Abstract
oskar (osk) mRNA is tightly localized to the posterior pole of the Drosophila oocyte, where the subsequent expression of Osk protein directs abdomen and germ-line formation in the developing embryo. Misplaced expression of Osk protein leads to lethal body patterning defects. The Osk message is translationally repressed before and during the localization process, ensuring that Osk protein is only expressed after the mRNA has reached the posterior. An ovarian protein, Bruno (Bru), has been implicated as a translational repressor of osk mRNA. Here we report the isolation of a cDNA encoding Bru using a novel approach to the expression cloning of an RNA-binding protein, and the identification of previously described mutants in the arrest (aret)-locus as mutants in Bru. The mutant phenotype, along with the binding properties of the protein and its pattern of accumulation within the oocyte, indicate that Bru regulates multiple mRNAs involved in female and male gametogenesis as well as early in embryogenesis. Genetic experiments provide further evidence that Bru functions in the translational repression of osk. Intriguingly, we find that Bru interacts physically with Vasa (Vas), an RNA helicase that is a positive regulator of osk translation. Bru belongs to an evolutionarily conserved family of genes, suggesting that Bru-mediated translational regulation may be widespread. Models for the molecular mechanism of Bru function are discussed.
Keywords: bruno, arrest, oskar, vasa, translational repressor, RNA-binding protein
The earliest stages of embryonic development generally do not rely on zygotic gene expression; instead, the protein products of maternally provided mRNAs support and direct early development. Many of these transcripts do not become translationally active until after fertilization and thus supply proteins only when they are needed. Both global and selective mechanisms are known to be involved in the translational regulation of maternal messages. For example, increased activity of the translational apparatus accompanies egg activation in sea urchins and causes a global enhancement of maternal mRNA translation (for review, see Davidson 1986). A more selective form of regulation involves cytoplasmic polyadenylation, which is initiated by cis-acting regulatory elements present in particular transcripts. These sequences direct the extension of the poly(A) tails of specific maternal mRNAs after fertilization, allowing them to become more efficiently translated (for review, see Wickens et al. 1996).
Selective forms of translational control are crucial in early development, as some proteins that direct key developmental events must appear only at appropriate times or places. For instance, developmental timing directed by c-mos in Xenopus requires translational regulation of the maternal c-mos mRNA (Gebauer et al. 1994; Sheets et al. 1995). Similarly, a number of mRNAs that encode proteins directing body patterning in Drosophila have been found to be translationally regulated (Wharton and Struhl 1991; Gavis and Lehmann 1994; Sallés et al. 1994; Kim-Ha et al. 1995; Markussen et al. 1995; Rongo et al. 1995). Although some of these mRNAs, including c-mos and Drosophila bicoid, are known to be regulated by cytoplasmic polyadenylation following fertilization (Gebauer et al. 1994; Sallés et al. 1994; Sheets et al. 1995), the mechanisms governing the complex translational control of other mRNAs are not yet understood.
The Drosophila oskar (osk) mRNA provides a particularly interesting example of a translationally regulated maternal transcript. The Osk protein normally appears only at the posterior pole of the oocyte, where it acts in the localized accumulation of factors required for both germ cell formation and posterior body patterning of the embryo (for review, see St Johnston and Nüsslein-Volhard 1992). Restriction of Osk protein to a single location is achieved, in part, by a coordinated program of mRNA localization and translational control (Ephrussi et al. 1991; Kim-Ha et al. 1991, 1995; Markussen et al. 1995; Rongo et al. 1995). Early in oogenesis, when the mRNA appears throughout the oocyte, translation is repressed. Later, when the mRNA becomes localized to the posterior pole of the oocyte, translation is activated. Thus, translational control of osk is elaborate, encompassing both repression and activation and being coupled to the subcellular localization of the mRNA.
