Abstract
We identified two additional genes of Helicobacter pyloriencoding Ccrp proteins. All four Ccrps have different multimerization and filamentation properties and different types of smallest subunits and do not copurify, suggesting a system of individual Ccrp filaments. Despite the presence of morphologically unaltered flagella, all ccrpmutants displayed significantly reduced motility.
TEXT
Helicobacter pyloriis a Gram-negative, highly motile, microaerophilic, spiral-shaped organism, which colonizes the stomachs of at least half of the world's human population (11). The cell shape of H. pylorihas always been held as an important pathogenicity factor. The cell shape of H. pyloriis apparently controlled by two unrelated mechanisms that operate at two levels: peptidases influence cell shape by causing peptidoglycan relaxation (5, 26), whereas so-called coiled-coil-rich proteins (Ccrp) compose an intracellular scaffold (30). In Caulobacter crescentus, the coiled-coil-rich protein crescentin is essential for the generation of cell curvature (2), likely through mechanical control of cell growth (6). In Streptomyces coelicolor, the filament-forming Ccrp FilP determines cell rigidity but not cell shape (4). Recently, Ccrp RsmP was shown to be essential both for viability and for rod shape determination in Corynebacterium glutamicum(8). H. pyloripossesses two Ccrp proteins (Ccrp59 and Ccrp1143), which are essential for the maintenance of proper cell shape (30).
In this work, a close inspection of the genome of H. pylori26695 revealed the presence of genes encoding proteins rich in putative heptad repeat regions, located adjacent to the previously characterized ccrp59and ccrp1143genes: HP0058 lies upstream of ccrp59, and HP1142 lies downstream of ccrp1143.This gene arrangement is conserved in all strains analyzed (26695, J99, HAPG1, 1061, G27, and B128). Concerning HP0058, we have identified a sequencing error (creating a wrong annotation) in the presumed intergenic region upstream of gene HP0058: the C stretch at bp position 62013 in the genome of strain 26695 is composed of 17 C's rather than the reported 15 C's. Because of this frameshift, HP0058 begins at bp position 61943, encoding an approximately 48-kDa protein. Indeed, we found that only the long version of HP0058 is expressed in H. pyloricells. Based on their putative coiled-coil-rich structure, we designate the H. pyloriHP0058 and HP1142 gene products Ccrp58 and Ccrp1142, and the genes will be referred to as ccrp58or ccrp1142here.
Both new ccrpgenes are also highly heterogenic between H. pyloristrains (18, 22). It has been suggested that the genes are submitted to specific selection pressure, making them evolve rapidly. By using BLASTn (NCBI) and the sequence of strain 26695 as a reference, the maximum identity of the ccrp58and ccrp1142genes of all sequenced H. pyloristrains decreased to 84% and 89%, respectively. However, genes were found in all sequenced strains available. This hypervariability may contribute to the highly different morphologies of H. pyloristrains.
To study the functions of Ccrp58 and Ccrp1142, we inactivated both genes separately in the H. pyloristrains 26695 and KE88-3887 (KE) and analyzed their role in cell shape determination. The strains, plasmids, and primers used are listed in Tables 1and 2. Mutants were derived as described previously (19, 28, 29). Growth analysis of both mutants revealed that the inactivation of either gene affected the growth rate of H. pylori(not shown). While less than 15% of 26695 wild-type cells were found to be straight (Fig. 1A), the percentage of straight cells in the population was 50% in the ccrp58mutant (n= 400) and 40% in the ccrp1142mutant (n= 130) (Fig. 1B and C). With regard to cell morphology, the corresponding mutants in strain KE behaved similarly (not shown). Because deletion of ccrp59leads to the formation of 100% straight cells (30), a polar effect of the ccrp58disruption could be excluded.
Table 1.
