Abstract
We report the first case of a prosthetic joint infection caused by Gemella sanguinis. This report includes a description of a “pseudosatelliting” phenomenon by G. sanguinis, the use of gene sequencing for pathogen identification, and successful use of debridement, retention, and chronic antibiotic suppression to preserve the prosthesis.
CASE REPORT
A 52-year-old man with a history of type II diabetes mellitus and avascular necrosis of the femoral heads (status post-bilateral hip arthroplasties 14 years ago) presented with 3 days of severe left hip pain. He described subjective fevers and chills but denied any recent trauma or skin breakdown. He underwent a dental cleaning approximately 3 months prior to presentation. On examination, he was afebrile with a blood pressure of 130/70 and a heart rate of 98 with a regular rhythm without murmurs. There was tenderness with palpation of the left hip but no appreciable skin break, erythema, or swelling. His musculoskeletal exam was notable for marked limitation and severe pain on both active and passive range of motion of his left hip. Radiography of the hip revealed a well-seated prosthesis with no lucency of the surrounding bone. His white blood cell count was 13.8 × 103/mm3 (normal range, 4.0 × 103/mm3 to 11.0 × 103/mm3) with 86% polymorphonuclear leukocytes; his erythrocyte sedimentation rate was 44 mm/h (normal range 0 to 15 mm/h), and his C-reactive protein was 11 mg/liter (normal range 0 to 5.0 mg/liter). Two sets of blood cultures were obtained and had no growth. An arthrocentesis of the left hip yielded 20 ml of purulent fluid with a total white cell count of 198 × 103/mm3. Gram staining of the fluid revealed 4+ polymorphonuclear cells and 4+ Gram-positive cocci in pairs and clusters. Empirical treatment with vancomycin was initiated.
The patient was taken to the operating room the following day for an arthrotomy with debridement, washout of the left hip, and polyethylene liner exchange. Cultures from the arthrocentesis and surgical specimen initially grew poorly on sheep blood agar plates after 24 h incubation at 35°C. More notable growth was observed on a chocolate agar plate. A Gram stain of growth from those plates revealed Gram-positive cocci in pairs. A catalase test was negative. l-Pyrrolidonyl-β-naphthylamide (PYR) and leucine aminopeptidase (LAP) tests were both positive, there was no growth in 6.5% NaCl medium, and the bile esculin test was negative. Given the biochemical results, a subculture from the initial plate was plated on sheep blood agar with a Staphylococcus aureus streak. Colonies were noted to satellite around a staphylococcal streak at 24 h, suggestive of Abiotrophia/Granulicatella (formerly called nutritionally variant streptococci) (Fig. 1). Due to inconsistencies in results of morphological and biochemical testing, the isolate was submitted to Mayo Medical Laboratory (Rochester, MN) for identification. The patient was continued on vancomycin therapy and discharged to a rehabilitation facility.
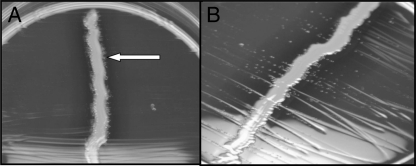
Fig. 1.
Gemella sanguinis cultured on sheep blood agar plate around a staphylococcal streak. (A) At 24 h, colonies are noted in proximity to the staphylococcal streak (arrow). (B) At 48 h, colonies are widespread.
Partial 16S rRNA sequencing at Mayo identified the pathogen as Gemella sanguinis. Although there is no Clinical and Laboratory Standards Institute (CLSI) standard method and interpretation of susceptibility testing for Gemella, susceptibility testing was performed using the CLSI method for viridans group streptococci on Mueller-Hinton agar supplemented with 5% sheep's blood and incubated at 35°C with 5% CO2 for 24 h. By Etest, the MICs were 2 μg/ml for vancomycin, 0.047 μg/ml for penicillin, and 0.023 μg/ml for ceftriaxone. By disk diffusion, zones of inhibition were 6 mm for levofloxacin and >25 mm for rifampin, erythromycin, and tetracycline. In general, zone sizes of >25 mm for these three antibiotics were in the susceptible range for Gram-positive organisms, including Staphylococcus, Streptococcus, and Enterococcus. Based on the in vitro activity, pharmacokinetics, toxicity, and convenience of use of tested agents, the patient was switched from vancomycin to ceftriaxone (2 g every 24 h [q24h] intravenously [i.v.]) to complete a total of 6 weeks of i.v. antibiotics. At outpatient follow-up at the completion of i.v. antibiotics, the patient reported a marked improvement in symptoms, and laboratory testing showed normalization of inflammatory markers. He was transitioned to chronic suppressive therapy with oral doxycycline. At a 6-month follow-up, the patient had regained baseline function of his hip and inflammatory markers were still normal.
