Abstract
Genome data of the extreme acidophilic verrucomicrobial methanotroph Methylacidiphilum fumariolicumstrain SolV indicated the ability of autotrophic growth. This was further validated by transcriptome analysis, which showed that all genes required for a functional Calvin-Benson-Bassham (CBB) cycle were transcribed. Experiments with 13CH4or 13CO2in batch and chemostat cultures demonstrated that CO2is the sole carbon source for growth of strain SolV. In the presence of CH4, CO2concentrations in the headspace below 1% (vol/vol) were growth limiting, and no growth was observed when CO2concentrations were below 0.3% (vol/vol). The activity of the key enzyme of the CBB cycle, ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), measured with a 13C stable-isotope method was about 70 nmol CO2fixed · min−1· mg of protein−1. An immune reaction with antibody against the large subunit of RuBisCO on Western blots was found only in the supernatant fractions of cell extracts. The apparent native mass of the RuBisCO complex in strain SolV was about 482 kDa, probably consisting of 8 large (53-kDa) and 8 small (16-kDa) subunits. Based on phylogenetic analysis of the corresponding RuBisCO gene, we postulate that RuBisCO of the verrucomicrobial methanotrophs represents a new type of form I RuBisCO.
INTRODUCTION
Methanotrophs are a unique group of microorganisms within the methylotrophs that oxidize methane (CH4) to carbon dioxide (CO2). Until 2007, the phylogenetic distribution of the aerobic methanotrophs was limited to the α and γ subclasses of the proteobacteria (16). In 2007, novel thermoacidophilic aerobic methanotrophs were discovered in geothermal areas in New Zealand, Russia, and Italy (9, 18, 23). These methanotrophs represented a distinct phylogenetic lineage within the Verrucomicrobia, for which the genus name Methylacidiphilumwas proposed (22).
Recently, methanotrophy was discovered in a member of the NC10 phylum. It was shown that “CandidatusMethylomirabilis oxyfera,” enriched under strict anoxic conditions, produces its own oxygen from nitrite (12). This oxygen is then used for CH4oxidation in a biochemical pathway comparable to those of aerobic methanotrophs.
During the aerobic oxidation of CH4and methanol by proteobacterial methanotrophs, formaldehyde is produced. This central metabolite can be further oxidized to CO2or directly assimilated via intermediates of the central metabolism. Based on the pathway used for formaldehyde assimilation, methanotrophs were divided into type I and type II. Type II methanotrophs use the serine pathway, in which formaldehyde and CO2are utilized in a one-to-one ratio to produce acetyl coenzyme A (acetyl-CoA) for biosynthesis (8), while type I methanotrophs use the ribulose monophosphate pathway for the assimilation of formaldehyde to form glyceraldehyde-3-phosphate as an intermediate of central metabolism (16). In the latter pathway, all cellular carbon is assimilated at the oxidation level of formaldehyde. Genome data of some proteobacterial methanotrophs (Methylococcus capsulatusBath, Methylocella silvestrisBL2 [7, 31]) and nonproteobacterial aerobic methanotrophs (Methylacidiphilum infernorumV4, Methylacidiphilum fumariolicumSolV, and “CandidatusMethylomirabilis oxyfera” [12, 17, 22]) revealed the presence of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), the key enzyme of the Calvin-Benson-Bassham (CBB) cycle. M. capsulatusBath was found to contain RuBisCO in an active form (27), and genome analysis suggested that a variant of the CBB cycle may operate (19, 31). Although hydrogen seems to support moderate growth with CO2on agar plates for M. capsulatusBath and some other methanotrophs (3), autotrophic growth in liquid cultures has not been reported. Marker exchange mutagenesis deleting the genes encoding RuBisCO may give definite answers on the exact role of RuBisCO, but unfortunately, a good genetic system for manipulation of these bacteria is lacking.
Analyses of the complete genome sequence of M. infernorumstrain V4 (17) and a draft genome of M. fumariolicumstrain SolV showed that these two verrucomicrobial methanotrophs lack the key enzymes for both the ribulose monophosphate and serine pathways (22). However, in this study, we show that a complete set of genes encoding the enzymes of the CBB cycle are present, which suggests that these methanotrophs may be able to fix CO2, probably using CH4mainly as an energy source. The CBB cycle has been associated with a large use of ATP per mole of CO2fixed (8) and was thus never considered to be a likely pathway to support growth on CH4. In the present paper, we show, by applying 13CH4or 13CO2in growth experiments, that CO2is the only carbon source for M. fumariolicumstrain SolV during growth on CH4. With a transcriptome study of strain SolV, we show that all genes necessary for a complete CBB cycle are transcribed. The large and small subunits of RuBisCO turned out to be highly expressed. In addition, we developed a novel 13C stable-isotope enzyme assay to demonstrate the activity of RuBisCO.
MATERIALS AND METHODS
Organism and medium composition for growth.
The M. fumariolicumstrain SolV used in this study was originally isolated from the volcanic region Campi Flegrei, near Naples, Italy (23). The composition and preparation of the growth medium were described previously (20).
Transcriptome analysis.
The available draft genome of strain SolV (23) was improved by adding data (30 × 10675-nucleotide reads) from an Illumina sequencing run. Genes encoding the enzymes of the CBB cycle were identified by BLAST searches, and their DNA and protein sequences were used for transcriptome analysis. Exponentially growing cells were harvested by centrifugation, 3.1 mg (dry weight) cells was used for isolation of mRNA, and subsequent synthesis of cDNA (328 ng) was done as described before (12). The cDNA was used for single-end Illumina sequencing (12). Expression analysis was performed with the RNA-Seq analysis tool from the CLC Genomics Workbench software (version 4.0; CLC bio, Aarhus, Denmark).
Gas and protein analysis.
Gas samples (100 μl) were analyzed for CH4and CO2on an Agilent series 6890 gas chromatograph (GC) equipped with Porapak Q and molecular sieve columns and a thermal conductivity detector (13). To quantify the molecular masses for 12CH4and 13CH4(16 and 17 Da) and for 12CO2and 13CO2(44 and 45 Da), the same gas chromatograph was coupled with a mass spectrometer (GC-MS) (Agilent 5975C GC MSD; Agilent, Santa Clara, CA) (12). All gas concentrations are given in volume percentages (volume/volume). Protein concentrations were measured using the Bio-Rad protein assay kit (Bio-Rad Laboratories B.V., Veenendaal, the Netherlands).
13/12C IRMS analysis.
The 13/12C ratio of biomass samples was determined using isotope ratio mass spectrometry (IRMS). The cells were harvested by centrifugation, and the pellet was washed with acidified water (pH 3) and dried overnight in a vacuum oven at 70°C. The dried material (about 0.04 mg) was analyzed on a Thermo Fisher Scientific EA 1110 CHN element analyzer coupled with a Finnigan DeltaPlus mass spectrometer.
Batch cultivation.
