Abstract
Pseudomonas aeruginosautilizes preferentially C4-dicarboxylates such as malate, fumarate, and succinate as carbon and energy sources. We have identified and characterized two C4-dicarboxylate transport (Dct) systems in P. aeruginosaPAO1. Inactivation of the dctA(PA1183) gene caused a growth defect of the strain in minimal media supplemented with succinate, fumarate or malate, indicating that DctA has a major role in Dct. However, residual growth of the dctAmutant in these media suggested the presence of additional C4-dicarboxylate transporter(s). Tn5insertion mutagenesis of the ΔdctAmutant led to the identification of a second Dct system, i.e., the DctPQM transporter belonging to the tripartite ATP-independent periplasmic (TRAP) family of carriers. The ΔdctA ΔdctPQMdouble mutant showed no growth on malate and fumarate and residual growth on succinate, suggesting that DctA and DctPQM are the only malate and fumarate transporters, whereas additional transporters for succinate are present. Using lacZreporter fusions, we showed that the expression of the dctAgene and the dctPQMoperon was enhanced in early exponential growth phase and induced by C4-dicarboxylates. Competition experiments demonstrated that the DctPQM carrier was more efficient than the DctA carrier for the utilization of succinate at micromolar concentrations, whereas DctA was the major transporter at millimolar concentrations. To conclude, this is the first time that the high- and low-affinity uptake systems for succinate DctA and DctPQM have been reported to function coordinately to transport C4-dicarboxylates and that the alternative sigma factor RpoN and a DctB/DctD two-component system regulates simultaneously the dctAgene and the dctPQMoperon.
INTRODUCTION
Pseudomonas aeruginosais a versatile ubiquitous Gram-negative bacterium that has a phenomenal capacity to adapt to different environments and utilizes a wide variety of different organic molecules as carbon and energy sources (32). The 6.2-Mb genome of P. aeruginosaPAO1 contains a large number of genes for catabolism, nutrient transport, and metabolic regulation (46). P. aeruginosapreferentially utilizes tricarboxylic acid (TCA) cycle intermediates such as the C4-dicarboxylates malate, fumarate and, in particular, succinate as carbon and energy sources (24, 28).
In various bacteria, carriers and sensors have been described to be involved in C4-dicarboxylate utilization. In rhizobia, a C4-dicarboxylic acid transport (Dct) system has been described in detail (58). It is composed of three genes clustered together: the dctAgene coding for a C4-dicarboxylate transport protein, belonging to the dicarboxylate/cation symporter (DAACS) family (43), and the dctBand dctDgenes coding for a two-component regulatory system, which responds to C4-dicarboxylates and regulates dctAexpression (6, 36, 58). In Rhizobium leguminosarumand Sinorhizobium meliloti, a functional DctA transporter is essential for symbiotic nitrogen fixation (7, 36). In the presence of C4-dicarboxylates, the DctB membrane sensor is activated by autophosphorylation and then transfers a phosphate group to the response regulator DctD (12). Once DctD is activated, it binds to an upstream activator sequence (UAS) present in the dctApromoter region, enabling RNA polymerase with the sigma factor σ54(RpoN) to transcribe the gene (21). In the absence of substrates, it has been proposed that the DctA and DctB proteins interact with each other in the cytoplasmic membrane, leading to inhibition of DctB autophosphorylation and consequently to low expression of the C4-dicarboxylic acid transport system (12, 22, 23, 52). The Gram-positive bacteria Bacillus subtilisand Corynebacterium glutamicumcontain a similar DctA transporter (2, 48). In Escherichia coli, C4-dicarboxylates are utilized under aerobic and anaerobic growth conditions. During aerobic growth, DctA is the main C4-dicarboxylate transporter, whereas a second carrier termed DcuA might further contribute to fumarate and succinate uptake (4, 14). During anaerobic growth, C4-dicarboxylate transport is performed by the DcuA, DcuB, DcuC, and CitT carriers (13, 34, 42, 59). Furthermore, a quintuple mutant (dctA, dcuA, dcuB, dcuC, and citT), which is deficient in all known C4-dicarboxylate transport functions, presents residual growth on succinate, indicating the presence of additional unidentified transporter(s) (18).
In the purple photosynthetic bacterium Rhodobacter capsulatus, the tripartite ATP-independent periplasmic (TRAP) carrier has been identified and characterized as a C4-dicarboxylate transporter (8, 40). The TRAP transporter is encoded by three genes clustered together: the dctPgene coding for a C4-dicarboxylate-binding protein and the dctQand dctMgenes coding for a C4-dicarboxylate transporter (8). Furthermore, the dctSRoperon, which is adjacent and divergent to the dctPQMoperon, encodes a two-component regulatory system controlling the expression of the dctPQMoperon (15). Similarly, studies on fumarate respiration in Wolinella succinogenesshowed that the C4-dicarboxylate transport was catalyzed by a DctPQM transporter (50). In Pseudomonas chlororaphisstrain O6, the expression of dctAis activated by succinate (31) and a dctAmutant does not grow on succinate or fumarate but can grow on malate (30, 53, 58).
In the present study, we report the identification and characterization of two Dct systems involved in C4-dicarboxylate uptake in P. aeruginosaPAO1. DctA was found to be the major carrier at high succinate concentrations, whereas the DctPQM transporter, belonging to the TRAP family of carriers, was efficient at low succinate concentrations. In addition, we demonstrate that both DctPQM and DctA are positively regulated by σ54(RpoN), DctA, and the DctB/DctD two-component system.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in the present study are listed in Table 1and oligonucleotides are listed in Table S1 in the supplemental material. Growth and β-galactosidase experiments were performed in nutrient yeast broth (NYB) (45) or in basalt salt medium (BSM) (44) supplemented with different carbon sources (succinate, fumarate, malate, oxaloacetate, citrate, glucose, or mannitol) to a final concentration of 40 mM or with succinate to a final concentration of 5 μM. Growth was performed in 50-ml Erlenmeyer flasks filled with 20 ml of medium, with shaking at 180 rpm and at 37°C. Nutrient agar (NA) was used as a solid medium. When required, antibiotics were added to these media at the following concentrations: 100 μg of ampicillin/ml, 25 μg of tetracycline/ml, and 10 μg of gentamicin (Gm)/ml for E. coliand 300 μg of carbenicillin/ml, 50 μg of gentamicin/ml, and 125 μg of tetracycline/ml for P. aeruginosa.
Table 1.
