer et al. 1994; Miyamoto et al. 1995b; Zhu and Assoian 1995). These proteins can also be activated by simply clustering integrins, demonstrating that integrins are responsible for transmitting the ECM signal. There appears to be a variety of possible routes from the integrins to MAPK, which involve focal adhesion kinase (FAK), Shc, Ras, Rho, and Raf (Schlaepfer et al. 1994; Chen et al. 1996; Renshaw et al. 1996; Schlaepfer and Hunter 1996; Wary et al. 1996; Lin et al. 1997b). The pathway involving Shc is unique in that it is initiated by specific integrin α subunits (Wary et al. 1996). MAPK and related kinases link signaling to gene regulation by translocating into the nucleus, and they may provide this function for integrin signaling pathways, because integrin clustering has been shown to induce expression of immediate-early genes (Yurochko et al. 1992; Wary et al. 1996). These studies demonstrate the ability of integrins to transmit signals from the ECM to the nucleus, but it is not yet clear how much we can extrapolate from this rapid signaling event, which is over within an hour, to signaling during developmental events in which integrins are continuously in contact with the ECM.
When cells are cultured in continuous contact with an ECM, the composition of the ECM can dramatically affect the proliferation or differentiation of the cells (for review, see Adams and Watt 1993; Roskelley et al. 1995; Juliano 1996; Sastry and Horwitz 1996). Integrins are also known to be important for these longer term examples of ECM signaling, but it is not yet certain whether it is integrin adhesion or signaling that is required. If the role of the ECM could be replaced experimentally by clustering of integrins, then this would confirm an integrin signaling pathway. The integrin-dependent interactions with the ECM have been shown to be essential for the transmission of signals initiated by other signaling molecules, such as mitogen stimulation of proliferation and prolactin stimulation of mammary epithelial cell differentiation. In both cases, integrin function is required for an early step in the transmission of the signal. In the absence of adhesion to the correct substrate, the mitogen signal is arrested between Ras and MAPK kinase (Lin et al. 1997a; Renshaw et al. 1997) and prolactin fails to activate its receptor (Edwards et al. 1998).
Integrins could have active or passive roles in the transmission of the signals sent by the ECM during continuous contact with cells. There could be an integrin-specific signaling cascade that synergizes with these other pathways to promote proliferation or differentiation. As integrins recruit large numbers of signaling proteins to sites of adhesion (Miyamoto et al. 1995b; Plopper et al. 1995), a more passive model for integrins in signaling is that they are required to organize effective intracellular signaling centers composed of cytoskeletal and signaling components. Even more passive, integrin-mediated adhesion to the ECM could be essential for other types of cell surface receptors to bind to ECM ligands and transduce signals, such as the recently identified receptor tyrosine kinases that are activated by binding to collagen (Shrivastava et al. 1997; Vogel et al. 1997).
Integrin function is also important for the assembly of the ECM, and recent genetic evidence suggests that it is this function rather than integrin signaling that is required for keratinocyte differentiation (Bagutti et al. 1996). So far, genetic analysis of integrin function in Drosophila and mice has shown that integrins are essential for normal development, with a clear requirement for integrins in cell–ECM adhesion, but has yet to provide strong support for the role of integrin signaling in embryonic cellular differentiation (for review, see Brown 1993; Brakebusch et al. 1997). However, it may be that integrin signaling pathways are only required for particular aspects of cellular differentiation, and integrin target genes that absolutely require integrin function for their normal expression have not been identified yet. Therefore, to address the role of integrins in cellular differentiation of the Drosophila embryo, we have continued to look for such genes.
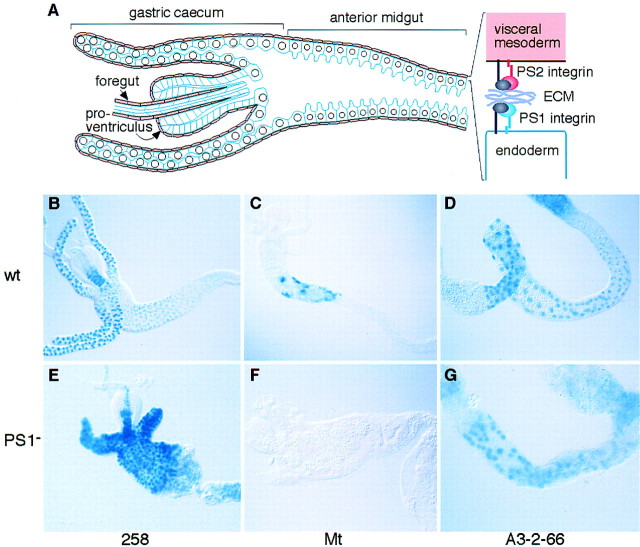
To identify target genes of a putative integrin signaling pathway in Drosophila, we have searched for genes that are expressed in the late stages of embryonic differentiation and examined their expression in embryos mutant for different integrin subunits. The integrin subunits identified in Drosophila consist of a highly diverged β subunit, βν, and three position-specific (PS) integrin heterodimers, PS1 (αPS1βPS), PS2 (αPS2βPS), and PS3 (αPS3βPS), which are most similar to vertebrate β1 integrin heterodimers (Brown 1993; Yee and Hynes 1993; Stark et al. 1997). In this work, we have focused on integrin function during the formation of the larval midgut, in which all five integrin subunits are expressed. However, only mutations in the PS1 and PS2 integrins have strong phenotypes in this tissue (Brabant and Brower 1993; Reuter et al. 1993; Brown 1994; Brower et al. 1995; Stark et al. 1997), causing a failure in the morphogenesis of the midgut and gastric caeca (four blind-ended tubes that evaginate from the anterior midgut). These two integrins are expressed in the complementary cell layers of the gut, with PS1 expressed in the endoderm epithelia, and PS2 in the surrounding layer of visceral muscles (Fig. 1A).
Figure 1.
Loss of PS1 integrin results in changes in gene expression in the gut epithelia.(A) Schematic drawing of a portion of the midgut, showing the large endodermal cells outlined in blue, which express the PS1 integrin, surrounded by a thin layer of visceral muscles (pink), which express the PS2 integrin. (B–G) Midguts from wild-type and integrin mutant embryos that were dissected and stained for β-galactosidase produced by enhancer traps (258 and A3-2-66) or a gene construct (Mt). Relative to wild type (B–D), the absence of the PS1 integrin leads to an increase in 258 expression in the midgut (E), a decrease in Mt expression (F), and no change in A3-2-66 expression (G). Because the expression of these markers changes during early larval development, the embryos were carefully staged to avoid differences as a result of developmental arrest.