The effect of CO2concentration on the growth of strain SolV was studied in batch cultures using 1-liter serum bottles containing 50 ml of medium and sealed with red butyl rubber stoppers. Incubations were performed in duplicate and at 55°C with shaking at 180 rpm, and mixtures contained 5% CH4and air as the only source of O2and CO2. To remove dissolved CO2originating from the inoculum, the cell suspension was flushed with sterile air for 10 min before inoculation.
13CH4experiments were done in 150-ml serum bottles containing 10 ml of culture medium, 40% CO2, and 5 ml of 13CH4(99 atom% 13C; Sigma-Aldrich). The bottles were inoculated with 0.25 ml of a culture of strain SolV. Initial and final gas concentrations and amounts, as well as mass ratios, were verified by gas chromatographic analysis using a pressure lock syringe. Recovery of 13C was calculated from the 13C/12C ratio of CO2and the total amount (moles) of gases in the bottle. For calculating CO2in the liquid phase, a partition coefficient of 1.066 (gas/liquid ratio) at 21°C was assumed (32). At the end of the experiment, the biomass was used for the IRMS analysis.
Chemostat cultivation.
13CH4- and 13CO2-labeling experiments were performed in continuous chemostat cultures under N2-fixing conditions as described before (20). In the first experiment, 13CH4(99 atom% 13C; Sigma-Aldrich) replaced the unlabeled CH4in the inlet gas mixture of the chemostat. After a steady state was obtained, the 13C-labeling percentage of the gases in the outlet of the chemostat was determined. The ingoing gas now contained 4.7% 13CH4and 3.5% CO2at a flow rate of 10.7 ml · min−1. The outgoing gas (10.3 ml · min−1) contained 2.5% 13CH4, 3.1% 12CO2, and 2.2% 13CO2.
In the second experiment, 13CO2replaced the unlabeled CO2. 13CO2was prepared by pumping a 0.6 M NaH13CO3(99% 13C) solution into a stirred solution (450 ml) of 1.2 M HCl in a closed 500-ml serum bottle. The argon gas supply of the chemostat was led via the headspace of this bottle, and once the 13CO2concentration was constant, it was supplied into the chemostat. The ingoing gas contained 4.6% CH4, 0.07% 12CO2, and 3.6% 13CO2at a flow rate of 10.4 ml · min−1. The outgoing gas, with a flow rate of 10.3 ml · min−1, contained 2.3% CH4, 1.95% 12CO2, and 3.2% 13CO2. During the experiments, samples of chemostat culture were collected and used for the IRMS analysis of the biomass.
Assuming that carbon in new biomass is labeled according to the label percentage of the CO2present (XCO2) in the chemostat (i.e., gas outlet), the 13C label percentage of biomass in the chemostat will develop over time according to the formula: XCO2× (1 − e−Dt), in which Drepresents the dilution rate (h−1) of the reactor and trepresents time (h).
The “old” biomass in the chemostat at the moment that we started the calculations washed out according to the formula Xold× (e−Dt). Xoldis the 13C label percentage of biomass of the first sample taken, at the moment that the 13CO2gas concentrations in the outlet reached steady state. This value was still close to the natural abundance of 13C in the biomass, as only a little 13CO2was incorporated. The sum of new and old biomass percentages was taken as the predicted average 13C label percentage of the chemostat biomass in the course of time during the supply of labeled gases: Xt= XCO2× (1 − e−Dt) + Xold× (e−Dt).
Recovery calculation of 13C from 13CH4in CO2and biomass.
The 13C recovery in biomass (see Table S1 in the supplemental material) was calculated using the following formula: mole fraction of formaldehyde × 0.34 × 100 + mole fraction of CO2× 0.34 × 42. In this formula, the mole fraction refers to the incorporation ratio of formaldehyde and CO2into biomass, depending on the carbon assimilation pathway. The values 100 and 42 refer to the labeling percentages of formaldehyde and CO2, respectively. The value 0.34 refers to the biomass produced according to the observed stoichiometry of CH4oxidation (see Results).
Cell extracts.
Cells of an exponentially growing culture (9 liters, optical density at 600 nm [OD600] = 0.56) were collected by centrifugation (4,000 × g, 4°C, 10 min) and washed two times with 70 ml buffer {25 mM PIPES [piperazine-N,N′-bis-(2-ethanesulfonic acid)]–NaOH, pH 7.5{. Finally, the cell pellet (9.6 g [wet weight]) was resuspended in 10 ml of PIPES-NaOH buffer. To this suspension (pH 7), one tablet of protease inhibitor cocktail (Boehringer, Mannheim, Germany) and 1 mg DNase I were added. Cells were broken by passing the cell suspension 3 times through a French press at 20,000 lb/in2. Unbroken cells and cell debris were removed from the resulting crude extract by centrifugation at 12,000 × gfor 20 min (4°C, Sorvall SS-34 rotor). This resulted in a turbid supernatant, with increasing turbidity toward the bottom of the tube. The turbid cell extract was centrifuged again at 48,000 × gfor 40 min (4°C). This resulted in clear reddish supernatant and a yellowish pellet. The clarified cell extract obtained was further ultracentrifuged, using a Sorvall Discovery with a 70.1 Ti rotor (150,000 × g, 4°C, 60 min). This resulted in clear reddish supernatant and a tiny yellow transparent pellet.
Protein gel electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and native PAGE were performed using a Mini-Protean 3 cell (Bio-Rad) according to the method of Laemmli (21). Molecular weight standards were obtained from Fermentas and Invitrogen. For SDS-PAGE, protein samples (25 μg) were incubated at 95°C for 10 min in loading buffer (6.5% [vol/vol] glycerol, 0.016 M Tris-HCl [pH 6.8], 0.5% [wt/vol] SDS, 0.003% [wt/vol] bromophenol blue, and 1.3% [vol/vol] β-mercaptoethanol). For native PAGE, loading buffer without SDS and β-mercaptoethanol was used. Proteins in the gel were stained overnight with Coomassie brilliant blue G-250.
Protein bands from native gels were cut out and further analyzed by SDS-PAGE; bands (8.5 mm3) were incubated in 100 μl SDS-PAGE loading buffer at 95°C for 10 min and incubated further overnight at 4°C with gentle shaking. The eluted proteins were 10-fold concentrated and washed several times with Tris-HCl buffer (0.5 M, pH 6.8) using a 3K Vivaspin column (Sartorius Stedim Biotech) before being loaded on the SDS-PAGE gel.
Molecular mass determination of RuBisCO.
For mass determination of RuBisCO, native PAGE (see above) and size exclusion chromatography were applied. Clarified cell extract of strain SolV was passed through a column (16 mm by 130 cm) of Sephacryl S-300 HR (VWR). The column was preequilibrated with 20 mM phosphate buffer-100 mM NaCl (pH 7.2). After loading, the column was eluted with the same buffer at 0.5 ml · min−1, and column effluent was monitored at 280 nm. Protein fractions were collected using an automated fraction collector. Peak fractions were then subjected to SDS-PAGE analysis. Calibration proteins obtained from Sigma were albumin egg (45 kDa), albumin bovine (66 kDa), alcohol dehydrogenase (150 kDa), apoferritin (443 kDa), thyroglobulin (669 kDa), and blue dextran (2,000 kDa).