Strains and plasmids used in this study
| Strain, bacteriophage, or plasmid | Genotype or relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1Δ(lacZYA-argF)U169[φ80dlacZΔM15]F−NaIr | 38 |
| HB101 | proA2 hsdS20(rB−mB−) recA13 ara-14 lacYI galK2 rpsL20 supE44 xyl-5 mtl-1F− | 38 |
| S17-1/λpir | pro thi hsdR recAchromosome::RP4-2 Tc::Mu Km::Tn7/λpir; TprSmr | 27, 41 |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR176 supE44 relA1 lac[F′ proAB lacIqZΔM15::Tn10(53)] | Stratagene |
| P. aeruginosa | ||
| PAO1 | Wild type | 17 |
| PAO6358 | PAO1 containing a 900-bp deletion in the rpoNlocus | 16 |
| PAO6592 | PAO1 containing a 1,296-bp deletion in the dctAlocus | This study |
| PAO6705 | PAO1 containing a 1,798-bp deletion in the PA5165 (dctB) locus | This study |
| PAO6706 | PAO1 containing a 1,377-bp deletion in the PA5166 (dctD) locus | This study |
| PAO6707 | PAO1 containing a double deletion in the PA5165 locus (1,798 bp) and in the PA5166 locus (1,377 bp) | This study |
| PAO6708 | PAO1 containing a 2,899-bp deletion in the PA5167-PA5169 (dctPQM) operon | This study |
| PAO6709 | PAO1 containing a double deletion in the dctAlocus (1,296 bp) and in the dctPQM(PA5167-PA5169) operon (2,899 bp) | This study |
| PA6710 | PAO6592 with PA5168::Tn5Gm; Gmr | This study |
| PA6815 | PAO1 with PA5168::Tn5Gm; Gmr | This study |
| Bacteriophage | ||
| E79tv-2 | Temperate, transducing variant of E79 | 29 |
| Plasmids | ||
| pLM1 | Tn5Gm delivery vector; GmrApr | 9 |
| pMMB67HE | IncQ expression vector carrying an inducible tacpromoter; Ap/Cbr | 11 |
| pME3087 | Suicide vector for allelic replacement; Tcr; ColE1 replicon | 51 |
| pME6015 | Cloning vector for translational lacZfusions; Tcr | 39 |
| pME6016 | Cloning vector for transcriptional lacZfusions; Tcr | 39 |
| pME9506 | Plasmid carrying a transcriptional dctA-lacZfusion | This study |
| pME9507 | Plasmid carrying a translational dctA′-′lacZfusion | This study |
| pME9508 | Plasmid carrying a translational dctA′-′lacZfusion in which the rpoNputative box of the dctApromoter TGGCAC-N5-CTGCA was replaced with TACCAC-N5-CTTTA | This study |
| pME10031 | Suicide construct used for deletion of the PA5165 gene; Tcr | This study |
| pME10032 | Suicide construct used for deletion of the PA5166 gene; Tcr | This study |
| pME10033 | Suicide construct used for deletion of the PA5167-PA5169 operon; Tcr | This study |
| pME10034 | Plasmid carrying a transcriptional dctP-lacZfusion | This study |
| pME10035 | Plasmid carrying a translational dctP′-′lacZfusion | This study |
| pME10036 | Plasmid carrying a translational dctP′-′lacZfusion in which the rpoNputative box of the dctPQMpromoter TGGCAC-N5-TTGCT was replaced with TACCAC-N5-TTTTT | This study |
| pME10037 | pMMB67HE with dctA | This study |
| pME10038 | pMMB67HE with PA5165 | This study |
| pME10039 | pMMB67HE with PA5166 | This study |
| pME10040 | pMMB67HE with PA5167-PA5169 | This study |
| pME10041 | Suicide construct used for deletion of the dctAgene; Tcr | This study |
Nalr, nalidixic acid resistance; Gmr, gentamicin resistance; Tcr, tetracycline resistance; Apr, ampicillin resistance; Smr, streptomycin resistance; Cbr, carbenicillin resistance; Tpr, trimethoprim resistance.
Constructions of plasmids and gene replacement mutants.
DNA cloning and plasmid preparation were performed according to standard methods (38). Large-scale preparations were performed using JETstar 2.0 (Genomed). Restriction and DNA-modifying enzymes were used according to the instructions of the manufacturers. Transformation of E. coliDH5α (for cloning) and E. coliXL1-Blue (for mutagenesis) and P. aeruginosawas carried out by electroporation (33).
A translational dctA′-′lacZfusion was constructed by amplifying a 397-bp PCR fragment with primers Dct1 and Dct2b. This fragment was digested with EcoRI and PstI and cloned into the corresponding sites of pME6015. In the resulting plasmid pME9507 the promoter region of the dctAgene and the sequence encoding the first seven amino acids of dctAwere fused to the ′lacZreporter. A transcriptional dctA-lacZfusion was constructed using the primer pair Dct1 and Dct3x to amplify a 248-bp fragment containing the dctApromoter region. The PCR fragment was digested with EcoRI and PstI and ligated into the corresponding sites of pME6016, resulting in pME9506.
A translational dctP′-′lacZfusion (pME10035) was constructed by inserting a 334-bp fragment, carrying the proximal part of the PA5167 gene, into the ′lacZgene of pME6015 previously digested EcoRI-BamHI. The 334-bp fragment was obtained by PCR using the EcoRI-tagged primer TRAP-trsc1 and the BamHI-tagged primer TRAP-trnl2 to amplify the PAO1 genome carrying the promoter region of the dctPQM(PA5167-PA5169) operon, the Shine-Dalgarno sequence, and the first three codons of the PA5167 gene. A transcriptional dctP-lacZfusion was constructed by using the primer pair TRAP-trsc1 and TRAP-trsc2 to amplify a 171-bp fragment containing the dctPpromoter region. The PCR fragment was digested with EcoRI and PstI and ligated into the corresponding sites of pME6016, resulting in pME10034.
The mutations in the RpoN box sequence T(GG→AC)CACAGCCTCT(GC→TT)A and T(GG→AC)CACAGGGCTT(GC→TT)T of the dctAand dctPpromoter were introduced into pME9507 and pME10035, respectively, according to the QuikChange site-directed mutagenesis protocol with the mutagenesis primer pairs SDM1-SDM2 and SDMtrap1-SDMtrap2, respectively. The parental DNA template was digested with DpnI, and the mutated plasmid was transformed into E. coliXL1-Blue, generating pME9508 and pME10036.
For the inactivation of the dctAgene in the P. aeruginosaPAO1 chromosome, a 597-bp fragment containing the upstream region and the first two codons of dctAand a 600-bp fragment containing the dctAterminator were amplified by PCR using the primer pairs Mut1/Mut2 and Mut3/Mut4, respectively. These products were digested with EcoRI-BglII and BglII-HindIII, respectively, and cloned into the corresponding sites of the suicide vector pME3087, yielding plasmid pME10041. Plasmid pME10041, carried by E. coliDH5α, was then introduced into P. aeruginosaPAO1 by triparental mating, using the helper strain E. coliHB101(pRK2013). Merodiploids were resolved as previously described (57). The resulting strain, PAO6592, carried an in-frame ΔdctAmutation.
For inactivation of the PA5165 (dctB) locus, a 515-bp fragment overlapping the PA5165 upstream region and the first three codons and a 521-bp fragment overlapping the last codons of PA5165 and the downstream region were amplified by PCR using primer pairs p5165.1/p5165.2 and p5165.3/p5165.4, respectively. These products were digested with EcoRI-BglII and BglII-HindIII, respectively, and cloned into pME3087, yielding plasmid pME10031. Plasmid pME10031 was then used as described above to produce strain PAO6705 (ΔdctB).
A ΔPA5166 (ΔdctD) mutant of PAO1, constructed by amplifying a 506-bp fragment overlapping the ATG start codon of PA5166, and a 509-bp fragment overlapping the TGA stop codon of PA5166 were amplified by PCR using the primer pairs p5166.1/p5166.2 and p5166.3/p5166.4, respectively. These products were digested with EcoRI-BglII and BglII-HindIII, respectively, and cloned into pME3087, yielding plasmid pME10032. Plasmid pME10032 was then introduced into P. aeruginosaPAO1 as described above; after excision of the integrated plasmid, strain PAO6706 (ΔdctD) was obtained.