Prosthetic joint infections (PJI) are uncommon but serious complications of total hip arthroplasties. Infections occur in 0.3 to 1.7% of hip arthroplasties (3, 9), and symptoms may include fever, pain, swelling, warmth, and erythema overlying the infected joint. Staphylococci (Staphylococcus aureus and coagulase-negative Staphylococcus species) account for the majority of prosthetic hip infections (5), yet other pathogens, including enterococci, Escherichia coli, group B Streptococcus, and Klebsiella spp., have been described (3). Species within the genus Gemella have rarely been implicated as the causal organisms in prosthetic joint infections. We report here the case of a prosthetic joint infection caused by Gemella sanguinis, the description of a “pseudosatelliting” phenomenon of Gemella spp., and the first case of a prosthetic joint infection caused by Gemella spp. successfully treated by debridement and retention of the prosthesis followed by chronic suppressive antibiotic therapy.
The genus Gemella consists of catalase-negative Gram-positive cocci which appear as smooth, non- or alpha-hemolytic colonies on blood agar, resembling viridans group streptococcus (4). The biochemical properties of Gemella spp. are identical to that of Abiotrophia/Granulicatella. Slow growth of some Gemella strains may cause confusion with Abiotrophia/Granulicatella; thus testing for satelliting behavior is performed to differentiate the two (8). In our case, the observation of satellite growth of colonies around a Staphylococcus aureus streak on blood agar at 24 h resulted in initial misidentification of the organism as Abiotrophia/Granulicatella. The presence of Gram-positive cocci in pairs and clusters in the initial arthrocentesis specimen would be unusual for Abiotrophia/Granulicatella and more fitting of Gemella haemolysans (though not G. sanguinis); however, growth from culture plates showed Gram-positive cocci in pairs only. Ultimately, 16S rRNA gene sequencing identified the bacterium from the patient's joint as G. sanguinis. Repeat subculturing revealed that though the aforementioned satelliting phenomenon was seen 24 h into the incubation, after 48 h, colonies had widespread growth, not consistent with the satelliting phenomenon of Abiotrophia/Granulicatella (Fig. 1). To our knowledge, this “pseudosatelliting” effect of Gemella has not been previously reported. While Abiotrophia/Granulicatella and Haemophilus spp. satellite around a staphylococcal streak due to its pyridoxal- or X/V factor-rich environment, it is unclear why our Gemella isolate demonstrated such behavior. We postulate a similar but transient nutrient benefit as the mechanism for this phenomenon at 24 h but not at 48 h. Gemella spp. may also be misidentified as viridans group streptococci due to similarities in colony morphology and biochemical characteristics. The distinction between the two is important because infections, such as infective endocarditis, caused by Gemella spp. are thought to be more difficult to treat than those caused by viridans group streptococci and thus more aggressive antibiotic therapy has been recommended (1). Due to the difficulty in phenotypic identification and to evidence that biochemical methods may be suboptimal (11), molecular diagnostics such as 16S rRNA gene sequencing may serve an important role in the identification of organisms like Gemella spp., when traditional methods may produce ambiguous results.
Most species of Gemella are found in the flora of the oral cavity and the upper respiratory and intestinal tracts in healthy individuals. Clinically relevant species include Gemella morbillorum, G. haemolysans, Gemella bergeri, and G. sanguinis. These organisms have been implicated most commonly in endovascular infections such as endocarditis and less frequently in meningitis or septic arthritis (4).
There have been three published reports of prosthetic joint infections by Gemella spp.: two caused by G. morbillorum (7, 10) and one by G. haemolysans (6). Of particular note is that one of the cases (7) also describes a patient with a remote hip arthroplasty who presented with Gemella hip prosthetic joint infection 3 months after a dental procedure, circumstances similar to our case. Several case reports of Gemella endovascular or joint infection speculate on an oropharyngeal source. However, there are no convincing data linking dental procedures to prosthetic joint infection (2).