MALDI-TOF MS analysis.
Identification of the protein bands on the SDS-PAGE gels was performed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). Gel spots (about 5 mm3) were destained (solutions contained acetonitrile [30, 50, and 100%, vol/vol], diluted with 25 mM ammonium bicarbonate [NH4HCO3]), and dried, and the proteins were in-gel digested with 5 μl of trypsin (the solution contained 15 ng trypsin · μl−1in a mixture of 25 mM NH4HCO3and 5 mM n-octyl-β-d-glucopyranoside) on ice for 1 h. The excess trypsin solution was removed, and the gel spot was covered with a solution containing 25 mM NH4HCO3and 5 mM n-octyl-β-d-glucopyranoside and was incubated overnight at 37°C. The peptides were extracted by adding 4 μl of a mixture containing (all volume/volume) 50% acetonitrile, 0.5% trifluoroacetic acid (TFA), and 49.5% 5 mM n-octyl-β-d-glucopyranoside, followed by 1 h of incubation at room temperature. The liquid was sonicated for 2 min in a water bath and dried in a vacuum centrifuge. The peptides were solved in 4 μl of TFA (0.1%). The dissolved peptides (3 μl) were mixed with 0.3 μl of matrix solution (20 mg α-cyano-4-hydroxycinnamic acid in 0.5 ml 0.1% TFA and 0.5 ml acetonitrile), spotted on a stainless steel target plate, and analyzed on a Biflex III MALDI-TOF mass spectrometer (Bruker, Bremen, Germany) operating in the reflectron mode. Data analysis was performed using X-TOF software (Bruker, Bremen, Germany), and the Mascot search engine (Matrix Science Ltd., Boston, MA) was used to identify the proteins.
Immunoblotting.
The proteins from SDS-PAGE and native-PAGE gels were transferred to a nitrocellulose membrane by electroblotting at 100 mA for 45 min. The obtained blots were then prepared for immune reaction as previously described (30). The polyclonal anti-RuBisCO, raised in a rabbit using a synthetic peptide, was used as the primary antibody (product AS03 037; Agrisera, Vännäs, Sweden). This antibody was 3,300-fold diluted in blocking buffer. The monoclonal mouse anti-rabbit IgG alkaline phosphatase conjugate (Sigma catalog no. A2556) was used as the secondary antibody.
Activity assay for RuBisCO.
(i) Carboxylase activity.As an alternative to the radioisotope method, we developed a stable-isotope method for measuring RuBisCO activity. This was based on the incorporation of 13CO2into 3-phosphoglycerate (3-PGA) and subsequent destruction of 3-PGA by acid permanganate oxidation. The 13CO2liberated was quantified by GC-MS as described above. The destruction of 3-PGA to liberate the carboxyl group was performed analogously to the destruction of lactate with permanganate and phosphoric acid (H3PO4), as previously described (4), which produces CO2and acetate. We used 1% potassium permanganate (KMnO4) in 0.1 M H3PO4instead of 5% KMnO4in 0.07 M H3PO4, and the temperature was lowered from 80°C to 50°C. Under such conditions, 15 min of incubation yielded 1 mol of CO2per mol of 3-PGA. This was verified by a series of 0.1 to 2 μmol of 3-PGA in 3-ml Exetainer vials (Labco Limited, High Wycombe, United Kingdom). Ice-cold KMnO4(500 μl) was added, and the vial was immediately closed with a screw cap with a rubber seal and incubated at 50°C. A linear relation between the amount of 3-PGA and CO2produced was measured (GC-MS analysis). Under stronger oxidative conditions, the initial production of CO2was more rapid but followed by a continuous slow evolution of CO2. Apparently, the oxidation product of 3-PGA is prone to further oxidation. The final destruction conditions applied were 0.1% KMnO4in 0.1 M H3PO4for 25 min at 50°C.
To limit the background production of CO2and increase the sensitivity of the method, we used 20 mM phosphate buffer in the assay mixtures to replace the commonly used organic buffers. The only organic material present in the assay mixture now originated from the RuBisCO-containing samples. Apart from some CO2from air, the major part of CO2(>80%) present in the Exetainer vial after destruction resulted from the RuBisCO assay samples. As it reflects the sample amount, this CO2could act as an internal standard when the ratio of 13CO2to 12CO2was taken. This resulted in better time curves than those obtained using the absolute amounts of 13CO2produced in the destruction vial. Moreover, the GC-MS response for CO2was varied over time, while the ratio of 13CO2to 12CO2was very constant. The ratio multiplied by the average total amount of CO2in the destruction bottles from a time series was used to calculate the amount of 13CO2produced. Time series were linear for 2 min. The standard RuBisCO assay was performed in a 2-ml vial (VersaVial; Alltech) containing 200 μl of Mg-phosphate buffer (20 mM potassium phosphate, 10 mM MgCl2, pH 6.9). Cell extracts diluted in 25 mM PIPES-NaOH, pH 7.5, to a total volume of 25 μl and NaH13CO3(99% 13C) (20 μl of a 100 mM stock) were added. The vial was closed immediately with a thin rubber cap (Alltech) to prevent the loss of CO2. The final pH in this mixture was pH 7.25 (at 50°C), and the CO2gas concentration was calculated to be 1.5%. The vial was preincubated for 10 min at 50°C in a water bath, and the RuBisCO activity assay was started by adding 5 μl of stock solution of ribulose-1,5-bisphosphate (25 mM) by a syringe through the rubber cap. The mixture was vortexed for 2 s and incubated further at 50°C. Samples of 50 μl were taken through the stopper by a syringe every 30 s for 2 min. Samples were mixed with 20 μl of 0.5 M HCl in 3-ml Exetainer tubes and dried under vacuum at 45°C. After the destruction with 0.5 ml permanganate solution as described above and cooling down to room temperature, the vials were stirred on a vortex device to allow CO2to equilibrate. The gas phase was measured for 13CO2and 12CO2by GC-MS analysis.
(ii) Oxygenase activity.The oxygenase reaction of RuBisCO was assayed in a Strathkelvin RC350 respiration cell at a working volume of 0.7 ml. The assay buffer contained 50 mM HEPES and 10 mM MgCl2(pH 7.2) and was air saturated at 50°C. Unlabeled NaHCO3was added in the range of 0 to 10 mM, and after the addition of cell extract (12,000 × gsupernatant, 1 mg protein), the background O2consumption was followed for 10 min before the addition of ribulose 1,5-bisphosphate (0.4 mM). After this addition, consumption rates increased immediately but then slowed down.
pH optimum for RuBisCO assay.