Plasmids and strains used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Plasmids | ||
| pTnMax5 | lacIqtnpR tnpA res orifdcatGC, Cmr | 17 |
| pZERO-2 | Cloning vector, MCS in lacZ′ neo, Kmr | Invitrogen |
| p0058PCAT | pZERO-2, ΔHP0058::Pcat, CmrKmr | This study |
| p0058StrepPCAT | pZERO-2 carrying 500 bp of the C terminus of HP0058 fused with a Strep tag, a chloramphenicol resistance cassette, and 500 bp of the N terminus of ccrp59 | This study |
| p1142PCAT | pZERO-2, ΔHP1142::Pcat, CmrKmr | This study |
| pASK-IBA7 | Expression vector, tetR Ptetbla, Apr | IBA |
| pIBA7-1142 | pASK-IBA7 carrying the HP1142 coding sequence under the control of the tetpromoter cloned in the BsaI site | This study |
| pIBA7-0059 | pASK-IBA7 carrying the HP0059 coding sequence under the control of the tetpromoter cloned in the BsaI site | 30 |
| pETDuet-1 | bla | Novagen |
| pETDuet-1143 | pETDuet-1 carrying the HP1143 coding sequence under the control of the T7promoter cloned between the NcoI and BamHI sites | 30 |
| pETDuet-0058 | pETDuet-1 carrying the HP0058 coding sequence under the control of the T7promoter cloned between the EcoRI and PstI sites | This study |
| Strains | ||
| E. coli strains | ||
| BL21 | F−dcm ompT hsdS(rB−mB−) gal | Stratagene |
| BL21-Ccrp58 | BL21 carrying plasmid pETDuet-0058 | This study |
| BL21-Ccrp59 | BL21 carrying plasmid pIBA7-0059 | 30 |
| BL21-Ccrp1143 | BL21 carrying plasmid pETDuet-1143 | 30 |
| BL21-Ccrp1142 | BL21 carrying plasmid p pIBA7-1142 | This study |
| DH5α | F−φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK−mK+) phoA supE44λ−thi-1 gyrA96 relA1 | Bethesda Research Laboratories |
| H. pyloristrains | ||
| 26695 | wt, containing the entire cagPAI | 27 |
| KE88-3887 | Piglet-passaged strain 26695 | 15 |
| KE-Ccrp58Strep | KE88-3887, Ccrp58 fused to Strep tag, Cmr | This study |
| KE-59PCAT | KE88-3887, ΔHP0059::Pcat, Cmr | 30 |
| KE-1143PCAT | KE88-3887, ΔHP1143::Pcat, Cmr | 30 |
| KE-58PCAT | KE88-3887, ΔHP0058::Pcat, Cmr | This study |
| KE-1142PCAT | KE88-3887, ΔHP1142::Pcat, Cmr | This study |
| 26695-59PCAT | 26695, ΔHP0059::Pcat, Cmr | 30 |
| 26695-1143PCAT | 26695, ΔHP1143::Pcat, Cmr | 30 |
| 26695-58PCAT | 26695, ΔHP0058::Pcat, Cmr | This study |
| 26695-1142PCAT | 26695, ΔHP1142::Pcat, Cmr | This study |
MCS, multiple cloning site; wt, wild type; PAI, pathogenicity island.
Table 2.
Oligonucleotides used in this studyb
| Genea | Application | Primer | Sequence (5′→3′) |
|---|---|---|---|
| HP0058 | Mutagenesis | 0058-L3 | GCTAACTAACAAGATCACCG |
| PCAT-0058-R2 | 1-CGCTATGAGTTGTTGCTACA | ||
| CAT-0058-L1 | 2-GCGCTTATGACTATACATGC | ||
| 0059-R1 | CGGTGATCTTGTTAGTTAGC | ||
| HP1142 | Mutagenesis | 1143-L1 | AAGCGACATGCGAGAGATTG |
| PCAT-1142-R1 | 1- CCACTGCTTACATTCTGCTG | ||
| CAT-1142-L1 | 2- GAAGATGGTCAATTAGTAGG | ||
| 1142-R2 | TATAATGAGCTTCATGACCG | ||
| HP0058 | Strep tag fusion | 58s-L2 | ATGGTAGGTCTCAGCGCTTGTTAAGAGAAAAAGAAAATCTCAATA |
| 58Strep-PCAT | GGCGGATTAACAAAAACCGGACTATTTTTCAAATTGCGGGTG | ||
| 59PCAT | TGGCAGGGCGGGGCGTAAATGGGA | ||
| 59_dw | GCTCTTGTTTAAGGCTATCC | ||
| HP0058 | Expression | 58_up | TCAGAATTCATGATGGGTGCTCATATTATAG |
| 58_Strepdw | TCACTGCTATTTTTCGAACTGCGGGTGGCTCCACCCGCCTGATCCCATATCCACGATAGT | ||
| HP1142 | Expression | pASK7-1142-L1 | ATGGTAGGTCTCAGCGCGTGAGCGTGAATAGTAATGGCAAT |
| pASK7-1142-R1 | ATGGTAGGTCTCATATCACTTCATTCTCATCATCATTTTATAATG | ||
| Pcat | catgene with promoter | CATS1 | TCCGGTTTTTGTTAATCCGCC |
| CATAS1 | TTACGCCCCGCCCTGCCA |
Gene numbers refer to the H. pylori26695 genome sequence (27).