We have successfully identified two genes that are regulated by PS1 integrin function in the midgut endodermal cells of the developing embryo. With these genes in hand, we have performed several experiments aimed at distinguishing between two possible ways that the integrin could be required for normal differentiation; as an adhesive molecule whose function is required for other signals to be received, or as a signaling molecule that initiates an intracellular pathway that regulates gene expression. Using a novel approach to send integrin signals in the absence of integrin adhesion, we show that the PS integrins function as signaling receptors, transducing signals from the extracellular matrix to inside the cell that result in changes in gene expression, independent of their role in adhesion.
Results
Identification of genes that require PS1 integrin function for their normal pattern of expression in the Drosophila midgut
To investigate whether PS integrin function is required for cellular differentiation during embryogenesis, we examined whether PS integrin mutations alter the expression of enhancer trap lines and constructs that are expressed in endodermal cells during the late stages of midgut development. Each of these constructs expresses β-galactosidase (in most cases targeted to the nucleus) under the control of adjacent regulatory elements, and thus, histochemical staining provides a simple assay for the expression of a variety of different genes. We found that PS1 integrin function is required for the normal expression of two of the genes tested (Fig. 1). The enhancer trap insertion in line 258 (Murakami et al. 1994) is expressed at high levels in the gastric caeca and at low levels in the anterior part of the midgut (Fig. 1B). In the absence of PS1, 258 is now expressed at high levels in the anterior midgut, similar to the level of expression in the gastric caeca, which does not change (Fig. 1E). Thus, the PS1 integrin is required for the repression of 258 expression in the anterior midgut. Conversely, lack of PS1 results in reduced expression of the gene construct Mt (Fig. 1C,F). This construct consists of a promoter fragment from a major midgut specific trypsin gene, Antryp1 from the mosquito Anopheles gambiae, fused to lacZ (construct ty1cBst), and is specifically expressed in the anterior part of the Drosophila larval midgut (Skavdis et al. 1996). Other enhancer traps do not change in the absence of PS1, such as the insertion in line A3-2-66 expressed in the large flat cells (Hoppler and Bienz 1995; Fig. 1D,G). Because all of the lines examined produced mRNAs with a similar structure, which encode β-galactosidase, the differences in expression caused by the absence of PS1 function are most likely to reflect the transcriptional control of these loci, rather than the stability of the gene product. The demonstration that PS1 function is required to suppress the transcription of 258 while stimulating the expression of the Mt construct, shows that the PS1 integrin specifically modulates gene expression, rather than generally up or down-regulating levels of gene expression. These represent the first examples of genes that are transcriptionally regulated by integrins in Drosophila (integrin target genes), and therefore provide us with an assay to determine what aspect of integrin function is required to regulate genes during cellular differentiation.
Loss of integrin-mediated adhesion between the endoderm and visceral mesoderm is not the cause of the changes in endodermal cell gene expression
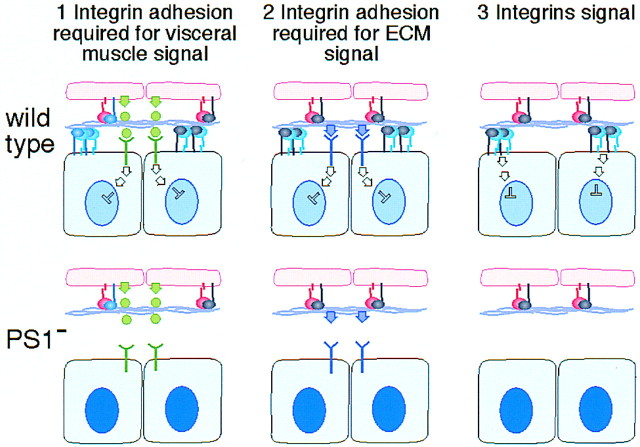
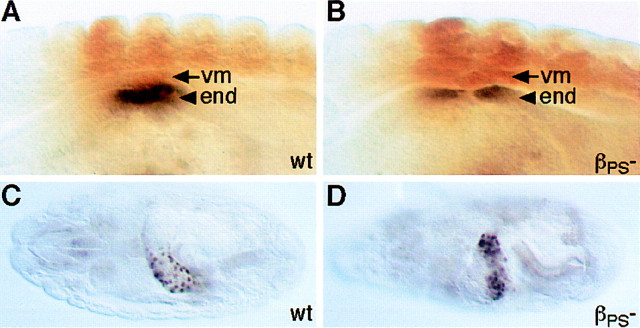
We can imagine three ways that the PS1 integrin could be required for normal patterns of gene expression in the midgut endoderm (Fig. 2):(1) PS1 could be required to hold the endoderm and visceral muscles in close proximity so that secreted signals sent by the visceral mesoderm are received by the endoderm; (2) PS1 could be required to hold the endoderm close to the ECM, or lead to the correct assembly of the ECM, so that signaling molecules within the matrix can bind to receptors on the endodermal cell surface; and (3) PS1 could send intracellular signals. There is good precedent for the first possibility because the visceral muscles are known to send signals to the endoderm. The visceral muscles secrete Decapentaplegic (Dpp; a member of the TGF-β family), which induces a new cell fate in the endoderm, as revealed by the expression of the homeobox gene labial (Immergluck et al. 1990; Panganiban et al. 1990). To test whether the disruption of integrin-mediated adhesion between these two cell layers disrupts this known example of signaling between them, we examined the expression of Labial in embryos mutant for the PS integrins. We found that Labial is expressed normally in the integrin mutant embryos (Fig. 3). This demonstrates that the loss of PS integrin adhesion between the two layers does not necessarily disrupt signaling between them. However, it remains possible that this signal is sent prior to the loss of attachment between these cell layers, and that other later signals would be hindered. Therefore, we tested this first possibility in an alternative way.
Figure 2.
Possible models for integrin regulation of gene expression. Each section of the diagram shows two blue endodermal cells expressing the αPS1βPS integrin (blue and gray) attaching via the extracellular matrix (purple) to two pink visceral mesodermal cells that express the αPS2βPS integrin (pink and gray). In the first two models, the PS1 integrin holds the endoderm in close proximity to the visceral mesoderm so that either (1) secreted signals from the visceral mesoderm are received by a receptor on the endodermal cell surface (in green), or (2) an ECM component signals to a nonintegrin receptor (in purple). In the third model, PS1 integrin adhesion to the ECM also sends signals. The outcome of the signal is depicted as the repression of 258 expression (light blue nucleus). In the PS1 mutant embryos (bottom), all three types of signal can be disrupted by the loss of PS1 integrin function, resulting in the increased expression of 258 (dark blue nucleus).