The effect of pH on the activity was determined by setting the initial pH value of the Mg-phosphate buffer at values of 6.45, 6.6, 6.85, and 7.05. NaH13CO3was added at 7, 9, 12, and 16 mM final concentrations, respectively. The final pH was verified at 50°C in an upscaled situation (10-ml tubes) to be 7.0, 7.22, 7.5, and 7.75, respectively. Taking a dissociation constant (pKa) of 6.1 for carbonic acid (H2CO3) at 50°C, the CO2percentage in the gas phase was calculated to be between 1.4 and 1.7%.
Phylogenetic analysis.
Representative cbbLand cbbSsequences, encoding the large and small subunits of RuBisCO, were obtained from GenBank and aligned using MUSCLE (10) in MEGA 5.0 (www.megasoftware.net). Phylogenetic trees were calculated using the neighbor-joining method (25) with 1,000 bootstraps (14) to infer the evolutionary relationship. Positions containing alignment gaps and missing data were eliminated only in pairwise sequence comparisons (pairwise deletion option). The Dayhoff matrix-based method was used to compute the evolutionary distances.
Nucleotide sequence accession numbers.
The M. fumariolicumSolV genes reported in this study were extracted from the draft genome and submitted to GenBank under accession numbers JF706245to JF706259and JF714482.
RESULTS
Growth of strain SolV at different CO2concentrations.
Standard batch incubations of M. fumariolicumstrain SolV with CO2and CH4, both 5%, resulted in exponential growth within 1 day. However, incubations without CO2did not result in any growth on CH4, even after 40 days of incubation, indicating that CO2was necessary for growth. This dependency was further investigated in a series of batch incubations in 1-liter bottles with air as the only source of CO2(0.04%) and with 5% CH4. In all incubations, we observed CO2production from CH4during the initial period, at a rate directly depending on the inoculum size. As the CH4oxidation was not coupled to growth, the CO2production rate decreased with time (see Fig. S1A in the supplemental material). Growth resumed only after CO2had reached levels above 0.3% (Fig. S1B and S1C). This indicated that the growth of strain SolV is dependent on a minimum concentration of CO2in the headspace.
In order to circumvent endogenous CO2production and to test the effect of nongrowth conditions on the viability of strain SolV, a culture was starved for CH4for 35 days (at 55°C). When CH4was subsequently added, CO2production was hardly measurable, and no growth was observed for 7 days. Thereafter, when CO2was added, growth resumed within 3 days (Fig. S2).
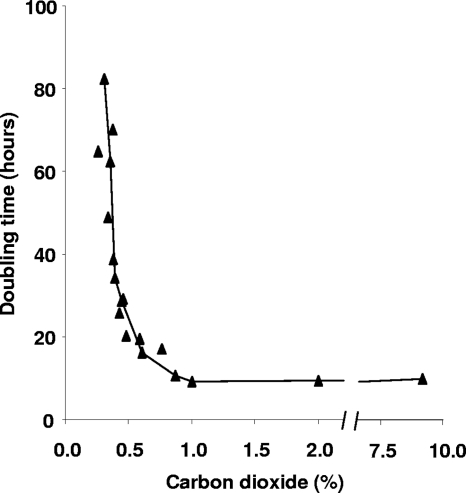
From various growing cultures of strain SolV, the growth rate was determined at different prevailing CO2concentrations. This was done for time intervals of about 6 h, in which CO2concentrations in the headspace did not change more than 15%. The results clearly showed the dependency of the growth rate on CO2concentration (Fig. 1). The lowest CO2concentration at which growth was observed was 0.3% (doubling time, about 80 h), and the growth rate increased toward its maximum (doubling time, 10 h) at 1% CO2.
Fig. 1.
Doubling time of M. fumariolicumstrain SolV at different CO2concentrations (triangles). The growth rates of various growing cultures of strain SolV were determined at different prevailing CO2concentrations. This was done for time intervals of about 6 h, in which CO2concentrations in the headspace did not change more than 15%.
13C-labeling experiments in chemostat cultures.
Labeling experiments were performed to determine whether CO2was the only carbon source in M. fumariolicumstrain SolV. A CH4-limited chemostat culture at a dilution rate (D) of 0.016 h−1was used for this purpose. At steady state, gas concentrations in the inlet and outlet were analyzed, and the CH4consumption rate (ml · min−1± standard error of the mean [SEM]) was calculated to be 0.254 ± 0.009 (n= 4). The CO2production rate (ml · min−1± SEM) was 0.167 ± 0.01 (n= 4). This results in the following stoichiometry for carbon: 1 CH4→ 0.66 CO2+ 0.34 biomass (CH2O). The biomass carbon can be derived either from CH4oxidation intermediates or from CO2.
In the first experiment, the unlabeled CH4supply of the chemostat was changed to 13CH4. The labeling percentages of the gases in the chemostat headspace reached steady state after 1.5 h (42% 13C for CO2and >99% 13C for CH4), and the 13CH4consumption and 13CO2production rates (ml · min−1± SEM) were calculated (0.254 ± 0.009 and 0.226 ± 0.002, respectively), thus resulting in a recovery (mean ± SEM) of 89% ± 3.3% of the 13CH4consumed.
Even when all 13CH4consumed is converted into 13CO2and all biomass is derived from CO2, a total recovery in 13CO2is not expected, as part of the 13CO2produced in the chemostat is incorporated into the biomass. With the above stoichiometry for carbon and a measured 42% 13C-labeling percentage for CO2in the chemostat, still 14% (measured 13C-labeling percentage × moles of biomass) of the label will be incorporated into the biomass, and so only 86% can be recovered as 13CO2(see Table S1 in the supplemental material).
The measured 13C-labeling percentage of CO2in the chemostat was in good agreement with the calculated value of about 40%. This was calculated on the basis of the unlabeled CO2in the inlet gas and the amount of 13CH4converted into 13CO2.
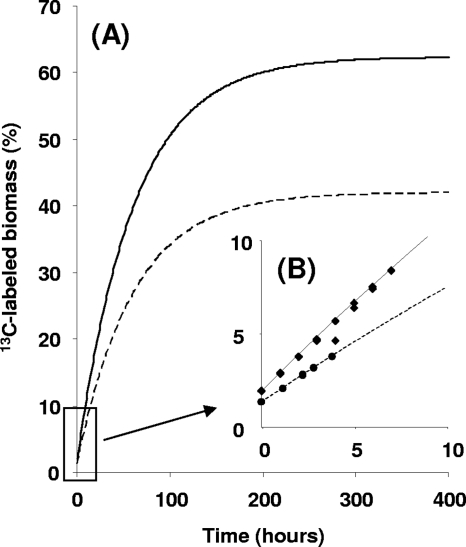
The increase in the 13C percentage of the biomass in the chemostat culture over the course of time (Fig. 2A) was predicted under the assumption that all biomass was derived from CO2, at the current 13C label percentage of 42%. Before the reactor was supplied with the 13C label, the percentage was 1.1, close to the natural abundance of 13C in CO2gas (1.17%). Measured values of the increase of the 13C percentage of the biomass followed exactly the predicted curve (Fig. 2B).