The 5′ extensions used for the fusion of PCR products to the catgene by megaprimer PCR are labeled as follows: 1 (5′-GGCGGATTAACAAAAACCGGA), complementary to the 5′ region of the catgene with promoter; 2 (5′-TGGCAGGGCGGGGCGTAA), complementary to the 3′ end of the catgene. The BsaI restriction site used for the protein expression via the IBA system is underlined.
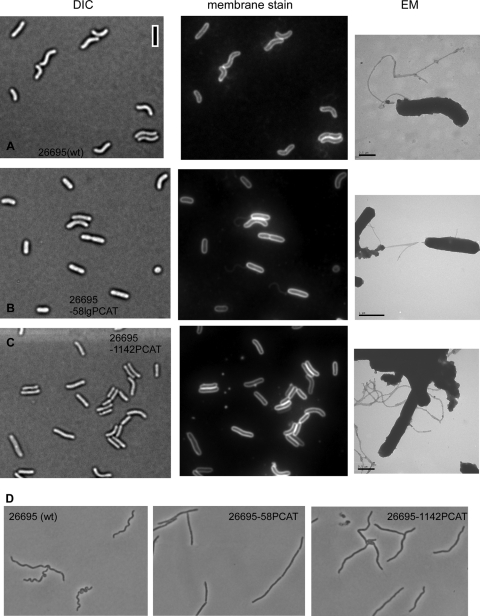
Fig. 1.
Analysis of cell morphology of H. pyloristrain 26695. Wild type (A), ccrp58mutant (B), and ccrp1142mutant (C). Nomarski DIC images (left), fluorescent micrographs of FM4-64 membrane staining (middle), transmission electron microscopy images of phosphotungstic acid (PTA)-stained cells (right). Wild-type (wt) and mutant strains are indicated in the images. Black bar, 2 μm. All images are equally scaled. (D) Phase contrast of wild-type and mutant cells treated with the filamenting drug aztreonam.
The gene HP1141 downstream of ccrp1142encodes methionyl-tRNA formyltransferase, whose function is essential in some bacteria (3, 10). The deletion of ccrp1142produced viable cells without any detectable growth defect, demonstrating that this deletion did not affect the expression of gene HP1141. To specify the effect of these deletions on cell morphology, we used a cell filamentation assay with aztreonam as previously described (26). This inhibitor of the septal peptidoglycan synthesis forces cells into long chains and therefore facilitates the determination of helicity. Accordingly, helical wild-type cells of strain 26695 formed polymorphic spiral chains, without regular pitch (Fig. 1D). Aztreonam-treated ccrp58and ccrp1142mutant strains showed a remarkable homomorphous phenotype of almost straight chains (Fig. 1D). This phenotype was more pronounced in the ccrp58mutant strain, which is in agreement with the somewhat higher impact of ccrp58deletion on cell morphology. Thus, both novel ccrpgenes play an important role in cell shape maintenance.