As the specific translational control of osk mRNA is essential in normal development (Kim-Ha et al. 1995), we are interested in defining the cis-acting sequences and trans-acting factors involved in this process. We previously identified an ovarian protein, Bruno (Bru), implicated in the translational repression of osk early in oogenesis. Bru binds specifically to sequences, termed Bru response elements, or BREs, found in the 3′ untranslated region (3′ UTR) of osk mRNA. An osk transgene in which point mutations have been introduced into all potential BREs (oskBRE−) produces transcripts that can no longer be bound by Bru in vitro; although these transcripts localize in an apparently normal fashion to the posterior of the oocyte, they are translated prematurely, suggesting that the binding of Bru to a wild-type osk transcript functions to repress osk translation. The premature translation of oskBRE−, occurring well before the message is fully localized, results in the production of Osk protein throughout the cytoplasm of early oocytes and leads to a lethal maternal-effect defect: In an osk mutant background, an oskBRE− transgene causes the formation of bicaudal embryos (embryos in which the anterior structures are replaced by mirror-image duplications of abdominal segments; Kim-Ha et al. 1995).
Here we report the cloning and molecular characterization of Bru, and the identification of previously described mutants in the arrest (aret) locus (Schüpbach and Wieschaus 1991; Castrillon et al. 1993) as mutants in Bru. The mutant phenotype, along with the binding properties of the protein and its pattern of accumulation within the oocyte, indicate that Bru regulates multiple mRNAs involved in gametogenesis and early embryogenesis. Genetic experiments provide further evidence that Bru is involved in the translational repression of osk. Intriguingly, we find that Bru interacts physically with Vasa (Vas), an RNA helicase (Liang et al. 1994) that is a positive regulator of osk translation (Markussen et al. 1995; Rongo et al. 1995). Bru belongs to an evolutionarily conserved family of genes, suggesting that Bru-mediated translational regulation may be widespread.
Results
Isolation of bru using a novel approach to expression cloning
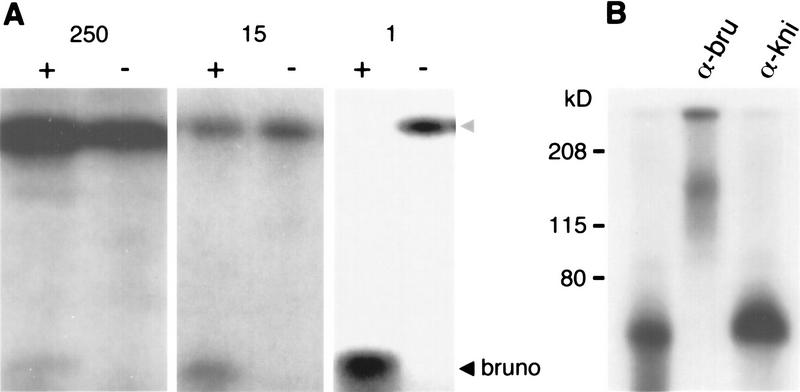
Bru was originally identified in UV cross-linking experiments as an ovarian protein that binds specific sequences (BREs) in the 3′ UTR of osk mRNA (Kim-Ha et al. 1995). Although Bru in solution in an ovarian extract readily binds an RNA probe containing tandemly repeated BREs (BRE+ RNA) (see Fig. 2B; below), a blot of such an extract probed with BRE+ RNA does not show binding (data not shown). Furthermore, we failed to identify any positive clones in a standard screen on nitrocellulose filters of an ovarian cDNA expression λ phage library probed with BRE+ RNA. These results suggest that the immobilization of Bru on nitrocellulose interferes with its ability to bind its target RNA sequence. Consequently, we designed an expression screen based on the binding of Bru to its target sequences in solution. We constructed an ovarian cDNA expression library in a plasmid vector, transformed it into Escherichia coli, and propagated pools of clones as liquid bacterial cultures. Expression of the ovarian proteins was induced, and a cellular lysate of each pool was tested in a UV cross-linking assay for the presence of a protein that would specifically bind BRE+ RNA. In 26 pools representing a total of 6500 clones, 1 pool was found to contain a protein of ∼20 kD with such a binding activity (Fig. 1A, left). This pool was subdivided into less complex pools, and a lysate containing the binding activity was again identified (Fig. 1A, middle). Cultures of individual bacteria from this pool were then assayed (Fig. 1A, right), and the plasmid encoding the binding activity was purified. This clone had an 0.8-kb insert; longer cDNAs were obtained through a standard hybridization screen of an ovarian cDNA library.