Analogous procedures were used to generate a P. aeruginosaPAO1 mutant (PAO6708) deleted in the PA5167-PA5169 (dctPQM) operon by using pME10033, a plasmid derived from the suicide vector pME3087, which had been digested with EcoRI-HindIII for insertion of a PCR fragment. The following primer pairs were used to create this fragment: pTRAP.1/pTRAP.2 (digested EcoRI-BglII) and pTRAP.3/pTRAP.4 (digested BglII-HindIII).
A double mutant (PAO6707) with the PA5165 dctBand the PA5166 dctDgenes deleted and a double mutant (PAO6709) with the PA5167-PA5169 (dctPQM) operon and dctAgene deleted were obtained as follows. Plasmid pME10031 and plasmid pME10033 were crossed into PAO6706 and PAO6592, respectively, as described above, yielding strains PA6707 (PAO1 ΔdctBD) and PA6709 (PAO1 ΔdctPQMΔdctA).
In all of the mutants described here, the deletions were confirmed by PCR, and the PCR fragments were checked by sequencing.
The deletions in strains PAO6592, PAO6705, PAO6706, and PAO6709 were complemented with fragments carrying, respectively, the dctA, dctB, dctD, and dctPQMgenes. The fragments had been amplified by PCR with primer pairs Cd.1-Cd.2, C65.1-C65.2, C66.1-C66.2, and Ct.1-Ct.2, respectively, and subcloned into pMMB67HE under the control of an inducible tacpromoter (resulting in plasmids pME10037 to pME10040). All plasmids were verified by sequencing.
Strain PAO6815 (dctQ::Tn5Gm) was obtained by transduction with phage E79tv-2(9). The phage preparations used for transduction were obtained as described previously (9). As a donor, strain PAO6710 was used, and the recipient PAO1 was infected with the phage, selecting for gentamicin resistance (50 μg/ml). Transductants were purified several times on selective medium and screened by PCR with the primers pTRAP.1 and tnpRL17-1.
β-Galactosidase assays.
These were performed as described previously (26), with P. aeruginosastrains grown in BSM medium containing a unique carbon source (fumarate, succinate, malate, oxaloacetate, citrate, glucose, or mannitol) or in rich NYB medium. The data are mean values of three independent samples ± the standard deviations.
Transposon mutagenesis.
About 5,000 random Tn5Gm insertions in strain PAO6592 (ΔdctA) were generated with plasmid pLM1 (Table 1), selecting for gentamicin resistance on NA amended with NYB. The growth of each mutant candidate was tested first on plates containing BSM minimal medium supplemented with 40 mM succinate. Then, replica plating onto BSM glucose (40 mM) and onto NA plates revealed auxotrophs which were discarded. Candidates that grew more slowly than strain PA6592 in the presence of succinate as the sole carbon source were retested in liquid media (BSM succinate, BSM glucose, and NYB). Genomic DNA of confirmed candidates was extracted by using the Wizard Genomic DNA purification kit (Promega), restricted with BamHI, self-ligated, and introduced into E. coliS17-1/λpirby electroporation, with selection for gentamicin resistance. After isolation of the plasmid containing Tn5Gm and flanking host sequences, the transposon insertion site was determined by nucleotide sequencing with the transposon-specific primer tnpRL17-1 (9) and was localized on the PAO1 chromosome (55) using BLASTN analysis (1).
Competition experiments.
A 20-ml culture in BSM containing 40 mM or 5 μM succinate was inoculated with a 50:50 mixture of exponential-phase cultures of the strains PAO6815 (dctQ::Tn5Gm) and PAO1 (wild-type), PAO6592 (ΔdctA) and PAO1 (wild type [WT]), or PAO6845 and PAO6592; the inoculum consisted of 100 CFU/ml. Serial dilutions were plated onto NA, NA-gentamicin, and BSM succinate plates to confirm the inoculum at t0. After overnight growth, 100 cells of the BSM–40 mM succinate cultures (dctQ::Tn5Gm versus WT, ΔdctAversus WT, and dctQ::Tn5Gm versus ΔdctAstrains) or 100 cells of the BSM 5 μM succinate cultures (dctQ::Tn5Gm versus WT, ΔdctAversus WT and dctQ::Tn5Gm versus ΔdctAstrains) were reinoculated into fresh 20-ml cultures of BSM containing 40 mM or 5 μM succinate, respectively. Growth of the strains was determined at 3 and 5 h after reinoculation by appropriate serial dilutions and plating onto NA, NA-gentamicin, and BSM succinate plates, as described above. Strain PAO6592 was recognized by its poor growth on succinate and PAO6815 by its gentamicin resistance (Gmr). The competitive index (CI) was defined as the mutant/WT or mutant/mutant ratio divided by the corresponding ratio in the inoculum (10, 25, 47). CI values are the means of three independent experiments ± the standard deviation. Each CI value was analyzed with a Student ttest using as null hypothesis that the mean index was not significantly different from 1.0 (P= 0.05) (25).
RESULTS
Identification of the C4-dicarboxylate transporter DctA in P. aeruginosaPAO1.
In the P. aeruginosaPAO1 genome, the gene PA1183 is annotated as dctAand consists of a 1,311-bp open reading frame encoding a predicted C4-dicarboxylate transport protein of 436 amino acid residues (55). P. aeruginosaPAO1 DctA has 74% amino acid sequence identity with Escherichia coliDctA (NP_417985.1), 79% amino acid sequence identity with P. chlororaphis06 DctA (AAO60164.1), 55% amino acid sequence identity with R. leguminosarumDctA (YP-002282214), and 54% amino acid sequence identity with S. meliloti1021 DctA (NP_438063).
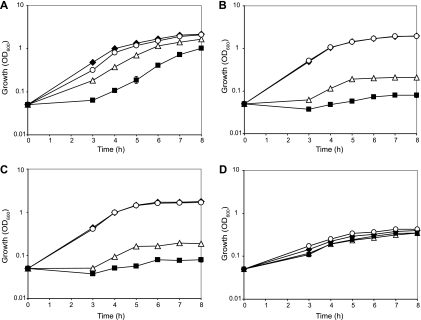
To confirm that dctAis involved in C4-dicarboxylic acid transport in P. aeruginosaPAO1, we constructed a dctAdeletion mutant (PAO6592) and tested its growth in BSM minimum medium supplemented with 40 mM the TCA cycle intermediates succinate, fumarate, malate, or oxaloacetate as the sole carbon source. The growth rate of the mutant on succinate was reduced ∼2-fold compared to the wild-type growth rate (Fig. 1A). Furthermore, growth of PAO6592 was severely impaired on fumarate and malate but similar to that of the wild-type strain on oxaloacetate (Fig. 1B, C, and D). In contrast, in rhizobia and in P. chlororaphisO6, the growth of a dctAmutant is strongly impaired on succinate (6, 31, 36). Unlike P. aeruginosaPAO6592, the dctAmutant of P. chlororaphisO6 is still able to grow on malate (31). The complementing plasmid pME10037, carrying dctAunder the control of the inducible tacpromoter, fully restored the growth of the mutant PAO6592 in succinate, fumarate and malate media (see Fig. S1 in the supplemental material). These data indicate that DctA is specifically involved in the utilization of C4-dicarboxylates, i.e., succinate, fumarate, and malate, but not in the utilization of oxaloacetate. The residual growth of the dctAmutant in the succinate, fumarate, and malate media suggests the presence of additional C4-dicarboxylate transport system(s).
Fig. 1.