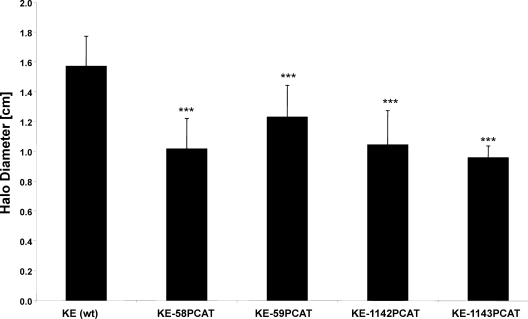
Subsequently, we investigated the influence of the deletion of ccrpgenes on flagellum formation using the fluorescent membrane stain FM4-64 at a final concentration 1 nM (the flagella of H. pyloriare covered by a membranous sheath [9]). Fluorescence microscopy was performed on a Zeiss Axioplan2 with a digital AxioCam MRm camera (30). However, flagella are not visible in all cells, as they can shear off during the preparation of cells or sit in a focal plane different from that of the cell body. Nevertheless, no defect in flagellum formation was found for any of the ccrpmutants (Fig. 1B and C and data not shown). To verify this finding, we analyzed the presence of flagella by transmission electron microscopy using a Philipps/FEI CM10 (80000V) electron microscope (31). Polar flagella were readily visible in the wild type as well as in all ccrpmutants (Fig. 1, right, and data not shown). Deletion of ccrpgenes therefore has no visible effect on the formation of flagella in H. pylori.It is believed that the helical shape of H. pylorienables the bacteria to penetrate the mucin web in the stomach (1, 24). Because strain 26695 is flagellated but only moderately motile (16), we tested swimming motility in strain KE, which is a highly motile variant of strain 26695, using soft-agar assays (23). We observed that all four ccrpmutants had significantly impaired motility (Fig. 2; P< 0.001, analysis of variance [ANOVA], Tukey's test). Surprisingly, the level of motility reduction did not strictly correlate with the loss of spiral cell shape. The deletion of the ccrp59gene, which leads to 100% straight cells, caused the least pronounced motility defect, whereas the smallest halo diameters were found in the ccrp58and the ccrp1143mutant strains. Possibly, a rod shape is more advantageous for motility than an irregular helical shape or slight curvature but is less advantageous than a helical shape. Alternatively, Ccrps may be involved in other cellular processes necessary for motility.
Fig. 2.
Motility phenotype of all Ccrp mutants in soft agar depicted as mean halo diameter of at least three independent experiments totaling 200 stabs per strain in soft agar after 5 days. Stars indicate significant differences from wild type (P< 0.001, ANOVA with Tukey test).
Eukaryotic intermediate filament (IF) proteins are involved in intracellular trafficking and positioning of cellular organelles (12). Thus, it may be possible that Ccrp proteins adopt some of these properties in prokaryotic cells. Furthermore, although flagella were clearly visible in the Ccrp mutants, we cannot exclude a possible defect in flagellum motion. Interestingly, cells with mutations in recently identified H. pylorigenes, which promote the helical cell shape by causing peptidoglycan relaxation, were not or only minimally reduced in their ability to swim directionally in a similar soft-agar assay (5, 26). However, Ccrp proteins of H. pylori, besides their function in cell shape maintenance, clearly influence motility.
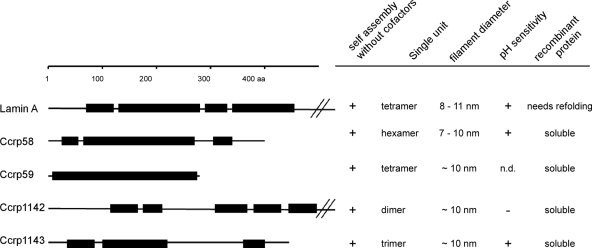
We purified Ccrps as C- (Ccrp58) or N-terminally (Ccrp1142, Ccrp59, and Ccrp1143) Strep-tagged versions as described previously (30) (plasmids shown in Table 1). Contrarily to IF proteins that are generally insoluble, recombinant versions of all Ccrps could be purified as soluble proteins and were analyzed by gel filtration using either a Superose6 10/300GL column (Tricorn) or a Biosep-SEC-S4000 (Phenomex) in Strep tag washing buffer (buffer-W; IBA GmbH) yielding Stokes radii. Furthermore, we performed sucrose gradient sedimentation experiments using ultracentrifugation (Beckman SW-41 rotor, 13,000 × g, 4°C, 15 h) through linear 5 to 15% sucrose gradients. Because Ccrp proteins have been shown to be filamentous (30), the native weight (M) must be deduced from the combination of the Stokes radius (Rs) and the sedimentation coefficient using the equation M= 3.909s× Rs. Accordingly, the calculated native masses of Ccrp58, Ccrp59, Ccrp1142, and Ccrp1143 are 295 kDa, 127.8 kDa, 184 kDa, and 170 kDa, respectively. The smallest units of Ccrp58, Ccrp59, Ccrp1142, and Ccrp1143 are therefore likely a hexamer (annotated molecular mass of 48 kDa), a tetramer (32 kDa), a dimer (88 kDa), and a trimer in solution (50.5 kDa), respectively. Whereas a dimeric structure is a precursor of a tetramer, the trimeric structure is found in collagen and could be seen as a prestage of the hexamer structure. These results demonstrate that H. pyloriCcrps have different multimeric single units as building blocks of the filaments. The biochemical properties of all four Ccrps are summarized in Fig. 3.