Figure 3.
Loss of integrin-mediated adhesion does not hinder the induction of endodermal Labial expression by Dpp secreted from the visceral mesoderm. (A) In wild-type embryos at stage 13, Labial expression (shown in black) is induced in those endodermal cells (arrowhead) in direct contact with visceral mesodermal cells (arrow) that express Decapentaplegic (not shown) under the control of the transcription factor Ultrabithorax (shown in brown). (B) This induction of Labial expression still occurs in the absence of PS integrin-mediated adhesion (lacking the βPS subunit). Examination of older embryos (stage 16) shows that Labial expression is still maintained in the absence of PS integrins (D), as it is in the wild type (C).
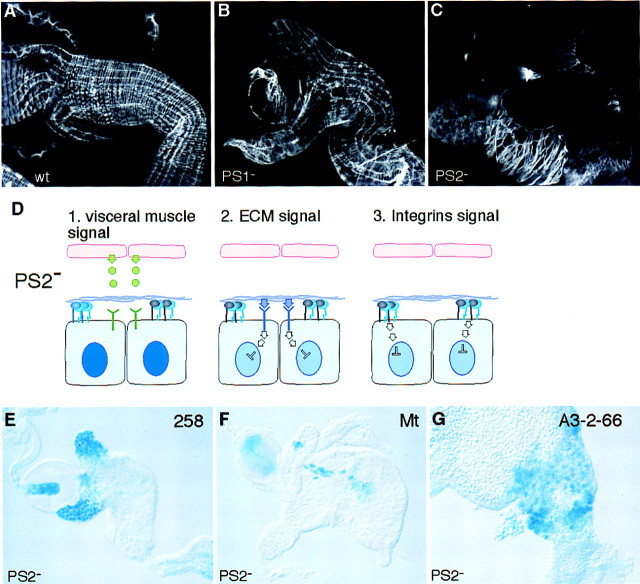
Both PS1 and PS2 integrins are required for the close apposition of the visceral muscles to the endoderm, as shown in Figure 4A–C, in which embryos lacking PS2 can be seen to have an even more extensive detachment of the visceral muscles from the endoderm than embryos lacking PS1. Therefore, if expression of the integrin target genes is regulated by a factor secreted by the visceral mesoderm, then we would expect the PS2 mutant embryos to show the same changes in gene expression as the PS1 mutant embryos, whereas if these genes are regulated by the ECM through the integrins or other receptors, then we would expect to see no change in the expression of these genes (Fig. 4D). We found that the loss of PS2 integrin function in the visceral muscles does not cause any changes in the expression of the two genes that are altered by the loss of PS1, 258 and Mt (Fig. 4E,F), nor the gene that is not affected by the loss of PS1, A3-2-66 (Fig. 4G). In the absence of both PS1 and PS2 integrins (embryos lacking the common βPS subunit), the morphological defects are too severe at this late stage to reliably examine the expression of these genes; substantial cell death occurs in the absence of both integrins at this stage, but not when just one or other integrin is absent (data not shown).
Figure 4.
Integrin-mediated adhesion between the endodermal and the visceral mesodermal cells is not required to regulate gene expression in the endoderm. (A–C) Dissected guts stained for actin with phalloidin conjugated to rhodamine to show the visceral mesoderm surrounding the gut. The continuous layer of visceral muscle seen in the wild type (A) is moderately disrupted in embryos that lack the PS1 integrin (B), and severely disrupted in embryos that lack the PS2 integrin (C). In D, the predicted result of the disruption of PS2 integrin-mediated adhesion of the visceral muscle to the endoderm is shown for each of the three models. If the signal is sent from the visceral mesoderm (model 1), then signaling will be lost in the PS2 mutant, whereas if the ECM provides the signal (models 2 and 3) then the signaling will be maintained. The latter is true as the absence of PS2 integrin does not change the expression pattern of any of the markers of (cf. E–G with Fig. 1A–C).
These results rule out the possibility that the changes in gene expression are an indirect consequence of the loss of integrin-mediated adhesion between the two cell layers of the midgut. Furthermore, these results show that the extracellular ligands required by PS1 in the endodermal cells to regulate gene expression cannot be transmembrane proteins on the surface of the visceral muscles and therefore are most likely to be ECM components. This is consistent with the evidence showing that laminin is a key ligand for PS1 (Gotwals et al. 1994; Prokop et al. 1998).
An integrin chimera that signals without mediating adhesion can regulate gene expression
Having ruled out the first of the three possible ways that PS1 could be required for normal patterns of gene expression, there remain two possible models; a requirement for PS1 adhesion to the ECM to allow other signals to be received, versus direct signaling by the PS1 integrin. Generating a PS1 integrin that lacks the ability to mediate adhesion but still retains signaling function would allow us to distinguish between the two models. A powerful technique in Drosophila to generate ligand independent, constitutively active, forms of transmembrane signaling proteins is to make chimeras containing the cytoplasmic domain of the test transmembrane protein fused to the extracellular and transmembrane domains of mutant forms of the Torso receptor tyrosine kinase (Dickson et al. 1992; Nellen et al. 1996). The mutant extracellular domain of Torso is derived from a dominant gain-of-function mutant allele (4021), which has a change in the extracellular domain from a tyrosine to a cysteine that allows the protein to form active signaling oligomers, independent of ligand binding (Sprenger and Nüsslein-Volhard 1992). Clustering chimeric proteins containing the cytoplasmic domain of integrin β subunits has been shown to lead to increased tyrosine phosphorylation of intracellular proteins such as FAK in vertebrate cells (Akiyama et al. 1994; Lukashev et al. 1994), but it is not known whether this fully mimics integrin signaling, especially as other experiments have shown that α subunits are important and, in some cases, sufficient for signaling (Huhtala et al. 1995; Sastry et al. 1996; Wary et al. 1996; Wei et al. 1998).