Figure 2.
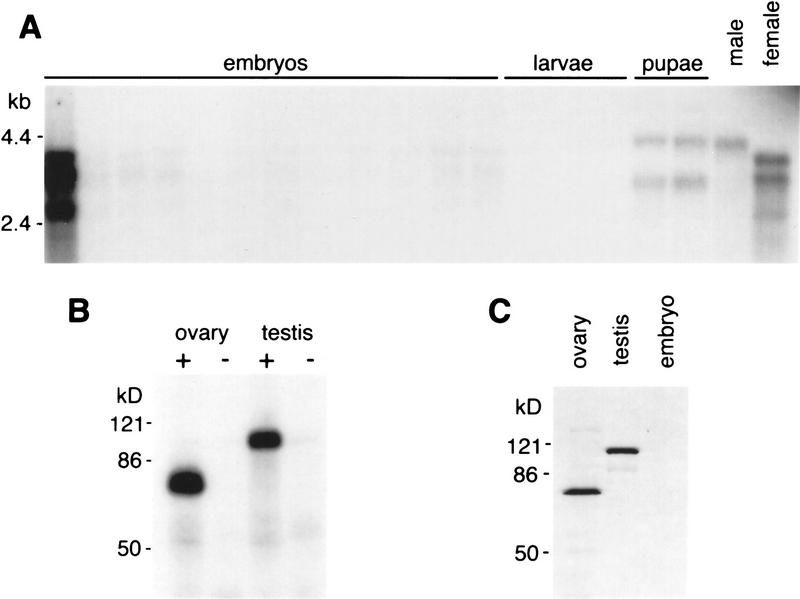
bru encodes sex-specific transcripts and protein isoforms. (A) Northern blot hybridized with a bru cDNA probe. (From left to right) Embryonic RNA at 2-hr intervals from 0 to 24 hr after egg laying (first lane, 0–2 hr; second lane, 2–4 hr; etc.); larval RNA from first, second and third instar; pupal RNA in early and late stages of pupal development; and adult RNA from whole males and females. (B) UV cross-linking assays of Drosophila ovary and testis protein extracts with BRE+ and BRE− probes as in Fig. 1A. (C) Western blot of Drosophila ovary, testis and 0- to 2-hr embryo protein extracts probed with α-BruB antibodies; similar results are obtained with α-BruA antibodies (see Materials and Methods). Both isoforms of Bru migrate more slowly than their predicted size.
Figure 1.
(A) Identification of a cDNA encoding a BRE-binding activity. Bacterial extracts containing pools of cDNA-encoded ovarian proteins were monitored in UV cross-linking assays for binding to radiolabeled RNA probes containing 16 tandemly repeated copies of either wild-type BREs (+) or BREs in which several nucleotides have been scrambled (−). Only the positive pools containing the binding activity are shown. Complexities of each pool are as follows: (left) 250 clones; (middle) 15 clones; (right) 1 clone. The cDNA-encoded fragment of Bru at the bottom of the gel is progressively more abundant as the complexity of the pool drops. The strong band at the top of the gels (shaded arrowhead) is an E. coli protein that binds nonspecifically to both probes; this protein is not able to compete successfully for the wild-type probe when Bru is abundant (right). (B) The isolated cDNA encodes the ovarian protein Bru. Ovary extracts were cross-linked to a BRE+ RNA probe, followed by the addition of antibodies as indicated. The mobility of the Bru–BRE+ cross-linked product (lane 1, ∼70 kD) is shifted in the presence of antibodies raised against the cDNA-encoded protein (α-BruA; see Materials and Methods; lane 2) but not in the presence of antibodies raised against other Drosophila proteins, including Knirps (lane 3), Bicoid, or Exuperantia (data not shown).