Growth properties of the wild-type PAO1 and of mutant strains in minimal medium supplemented with C4-dicarboxylates. Growth curves of the wild-type PAO1 (⧫), the ΔdctAmutant PAO6592 (▵), the ΔdctPQMmutant PAO6708 (○), and the ΔdctAΔdctPQMmutant PAO6709 (▪) in BSM minimal medium containing succinate (A), fumarate (B), malate (C), or oxaloacetate (D) as the unique carbon source (40 mM) are shown. Each value is the average of three different cultures ± the standard deviation. In some instances, the standard deviation bars are smaller than the symbols used.
Search for a second C4-dicarboxylate transporter in a PAO1 ΔdctAbackground.
To identify new C4-dicarboxylate transport system(s) in P. aeruginosaPAO1, we carried out transposon mutagenesis in the ΔdctAmutant PAO6592. Approximately 5,000 Tn5insertion mutants were generated. Of the three mutants displaying a severe growth defect on minimal medium supplemented with succinate, one was further studied. Its Tn5insertion was mapped to the PA5168 gene. This gene is proposed to be a probable dicarboxylate transporter gene showing 47% similarity to the dctQgene product of R. capsulatus(55). Furthermore, the PA5168 gene is annotated as being part of the PA5167-PA5169 operon, encoding a TRAP-type C4-dicarboxylate transport system. The flanking genes PA5167 and PA5169 are proposed, in the Pseudomonasdatabase, to encode a probable periplasmic C4-dicarboxylate-binding protein with 67% similarity to the dctPgene product of R. capsulatusand to encode a probable C4-dicarboxylate-transporter with 72% similarity to the dctMgene product of R. capsulatus, respectively (55).
To demonstrate that the product of the PA5167-PA5169 (dctPQM) operon is involved in C4-dicarboxylate transport in P. aeruginosaPAO1, we constructed the deletion mutants PAO6708 (ΔdctPQM) and PAO6709 (ΔdctPQMΔdctA) and tested their growth rates in BSM supplemented with succinate, fumarate, malate, or oxaloacetate as the sole carbon source. The growth rate of the ΔdctPQMmutant on succinate, fumarate, malate, and oxaloacetate was similar to that of the wild-type strain (Fig. 1). The ΔdctPQMΔdctAdouble mutant had an 8-fold-lower growth rate compared to the wild-type strain on succinate and could not grow on fumarate and malate (Fig. 1Ato C) but grew normally on oxaloacetate (Fig. 1D). The complementing plasmid pME10040, carrying the dctPQMoperon under the control of the inducible tacpromoter, restored the growth of the ΔdctPQMΔdctAmutant to the level of the ΔdctAmutant on succinate (see Fig. S1 in the supplemental material). Taken together, these results indicate that the dctPQMoperon and the dctAgene specify major systems required for the utilization of C4-dicarboxylates, i.e., succinate, fumarate, and malate, but not for the utilization of oxaloacetate.
dctAand dctPQMexpression depends on the growth phase and carbon sources.
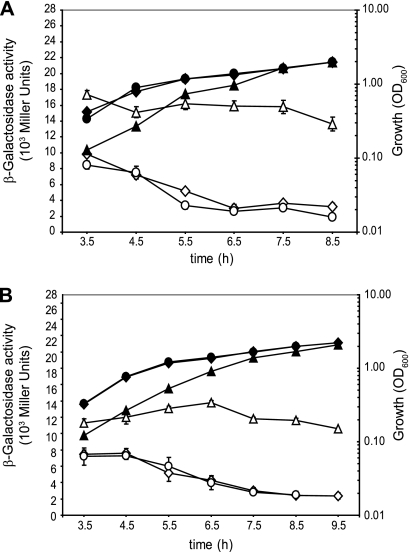
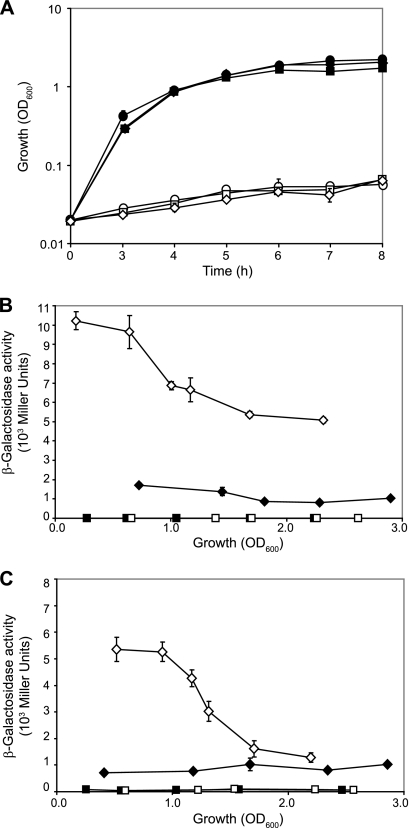
The expression of the dctAgene and the dctPQMoperon in P. aeruginosaPAO1 was monitored in succinate minimal medium by using translational dctA′-′lacZand dctP′-′lacZfusions (carried by plasmids pME9507 and pME10035, respectively). The expression of both reporter fusions was maximal in early exponential growth phase and declined with cell density to reach a plateau in the stationary growth phase (Fig. 2). As a control, a similar expression pattern for dctAwas observed with the use of a chromosomal dctA′-′lacZfusion (data not shown).
Fig. 2.
Cell population density-dependent β-galactosidase expression (open symbols) of a dctA′-′lacZfusion (pME9507) (A) and a dctP′-′lacZfusion (pME10035) (B) in wild-type PAO1 (diamonds), ΔdctAmutant PAO6592 (triangles), and ΔdctPQMmutant PAO6708 (circles). Strains were cultivated in BSM amended with 40 mM succinate. Cell growth was monitored by measuring the optical density at 600 nm (OD600) (solid symbols). Each value is the average of three different cultures ± the standard deviation.
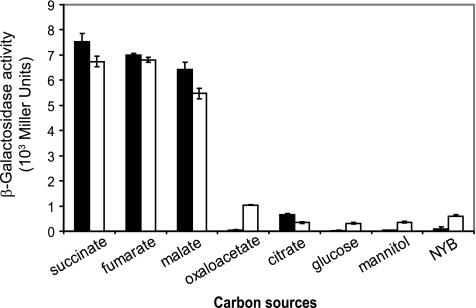
The expression of the dctAgene and the dctPQMoperon was induced by the C4-dicarboxylates succinate, fumarate, and malate but was low in the presence of oxaloacetate, citrate, glucose, mannitol, or NYB, as revealed by using translational dctA′-′lacZand dctP′-′lacZfusions (Fig. 3).
Fig. 3.
β-Galactosidase activity of a dctA′-′lacZ(▪) and a dctP′-′lacZ(□) fusion carried by plasmid pME9507 and pME10035, respectively, in wild-type PAO1 in BSM minimal medium amended with succinate, fumarate, malate, oxaloacetate, citrate, glucose, or mannitol or in rich NYB medium. Strains were cultivated to an OD600of ∼0.8, and each value is the average of three different cultures ± the standard deviation.
The ΔdctAmutant outcompetes the dctQ::Tn5mutant at a low succinate concentration.