Fig. 3.
Architectures and biochemical properties of Ccrp proteins. The tripartite building plan of a human IF protein nuclear lamin A is depicted at the top. The scale bar refers to amino acid residues. Boxes represent domains in coiled-coil conformation and lines noncoiled-coil sequences. Long head or tail domains are in some cases truncated. n.d., not determined.
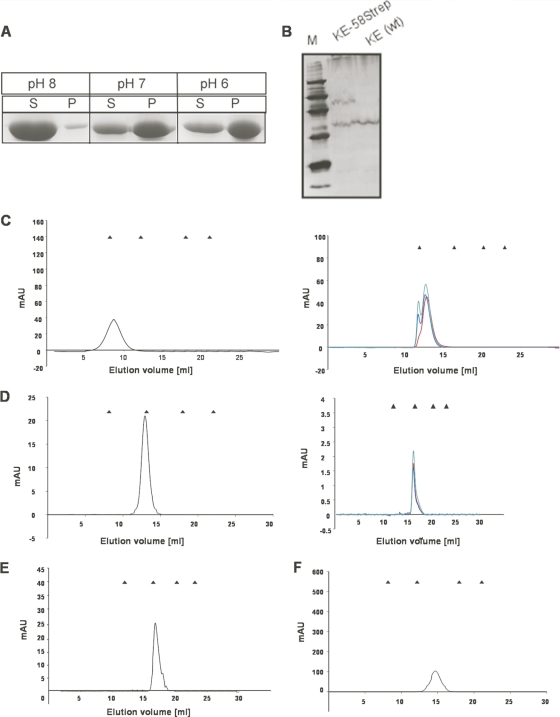
Interestingly, we observed an increase of large assemblies of Ccrp58 and Ccrp1142 over time (Fig. 4C and D, right), as gel filtration experiments performed with the Biosep-SEC-S4000 column showed that the elution peak of the same sample changed to larger sizes after 2 (Fig. 4C and D, blue curves) and 4 weeks (Fig. 4C and D, green curves), compared to the apparent size derived directly after Strep tag purification (Fig. 4C and D, red curves). Furthermore low-spin centrifugation (13,000 rpm) revealed that Ccrp58 was found mostly in the supernatant at pH 8, whereas decreasing the pH increased sedimentation, and at pH 6, most of Ccrp58 was present in the pellet fraction (Fig. 4A).
Fig. 4.
(A) Spin-down assay of Ccrp58 after purification from E. coli. Coomassie-stained SDS-PAGE of spin-down assays of 20-μl fractions. S, supernatants; P, pellets resuspended in 20 μl SDS loading buffer. Different pH levels are indicated. (B) Western blot of 20 μg of whole-cell extracts of H. pyloriwild-type cells (KE) and of the mutant, in which Ccrp58 is fused to the Strep tag (KE-58Strep) as indicated. M, marker; marker protein sizes are indicated. The antiserum recognizes the tagged version of Ccrp58 in strain KE-58Strep, as well as a nonspecific band, which can also be seen with the parent strain KE. (C to F) Analytical gel filtration of Ccrp proteins. Elution profiles of analytical gel filtration of Ccrp58 (C) and Ccrp1142 (D) using a Superose6 10/300 column (left) and a Biosep-SEC-S4000 column (right). In the right panels, the red curves display gel filtration immediately after purification, the blue curves after 2 weeks, and the green curves after 4 weeks after purification. Elution profiles of Ccrp59 (E) and Ccrp1143 (F) using a Superose6 10/300 column. The triangles above each elution profile indicate the elution times of the standard proteins (Bio-Rad) as follows: thyroglobulin (670 kDa, 19 S), bovine gamma-globulin (158 kDa, 7.4 S), chicken ovalbumin (44 kDa, 3.5 S), equine myoglobin (17 kDa, 2.0 S), vitamin B12(1.35 kDa). mAU, milliabsorbance units.
