TEXT
The extracellular signal-regulated kinase (ERK) is a member of the evolutionarily conserved mitogen-activated protein kinase (MAPK) family and is a key regulator of many cellular processes, including growth, differentiation, cell survival, and the immune response (2). The misregulation of ERK signaling is implicated in several disorders, including cancer, immune disease, and neurodegenerative disease (2). ERK phosphorylates substrates that reside throughout the cell; however, many ERK targets are nuclear and mediate changes in gene expression (2, 7). While a number of studies have addressed the spatial regulation of ERK in cells, and in particular how it gets targeted to the nucleus (13), our understanding of the molecular mechanisms involved remains incomplete. In this issue, Plotnikov and coworkers report that casein kinase 2 (CK2) directly phosphorylates ERK and promotes its nuclear import, thus uncovering a novel point of regulation in the ERK MAPK pathway (8).
The ERK MAPK pathway is activated by diverse stimuli, including growth factors, hormones, and cytokines, and similar to other MAPK pathways features a multitiered signaling module. This consists of RAS GTPase, RAF (a MAPK kinase kinase), and MEK, which activates ERK by phosphorylation on Thr and Tyr residues within a Thr-Glu-Tyr motif located in its activation loop (2). The components of MAPK pathways are often found associated with regulatory or scaffold proteins that can enhance signaling specificity and localize the pathway to distinct cell compartments (2). The functional importance of spatially and temporally regulating ERK activation was originally observed in rat PC12 cells, where it was found that the transient activation of ERK promoted cell proliferation, whereas sustained activation led to a significant accumulation of nuclear ERK and cell differentiation (2, 7). Subsequent research in other cell types suggested that one mechanism for the differential functional effects of transient versus sustained ERK activity was that the transcription factor targets of ERK are able to interpret the duration of ERK signaling. For example, the transient accumulation of active ERK in the nucleus promotes the expression of the immediate-early gene c-fos but as ERK activity declines c-Fos protein is degraded and there is minimal expression of c-Fos target genes. In contrast, sustained levels of active ERK in the nucleus result in the phosphorylation and stabilization of the c-Fos protein, thereby promoting the expression of c-Fos-dependent genes (7). These and many other studies have clearly demonstrated an essential role for nuclear ERK in cell function and underscore the importance of uncovering the regulatory mechanisms controlling ERK nuclear import and activity.
A model of how ERK nuclear localization might be regulated is emerging. In quiescent cells, ERK is found mainly in the cytoplasm due to its association with anchoring proteins, which prevent its nuclear translocation (Fig. 1). A major cytoplasmic anchor for ERK is its upstream activator MEK; however, scaffold proteins (e.g., PEA-15, Sef1, and hScrib) and MAPK phosphatases may also act as anchors (2, 13). In response to extracellular cues, the MAPK pathway is triggered and MEK phosphorylates the Thr-Glu-Tyr motif in ERK, causing it to dissociate from the anchor proteins and move into the nucleus (2, 13). Several studies indicate that both passive diffusion and active processes contribute to ERK translocation into the nucleus (13). There is no recognizable nuclear localization sequence (NLS) present in ERK, but mutation studies have implicated the MAPK insert domain (KID) in both passive and active nuclear translocation (12). This is supported by evidence suggesting that the KID region is part of a substrate docking site that can interact with the nuclear pore components Nup153 and Nup214 (12, 13). Chuderland and colleagues followed up on these observations and found that the mutation of two Ser residues within a Ser-Pro-Ser (SPS) motif, present in the KID, blocked nuclear translocation of ERK (3). Furthermore, the nuclear accumulation of ERK following cell stimulation correlated with the phosphorylation of these Ser residues and was enhanced when they were mutated to phosphomimetic residues. The phosphorylated SPS motif was found to be required for both passive translocation of ERK to the nucleus and facilitated import via its binding to the nuclear importin Imp7 (3).