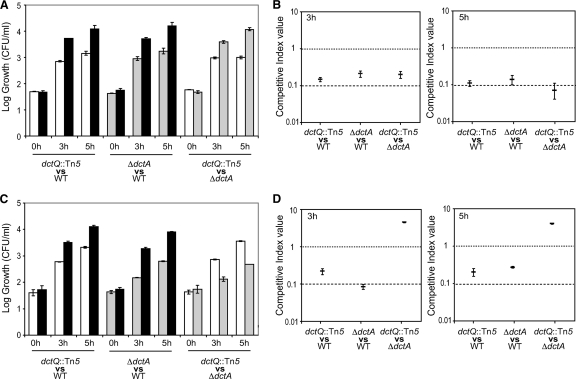
To investigate the specific functions of the DctA and DctPQM transporters, we performed growth competition experiments in minimal medium supplemented with either 5 μM or 40 mM succinate. In 5 μM succinate, as expected, the wild-type PAO1 had a competitive advantage over both the dctQ::Tn5PAO6815 and the ΔdctAPAO6592 mutants after 3 and 5 h of reincubation (Fig. 4Aand B). Interestingly, under these conditions, the PAO1ΔdctAmutant showed a competitive advantage over the PAO1dctQ::Tn5mutant after 3 and 5 h, demonstrating that the DctPQM transporter is more efficient than the DctA transporter for utilization of succinate in the micromolar range.
Fig. 4.
Competitive abilities of wild-type PAO1 and ΔdctAand dctQ::Tn5mutant strains. Cultures were generated from mixed inoculations of wild-type PAO1 (▪) and dctQ::Tn5mutant PAO6815 (□), wild-type PAO1 and ΔdctAmutant PAO6592 (▩), and dctQ::Tn5and ΔdctAmutants. The cultures were grown in BSM medium supplemented with 5 μM (A and B) or 40 mM (C and D) succinate, and each population size was recorded after 0 h of cell growth or after 3 or 5 h of cell growth after reinoculation (see Materials and Methods) (A and C). Competition index values are generated for each competition experiment in 5 μM (B) or 40 mM (D) succinate and defined as the mutant/wild-type or mutant/mutant ratio within the different time points, divided by the initial ratio (0 h) in the inoculum (see Materials and Methods). The competitive index values are mean values of three different cultures. Error bars represent the standard deviation.
A similar experiment was performed in minimal medium supplemented with 40 mM succinate. Again, the wild-type PAO1 had a competitive advantage over the dctQ::Tn5and the ΔdctAmutants strains after 3 and 5 h of reincubation (Fig. 4Cand D). However, now the dctQ::Tn5mutant outcompeted the ΔdctAmutant, indicating that the DctA transporter is more efficient than the DctPQM transporter for utilization of succinate in the millimolar range.
The expression of dctAand dctPQMis negatively regulated by DctA and appears not to be regulated by DctPQM.
Previous work on rhizobia and E. colihas shown that the expression of dctAis increased in a ΔdctAmutant, suggesting that DctA controls its own synthesis (4, 20, 35, 55). To test the autoregulation of DctA and the possible regulation of the dctPQMoperon by DctA in P. aeruginosaPAO1, we measured the expression of a dctA′-′lacZand a dctP′-′lacZtranslational fusion in the wild-type PAO1 and in the ΔdctAmutant PAO6592 growing in BSM amended with 40 mM succinate. The expression of both reporter constructs was increased 2.5-fold in the ΔdctAmutant compared to the wild-type (Fig. 2). In contrast, both constructs gave similar β-galactosidase activities in the wild-type PAO1 and in the ΔdctPQMmutant PAO6708, indicating that DctPQM does not control its own synthesis nor that of DctA in this condition (Fig. 2). The same result was obtained with transcriptional reporter fusions (see Fig. S2 in the supplemental material).
Complementation of the ΔdctAmutant PAO6592 with the plasmid pME10037, carrying the dctAgene, restored the β-galactosidase activity of the dctA′-′lacZand dctP′-′lacZfusions to the wild-type level (see Fig. S3 in the supplemental material).
The expression of dctAand dctPQMis positively regulated by RpoN.
Previous studies in rhizobia and P. chlororaphishave demonstrated that the transcription of the C4-dicarboxylate transporter dctAis activated by the alternative sigma factor RpoN (30, 37). RpoN binds to promoters with the consensus sequence (TGGCAC-N5-TTGCW) at −24/−12 upstream of the transcription start site (3). In the dctAand dctPpromoter regions, highly conserved RpoN binding sites, TGGCACAGCCTCTGCA and TGGCACAGGGCTTGCT, respectively, were found. The expression of the translational dctA′-′lacZand dctP′-′lacZfusions was tested in the wild-type PAO1 and in the ΔrpoNmutant PAO6358 growing in NYB. Rich medium was used in this experiment because of the growth defect of the ΔrpoNmutant in minimal medium supplemented with succinate, fumarate, or malate (Fig. 5A). In the ΔrpoNmutant, dctAand dctPQMexpression was abolished (Fig. 5Band C). To confirm regulation by RpoN, we constructed dctA′-′lacZand dctP′-′lacZfusions carrying mutations in the RpoN-binding site (pME9508 and pME10036, respectively) and tested them in the PAO1 wild-type strain growing in minimal medium with succinate. As expected, the expression of both dctAand dctPmutated in the RpoN box was lost (Fig. 5Band C), showing that RpoN initiates the transcription of dctAand dctPQM.
Fig. 5.
Regulation of dctAand dctPQMgene expression by RpoN. (A) Growth of wild-type PAO1 (filled symbols) and the ΔrpoNmutant PAO6358 (open symbols) in BSM minimal medium amended with succinate (diamonds), fumarate (circles), or malate (squares) as aunique carbon source (40 mM). (B) Cell density-dependent β-galactosidase expression of a dctA′-′lacZtranslational fusion (pME9507) in the wild-type PAO1 (⧫) and the ΔrpoNmutant PAO6358 (▪) grown in NYB medium and in PAO1 wild-type (⋄) in BSM medium amended with 40 mM succinate. Values of a dctA′-′lacZtranslational fusion mutated in the RpoN-box (pME9508) are given for the PAO1 wild-type (□) grown in BSM medium amended with 40 mM succinate. (C) Cell density-dependent β-galactosidase expression of a dctP′-′lacZtranslational fusion (pME10035) in the wild-type PAO1 (⧫) and the ΔrpoNmutant (▪) grown in NYB medium and in the wild-type PAO1 (⋄) in BSM medium amended with 40 mM succinate. Values of a dctP′-′lacZtranslational fusion mutated in the RpoN-box (pME10036) are given for the wild-type PAO1 (□) grown in BSM medium amended with 40 mM succinate. Each value is the average of three different cultures ± the standard deviation. In some instances, the standard deviation bars are smaller than the symbols used.
Identification of a two-component system regulating C4-dicarboxylate utilization in P. aeruginosaPAO1.