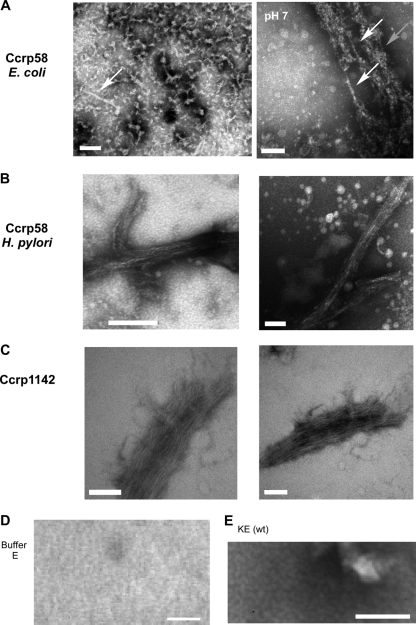
Electron microscopy analysis showed that purified Ccrp58 formed filaments with an average length of 50 nm (Fig. 5A) at pH 8. Additionally we could observe some longer filaments of 200-nm length (Fig. 5A, white arrow). A control (buffer E; IBA GmbH) showed a plain gray image without any structure (Fig. 5D). Whereas the addition of up to 10 mM magnesium chloride or calcium chloride had no effect on filament formation, the decrease of the pH from pH 8 to pH 7 resulted in the formation of much longer filaments of up to 1 μm (Fig. 5A right, white arrows) as well as of bundles of filaments (gray arrows). These results demonstrate that the assembly of Ccrp58 is dependent on pH and time, very similarly to the assembly of IF proteins (14).
Fig. 5.
Electron microscopic images of Ccrp58 and Ccrp1142 purified at pH 8. (A) Ccrp58-Strep expressed in E. coli. White arrows indicate single long filaments, and gray arrow indicates a bundle of filament. pH 7, image of the same purification fraction shown on the left adjusted to pH 7. (B) Ccrp58-Strep purified from H. pylori. (C) Strep-Ccrp1142 expressed in E. coli. (D) Electron microscopic image of solution buffer E. (E) Electron microscopic image of H. pyloriwild-type (KE) fraction after Strep tag purification. All images are from PTA-stained samples. White scale bars, 100 nm.
Ccrp1142 also forms filamentous structures in vitro, independent of any cofactor (Fig. 5C). In contrast to Ccrp58, but similar to Ccrp59 (30), Ccrp1142 already formed long bundles of filaments (Fig. 5C) at pH 8. Single filaments had a diameter of 10 nm, while the width of whole bundles was more than 100 nm. The length of the bundles could even reach more than 500 nm (Fig. 5C).
To obtain more information on the native architecture of Ccrp58 filaments, we generated a construct in which the 3′ end of ccrp58is fused to a Strep tag and an adjacent chloramphenicol resistance cassette (21), and primers listed in Table 2. This construct was integrated into the ccrp58locus in strain KE, and correct integration was confirmed by PCR and sequencing. The morphology of the Ccrp58-Strep fusion strain was as spiral as that of wild-type cells, showing that the fusion does not disturb function. Ccrp58-Strep could be efficiently purified from H. pyloricell extracts (Fig. 4B) using procedures used for E. colicell extracts (30). Electron microscopy revealed that Ccrp58 was also organized in filaments (Fig. 5B). Interestingly, even when these filaments were purified at pH 8, they were much longer than those observed after purification from E. coli. Apparent single filaments had a diameter of about 7 nm, but filaments generally assembled into large bundles with a diameter of about 100 nm and a length of more than 1 μm (Fig. 5B). H. pyloriwild-type cell extracts subjected to Strep tag purification and analyzed by electron microscopy did not show any filamentous structures, indicating that the observed filaments are indeed composed of Ccrp58-Strep (Fig. 5E).