Fig. 2.
(A) Predicted percentage of 13C-labeled biomass, for an M. fumariolicumstrain SolV chemostat culture supplied with 13CH4(dashed line) and 13CO2(solid line). (B) Measured percentages of 13C-labeled biomass in the first 10 h for a SolV culture supplied with 13CH4(circles) and 13CO2(squares), combined with the predicted percentages.
The experiment was repeated with 13CO2in the inlet gas of the chemostat and unlabeled CH4. The labeling percentage of CO2in the outlet increased to 62% after 1.5 h (steady state). Unlabeled CO2originated from oxidation of the unlabeled CH4. Again, the increase of the 13C percentage of the biomass followed the predicted curve (Fig. 2B), with the assumption that CO2was the only carbon source.
Within the first hours of the experiment, the increase of the 13C label percentage into biomass was assumed to be linear, and the 13C incorporation rate of the predicted curve for the 13CO2experiment was calculated to be 1.5 times higher than the predicted curve for the 13CH4experiment (Fig. 2B), in accordance with the 1.5-times-higher label percentage (62% versus 42%).
13C-labeling experiments in batch cultures.
The labeling study with 13CH4in the chemostat culture always resulted in high 13CO2production from 13CH4oxidation. This could have been circumvented by sparging much larger gas volumes through the reactor. This option was not used, since this is technically not feasible in our chemostat setup; instead, labeling experiments in two batch cultures with a high concentration (40%) of unlabeled 12CO2and a relatively limited amount (3.2%) of 13CH4were performed. If all 13CH4was converted to 13CO2, the CO2pool would be labeled for an additional 7.4% only (3.2%/40% × 100). Concentrations of the gases and 13C/12C mass ratios of CO2at the start of and after growth, when 80 to 95% of the added CH4was converted, were carefully measured. It could be calculated that in both bottles, at least 96% of the label of 13CH4was recovered as 13CO2. This pointed to complete oxidation of CH4and the sole use of CO2as the carbon source.
If we assume that carbon assimilation by fixation of CO2and that 0.34 mol CO2incorporated into the biomass per mole CH4converted, then close to 2% of the total 13C produced would have been incorporated. This makes the total 13C recovery at least 98%. At the end of the experiment, the 13C/12C ratio of the biomass in both bottles was determined by IRMS. The analysis showed that 4.6% of the biomass carbon was 13C labeled. This percentage was in very good agreement with the average measured 13C percentage of the CO2during growth (1.2% at the start of and 7.7% at the end of growth, so an average of 4.5%) and again confirms that CO2is the main carbon source.
RuBisCO in strain SolV.
The labeling experiments described above showed that M. fumariolicumstrain SolV fixes CO2, and the draft genome data of strain SolV contained a RuBisCO gene, encoding the key enzyme of the CBB cycle (see below). Therefore, cell extracts of strain SolV were tested for the presence of RuBisCO activity and analyzed by SDS-PAGE (13% gel) and native PAGE (5% gel).
For the activity assay, we developed a method based on the 13CO2incorporation into 3-PGA and its subsequent destruction in closed vials with acid permanganate solution. 13CO2concentrations in the vial were quantified on the basis of 13C/12C ratios as measured by GC-MS.
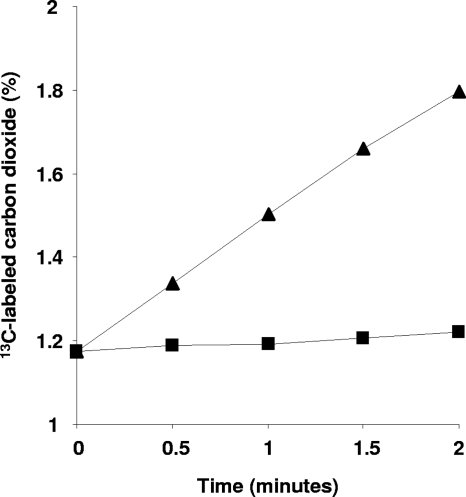
Activity was proportional to the amount of turbid cell extract added (concentrations of 10 to 250 μg protein per 250 μl assay mixture were tested) and linear for 2 min (Fig. 3). No 13CO2was incorporated without extract or the substrate ribulose-1,5-biphosphate (0.5 mM). Activity was optimal between pHs 7.2 and 7.5. At pHs 7.7 and 7.0, activity dropped by about 10%. When kept on ice, the activity in extracts was stable for at least a month. The specific activity of the turbid cell extract was 55 to 70 nmol CO2fixed · min−1· mg of protein−1. The oxygenase activity of RuBisCO measured at an oxygen concentration of around 150 μM was about 4.5 nmol O2· min−1· mg of protein−1, and this activity was inhibited by the addition of NaHCO3(50% inhibition at 4 mM NaHCO3[results not shown]). Virtually all the RuBisCO activity (>90%) appeared to be present in the clarified cell extract (48,000 × gsupernatant). The corresponding pellet contained only very small amounts of RuBisCO activity that could be attributed to a residual soluble fraction (Fig. 3).
Fig. 3.
13CO2incorporation into 3-PGA and its subsequent destruction in closed vials with acid permanganate solution. 13CO2concentrations quantified in the vial represent M. fumariolicumstrain SolV RuBisCO activity in turbid cell extract (12,000 × gsupernatant [triangles]) and in the pellet after centrifugation at 48,000 × g(squares).