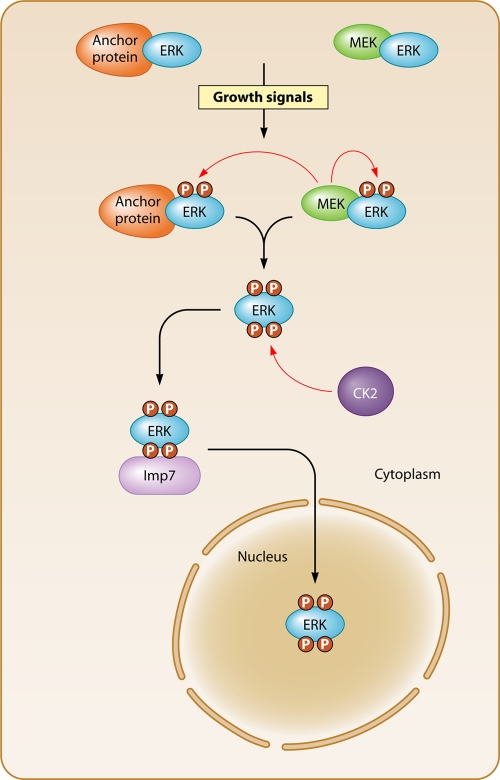
Fig. 1.
CK2 regulates ERK nuclear import. In the absence of growth-promoting signals, ERK mainly resides in the cytoplasm associated with MEK and other anchoring proteins. Growth signals stimulate the RAS-RAF-MEK pathway, leading to the phosphorylation of the ERK activation loop and its dissociation from the anchoring proteins. This allows CK2 to phosphorylate the ERK NTS, which provides a binding site for Imp7. The ERK-Imp7 complex is imported into the nucleus, where ERK targets its nuclear substrates, including transcriptional regulatory proteins.
An important conclusion arising from the characterization of this putative phosphorylation-dependent nuclear translocation signal (NTS) in ERK was that one or more intracellular protein kinases could directly control ERK nuclear import and function. Plotnikov and coworkers (8) propose that CK2 is the major protein kinase responsible for phosphorylating the NTS of ERK. They noted that there were acidic residues downstream of the SPS motif, indicative of a CK2 consensus phosphorylation site, which led them to examine the effect of pharmacological inhibition and RNA interference (RNAi) knockdown of CK2 on ERK nuclear translocation. Inhibition of CK2 abrogates SPS motif phosphorylation and nuclear translocation of ERK but does not appear to affect ERK activation, as ERK was still capable of phosphorylating substrates localized in the cytoplasm. The authors also demonstrate the direct phosphorylation of the SPS motif in vitro by recombinant CK2 and were able to isolate a protein complex containing ERK and CK2 from cells. The study goes on to dissect the relative importance of the two Ser residues (Ser 244 and Ser246) within the SPS motif. The phosphorylation of Ser246 appears sufficient for ERK nuclear translocation, but dual phosphorylation on both Ser residues enhances the kinetics of nuclear import. Indeed, the phosphorylation of Ser246 is a prerequisite for Ser244 phosphorylation. While the study provides compelling evidence for CK2 as the predominant kinase responsible for Ser246 and Ser244 phosphorylation, it also suggests that ERK autophosphorylation of Ser244 may play a contributing role (8).
CK2 forms a heterotetramer of two regulatory and two catalytic subunits that is present throughout the cell and appears to be constitutively active (4). This leads to the question of why only activated ERK seems to be phosphorylated by CK2. The study by Plotnikov et al. demonstrates in vitro that MEK association with ERK reduces CK2 phosphorylation of the SPS motif, suggesting that the cytoplasmic anchor proteins to which ERK binds, such as MEK, may be protecting it from CK2 activity (8). Putting this work in the context of the model of ERK nuclear translocation described above, it can be proposed that in the absence of growth stimuli ERK is retained in the cytoplasm by MEK and various anchoring proteins, which protect it from phosphorylation by CK2 (Fig. 1). Upon stimulation of cells, MEK phosphorylates and activates ERK, which releases it from the anchoring proteins, allowing CK2 to phosphorylate it. The phosphorylated NTS of ERK provides a negatively charged surface that associates with Imp7 to facilitate nuclear import (Fig. 1). Previous studies have demonstrated that the phosphorylation of ERK by MEK can be uncoupled from nuclear translocation, and the study by Plotnikov et al. supports this, as NTS phosphorylation by CK2 is independent of ERK phosphorylation by MEK (8, 13). What is unclear is whether the phosphorylation of the NTS in ERK is solely required for its nuclear import or whether it can regulate ERK function within the nucleus, perhaps by affecting its targeting to particular transcription factor substrates or its interactions with other proteins that anchor ERK in the nucleus.