These findings raise several possibilities: the simplest explanation might lie with the internal pH of E. coli, which is pH 7.8 (20) and thus similar to the standard purification conditions, whereas the pH in the cytoplasm of H. pyloriis pH 6.8 (25). Additionally, Ccrp58 could be modified in vivo, e.g., phosphorylated, as has been shown for IF proteins, which contain numerous phosphorylation sites involved in their assembly/disassembly and subcellular organization (13). Furthermore, copolymerization of Ccrps could act as facilitation factor. Hence, to address this question we analyzed Ccrp58 eluates from H. pyloricells by mass spectrometry (nano-liquid chromatography–tandem mass spectrometry [nLC-MS/MS]) for the presence of the other Ccrp proteins according to the method of Defeu Soufo et al. (7). As a control, extracts from H. pyloricells lacking the Strep fusion were run over Strep-tactin columns. Only a small amount of Ccrp1143 could be detected by this comparative analysis within the Ccrp58-Strep eluate. None of the other Ccrp proteins was found to coelute with Ccrp58-Strep. These data suggest that rather than being part of a mixed filamentous structure, Ccrp58 forms individual filaments in vivo. We therefore suggest the speculative model that all Ccrp proteins build up individual filaments. In this context it might be possible that different Ccrp polymers with different degrees of curvature may generate different degrees of helicity. However, whether the other Ccrp proteins generate mixed or individual filaments remains to be investigated.
In conclusion, H. pyloricontains a complex cytoskeleton that affects cell morphology as well as motility.
Acknowledgments
We thank Ali Al-Ahmad for performing the statistical analysis and Maren Lingnau for technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft(WA2574/1-1, WA2574/1-2, and FOR 929).
Footnotes
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Andersen L. P. 2007. Colonization and infection by Helicobacter pyloriin humans. Helicobacter 12(Suppl. 2):12–15 [DOI] [PubMed] [Google Scholar]
- 2. Ausmees N., Kuhn J. R., Jacobs-Wagner C. 2003. The bacterial cytoskeleton: an intermediate filament-like function in cell shape. Cell 115:705–713 [DOI] [PubMed] [Google Scholar]
- 3. Baba T., et al. 2006. Construction of Escherichia coliK-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bagchi S., Tomenius H., Belova L. M., Ausmees N. 2008. Intermediate filament-like proteins in bacteria and a cytoskeletal function in Streptomyces. Mol. Microbiol. 70:1037–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonis M., Ecobichon C., Guadagnini S., Prevost M. C., Boneca I. G. 2010. A M23B family metallopeptidase of Helicobacter pylorirequired for cell shape, pole formation and virulence. Mol. Microbiol. 78:809–819 [DOI] [PubMed] [Google Scholar]
- 6. Cabeen M. T., et al. 2009. Bacterial cell curvature through mechanical control of cell growth. EMBO J. 28:1208–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Defeu Soufo H. J., et al. 2010. Bacterial translation elongation factor EF-Tu interacts and colocalizes with actin-like MreB protein. Proc. Natl. Acad. Sci. U. S. A. 107:3163–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fiuza M., et al. 2010. Phosphorylation of a novel cytoskeletal protein (RsmP) regulates rod-shaped morphology in Corynebacterium glutamicum. J. Biol. Chem. 285:29387–29397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geis G., Suerbaum S., Forsthoff B., Leying H., Opferkuch W. 1993. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J. Med. Microbiol. 38:371–377 [DOI] [PubMed] [Google Scholar]
- 10. Gerdes S. Y., et al. 2003. Experimental determination and system level analysis of essential genes in Escherichia coliMG1655. J. Bacteriol. 185:5673–5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Go M. F. 2002. Review article: natural history and epidemiology of Helicobacter pyloriinfection. Aliment. Pharmacol. Ther. 16(Suppl. 1):3–15 [DOI] [PubMed] [Google Scholar]