Our patient was initially treated with vancomycin with good clinical response and switched to ceftriaxone to complete a 6-week i.v. antibiotic course. The antimicrobial susceptibility patterns of Gemella spp. are similar to that of viridans group streptococci, typically exhibiting susceptibility to most beta-lactams, vancomycin, and macrolides, intrinsic resistance to sulfonamides and trimethoprim, and low-level resistance to aminoglycosides (4). Because the strain in our case had a large (>25 mm) zone of inhibition to tetracycline on disk diffusion testing, we chose to use doxycycline for chronic oral suppressive therapy.
All three previous reports of Gemella PJI were managed by joint replacement by a two-stage procedure. This is the first description of a Gemella infection successfully treated with debridement and retention, albeit followed by chronic suppression. Consideration was given to use of a rifampin-containing regimen to improve the possibility of a microbiological cure and abrogate the need for chronic suppression, i.e., a true debridement and retention approach. However, unlike staphylococcal PJI, in which there is a well-defined role for rifampin due to its activity against bacteria embedded in biofilm, there are very limited data available on the treatment of Gemella PJI and it was concluded that the risk/benefit equation favored chronic suppression.
In conclusion, we report a case of prosthetic joint infection involving G. sanguinis and the first Gemella PJI treated successfully with debridement and retention followed by chronic suppression. We also report the phenomenon of pseudosatelliting of this species seen on blood agar, creating some confusion in the initial identification of the organism. Given the increasing number of joint arthroplasties and thus prosthetic joint infections, further characterization of the extent of Gemella species involvement in PJI is warranted, including evaluation of antibiotic resistance patterns and response to treatment. Gram-positive cocci that grow slowly should raise concern for Gemella species in the setting of prosthetic joint infection. As in our case, molecular methods may be helpful in identifying this pathogen.
Acknowledgments
We thank Matthew MacKechnie for technical laboratory assistance.
Footnotes
Published ahead of print on 27 July 2011.
REFERENCES
- 1. Baddour L. M., et al. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434 doi: 10.1161/CIRCULATIONAHA.105.165564 [DOI] [PubMed] [Google Scholar]
- 2. Berbari E. F., et al. 2010. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case-control study. Clin. Infect. Dis. 50:8–16 doi: 10.1086/648676 [DOI] [PubMed] [Google Scholar]
- 3. Choong P. F., Dowsey M. M., Carr D., Daffy J., Stanley P. 2007. Risk factors associated with acute hip prosthetic joint infections and outcome of treatment with a rifampin based regimen. Acta Orthop. 78:755–765 doi:10.1080/17453670710014527 [DOI] [PubMed] [Google Scholar]
- 4. Collins M. D. 2006. The genus Gemella, p. 511–518In Dworkin M., Falkow S., Rosenberg E., Schleifer K., Stackebrandt E.(ed.), The prokaryotes, 3rd ed., vol. 4 Springer-Verlag, New York, NY [Google Scholar]
- 5. Del Pozo J. L., Patel R. 2009. Clinical practice. Infection associated with prosthetic joints. N. Engl. J. Med. 361:787–794 doi:10.1056/NEJMcp0905029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eggelmeijer F., Petit P., Dijkmans B. A. 1992. Total knee arthroplasty infection due to Gemella haemolysans. Br. J. Rheumatol. 31:67–69 [DOI] [PubMed] [Google Scholar]
- 7. Medina-Gens L., Bordes-Benítez A., Saéz-Nieto J. A., Pena-López M. J. 2007. Infection of a total hip arthroplasty due to Gemella morbillorum. Enferm. Infecc. Microbiol. Clin. 25:553. [DOI] [PubMed] [Google Scholar]
- 8. Murray P. R., Baron E. J. 2007. Manual of clinical microbiology. ASM Press, Washington, DC [Google Scholar]
- 9. Pulido L., Ghanem E., Joshi A., Purtill J. J., Parvizi J. 2008. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin. Orthop. Relat. Res. 466:1710–1715 doi:10.1007/s11999-008-0209-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Essen R., Ikavalko M., Forsblom B. 1993. Isolation of Gemella morbillorum from joint fluid. Lancet 342:177–178 [DOI] [PubMed] [Google Scholar]
- 11. Woo P. C., et al. 2003. Gemella bacteraemia characterised by 16S rRNA gene sequencing. J. Clin. Pathol. 56:690–693 [DOI] [PMC free article] [PubMed] [Google Scholar]