We constructed Torso/βcyt chimeras using the extracellular Torso domains from wild-type (TorsoWT) and the dominant 4021 allele (TorsoD), and as an additional control used a fusion of TorsoD to the cytoplasmic domain of Punt (Nellen et al. 1996), a receptor serine/threonine kinase. The chimeras were expressed in the midgut endodermal cells with the GAL4 system (Brand and Perrimon 1993), with the GAL4 line 48Y (Martin-Bermudo et al. 1997). Both the wild-type chimera (TorsoWT/βcyt) and the dominant chimera (TorsoD/βcyt) are expressed at similar levels in the midgut as well as some other tissues (Fig. 5A,B). To test the ability of these chimeric proteins to mimic integrin signaling, we examined their ability to substitute for the endogenous PS1 integrin and regulate the two integrin target genes. We found that TorsoWT/βcyt cannot substitute for PS1, as it does not change the overexpression of 258 caused by loss of PS1 (Fig. 5F,I). In contrast, the TorsoD/βcyt chimera can substitute for PS1, as it represses the expression of 258 in the anterior midgut (Fig. 5L). The constitutive signaling molecule is expressed throughout the gut, including in the gastric caeca. It does not repress 258 expression in the gastric caeca, but it does represses 258 in the portion of the midgut in which PS1 integrin function is normally required to repress 258 (indicated by black lines in the figure), and in addition, represses expression posterior to this region. The other control, TorsoD/Puntcyt is not able to repress 258 expression in the anterior midgut (Fig. 5P), demonstrating that repression requires the βPS cytoplasmic domain, and that the ability of TorsoD/βcyt to regulate integrin target genes is not caused by its extracellular domain fortuitously mimicking the adhesive function of the PS1 integrin. Similar experiments performed with the second integrin target gene Mt, also show that only the TorsoD/βcyt chimeric protein can successfully substitute for endogenous PS1 integrin function and induce expression of Mt in the absence of the endogenous PS1 integrin (Fig. 5D,G,J,M,Q). Widespread expression of TorsoD/βcyt only activates Mt expression in the region of the midgut in which it is normally expressed (Fig. 5M), demonstrating that it is not integrin signaling alone that specifies the spatial patterns of Mt and 258 expression. The ability of the TorsoD/βcyt chimera to substitute for the endogenous integrin rules out the possibility that the regulation of integrin target genes is an indirect consequence of integrin adhesion, and shows that it is due to a signaling pathway initiated by the 47-amino-acid βPS cytoplasmic domain.
Figure 5.
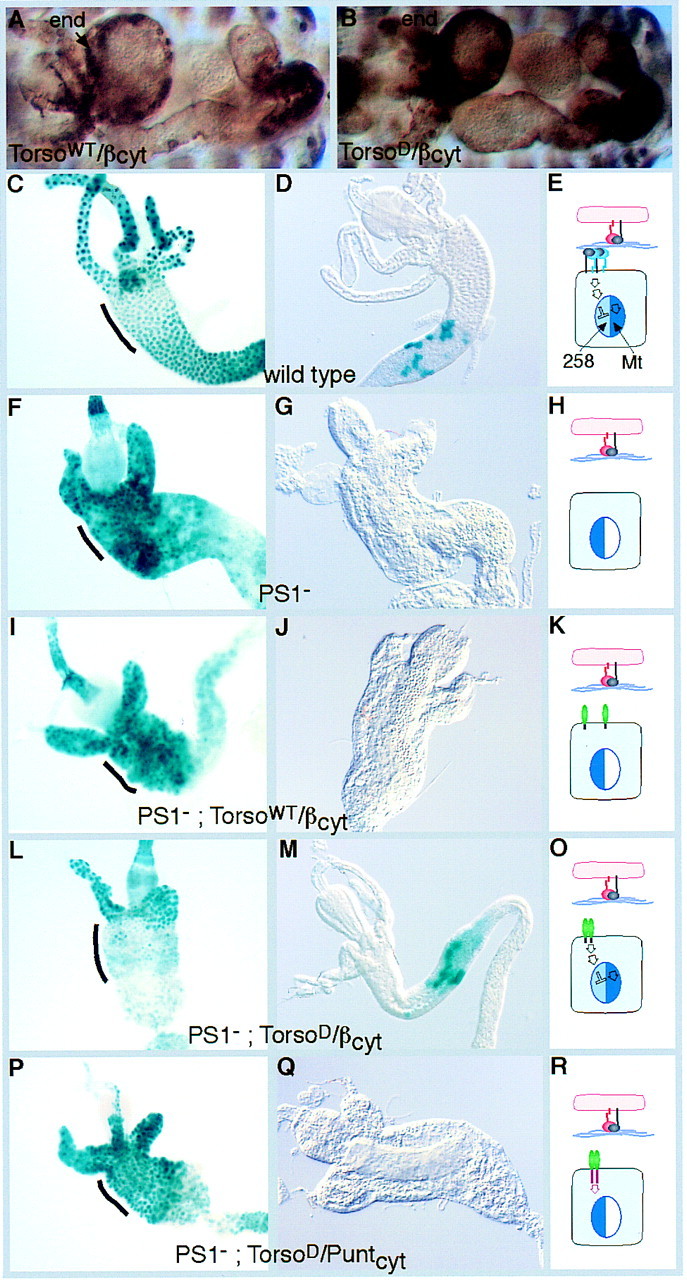
Dimerization of the βPS cytoplasmic tail is sufficient to send signals that regulate gene expression. Antibody staining of the chimeras shows that both wild-type and dominant forms are expressed at similar levels in the embryonic midgut endoderm (end; A,B) with the GAL4 line 48Y. The increase in 258 expression that occurs in the absence of PS1 (F vs. C) is suppressed by the dominant chimera TorsoD/βcyt (L), but not by the wild-type chimera TorsoWT/βcyt (I), nor a control chimera TorsoD/puntcyt (P). The region of the midgut that requires PS1 activity for the repression of 258 is marked with a black line in each panel. Similarly, the expression of the Mt transgene (D) requires PS1 integrin function (G), which can be functionally replaced with the dominant chimera TorsoD/βcyt (M), but not by the wild-type chimera TorsoWT/βcyt (J), nor TorsoD/puntcyt (Q). (Right) Diagrams of the postulated effects of the different genotypes on 258 and Mt expression are shown. (K,O,R) Torso domains are green: (K,O) the βcyt is gray; (R) the puntcyt is pink. Although TorsoD/βcyt sends similar signals to the endogenous PS1 integrin (E,O), neither the TorsoD/puntcyt signal (red arrow, R) nor the nonoligomeric TorsoWT/βcyt (K) alters 258 expression (left side of nucleus) or Mt expression (right side of nucleus).