The NTS of ERK acts as an autonomous nuclear import signal that, when fused to nonnuclear proteins, results in their nuclear accumulation (3), provoking the question of whether there is a widespread role for this novel NTS in the nuclear import of proteins. A number of imported proteins contain a similar NTS sequence, including AKT, p53, BRCA1, SMAD3, Jun N-terminal protein kinase 2 (JNK2), MEK1, and Drosha. The NTSs of MEK1 and SMAD3 are phosphorylated and that the mutation of the phosphorylation sites blocks the nuclear import of both these proteins (3). However, it is unclear if CK2 is involved, as the NTS sequences in MEK1 and SMAD3, unlike that of ERK, lack acidic residues downstream of the phosphorylation sites, which is a hallmark of the CK2 consensus site. Recently, the NTS present in the RNase III enzyme Drosha was reported to be phosphorylated by glycogen synthase kinase 3β, indicating that protein kinases in addition to CK2 can mediate the phosphorylation of NTS sequences (11). The use of similar mechanisms for nuclear entry by both MEK1 and ERK is intriguing, particularly if a protein kinase distinct from CK2 is regulating the MEK1 NTS. It is possible that NTS-regulated nuclear import of MEK1 allows it to either prolong ERK activation in the nucleus or, conversely, export ERK out of the nucleus, thereby temporally regulating ERK nuclear functions.
While the work by Plotnikov and coworkers supports a major role for CK2 phosphorylation of the NTS in ERK nuclear translocation, other mechanisms clearly exist. There is evidence that substrate docking sites on ERK that are distinct from the KID region are involved, while the role of ERK dimerization has been hotly debated (13). Other mechanisms may involve ERK association with proteins that have conventional NLSs, such as biliverdin reductase (6). A potential rate-limiting step in nuclear import of ERK is the expression levels of transport proteins such as Imp7 and nuclear pore components. It is reported that in some primary cell types the accumulation of activated ERK in the nucleus is restricted, while in transformed cells there is increased nuclear entry, and that these differences correlate well with the expression levels of importins and nuclear pore complex proteins (10). Interestingly, at least three components of the nuclear pore complex (Nup50, Nup153, and Nup214) are direct targets of ERK phosphorylation, and this appears to disrupt their binding to specific nuclear importins and to reduce the movement of these importins into the nucleus (1). ERK may therefore directly regulate nuclear pore permeability and permit the selective movement of importins and their cargo into the nucleus. It is not clear how this might impact ERK's own entry into the nucleus. The phosphorylation of the ERK NTS reduces its affinity for Nup153, and it is proposed that this facilitates ERK entry through the nuclear pore (3). Whether the phosphorylation of the NTS in ERK affects its own ability to phosphorylate Nup153 and modulate nuclear pore permeability more generally remains to be elucidated.