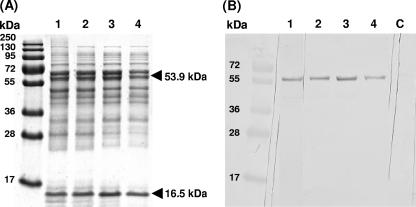
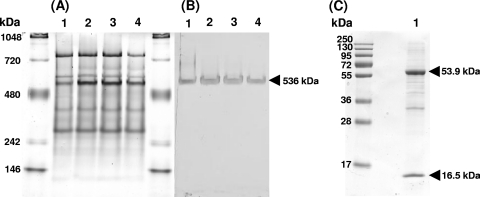
When the proteins in the crude extract were analyzed by 13% SDS-PAGE, a consistent pattern of proteins was observed, which did not change upon subsequent centrifuging steps (Fig. 4A). Dominant bands (indicated with arrows) were cut out of the gel and identified by tryptic digestion and MALDI-TOF MS peptide mass analysis. The results showed that the upper band corresponded to the large subunit of RuBisCO, with a predicted mass of 53,930 Da, and the lower band corresponded to the small subunit of RuBisCO, with a predicted mass of 16,550 Da (Fig. 4A). The pellets obtained after the different centrifugation steps did not contain these bands (data not shown). In addition to the MALDI-TOF MS identification, immunoblotting with an antibody against the RuBisCO large subunit was done, and only the 53,930-Da band specifically reacted with this antibody (Fig. 4B, lanes 1 to 4). After being washed extensively with PIPES-NaOH buffer, pellets of the 12,000 × gand 48,000 × gcentrifugation steps (yielding intact cells and membranes) showed only a weak reaction with this antibody. In contrast, after a washing of the 150,000 × gpellet, a clear immune response band remained visible (data not shown), suggesting that RuBisCO is present as a high-molecular-mass complex that partly sediments at this high centrifugation speed. This was verified by analyzing all supernatants on a 5% native-PAGE gel (Fig. 5A). The prominent band at about 536 kDa (Fig. 5A) showed an immunoreaction with the RuBisCO antibody (Fig. 5B). The corresponding protein band from an unstained native gel part was cut out of the gel. Proteins from the gel pieces were denatured, eluted, and loaded on a 13% SDS-PAGE gel. This resulted in only 2 protein bands with molecular weights corresponding to the large and small subunits of RuBisCO (Fig. 5C, lane 1). This was confirmed by the MALDI-TOF analysis and antibody reaction (data not shown). The native mass of RuBisCO was also determined by size exclusion chromatography, which showed a somewhat apparent lower molecular mass of about 482 kDa. Most likely, the native RuBisCO protein complex is composed of 8 small and 8 large subunits, with a calculated molecular mass of 563,840 Da.
Fig. 4.
(A) M. fumariolicumstrain SolV proteins in the crude extract were analyzed by 13% SDS-PAGE after subsequent centrifuging steps. (B) The proteins were then transferred onto a nitrocellulose membrane and tested for the presence of RuBisCO, using a peptide antibody against the large subunit of this enzyme. Lane numbers in both figures are the same. Lane 1, crude extract; lane 2, 12,000 × gsupernatant; lane 3, 48,000 × gsupernatant; lane 4, 150,000 × gsupernatant; lane C, control (crude extract without peptide antibody). The upper and lower bands indicated with arrows correspond to the large and small subunits of RuBisCO, respectively, identified by MALDI-TOF MS analysis.
Fig. 5.
(A) M. fumariolicumstrain SolV proteins in the crude extract were analyzed by 5% native PAGE after subsequent centrifuging steps. (B) The proteins were then transferred onto a nitrocellulose membrane and tested for the presence of RuBisCO, using the peptide antibody against the large subunit of this enzyme. Lane numbers in both figures are identical. Lane 1, crude extract; lane 2, 12,000 × gsupernatant; lane 3, 48,000 × gsupernatant; lane 4, 150,000 × gsupernatant. (C) The native band corresponding to the RuBisCO complex (indicated with an arrow in panel B) was analyzed on a 13% SDS-PAGE gel (lane 1).
Transcriptomics.
Analyses of the draft genome of M. fumariolicumstrain SolV revealed that the key genes needed for an operational ribulose monophosphate pathway, the hexulose-6-P synthase and hexulose-6-P isomerase genes, were absent (23). In addition, the crucial genes encoding key enzymes of the serine pathway, the malyl-coenzyme A lyase and glycerate kinase genes, were not found. However, the genes needed for a full operational CBB cycle were present. All these genes were also transcribed (Table 1), as was verified by transcriptome analysis of exponentially growing cells, especially the genes encoding the large and small subunits of RuBisCO, which were highly transcribed. These results, together with the physiological and biochemical experiments described above, fully support the conclusion that strain SolV makes use of the CBB cycle to fix CO2for carbon assimilation.
Table 1.
Genes involved in the Calvin-Benson-Bassham cycle in M. fumariolicumstrain SolVa
| Enzyme | Gene name | EC no. | GenBank accession no. | Relative expression mRNA |
|---|---|---|---|---|
| Ribulose 1,5-bisphosphate carboxylase, large subunit | cbbL | EC 4.1.1.39 | JF706253 | 21.6 |
| Ribulose 1,5-bisphosphate carboxylase, small subunit | cbbS | EC 4.1.1.39 | JF706253 | 14.4 |
| RuBisCO accessory protein CbbX, AAA ATPase | cbbX | JF706253 | 14.3 | |
| Ribulose-phosphate 3-epimerase | rpe | EC 5.1.3.1 | JF706259 | 1.1 |
| Phosphoribulokinase | prkB | EC 2.7.1.19 | JF706255 | 1.8 |
| JF706253 | 3.8 | |||
| Ribose 5-phosphate isomerase B | rpiB | EC 5.3.1.6 | JF706256 | 1.5 |
| Triosephosphate isomerase | tpiA | EC 5.3.1.1 | JF714482 | 1.2 |
| Putative phosphoketolase | JF706257 | 0.7 | ||
| Transketolase | tktA | EC 2.2.1.1 | JF706253 | 4.3 |
| Fructose-1,6-bisphosphatase II/sedoheptulose 1,7-bisphosphatase | glpX-SEBP gene | EC 3.1.3.11 | JF706249 | 1.3 |
| EC 3.1.3.37 | ||||
| Fructose-1,6-bisphosphatase | fbp | EC 3.1.3.11 | JF706254 | 2.5 |
| Fructose-bisphosphate aldolase, class I | fbaA | EC 4.1.2.13 | JF706258 | 0.5 |
| Fructose-bisphosphate aldolase, class II | fbA2 | EC 4.1.2.13 | JF706254 | 4.4 |
| Glyceraldehyde-3-phosphate dehydrogenase | gapA | EC 1.2.1.59 | JF714482 | 5.1 |
| Phosphoglycerate kinase | pgk | EC 2.7.2.3 | JF714482 | 1.8 |
| 6-Phosphogluconolactonase | nagB | EC 3.1.1.31 | JF706252 | 0.3 |
| 6-Phosphogluconate dehydrogenase | gnd | EC 1.1.1.44 | JF706256 | 1.2 |
| Glucose-6-phosphate 1-dehydrogenase | zwf | EC 1.1.1.49 | JF706252 | 1.0 |
| Glucose-6-phosphate isomerase | pgi | EC 5.3.1.9 | JF706248 | 0.4 |
| Fructose-6-phosphate aldolase 1 | mipB | EC 4.1.2.- | JF706251 | 0.8 |
The relative levels of transcription of the genes were obtained by a transcriptome analysis of an exponentially growing liquid culture (see Materials and Methods).
Phylogenetic analysis of RuBisCO.