Integrin regulation of gene expression does not require specific α subunit function
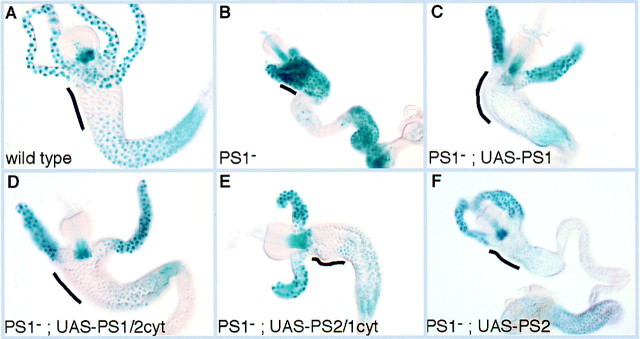
Whereas the βPS cytoplasmic domain alone can mimic PS1 integrin signaling when fused to TorsoD, in the intact integrin the α subunit will be required for interaction with the extracellular ligands to promote clustering and may also play a role inside the cell in the signaling pathway. It has been shown that only integrin heterodimers containing specific α subunits are competent to signal through the Shc adaptor protein to Ras (Wary et al. 1996). To test whether specific α subunits are required for signaling by PS integrin heterodimers, we examined the consequences of switching α subunits in the endodermal cells. We have found previously that αPS2 is not able to substitute for αPS1 function in the midgut when assayed by larval lethality (Martin-Bermudo et al. 1997). We first tested whether expression of UAS–αPS1 with the GAL4 driver can substitute for endogenous αPS1 function to repress the target gene 258 and found that it can (Fig. 6C). We then tested two chimeric α subunits, in which we have swapped the cytoplasmic domains between αPS1 and αPS2, and the normal αPS2 subunit, and found that all three could substitute for αPS1 and repress 258 expression (Fig. 6D–F). This shows that the α subunits do not provide specificity to this signaling event. In addition, it shows that the PS2 integrin is able to interact with enough ligands to become clustered and initiate a signaling pathway, even when it is expressed in an ectopic location.
Figure 6.
Specific α subunits are not required for integrin signaling in the midgut to regulate 258 gene expression. As shown before, low levels of 258 expression in the anterior midgut require PS1 integrin expression (A,B). Replacement of the endogenous αPS1 subunit with an αPS1 subunit provided by GAL4 driven expression produces the wild-type 258 expression pattern (C). Expression of chimeric α subunits containing the extracellular domain of αPS1 and the cytoplasmic domain of αPS2 (D), or the extracellular domain of αPS2 and the cytoplasmic domain of αPS1 (E), and expression of the entire αPS2 subunit (F), are all able to produce functional heterodimers that signal to repress 258 expression. The region of the midgut that requires the PS1 integrin for repression of 258 is indicated by the black lines.
Discussion
By identifying two genes that require integrins for their normal expression in Drosophila, we have been able to examine what aspects of integrin function contribute to tissue differentiation during embryonic development. As integrins are also required for adhesion between the embryonic cell layers during embryogenesis, it was essential to test whether it is integrin adhesion or signaling that is required for normal gene expression, because loss of adhesion could indirectly disrupt other signaling pathways. We have confirmed that integrins themselves initiate a signaling pathway that regulates gene expression through two key experiments. First, we have shown that although the disruption of integrin function in either the visceral mesoderm or the endoderm disrupts the adhesion between these two cell layers, changes in endodermal cell gene expression only occur when these cells themselves lack integrin function. This is consistent with the endodermal cell PS1 integrin signaling to regulate gene expression, and not with an indirect requirement for integrin function to hold these cell layers together so that other signals can be exchanged. Second, we show that integrins can regulate gene expression in the absence of an adhesive function, because the 47-amino-acid cytoplasmic tail of the integrin βPS subunit alone is sufficient to regulate gene expression when fused to the transmembrane and extracellular domains of a different transmembrane protein, the Torso receptor tyrosine kinase. Consistent with experiments showing that integrins must be dimerized or clustered to send signals (Yurochko et al. 1992; Huhtala et al. 1995; Miyamoto et al. 1995a), the cytoplasmic domain only regulates gene expression when fused to modified forms of the Torso protein that dimerize in the absence of ligand (derived from dominant alleles of torso), and not when fused to the wild-type monomeric form. Thus, oligomerizing the integrin βPS subunit cytoplasmic domain initiates a signaling pathway that regulates the expression of genes during the late steps of embryonic differentiation. Furthermore, our results show that the α subunits do not provide unique functions for this intracellular signaling pathway, suggesting that the role of the α is confined to extracellular ligand binding. These findings demonstrate that the integrins have a regulatory role in controlling differentiation during Drosophila embryogenesis, in addition to their essential structural role in linking together different cell layers.
Integrin dimerization vs. clustering
The ability of the chimeric protein consisting of a fusion between TorsoD and βPS to substitute for the wild-type integrin function in regulating gene expression raises the question of whether integrin dimerization is sufficient to send signals, rather than requiring higher order clusters. It is generally thought that receptor tyrosine kinases form dimers when they bind to ligands (for review, see Heldin 1995) although it is difficult to rule out the formation of higher order oligomers. The mutation that causes Torso to activate its pathway in the absence of ligand is a substitution of tyrosine to cysteine (Sprenger and Nüsslein-Volhard 1992), which may allow the formation of disulfide-linked dimers. We have found that the extracellular domain of this constitutively active receptor tryrosine kinase can substitute for the extracellular domain of integrins, to generate a molecule that constitutively sends integrin signals. This suggests that the level of oligomerization that is required for these two classes of signal-transducing receptors to initiate signaling pathways is equivalent, and therefore, that dimerization is sufficient for integrin signaling to regulate gene expression. In vivo, integrins can become dimerized or oligomerized by binding to the multivalent ECM. Experimentally, integrin signaling has been triggered by the formation of large clusters by crosslinking with polyclonal antisera or monoclonal antibodies linked to beads (e.g., Miyamoto et al. 1995a). Consistent with our results, dimerization of integrins with a monoclonal antibody has been shown to cause changes in gene expression in monocytes (Yurochko et al. 1992). However, integrin dimerization does not cause increases in intracellular tyrosine phosphorylation: Higher order clustering with a secondary antibody is required (Lukashev et al. 1994). This suggests that integrin signaling independently causes the major increases in the tyrosine phosphorylation of intracellular proteins and changes in gene expression.