What is the significance for cell function of this newly uncovered cross talk between CK2 and the ERK pathway? CK2 regulates many of the same cell processes as the ERK pathway, including cell proliferation and survival (4). CK2 expression and activity are upregulated in many cancers, and CK2 is reported to downregulate the activity of tumor suppressors and promote the activity of oncoproteins such as β-catenin, thereby contributing to tumorigenesis (4). It is well established that mutations in genes coding for components of the ERK pathway, including members of the epidermal growth factor (EGF) receptor family, RAS, RAF, and MEK, are key oncogenic events in many cancers (2). In addition, nuclear ERK is required to regulate cell proliferation and oncogenic transformation, and many of the nuclear targets of ERK, including transcription factors, coregulatory proteins, and chromatin-modifying proteins, are implicated in these processes (2, 13). Connections between CK2 and the ERK pathway have been reported previously. For example, the ERK MAPK scaffold protein kinase suppressor of Ras (KSR) can recruit CK2 to phosphorylate RAF and enhance its activity toward MEK (9). Recently, a complex of ERK and CK2 has been shown to mediate cross talk between growth factor receptor and Wnt signaling pathways (5). EGF stimulates ERK binding to CK2, allowing it to phosphorylate the CK2α catalytic subunit at Thr360 and Ser362. This enhances the ability of CK2 to phosphorylate α-catenin, which releases its inhibitory effect on β-catenin transactivation and promotes β-catenin-mediated cellular processes, including cell invasion (5). Taken together with the current study by Plotnikov et al., this suggests that under certain growth conditions there is reciprocal phosphorylation of ERK and CK2 occurring, which regulates both ERK nuclear translocation and the activity of other CK2-dependent pathways. Clearly further work needs to be undertaken to determine the impact of this coregulation on cell growth and if it can contribute to tumor formation.
The finding that CK2 regulates ERK nuclear import provides a new mechanism by which CK2 collaborates with oncogenic pathways in cells and provides further impetus to current efforts to find highly specific CK2 inhibitors. In addition to the development of ATP-competitive inhibitors of CK2, disruption of the ERK-CK2 complex may be an alternative therapeutic strategy for modulating ERK nuclear entry downstream of oncogenic signaling events.
Footnotes
Published ahead of print on 26 July 2011.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1. Ahn N. G. 2009. PORE-ing over ERK substrates. Nat. Struct. Mol. Biol. 16:1004–1005 [DOI] [PubMed] [Google Scholar]
- 2. Avruch J. 2007. MAP kinases: the first twenty years. Biochim. Biophys. Acta 1773:1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chuderland D., Konson A., Seger R. 2008. Identification and characterization of a general nuclear translocation signal in signaling proteins. Mol. Cell 31:850–861 [DOI] [PubMed] [Google Scholar]
- 4. Duncan J. S., Litchfield D. W. 2008. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim. Biophys. Acta 1784:33–47 [DOI] [PubMed] [Google Scholar]
- 5. Ji H., et al. 2009. EGF-induced ERK activation promotes CK2-mediated disassociation of α-catenin from β-catenin and transactivation of β-catenin. Mol. Cell 36:547–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lerner-Marmarosh N., Miralem T., Gibbs P. E. M., Maines M. D. 2008. Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proc. Natl. Acad. Sci. U. S. A. 105:6870–6875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy L. O., Blenis J. 2006. MAPK signaling specificity: the right place at the right time. Trends Biochem. Sci. 31:268–275 [DOI] [PubMed] [Google Scholar]
- 8. Plotnikov A., Chuderland D., Karamansha Y., Livnah O., Seger R. 2011. Nuclear ERK translocation is mediated by protein kinase CK2 and accelerated by autophosphorylation. Mol. Cell Biol. 31:3515–3530 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Ritt D. A., et al. 2007. CK2 is a component of the KSR1 scaffold complex that contributes to Raf kinase activation. Curr. Biol. 17:179–184 [DOI] [PubMed] [Google Scholar]
- 10. Smith E. R., et al. 2010. Nuclear entry of activated MAPK is restricted in primary ovarian and mammary epithelial cells. PLoS One 5:e9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang X., Li M., Tucker L., Ramratnam B. 2011. Glycogen synthase kinase 3 beta (GSK3β) phosphorylates the RNase III enzyme Drosha at S300 and S302. PLoS One 6:e20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yazicioglu M. N., et al. 2007. Mutations in ERK2 binding sites affect nuclear entry. J. Biol. Chem. 282:28759–28767 [DOI] [PubMed] [Google Scholar]
- 13. Zehorai E., Yao Z., Plotnikov A., Seger R. 2010. The subcellular localization of MEK and ERK-a novel nuclear translocation signal (NTS) paves a way to the nucleus. Mol. Cell. Endocrinol. 314:213–220 [DOI] [PubMed] [Google Scholar]