- 12. Goldman R. D., Grin B., Mendez M. G., Kuczmarski E. R. 2008. Intermediate filaments: versatile building blocks of cell structure. Curr. Opin. Cell Biol. 20:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helfand B. T., Chang L., Goldman R. D. 2003. The dynamic and motile properties of intermediate filaments. Annu. Rev. Cell Dev. Biol. 19:445–467 [DOI] [PubMed] [Google Scholar]
- 14. Herrmann H., Haner M., Brettel M., Ku N. O., Aebi U. 1999. Characterization of distinct early assembly units of different intermediate filament proteins. J. Mol. Biol. 286:1403–1420 [DOI] [PubMed] [Google Scholar]
- 15. Hoffman P. S., et al. 2003. Development of an interleukin-12-deficient mouse model that is permissive for colonization by a motile KE26695 strain of Helicobacter pylori. Infect. Immun. 71:2534–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Josenhans C., Eaton K. A., Thevenot T., Suerbaum S. 2000. Switching of flagellar motility in Helicobacter pyloriby reversible length variation of a short homopolymeric sequence repeat in fliP, a gene encoding a basal body protein. Infect. Immun. 68:4598–4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kahrs A. F., et al. 1995. An improved TnMax mini-transposon system suitable for sequencing, shuttle mutagenesis and gene fusions. Gene 167:53–57 [DOI] [PubMed] [Google Scholar]
- 18. Menard A., Danchin A., Dupouy S., Megraud F., Lehours P. 2008. A variable gene in a conserved region of the Helicobacter pylorigenome: isotopic gene replacement or rapid evolution. DNA Res. 15:163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pfeiffer J., Guhl J., Waidner B., Kist M., Bereswill S. 2002. Magnesium uptake by CorA is essential for viability of the gastric pathogen Helicobacter pylori. Infect. Immun. 70:3930–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richard H., Foster J. W. 2004. Escherichia coliglutamate- and arginine-dependent acid resistance systems increase internal pH and reverse transmembrane potential. J. Bacteriol. 186:6032–6041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sarkar G., Sommer S. S. 1990. The “megaprimer” method of site-directed mutagenesis. Biotechniques 8:404–407 [PubMed] [Google Scholar]
- 22. Saunders N. J., Boonmee P., Peden J. F., Jarvis S. A. 2005. Inter-species horizontal transfer resulting in core-genome and niche-adaptive variation within Helicobacter pylori. BMC Genomics 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schirm M., et al. 2003. Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori. Mol. Microbiol. 48:1579–1592 [DOI] [PubMed] [Google Scholar]
- 24. Slomiany B. L., Slomiany A. 1992. Mechanism of Helicobacter pyloripathogenesis: focus on mucus. J. Clin. Gastroenterol. 14(Suppl. 1):S114–S121 [PubMed] [Google Scholar]
- 25. Stingl K., Uhlemann Em E. M., Deckers-Hebestreit G., Schmid R., Bakker E. P., Altendorf K. 2001. Prolonged survival and cytoplasmic pH homeostasis of Helicobacter pyloriat pH 1. Infect. Immun. 69:1178–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sycuro L. K., et al. 2010. Peptidoglycan crosslinking relaxation promotes Helicobacter pylori's helical shape and stomach colonization. Cell 141:822–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tomb J. F., et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539–547 [DOI] [PubMed] [Google Scholar]
- 28. Waidner B., et al. 2002. Identification by RNA profiling and mutational analysis of the novel copper resistance determinants CrdA (HP1326), CrdB (HP1327), and CzcB (HP1328) in Helicobacter pylori. J. Bacteriol. 184:6700–6708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Waidner B., Melchers K., Stahler F. N., Kist M., Bereswill S. 2005. The Helicobacter pyloriCrdRS two-component regulation system (HP1364/HP1365) is required for copper-mediated induction of the copper resistance determinant CrdA. J. Bacteriol. 187:4683–4688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waidner B., et al. 2009. A novel system of cytoskeletal elements in the human pathogen Helicobacter pylori. PLoS Pathog. 5:e1000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zaar K., et al. 2002. Cellular and subcellular distribution of d-aspartate oxidase in human and rat brain. J. Comp. Neurol. 450:272–282 [DOI] [PubMed] [Google Scholar]