Signaling between integrins and the nucleus
A large number of signaling molecules have been observed to be activated in response to integrin adhesion and/or clustering (Clark and Brugge 1995; Juliano 1996; Sastry and Horwitz 1996; Schlaepfer and Hunter 1998). Some of these molecules have been identified in Drosophila, and they are currently being tested to determine whether they are part of this integrin signaling pathway that is required for midgut differentiation. Wary et al. (1996) have identified an integrin signaling pathway that is α subunit specific and mediated by interactions between the α subunit transmembrane and/or extracellular domain and the adaptor protein Shc. This pathway can be mimicked by clustering the α subunit alone, whereas clustering the β cytoplasmic domain does not initiate signaling through Shc. The fact that we can mimick signaling by clustering the βPS cytoplasmic domain and the α subunits do not provide specificity to the signaling, demonstrates that a different type of pathway is involved in gene regulation in the developing gut. It is not suprising that the pathway we have identified appears to be independent of the Shc pathway, because the αPS1 integrin is in the same subfamily as α6 (Martin-Bermudo et al. 1997), which does not signal through Shc. The αPS2 subunit is in the same family as α5, which does signal through Shc, suggesting that if this pathway operates in Drosophila, it is more likely to be operating through this integrin.
The pathway downstream of the integrin cytoplasmic domain could function in several ways to modify gene expression. One possibility is that there is an intracellular signaling cascade that brings about the modification of transcription factors, resulting in the repression of some genes such as 258 and the activation of others, such as the Mt construct. Alternatively, the integrin signaling activity could be confined to the plasma membrane and function by modifying other signaling pathways, either by promoting their organization into signaling complexes, or by modifying the initial steps in these pathways. These possibilities are consistent with results showing that there is reorganization of signaling molecules in response to integrin clustering (Miyamoto et al. 1995b), and that integrin function is required for other signaling receptors to transmit their signals along the appropriate pathway (Lin et al. 1997a; Renshaw et al. 1997; Edwards et al. 1998). Thus, the target gene 258 could be activated by the reception of a growth factor type signal, which is modified in the anterior midgut by integrin activity that could either block this signal close to the plasma membrane, or could initiate a signaling pathway that culminates in the binding of a repressor to the 258 gene. The interaction of integrin signaling with other pathways is also suggested by the fact that constitutive signaling with the TorsoD/βcyt chimera does not cause ectopic repression of 258 nor ectopic expression of Mt. The repression of 258 expression only occurs in the anterior midgut and not in the gastric caeca, and Mt is only expressed in the region of the midgut in which it is normally expressed. This suggests that some components of the pathway are differentially expressed in these different domains of the midgut, or that the expression of these genes is regulated by trans-acting factors expressed in specific subregions of the midgut.
Role of integrin signaling during midgut development
The requirement for integrin function in the expression of the target gene Mt suggests that the integrins are required for the latest stages of midgut differentiation. The promoter driving this construct, which is derived from a trypsin gene expressed in the mosquito midgut, is expressed at the very end of embryogenesis in Drosophila, and it most likely reflects the expression of homologous trypsin genes during the final stages of generating a functional larval midgut. Thus, integrin function is required for the endodermal cells to become fully functional, and suggests an important link between proper morphogenesis of this tissue, in part mediated by integrin adhesion to the ECM, and the differentiation of the organ. Experiments in vertebrate cell culture add support to the role of the ECM in the differentiation of the gut, because antibodies against laminin-1, a component of the basement membrane between epithelial and mesenchymal cells, can block the differentiation of the gut epithelium, as indicated by the absence of enterocytic markers such as lactase–phlorizin hydrolase and sucrase isomaltase (De Arcangelis et al. 1996).
However, the PS1 integrin is not universally required for the expression of final products of gut differentiation, because, for example, a gene expressed during the late differentiation of another group of specialized midgut cells, the large flat cells, is expressed normally in the absence of the PS1 integrin (enhancer trap A3-2-66). Yet, despite the normal expression of this gene, the large flat cells appear morphologically abnormal (M.D. Martin-Bermudo and N.H. Brown, unpubl.), suggesting that their differentiation is abnormal in the absence of PS1 integrin function. This suggests that differentiation is a complex process with multiple independent signals leading to the final patterns of gene expression, only some of which are integrin dependent. Alternatively, the expression of some genes could be regulated in a redundant fashion by more than one integrin, as another integrin β subunit, βν, is also expressed in the midgut (Yee and Hynes 1993). To resolve this question it will be helpful to identify additional integrin target genes, so as to be able to characterize the products of the genes that rely on feedback from integrin-mediated morphogenesis.
In conclusion, we have demonstrated that during normal development, integrin binding to the ECM is not only required to attach cells firmly to the basement membrane, but it is also essential for normal patterns of gene expression. More importantly, our results suggest that dimerization of the βPS subunit intracellular domain is sufficient to initiate a signaling pathway that can upregulate and downregulate gene expression. This shows that whereas integrin ligand binding is used for adhesion to the extracellular matrix, as signaling receptors, the integrins are formally equivalent to growth factor receptors, in that their ability to mediate adhesion is not required for integrins to regulate gene expression. Thus, these results have confirmed the importance of integrins in providing a vital link between cell adhesion during morphogenesis and cellular differentiation.
Materials and methods
Mutant alleles
The mutant alleles used are mewM6 (Brower et al. 1995), ifB4 (Brown 1994), and mysXG43 (Bunch et al. 1992).
Histochemical detection of β-galactosidase activity and antibody staining
The histochemical staining was performed on hand-dissected guts according to Murakami et al. (1994). Antibody staining of embryos was done by standard methods with anti-Ultrabithorax and anti-Labial antibodies (Panganiban et al. 1990), or the anti-myc tag monoclonal 9E10 (Oncogene Research Products) at 1:500, followed by enhancement with the Vectastain Elite ABC kit. Stained embryos and dissected guts were photographed with either a Zeiss Axiophot microscope and the images scanned with a Nikon Coolscan, or were photographed with a Spot digital camera on a Leica DMR microscope. The digital images were assembled with Adobe Photoshop 4.0, and labeled with FreeHand 8.0 on a Power Macintosh.