As a first step in assessing whether the clone encoded Bru, we tested the binding specificity of the bacterially expressed protein. Sequences resembling the osk BREs are found in the 3′ UTRs of a number of other ovarian transcripts involved in early development; at least one of these, gurken (grk) (Neuman-Silberberg and Schüpbach 1993), is also bound by Bru in vitro (Kim-Ha et al. 1995). Using a variety of probes derived from the osk and grk 3′ UTRs, we found that the bacterially expressed protein shows the same binding specificity as Bru (data not shown). We subsequently raised antibodies to a bacterially expressed protein fragment encoded by the cloned cDNAs (see Materials and Methods) and found that the migration of ovarian Bru in a gel is retarded by the addition of these antibodies (Fig. 1B). This result demonstrates that the clone isolated in our expression screen encodes the Drosophila protein identified biochemically as Bru.
bru has sex-specific isoforms
Northern analysis of RNA from adult flies probed with bru identifies three female-specific transcripts of ∼2.7, 3.3, and 3.7 kb, as well as a single male-specific message of ∼4.0 kb (Fig. 2A, right). These transcripts are present in ovaries and testes, respectively, but are not detectable by Northern analysis in the remaining somatic tissue (data not shown). The three ovarian mRNAs are abundant in ovaries and 0- to 2-hr embryos but extremely reduced or absent during the rest of embryogenesis and the larval stages of the life cycle (Fig. 2A); this expression pattern is typical of messages that are supplied maternally to the embryo but not expressed zygotically. In addition, two pupal transcripts are evident. One of these migrates slightly more slowly than the male-specific message, and one slightly faster than the 3.3-kb female-specific message (note that the pupal RNA samples were prepared from a mixture of both sexes). We do not yet know whether these pupal messages are unique alternative transcripts or whether their altered migration rates reflect poly(A) modifications of the similar adult mRNAs.
The presence of a testis-specific message suggested that Bru protein might be produced in testes as well as ovaries. We therefore assayed testis extracts for the presence of a protein that specifically binds a BRE+ RNA probe. In UV cross-linking experiments we confirmed that testis extracts do contain such a protein, but it is larger than the Bru protein found in ovary extracts (Fig. 2B). Western analysis of ovary and testis extracts reveals two sex-specific proteins that are recognized by anti-Bru antibodies (Fig. 2C) and correspond approximately in size to the two UV cross-linked forms of Bru. Consistent with the lack of Bru-binding activity in early embryo extracts (Kim-Ha et al. 1995), no Bru protein is detectable in 0- to 2-hr embryos (Fig. 2C), despite the abundant transcript seen at this time (Fig. 2A).
bru encodes a conserved RNA-binding protein
Two independent ovarian bru cDNAs that varied at their 5′ ends were sequenced and found to contain identical open reading frames encoding a protein of 604 amino acids (Fig. 3A). [Polyclonal antisera raised against two essentially nonoverlapping fragments of Bru protein that encompass most of the ovarian isoform (see Materials and Methods) both recognize a single predominant protein in ovary extracts (Fig. 2C), suggesting that the multiple ovarian transcripts are likely to encode the same protein.] Sequence analysis of the alternatively spliced male transcript indicates that it encodes a protein of 808 amino acids (Fig. 3A,B). The two predicted forms of Bru are largely similar; only the amino-terminal regions of the isoforms differ. The most notable features in the common portion of the proteins are three ribonucleoprotein-type RNA-binding domains (Bandziulis et al. 1989), two adjacent to each other in the central portion of the protein, and one at the carboxyl terminus (Fig. 3C). The cDNA identified in the original screen expresses only the carboxy-terminal RNA-binding domain along with a short stretch of upstream sequence (Fig. 3C; amino acids 416–604, Fig. 3A), indicating that this region is sufficient for the specific binding of BREs in vitro.
Figure 3.