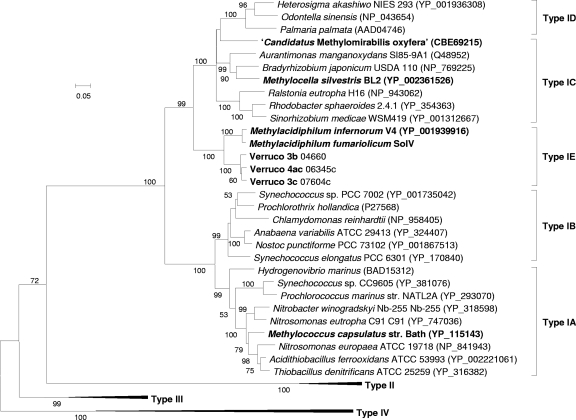
RuBisCO enzymes can be grouped according to their structures, catalytic properties, and gene arrangements into types I (A, B, C, and D), II, III, and IV (RuBisCO-like) (2). A neighbor-joining tree (Fig. 6) was constructed using representative cbbLsequences of each group. Also, for this analysis, the sequences of three (uncharacterized) mesophilic verrucomicrobial methanotrophs available from GenBank (accession numbers JF706245, JF706246, and JF706247) were added. The verrucomicrobial RuBisCO enzymes formed a distinct group in the phylogenetic tree. The RuBisCO operons in M. fumariolicumstrain SolV and M. infernorumstrain V4 are arranged in the gene order cbbL cbbS cbbX(see Fig. S3 in the supplemental material), which is typical for type IC (2). In both strains, no genes involved in carboxysome formation were detected. The remaining genes of the CBB cycle (Table 1) are spread through the genome of strain V4, sometimes with two or three CBB cycle genes clustered. BLASTP searches with the M. fumariolicumSolV RuBisCO large subunit in environmental data sets did not result in sequences fitting into the distinct verrucomicrobial group.
Fig. 6.
Phylogenetic analysis of the RuBisCO large subunit (CbbL). Representative amino acid sequences were obtained from GenBank. Bootstrap values (1,000 replicates) are shown at the branches. The bar represents 20% sequence divergence. The neighbor-joining tree is drawn to scale; the evolutionary distances were computed using the Dayhoff matrix-based method, and units are the numbers of amino acid substitutions per site. All positions containing alignment gaps and missing data were eliminated only in pairwise sequence comparisons (pairwise deletion option). There were a total of 539 positions in the final data set.
DISCUSSION
Carbon fixation by methanotrophs has been a topic of research for decades, and the assimilation via the CBB cycle by proteobacterial methanotrophs is unlikely, because CBB is associated with high ATP requirements (8). Although M. capsulatusBath possesses CBB cycle genes (31), physiological evidence for an active CBB cycle is lacking thus far. However, available genome data from verrucomicrobial methanotrophs (17, 23) point to an autotrophic lifestyle for these microbes.
In the present study, the transcriptome analysis of M. fumariolicumstrain SolV showed that all of the genes for the enzymes necessary for a CBB cycle are expressed, most prominently the gene encoding RuBisCO. The cbbRgene (encoding the RuBisCO operon transcriptional regulator) is present, but its expression is low (only 0.1). On the V4 genome, this gene seems to be coupled to nitrate transport and reduction (17). Whether this gene is involved in the regulation of the RuBisCO operon needs to be resolved.
On SDS-PAGE gels, RuBisCO was identified as one of the most dominant proteins in the cell extracts of strain SolV. Using a novel 13CO2incorporation assay, we showed the high specific activity of RuBisCO (70 nmol CO2fixed · min−1· mg of protein−1). This activity is similar to that reported for other autotrophic bacteria (5) and corresponds well with the maximum specific growth rate of 0.07 h−1(doubling time, 10 h), assuming that dry cells contain 50% carbon and 70% protein.
As RuBisCO was found only in the clarified cell extract, we conclude that RuBisCO is not densely packed into polyhedral inclusion bodies (carboxysomes), which generally sediment upon centrifugation at 10,000 × gand 40,000 × g(15, 24). In addition, the genome data did not show any proteins that could encode carboxysomal shell proteins. Carboxysomes are encountered in cyanobacteria and in a limited number of chemoautotrophs like sulfur- or sulfide-oxidizing and nitrifying bacteria (33). These compartments are thought to enhance the concentration of CO2for RuBisCO, which has a low affinity for CO2. Microorganisms, which contain carboxysomes, are able to grow at an ambient CO2concentration; however, our batch incubations have demonstrated that strain SolV needs a high CO2concentration for growth (above 0.3%), in agreement with a free form of RuBisCO. Furthermore, the volcanic regions from which the verrucomicrobial methanotrophs were isolated exhibit high CO2concentrations (6). In these environments, there is apparently no need to sequester CO2.
Based on the apparent molecular masses of the native RuBisCO protein and the two subunits, we predict that it forms an octomeric complex (L8S8). This form of RuBisCO is typical for all form I RuBisCOs, which are composed of four large-subunit dimers (L2) with small subunits (S) decorating the top and bottom of the L8 octomeric core (28).
Phylogenetic analysis and the gene arrangement of the RuBisCOs from two thermophilic Methylacidiphilumstrains positions them in a separate cluster within form I RuBisCOs, for which we propose the name type IE. Based on gene arrangement, the verrucomicrobial RuBisCO is most closely related to RuBisCO type IC (2). The cbbX cbbS cbbLoperon arrangement is typical for type IC and type IE and not typical for other form I enzymes. The IC form has medium to low affinity for CO2, indicating an adaptation to environments with medium to high CO2but with O2present, and microorganisms having type IC RuBisCO do not possess carboxysomes (2). This verrucomicrobial RuBisCO type has not been detected before by molecular approaches. This can be explained by the mismatches we observed when comparing the Methylacidiphilum-type cbbLsequence with all the available RuBisCO primers sets (1, 11, 26, 29).
The 13C label percentage in the biomasses of both the batch and the chemostat experiments was in complete accordance with the 13C label percentage of CO2in the cultures and confirms that biomass carbon was derived exclusively from CO2. If CO2is the only carbon source in M. fumariolicumstrain SolV, it can be anticipated that CH4is completely oxidized into CO2. This was demonstrated by the recovery study of 13CH4(see Results). If intermediates of CH4oxidation (which are fully 13C labeled) were incorporated, the recovery of label into CO2would have been much lower: about 66% for the ribulose monophosphate pathway and 79% or 76% for the serine pathway (see Table S1 in the supplemental material).
The autotrophic nature of verrucomicrobial methanotrophs has large consequences for their detection in the environment. The presence of active methanotrophs has been assessed by isotope-based techniques, like stable isotope probing and phospholipid fatty acid labeling, which rely on the incorporation of labeled CH4into DNA/RNA or lipids, respectively. Such methods overlook the involvement of these autotrophic methanotrophs, especially in environments with high CO2concentrations.
Conclusions.
M. fumariolicumstrain SolV is an autotrophic methanotroph. It fixes CO2via the CBB cycle, with CH4as the energy source. It uses a non-carboxysome-associated RuBisCO, in agreement with a high requirement for CO2. RuBisCOs in verrucomicrobial methanotrophs form a new group most closely related to type IC. The ultimate proof would require knockout and complementation studies, but this has to await the development of a genetic system.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Mosaicgrant 62000583from the Dutch Organization for Scientific Research (NWO).