Construction of genes encoding Torso/βcyt fusion proteins
The UAS–torsoWT/βcyt gene was constructed by combining, in a series of steps, the following five DNA fragments: (1) a KpnI–CelII (filled in) fragment from pUAST (Brand and Perrimon 1993) containing the UAS promoter and 36 nucleotides of HSP70 5′ untranslated sequence; (2) an XhoI (filled in) to SspBI fragment from pBD490 (B. Dickson and E. Hafen, pers. comm.) containing a signal sequence followed by a myc tag and the amino terminus of the Torso extracellular domain; (3) an SspBI–EcoRI (filled in) fragment from torsoWT–sev (Dickson et al. 1992) containing the rest of the Torso extracellular domain and the transmembrane domain; (4) a BamHI (trimmed with mung bean nuclease) to SpeI fragment from pβcyt (see below) containing the cytoplasmic domain of the integrin βPS subunit; (5) a SpeI–RsrII fragment containing the polyadenylation site of the rosy gene (Martin-Bermudo et al. 1997). The gene was cloned between KpnI and RsrII sites in a P-element vector containing the white gene as a selectable marker (pWhiteRabbit, N.H. Brown unpubl.). The plasmid pβcyt, which contains a BamHI site at the junction between the transmembrane and cytoplasmic domains of the βPS subunit, was generated by PCR, with the primer GGAGGATCCTCACTACGATCCAC and a primer in the vector, cloned as a BamHI–NotI fragment and checked by sequencing (the SpeI site used for fragment 4 is within the 3′ untranslated region of the βPS gene). The amino acid sequences at the junctions between transmembrane and cytoplasmic domains are -LLLWKLLTTIHDRR- in the βPS subunit and -LTFCRILTTIHDRR- in the TorsoWT/βcyt fusion (the Torso sequence is underlined and the junction amino acids RI come from the synthetic EcoRI site). To generate the UAS–torsoD/βcyt gene, a NgoMI–EcoRI fragment from UAS–torsoWT/βcyt was replaced with the corresponding fragment from torso4021–sev (Dickson et al. 1992). P-element transformants were obtained by standard methods, and several lines were obtained for each construct. Independent lines of the constructs were used; lines B, E2 and D1 for the UAS–torsoWT/βcyt gene and lines B and C for the UAS–torsoD/βcyt gene. They were expressed in the midgut by the GAL4 line 48Y, which is expressed in the midgut from stage 12 onwards (Martin-Bermudo et al. 1997). To unambiguously distinguish the mew mutant embryos, we used a balancer chromosome marked with yellow+ (Martin-Bermudo et al. 1997), for example, virgin females y mewM6 /FM6, y+; UAS–torsoD/βcyt were crossed to y+/Y; 24B; 258 males. In the offspring, all will express UAS–torsoD/βcyt under the control of 48Y and contain the 258 enhancer trap, and the 1/4 that are mutant for mew can be distinguished by their y mouth hooks.
To assess the role of the α subunits, we used the following UAS constructs: UAS–PS1 2.1, UAS–PS1/2cyt 2.1, UAS–PS2/1cyt 2.A, UAS–PS2 2A (Martin-Bermudo et al. 1997).
Acknowledgments
We thank J. Casanova for suggesting the Torso–integrin fusion. We also thank K. Basler, M. Bienz, B. Dickson, T. Kaufman, E. Hafen, R. Murakami, and I. Siden-Kiamos for fly strains and reagents, and S. Bray, A. Gonzales-Reyes, S. Gregory, C. Holt, T. Kouzarides, I. Palacios, D. St Johnston, and C. Streuli for helpful comments on the manuscript. This work was supported by grants from the Wellcome Trust; project grant 050301 and a senior fellowship to N.H.B.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL nb117@mole.bio.cam.ac.uk; FAX 44-1223-334089.
References
- Adams J, Watt F. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Akiyama SK, Yamada SS, Yamada KM, LaFlamme SE. Transmembrane signal transduction by integrin cytoplasmic domains expressed in single-subunit chimeras. J Biol Chem. 1994;269:15961–15964. [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signalling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Bagutti C, Wobus AM, Fässler R, Watt FM. Differentiation of embryonal stem cells into keratinocytes: Comparison of wild-type and β1integrin-deficient cells. Dev Biol. 1996;179:184–196. doi: 10.1006/dbio.1996.0250. [DOI] [PubMed] [Google Scholar]
- Brabant MC, Brower DL. PS2 integrin requirements in Drosophilaembryo and wing morphogenesis. Dev Biol. 1993;157:49–59. doi: 10.1006/dbio.1993.1111. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Hirsch E, Potocnik A, Fässler R. Genetic analysis of β1 integrin function: Confirmed, new and revised roles for a crucial family of cell adhesion molecules. J Cell Sci. 1997;110:2895–2904. doi: 10.1242/jcs.110.23.2895. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brower DL, Bunch TA, Mukai L, Adamson TE, Wehrli M, Lam S, Friedlander E, Roote CE, Zusman S. Nonequivalent requirements for PS1 and PS2 integrin at cell attachments in Drosophila: Genetic analysis of the αPS1integrin subunit. Development. 1995;121:1311–1320. doi: 10.1242/dev.121.5.1311. [DOI] [PubMed] [Google Scholar]
- Brown NH. Integrins hold Drosophilatogether. BioEssays. 1993;15:383–390. doi: 10.1002/bies.950150604. [DOI] [PubMed] [Google Scholar]
- ————— Null mutations in the αPS2 and βPSintegrin subunit genes have distinct phenotypes. Development. 1994;120:1221–1231. doi: 10.1242/dev.120.5.1221. [DOI] [PubMed] [Google Scholar]
- Bunch TA, Salatino R, Engelsgjerd MC, Mukai L, West RF, Brower DL. Characterization of mutant alleles of myospheroid, the gene encoding the β subunit of the DrosophilaPS integrins. Genetics. 1992;132:519–528. doi: 10.1093/genetics/132.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- Chen Q, Lin TH, Der CJ, Juliano RL. Integrin-mediated activation of mitogen-activated protein (MAP) kinase or extracellular signal-related kinase (MEK) is independent of Ras. J Biol Chem. 1996;271:18122–18127. doi: 10.1074/jbc.271.30.18122. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: The road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- De Arcangelis AP, Neuville R, Boukamel R, Lefebvre O, Kedinger M, Simon-Assmann P. Inhibition of laminin α1-chain expression leads to alteration of basement membrane assembly and cell differentiation. J Cell Biol. 1996;133:417–430. doi: 10.1083/jcb.133.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson B, Sprenger F, Hafen E. Prepattern in the developing Drosophilaeye revealed by an activated torso-sevenless chimeric protein. Genes & Dev. 1992;6:2327–2339. doi: 10.1101/gad.6.12a.2327. [DOI] [PubMed] [Google Scholar]
- Edwards GM, Wilford FH, Liu X, Hennighausen L, Djiane J, Streuli CH. Regulation of mammary differentiation by extracellular matrix involves protein-tyrosine phosphatases. J Biol Chem. 1998;273:9495–9500. doi: 10.1074/jbc.273.16.9495. [DOI] [PubMed] [Google Scholar]