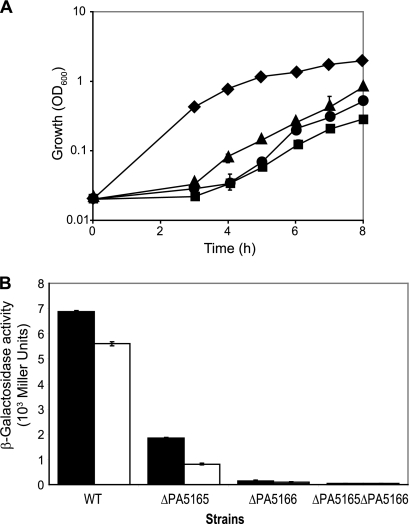
Promoters activated by the alternative sigma factor RpoN require another transcriptional regulatory protein for their activation (49). In the case of C4-dicarboxylates, RpoN requires the two-component system DctB/DctD for dctAregulation in rhizobia and DctS/DctR for dctPQMregulation in R. capsulatus. Therefore, to identify the two-component system(s) in P. aeruginosaPAO1 regulating dctAand dctPQM, we searched for dctB/dctD-like two-component systems in the Pseudomonasdatabase (55). Three dctB/dctDcandidate genes were identified: PA1336/PA1335 with 48 and 67% nucleotide sequence identity, PA5165/PA5166 with 49 and 67% nucleotide sequence identity, and PA5512/PA5511 with 51 and 69% nucleotide sequence identity to S. meliloti dctB/dctD, respectively (55), whereas no significant homology with R. capsulatus dctS/dctRwas observed. Interestingly, the candidate PA5165/PA5166 is located upstream of the dctPQMoperon. To determine which of the three candidate gene pairs might be relevant for C4-dicarboxylate uptake in PAO1, we constructed deletion mutants and tested them for their ability to grow on minimal medium supplemented with succinate. The ΔPA5165 ΔPA5166 double mutant (PAO6707) and the ΔPA5165 (PAO6705) and ΔPA5166 (PAO6706) single mutants were the only mutants showing a growth defect on succinate compared to the wild-type PAO1 (Fig. 6A). On fumarate and malate the same growth defects as on succinate were observed (data not shown). These results indicate that PA5165-PA5166 is involved in C4-dicarboxylate utilization. However, residual growth of the ΔPA5165 ΔPA5166 mutant suggests the existence of additional systems regulating C4-dicarboxylate utilization (Fig. 6A).
Fig. 6.
Regulation of succinate uptake by PA5165-PA5166 (DctB-DctD). (A) Growth properties of the wild-type PAO1 (diamonds), the ΔPA5165 mutant PAO6705 (triangles), the ΔPA5166 mutant PAO6706 (circles), and the ΔPA5165 ΔPA5166 mutant PAO6707 (squares) in BSM medium containing 40 mM succinate. Each value is the average of three different cultures ± the standard deviation. (B) β-Galactosidase activities of a dctA′-′lacZ(▪) and dctP′-′lacZfusion (□) (pME9507 and pME10035, respectively) in wild-type PAO1, ΔPA5165 mutant (PAO6705), ΔPA5166 mutant (PAO6706), and ΔPA5165 ΔPA5166 mutant (PAO6707). Cultures were grown aerobically to an OD600of ∼0.8 in BSM medium containing 40 mM succinate. Each value is the average of three different cultures ± the standard deviation. In some instances, the standard deviation bars are smaller than the symbols used.
The expression of dctAand dctPQMis activated by the two-component system PA5165-PA5166.
To examine further the regulatory role of the predicted two-component system PA5165-PA5166, we tested the expression of the translational dctA′-′lacZand dctP′-′lacZfusions in the wild-type PAO1, the ΔPA5165 and the ΔPA5166 single mutants and the ΔPA5165 ΔPA5166 double mutant. The expression of both reporter fusions was strongly reduced in the ΔPA5165 and the ΔPA5166 single mutants and abolished in the ΔPA5165 ΔPA5166 double mutant (Fig. 6B). The ΔdctBPAO6705 and ΔdctDPAO6707 mutants were complemented with the plasmids pME10038 and pME10039, respectively, restoring the growth (see Fig. S1 in the supplemental material) and β-galactosidase activity of the dctA′-′lacZand dctP′-′lacZfusions (see Fig. S3 in the supplemental material) to the wild-type level. We conclude from these data that PA5165-PA5166 is a DctB/DctD-like two-component system activating the expression of both the dctAgene and the dctPQMoperon.
DISCUSSION
P. aeruginosautilizes preferentially TCA cycle intermediates such as malate, fumarate, and succinate as carbon and energy sources (24, 28). However, their uptake in Pseudomonashad not been studied and, for this reason, we decided to investigate C4-dicarboxylate utilization in this species.
Using a genetic approach, we discovered that a low-affinity system (DctA) and a high-affinity system (DctPQM) together account for most of C4-dicarboxylate transport in strain PAO1. P. aeruginosais the first organism investigated that uses such a dual strategy. Even though growth of a ΔdctPQMΔdctAdouble mutant was dramatically reduced on succinate, residual growth was still observed, suggesting that succinate transport is catalyzed by multiple carriers in P. aeruginosaPAO1, whereas fumarate and malate transport are only catalyzed by the DctA and DctPQM carriers. In the PAO1 genome, two additional dctPQM-like operons (PA0884-PA0886 and PA3779-PA3781) are predicted (55), and whether they act as succinate transporters remains to be determined.
The expression of dctAand dctPQMwas growth phase dependent, being maximal in the early exponential growth phase and induced by succinate, malate, and fumarate (Fig. 2and 3). The dctApattern of expression in strain PAO1 is in contrast to the expression of the E.coli dctAgene, which is enhanced in the stationary phase (31). As in rhizobia and P. chlororaphis(30, 46), the alternative sigma factor RpoN activates the expression of the dctAgene and of the dctPQMoperon (Fig. 5) and, as in rhizobia and E. coli(4, 35, 36, 52, 53), DctA controls negatively its own synthesis, as well as the synthesis of DctPQM. Previous work on Rhizobiumand E. colihas proposed that in the absence of substrates, the DctA and DctB proteins interact with each other in the cytoplasmic membrane, leading to inhibition of the autophosphorylation of DctB and consequently to low expression of the C4-dicarboxylic acid transport system (58, 20, 56, 35, 54). Therefore, we propose that in the absence of DctA, DctB would be in a permanently active state in P. aeruginosa. The fact that the expression of a dctA′-′lacZand of the dctPQMoperon is constitutively activated in a dctAmutant is in favor of this model. Interestingly, in 40 mM succinate, DctPQM does not seem to regulate its own synthesis nor that of DctA, suggesting that DctPQM may not interact with DctB in this condition. To decipher the regulation of the Dct system in P. aeruginosaPAO1, we searched for a two-component system sensing C4-dicarboxylates and responding by activating the expression of dctAand dctPQM. We found that a ΔPA5165 ΔPA5166 mutant showed a growth defect on succinate and that the expression of dctAand dctPQMwas abolished in this mutant, indicating that PA5165-PA5166 is involved in C4-dicarboxylate utilization in PAO1 (Fig. 6).