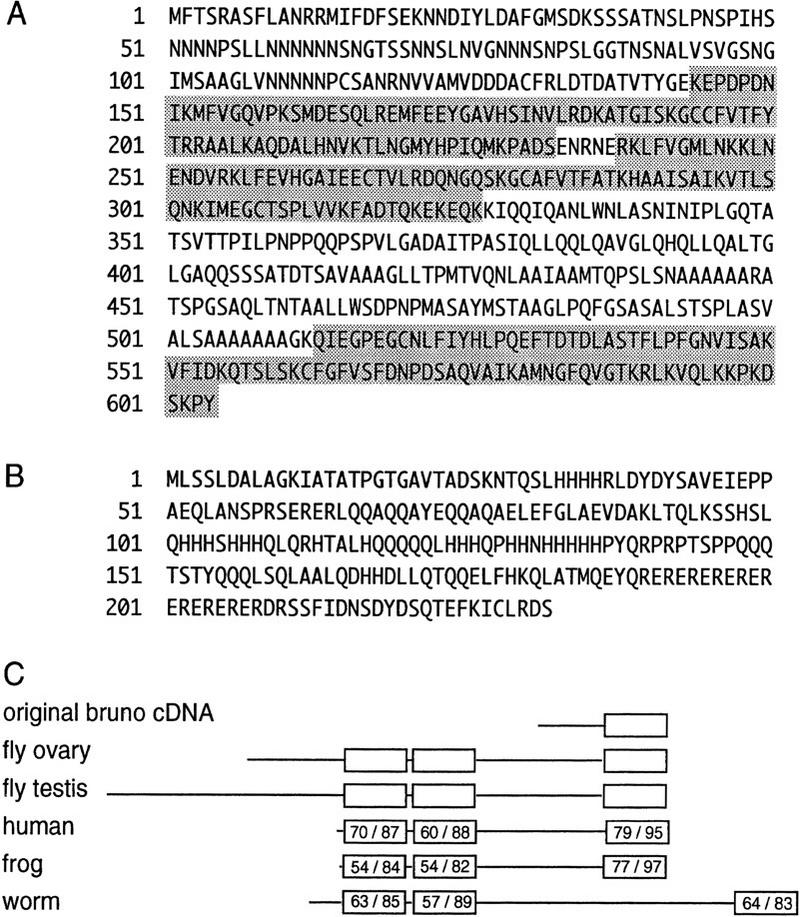
bru encodes two isoforms of an RNA-binding protein conserved in evolution. (A) Deduced peptide sequence of the longest open reading frame in the ovarian bru cDNAs. Predicted RNA-binding domains are shaded. (B) Deduced peptide sequence of the putative amino-terminal domain of testis-specific Bru; the remainder of the protein coincides with residues 29–604 of the ovary-specific form shown in A. (C) Schematic of the protein encoded by the original cDNA and Bru protein isoforms, followed by the amino acid sequence alignment of human RNA-binding protein CUG-BP (GenBank accession no. U63289; Timchenko et al. 1996), Xenopus etr-1 (no. U16800; Knecht et al. 1995), and C. elegans etr-1 (no. U53931; P. Good, pers. comm.) with Bru. With the exception of the fragment encoded by the original cDNA, all sequences represent putative full-length proteins. RNA-binding domains are indicated as boxes, with the percent identity/similarity to the corresponding RNA-binding domains in Bru indicated. There is no striking homology outside of these domains.
Database comparisons reveal that both the relative positioning and the sequence of the three Bru RNA-binding domains are highly conserved in evolution: They are homologous to those of the human CUG–BP (binding protein) gene, as well as the Xenopus and Caenorhabditis elegans etr-1 genes (Fig. 3C). CUG–BP was identified as a protein that binds CUG repeats and may be involved in the human disease myotonic dystrophy (Timchenko et al. 1996); Xenopus etr-1 was isolated as a neural-specific marker (Knecht et al. 1995).