We are grateful to Jelle Eygensteyn for help with the IRMS analyses, and we thank Mingliang Wu for help with the Western blotting and immunoblotting techniques, Naomi de Almeida for help with the SDS-PAGE and native-PAGE techniques, and Dick van Aalst for photography.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 1 July 2011.
REFERENCES
- 1. Alfreider A., Vogt C., Geiger-Kaiser M., Psenner R. 2009. Distribution and diversity of autotrophic bacteria in groundwater systems based on the analysis of RubisCO genotypes. Syst. Appl. Microbiol. 32:140–150 [DOI] [PubMed] [Google Scholar]
- 2. Badger M. R., Bek E. J. 2008. Multiple Rubisco forms in proteobacteria: their functional significance in relation to CO2acquisition by the CBB cycle. J. Exp. Bot. 59:1525–1541 [DOI] [PubMed] [Google Scholar]
- 3. Baxter N. J., et al. 2002. The ribulose-1,5-bisphosphate carboxylase/oxygenase gene cluster of Methylococcus capsulatus(Bath). Arch. Microbiol. 177:279–289 [DOI] [PubMed] [Google Scholar]
- 4. Bird I. F., Cornelius M. J., Keys A. J., Whittingham C. P. 1978. Intramolecular labeling of sucrose made by leaves from [14C] carbon dioxide or [3-14C] serine. Biochem. J. 172:23–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cannon G. C., Shively J. M. 1983. Characterization of a homogenous preparation of carboxysomes from Thiobacillus neapolitanus. Arch. Microbiol. 134:52–59 [Google Scholar]
- 6. Castaldi S., Tedesco D. 2005. Methane production and consumption in an active volcanic environment of Southern Italy. Chemosphere 58:131–139 [DOI] [PubMed] [Google Scholar]
- 7. Chen Y., et al. 2010. Complete genome sequence of the aerobic facultative methanotroph Methylocella silvestrisBL2. J. Bacteriol. 192:3840–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chistoserdova L., Kalyuzhnaya M. G., Lidstrom M. E. 2009. The expanding world of methylotrophic metabolism. Annu. Rev. Microbiol. 63:477–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dunfield P. F., et al. 2007. Methane oxidation by an extremely acidophilic bacterium of the phylum Verrucomicrobia. Nature 450:879–883 [DOI] [PubMed] [Google Scholar]
- 10. Edgar R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elsaied H. E., Kimura H., Naganuma T. 2007. Composition of archaeal, bacterial, and eukaryal RuBisCO genotypes in three Western Pacific arc hydrothermal vent systems. Extremophiles 11:191–202 [DOI] [PubMed] [Google Scholar]
- 12. Ettwig K. F., et al. 2010. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–550 [DOI] [PubMed] [Google Scholar]
- 13. Ettwig K. F., et al. 2008. Denitrifying bacteria anaerobically oxidize methane in the absence of Archaea. Environ. Microbiol. 10:3164–3173 [DOI] [PubMed] [Google Scholar]
- 14. Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791 [DOI] [PubMed] [Google Scholar]
- 15. Gonzales A. D., et al. 2005. Proteomic analysis of the CO2-concentrating mechanism in the open-ocean cyanobacterium SynechococcusWH8102. Can. J. Bot. 83:735–745 [Google Scholar]
- 16. Hanson R. S., Hanson T. E. 1996. Methanotrophic bacteria. Microbiol. Rev. 60:439–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hou S., et al. 2008. Complete genome sequence of the extremely acidophilic methanotroph isolate V4, Methylacidiphilum infernorum, a representative of the bacterial phylum Verrucomicrobia. Biol. Direct 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Islam T., Jensen S., Reigstad L. J., Larsen O., Birkeland N. K. 2008. Methane oxidation at 55°C and pH 2 by a thermoacidophilic bacterium belonging to the Verrucomicrobiaphylum. Proc. Natl. Acad. Sci. U. S. A. 105:300–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelly D. P., Anthony C., Murrell J. C. 2005. Insights into the obligate methanotroph Methylococcus capsulatus. Trends Microbiol. 13:195–198 [DOI] [PubMed] [Google Scholar]
- 20. Khadem A. F., Pol A., Jetten M. S. M., Op den Camp H. J. M. 2010. Nitrogen fixation by the verrucomicrobial methanotroph “Methylacidiphilum fumariolicum” SolV. Microbiology 156:1052–1059 [DOI] [PubMed] [Google Scholar]
- 21. Laemmli U. K. 1970. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 22. Op den Camp H. J. M., et al. 2009. Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia. Environ. Microbiol. Rep. 1:293–306 [DOI] [PubMed] [Google Scholar]
- 23. Pol A., et al. 2007. Methanotrophy below pH 1 by a new Verrucomicrobiaspecies. Nature 450:874–878 [DOI] [PubMed] [Google Scholar]
- 24. Price G. D., Coleman J. R., Badger M. R. 1992. Association of carbonic anhydrase activity with carboxysomes isolated from the cyanobacterium SynechococcusPCC7942. Plant Physiol. 100:784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saitou N., Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 26. Selesi D., Pattis I., Schmid M., Kandeler E., Hartmann A. 2007. Quantification of bacterial RubisCO genes in soils by cbbLtargeted real-time PCR. J. Microbiol. Methods 69:497–503 [DOI] [PubMed] [Google Scholar]
- 27. Stanley S. H., Dalton H. 1982. Role of ribulose-1,5-bisphosphate carboxylase/oxygenase in Methylococcus capsulatus(Bath). Microbiology 128:2927–2935 [Google Scholar]
- 28. Tabita F. R., Satagopan S., Hanson T. E., Kreel N. E., Scott S. S. 2008. Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. J. Exp. Bot. 59:1515–1524 [DOI] [PubMed] [Google Scholar]
- 29. Tourova T. P., Kovaleva O. L., Sorokin D. Y., Muyzer G. 2010. Ribulose-1,5-bisphosphate carboxylase/oxygenase genes as a functional marker for chemolithoautotrophic halophilic sulfur-oxidizing bacteria in hypersaline habitats. Microbiology 156:2016–2025 [DOI] [PubMed] [Google Scholar]
- 30. van Niftrik L., et al. 2009. Cell division ring, a new cell division protein and vertical inheritance of a bacterial organelle in anammox planctomycetes. Mol. Microbiol. 73:1009–1019 [DOI] [PubMed] [Google Scholar]
- 31. Ward N., et al. 2004. Genomic insights into methanotrophy: the complete genome sequence of Methylococcus capsulatus(Bath). PLoS Biol. 2:e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weiss R. F. 1974. Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar. Chem. 2:203–215 [Google Scholar]
- 33. Yeates T. O., Kerfeld C. A., Heinhorst S., Cannon G. C., Shively J. M. 2008. Protein-based organelles in bacteria: carboxysomes and related microcompartments. Nat. Rev. Microbiol. 6:681–691 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.