Since the PA5165-PA5166 system regulates the expression of dctA, belonging to the DAACS family, and that of dctPQM, belonging to the TRAP family, we next addressed the question whether the two-component system PA5165-PA5166 is similar to the two-component system DctB/DctD of rhizobia and E. colior to the two-component system DctS/DctR of R. capsulatus. The sensor-regulator pair DctB/DctD belongs to the NtrB/NtrC family, whereas the DctS/DctR system belongs to the FixL/FixJ family (19). The sensor kinase proteins of the NtrB family consist of a PAS periplasmic sensing domain at the N terminus, a histidine kinase A domain, and a histidine kinase-like ATPase domain at the C terminus. The response regulator proteins of the NtrC family are characterized by a CheY-homologous receiver domain in the N-terminus region, an AAA ATPase domain and an FIS helix-turn-helix motif in the C-terminus region. The sensor kinase proteins of the FixL family contain two transmembrane segments in the N-terminus region, a PAS domain, PAC motifs, a histidine kinase A domain, and a histidine kinase-like ATPase domain in the C terminus. The response regulator proteins of the FixJ family consist of a CheY-homologous receiver domain in the N-terminus region and a LuxR helix-turn-helix motif in the C-terminus region (5). The PA5165/PA5166 amino acid sequence was analyzed by using the SMART program (http://smart.embl.de/). PA5165 is predicted to contain transmembrane segments, a histidine kinase A domain, and a histidine kinase-like ATPase domain at the C terminus, whereas PA5166 consists of a CheY-homologous receiver domain at the N terminus, an AAA ATPase domain, and a FIS helix-turn-helix motif at the C terminus (see Fig. S4 and S5 in the supplemental material). Additionally, PA5165/PA5166 show sequence similarity to DctB/DctD of S. melilotiand NtrB/NtrC of P. aeruginosaand no significant sequence homology to DctR/DctS of R. capsulatus(seeFig. S4 and S5 in the supplemental material). We conclude that PA5165/PA5166 is a DctB/DctD two-component system.
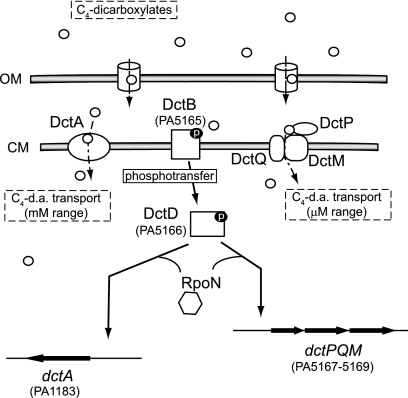
In conclusion, the DctA and DctPQM carriers function coordinately for C4-dicarboxylate uptake and dctAand dctPQMexpression is regulated by the same two-component system DctB/DctD (Fig. 7).
Fig. 7.
Model for C4-dicarboxylate transport in P. aeruginosaPAO1. C4-dycarboxylates (C4-d.a.) trigger the activation of the DctB/DctD two-component system (PA5165/PA5166) enabling RNA polymerase with the RpoN sigma factor to transcribe the dctAgene (PA1183) and the dctPQMoperon (PA5167-PA5169). DctA is the major transporter for utilization of succinate in the mM range, whereas DctPQM transporter is more effective in the μM range. →, Positive effect.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dieter Haas for critical reading of the manuscript and helpful discussions.
This study was supported by the Sandoz Family Foundation (Programme for academic promotion)and the Swiss National Foundation for Scientific Research(project 31003A-127587/1).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 1 July 2011.
REFERENCES
- 1. Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 2. Asai K., Baik S. H., Kasahara Y., Moriya S., Ogasawara N. 2000. Regulation of the transport system for C4-dicarboxylic acids in Bacillus subtilis. Microbiology 146:263–271 [DOI] [PubMed] [Google Scholar]
- 3. Barrios H., Valderrama B., Morett E. 1999. Compilation and analysis of sigma(54)-dependent promoter sequences. Nucleic Acids Res. 27:4305–4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies S. J., et al. 1999. Inactivation and regulation of the aerobic C4-dicarboxylate transport (dctA) gene of Escherichia coli. J. Bacteriol. 181:5624–5635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dixon R., Kahn D. 2004. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2:621–631 [DOI] [PubMed] [Google Scholar]
- 6. Engelke T., Jording D., Kapp D., Puhler A. 1989. Identification and sequence analysis of the Rhizobium meliloti dctAgene encoding the C4-dicarboxylate carrier. J. Bacteriol. 171:5551–5560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finan T. M., Wood J. M., Jordan D. C. 1983. Symbiotic properties of C4-dicarboxylic acid transport mutants of Rhizobium leguminosarum. J. Bacteriol. 154:1403–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forward J. A., Behrendt M. C., Wyborn N. R., Cross R., Kelly D. J. 1997. TRAP transporters: a new family of periplasmic solute transport systems encoded by the dctPQMgenes of Rhodobacter capsulatusand by homologs in diverse gram-negative bacteria. J. Bacteriol. 179:5482–5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fox A., et al. 2008. Emergence of secretion-defective sublines of Pseudomonas aeruginosaPAO1 resulting from spontaneous mutations in the vfrglobal regulatory gene. Appl. Environ. Microbiol. 74:1902–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freter R., Allweiss B., O'Brien P. C., Halstead S. A., Macsai M. S. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect. Immun. 34:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furste J. P., et al. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacPexpression vector. Gene 48:119–131 [DOI] [PubMed] [Google Scholar]
- 12. Giblin L., Boesten B., Turk S., Hooykaas P., O'Gara F. 1995. Signal transduction in the Rhizobium melilotidicarboxylic acid transport system. FEMS Microbiol. Lett. 126:25–30 [DOI] [PubMed] [Google Scholar]
- 13. Golby P., Kelly D. J., Guest J. R., Andrews S. C. 1998. Topological analysis of DcuA, an anaerobic C4-dicarboxylate transporter of Escherichia coli. J. Bacteriol. 180:4821–4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golby P., Kelly D. J., Guest J. R., Andrews S. C. 1998. Transcriptional regulation and organization of the dcuAand dcuBgenes, encoding homologous anaerobic C4-dicarboxylate transporters in Escherichia coli. J. Bacteriol. 180:6586–6596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamblin M. J., Shaw J. G., Kelly D. J. 1993. Sequence analysis and interposon mutagenesis of a sensor-kinase (DctS) and response-regulator (DctR) controlling synthesis of the high-affinity C4-dicarboxylate transport system in Rhodobacter capsulatus. Mol. Gen. Genet. 237:215–224 [DOI] [PubMed] [Google Scholar]
- 16. Heurlier K., Denervaud V., Pessi G., Reimmann C., Haas D. 2003. Negative control of quorum sensing by RpoN (sigma54) in Pseudomonas aeruginosaPAO1. J. Bacteriol. 185:2227–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holloway B. W., Krishnapillai V., Morgan A. F. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:73–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janausch I. G., Kim O. B., Unden G. 2001. DctA- and Dcu-independent transport of succinate in Escherichia coli: contribution of diffusion and of alternative carriers. Arch. Microbiol. 176:224–230 [DOI] [PubMed] [Google Scholar]
- 19. Janausch I. G., Zientz E., Tran Q. H., Kroger A., Unden G. 2002. C4-dicarboxylate carriers and sensors in bacteria. Biochim. Biophys. Acta 1553:39–56 [DOI] [PubMed] [Google Scholar]
- 20. Jording D., et al. 1992. Regulatory aspects of the C4-dicarboxylate transport in Rhizobium meliloti: transcriptional activation and dependence on effective symbiosis. J. Plant Physiol. 141:18–27 [Google Scholar]
- 21. Ledebur H., Gu B., Sojda J., III, Nixon B. T. 1990. Rhizobium melilotiand Rhizobium leguminosarum dctDgene products bind to tandem sites in an activation sequence located upstream of sigma 54-dependent dctApromoters. J. Bacteriol. 172:3888–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ledebur H., Nixon B. T. 1992. Tandem DctD-binding sites of the Rhizobium meliloti dctAupstream activating sequence are essential for optimal function despite a 50- to 100-fold difference in affinity for DctD. Mol. Microbiol. 6:3479–3492 [DOI] [PubMed] [Google Scholar]