- Gotwals PJ, Fessler LI, Wehrli M, Hynes RO. DrosophilaPS1 integrin is a laminin receptor and differs in ligand specificity from PS2. Proc Natl Acad Sci. 1994;91:11447–11451. doi: 10.1073/pnas.91.24.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Bienz M. Two different thresholds of wingless signalling with distinct developmental consequences in the Drosophilamidgut. EMBO J. 1995;14:5016–5026. doi: 10.1002/j.1460-2075.1995.tb00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtala P, Humphries M, McCarthy J, Tremble P, Werb Z, Damsky C. Cooperative signaling by α5β1 and α4β1integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol. 1995;129:867–879. doi: 10.1083/jcb.129.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Immergluck K, Lawrence PA, Bienz M. Induction across germ layers in Drosophila mediated by a genetic cascade. Cell. 1990;62:261–268. doi: 10.1016/0092-8674(90)90364-k. [DOI] [PubMed] [Google Scholar]
- Juliano R. Cooperation between soluble factors and integrin-mediated cell anchorage in the control of cell growth and differentiation. BioEssays. 1996;18:911–917. doi: 10.1002/bies.950181110. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T, Vale R. Guidebook to the extracellular matrix and adhesion proteins. Oxford, UK: Oxford University Press; 1993. [Google Scholar]
- Lawrence PA, Struhl G. Morphogens, compartments, and pattern-lessons from Drosophila. Cell. 1996;85:591–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- Lin TH, Chen Q, Howe A, Juliano RL. Cell anchorage permits efficient signal transduction between Ras and its downstream kinases. J Biol Chem. 1997a;272:8849–8852. [PubMed] [Google Scholar]
- Lin TH, Aplin AE, Shen Y, Chen QM, Schaller M, Romer L, Aukhil I, Juliano RL. Integrin mediated activation of MAP kinase is independent of FAK: Evidence for dual integrin signaling pathways in fibroblasts. J Cell Biol. 1997b;136:1385–1395. doi: 10.1083/jcb.136.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev ME, Sheppard D, Pytela R. Disruption of integrin function and induction of tyrosine phosphorylation by the autonomously expressed β1 integrin cytoplasmic domain. J Biol Chem. 1994;269:18311–18314. [PubMed] [Google Scholar]
- Martin-Bermudo MD, Dunin-Borkowski OM, Brown NH. Specificity of PS integrin function during embryogenesis resides in the α subunit extracellular domain. EMBO J. 1997;16:4184–4193. doi: 10.1093/emboj/16.14.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995a;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: Molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995b;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami R, Shigenaga A, Kawano E, Matsumoto A, Yamaoka I, Tanimura T. Novel tissue units of regional differentiation in the gut epithelium of Drosophila, as revealed by P-element-mediated detection of enhancer. Wilhelm Roux’s Arch Dev Biol. 1994;203:243–249. doi: 10.1007/BF00360519. [DOI] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Panganiban GEF, Reuter R, Scott M, Hoffmann FM. A Drosophila growth factor homolog, decapentaplegic, regulates homeotic gene expression within and across germ layers during midgut morphogenesis. Development. 1990;110:1041–1050. doi: 10.1242/dev.110.4.1041. [DOI] [PubMed] [Google Scholar]
- Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A, Martin-Bermudo MD, Bate M, Brown NH. Drosophilaembryos, the absence of the PS integrins or laminin A affects the extracellular adhesion of hemiadherens and neuromuscular junctions, but not their intracellular assembly. Dev Biol. 1998;196:58–76. doi: 10.1006/dbio.1997.8830. [DOI] [PubMed] [Google Scholar]
- Renshaw MW, Toksoz D, Schwartz MA. Involvement of the small GTPase Rho in integrin-mediated activation of mitogen-activated protein kinase. J Biol Chem. 1996;27:21691–21694. doi: 10.1074/jbc.271.36.21691. [DOI] [PubMed] [Google Scholar]
- Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter R, Grunewald B, Leptin M. A role for mesoderm in endodermal migration and morphogenesis in Drosophila. Development. 1993;119:1135–1145. doi: 10.1242/dev.119.4.1135. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signalling regulates tissue-specific gene-expression. Curr Opin Cell Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry SK, Horwitz AF. Adhesion-growth factor interactions during differentiation: An integrated biological response. Dev Biol. 1996;180:455–467. doi: 10.1006/dbio.1996.0319. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Lakonishok M, Thomas D, Muschler J, Horwitz A. Integrin α subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle cell proliferation and differentiation. J Cell Biol. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Integrin signalling and tyrosine phosphorylation: Just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by Grb2 binding to the focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Shrivastava A, Radiziejewski C, Campbell E, Kovac L, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- Skavdis G, Siden-Kiamos I, Muller HM, Crisanti A, Louis C. Conserved function of Anopheles gambiaemidgut-specific promoters in the fruitfly. EMBO J. 1996;15:344–350. [PMC free article] [PubMed] [Google Scholar]
- Sprenger F, Nüsslein-Volhard C. Torso receptor activity is regulated by a diffusible ligand produced at the extracellular terminal regions of the Drosophila egg. Cell. 1992;71:987–1001. doi: 10.1016/0092-8674(92)90394-r. [DOI] [PubMed] [Google Scholar]
- Stark KA, Yee GH, Roote CE, Williams EL, Zusman S, Hynes RO. A novel α integrin subunit associates with βPS and functions in tissue morphogenesis and movement during Drosophiladevelopment. Development. 1997;124:4583. doi: 10.1242/dev.124.22.4583. [DOI] [PubMed] [Google Scholar]
- Taipale J, Keskioja J. Growth factors in the extracellular matrix. FASEB J. 1997;11:51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–25. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:1733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Wei J, Shaw LM, Mercurio AK. Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the α6integrin subunit. J Biol Chem. 1998;273:5903–5907. doi: 10.1074/jbc.273.10.5903. [DOI] [PubMed] [Google Scholar]
- Yee GH, Hynes RO. A novel, tissue-specific integrin subunit, βγ, expressed in the midgut of Drosophila melanogaster. Development. 1993;118:845–858. doi: 10.1242/dev.118.3.845. [DOI] [PubMed] [Google Scholar]
- Yurochko AD, Liu YD, Eierman D, Haskill S. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci. 1992;89:9034–9038. doi: 10.1073/pnas.89.19.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: A link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]