- 23. Lee J. H., Hoover T. R. 1995. Protein cross-linking studies suggest that Rhizobium melilotiC4-dicarboxylic acid transport protein D, a sigma 54-dependent transcriptional activator, interacts with sigma 54 and the beta subunit of RNA polymerase. Proc. Natl. Acad. Sci. U. S. A. 92:9702–9706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu P. 1952. Utilization of carbohydrates by Pseudomonas aeruginosa. J. Bacteriol. 64:773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Macho A. P., Zumaquero A., Ortiz-Martin I., Beuzon C. R. 2007. Competitive index in mixed infections: a sensitive and accurate assay for the genetic analysis of Pseudomonas syringae-plant interactions. Mol. Plant Pathol. 8:437–450 [DOI] [PubMed] [Google Scholar]
- 26. Miller J. H. 1972. Experiments in molecular genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 27. Miller V. L., Mekalanos J. J. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio choleraerequires toxR. J. Bacteriol. 170:2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montie T. C. 1998. Pseudomonas. Plenum Press, Inc., New York, NY. [Google Scholar]
- 29. Morgan A. F. 1979. Transduction of Pseudomonas aeruginosawith a mutant of bacteriophage E79. J. Bacteriol. 139:137–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nam H. S., Anderson A. J., Yang K. Y., Cho B. H., Kim Y. C. 2006. The dctAgene of Pseudomonas chlororaphisO6 is under RpoN control and is required for effective root colonization and induction of systemic resistance. FEMS Microbiol. Lett. 256:98–104 [DOI] [PubMed] [Google Scholar]
- 31. Nam H. S., Spencer M., Anderson A. J., Cho B. H., Kim Y. C. 2003. Transcriptional regulation and mutational analysis of a dctAgene encoding an organic acid transporter protein from Pseudomonas chlororaphisO6. Gene 323:125–131 [DOI] [PubMed] [Google Scholar]
- 32. Ornston L. N. 1971. Regulation of catabolic pathways in Pseudomonas. Bacteriol. Rev. 35:87–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pessi G., Haas D. 2000. Transcriptional control of the hydrogen cyanide biosynthetic genes hcnABCby the anaerobic regulator ANR and the quorum-sensing regulators LasR and RhlR in Pseudomonas aeruginosa. J. Bacteriol. 182:6940–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pos K. M., Dimroth P., Bott M. 1998. The Escherichia colicitrate carrier CitT: a member of a novel eubacterial transporter family related to the 2-oxoglutarate/malate translocator from spinach chloroplasts. J. Bacteriol. 180:4160–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reid C. J., Poole P. S. 1998. Roles of DctA and DctB in signal detection by the dicarboxylic acid transport system of Rhizobium leguminosarum. J. Bacteriol. 180:2660–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ronson C. W., Astwood P. M., Downie J. A. 1984. Molecular cloning and genetic organization of C4-dicarboxylate transport genes from Rhizobium leguminosarum. J. Bacteriol. 160:903–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ronson C. W., Astwood P. M., Nixon B. T., Ausubel F. M. 1987. Deduced products of C4-dicarboxylate transport regulatory genes of Rhizobium leguminosarumare homologous to nitrogen regulatory gene products. Nucleic Acids Res. 15:7921–7934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY. [Google Scholar]
- 39. Schnider-Keel U., et al. 2000. Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescensCHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J. Bacteriol. 182:1215–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shaw J. G., Hamblin M. J., Kelly D. J. 1991. Purification, characterization and nucleotide sequence of the periplasmic C4-dicarboxylate-binding protein (DctP) from Rhodobacter capsulatus. Mol. Microbiol. 5:3055–3062 [DOI] [PubMed] [Google Scholar]
- 41. Simon R., O'Connell M., Labes M., Puhler A. 1986. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 118:640–659 [DOI] [PubMed] [Google Scholar]
- 42. Six S., Andrews S. C., Unden G., Guest J. R. 1994. Escherichia colipossesses two homologous anaerobic C4-dicarboxylate membrane transporters (DcuA and DcuB) distinct from the aerobic dicarboxylate transport system (Dct). J. Bacteriol. 176:6470–6478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slotboom D. J., Konings W. N., Lolkema J. S. 1999. Structural features of the glutamate transporter family. Microbiol. Mol. Biol. Rev. 63:293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sonnleitner E., Abdou L., Haas D. 2009. Small RNA as global regulator of carbon catabolite repression in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 106:21866–21871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stanisich V. A., Holloway B. W. 1972. A mutant sex factor of Pseudomonas aeruginosa. Genet. Res. 19:91–108 [DOI] [PubMed] [Google Scholar]
- 46. Stover C. K., et al. 2000. Complete genome sequence of Pseudomonas aeruginosaPAO1, an opportunistic pathogen. Nature 406:959–964 [DOI] [PubMed] [Google Scholar]
- 47. Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. 1987. Use of phoAgene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. U. S. A. 84:2833–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Teramoto H., Shirai T., Inui M., Yukawa H. 2008. Identification of a gene encoding a transporter essential for utilization of C4-dicarboxylates in Corynebacterium glutamicum. Appl. Environ. Microbiol. 74:5290–5296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thony B., Hennecke H. 1989. The −24/−12 promoter comes of age. FEMS Microbiol. Rev. 5:341–357 [DOI] [PubMed] [Google Scholar]
- 50. Ullmann R., Gross R., Simon J., Unden G., Kroger A. 2000. Transport of C4-dicarboxylates in Wolinella succinogenes. J. Bacteriol. 182:5757–5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Voisard C., et al. 1994. Biocontrol of root diseases by Pseudomonas fluorescensCHAO: current concepts and experimental approaches. VCH Publishers, Weinheim, Germany [Google Scholar]
- 52. Wang Y. K., Park S., Nixon B. T., Hoover T. R. 2003. Nucleotide-dependent conformational changes in the sigma54-dependent activator DctD. J. Bacteriol. 185:6215–6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Watson R. J., Chan Y. K., Wheatcroft R., Yang A. F., Han S. H. 1988. Rhizobium melilotigenes required for C4-dicarboxylate transport and symbiotic nitrogen fixation are located on a megaplasmid. J. Bacteriol. 170:927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watson R. J. 1990. Analysis of the C4-dicarboxylate transport genes of Rhizobium meliloti: nucleotide sequence and deduced products of dctA, dctB, and dctD. Mol. Plant-Microbe Interact. 3:174–181 [DOI] [PubMed] [Google Scholar]
- 55. Winsor G. L., et al. 2009. PseudomonasGenome Database: facilitating user-friendly, comprehensive comparisons of microbial genomes. Nucleic Acids Res. 37:D483–D488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yarosh O. K., Charles T. C., Finan T. M. 1989. Analysis of C4-dicarboxylate transport genes in Rhizobium meliloti. Mol. Microbiol. 3:813–823 [DOI] [PubMed] [Google Scholar]
- 57. Ye R. W., et al. 1995. Anaerobic activation of the entire denitrification pathway in Pseudomonas aeruginosarequires Anr, an analog of Fnr. J. Bacteriol. 177:3606–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yurgel S. N., Kahn M. L. 2004. Dicarboxylate transport by rhizobia. FEMS Microbiol. Rev. 28:489–501 [DOI] [PubMed] [Google Scholar]
- 59. Zientz E., Six S., Unden G. 1996. Identification of a third secondary carrier (DcuC) for anaerobic C4-dicarboxylate transport in Escherichia coli: roles of the three Dcu carriers in uptake and exchange. J. Bacteriol. 178:7241–7247 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.