Bru protein colocalizes with osk and grk transcripts in oogenesis
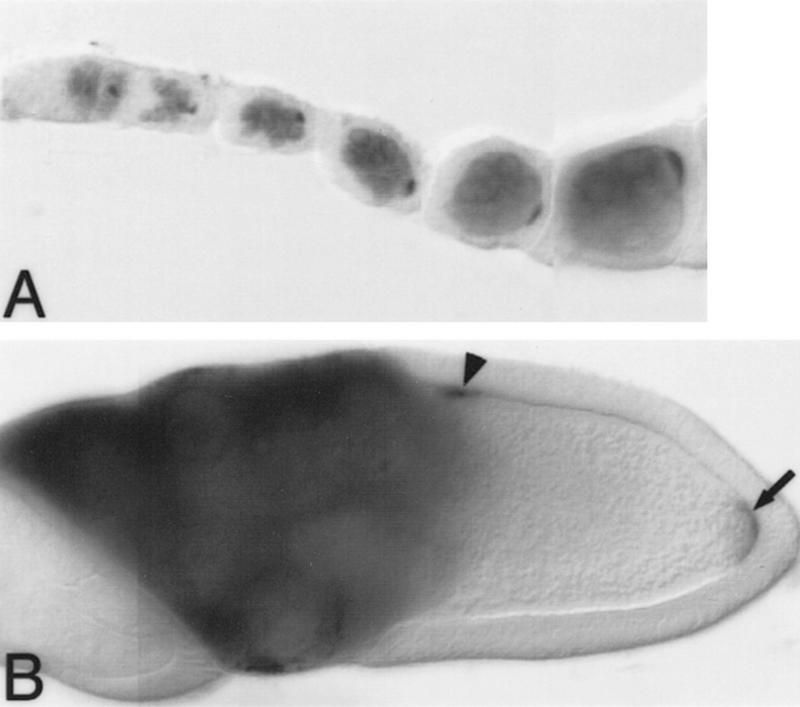
During oogenesis, bru mRNA is first expressed in all of the germ cells in region 2A of the germarium and continues to be found throughout the cytoplasm of both the nurse cells and oocyte as oogenesis progresses (data not shown). Bru protein is also expressed throughout the nurse cells. In contrast, the distribution of Bru protein in the oocyte is highly restricted, showing striking colocalization with osk mRNA: At stages when osk transcripts accumulate in discrete regions of the oocyte, Bru protein is highly concentrated in the same regions. Bru protein first appears in all germ cells in region 2A of the germarium and rapidly becomes concentrated in the presumptive oocyte. Bru quickly resolves as a crescent at the oocyte posterior, following a dynamic pattern similar to that of osk mRNA (Ephrussi et al. 1991; Kim-Ha et al. 1991; Fig. 4A,B), including a transient accumulation at the anterior of the oocyte infrequently detected during stages 7 and 8 of oogenesis. In early embryos, however, although osk mRNA continues to be localized to the posterior pole, Bru protein is no longer detectable in whole-mounted tissue (data not shown), consistent with its absence on a Western blot at this time (Fig. 2C).
Figure 4.
Bru protein colocalizes with osk and grk mRNAs during oogenesis. Distribution of Bru protein in whole-mounted preparations of developing egg chambers. Each egg chamber contains a cluster of nurse cells on the left, and a single oocyte on the right, oriented with the posterior of the oocyte to the right. Bru protein is visualized as a dark stain. (A) Early oogenesis. Bru protein can be seen in all of the germ cells, beginning in stage 2A of the germarium, and is clearly accumulating preferentially in the presumptive oocyte by stage 2B. As with osk mRNA, Bru can be seen as a posterior crescent within the oocyte even in the early stages of oogenesis. Note that Bru protein is also maintained throughout the nurse cells, the site of osk mRNA synthesis, as oogenesis progresses. (B) Stage 10 egg chamber. As with osk mRNA (Ephrussi et al. 1991; Kim-Ha et al. 1991), the posterior localization of Bru in the oocyte becomes more striking in later egg chambers (arrow). Additionally, in stage 10 egg chambers Bru mimics the anterodorsal pattern of grk mRNA localization over the oocyte nucleus (arrowhead; Neuman-Silberberg and Schüpbach 1993).
bru protein is also localized to a distinct anterodorsal zone in stage 10 oocytes, a region where osk mRNA does not appear (Fig. 4B). This localization is intriguing, as it coincides with the position of grk mRNA (Neuman-Silberberg and Schüpbach 1993). Although Bru protein binds in vitro to grk mRNA, the significance of this interaction is unknown.
aret mutants have molecular lesions in bru
We mapped bru by in situ hybridization to region 33D on the left arm of the second chromosome. The coding region of an ovarian bru cDNA was subsequently mapped to genomic P1 clones from the region, and the intron/exon junctions determined by a combination of Southern hybridizations and DNA sequencing. The coding sequence of the female transcripts is contained in nine exons spread across ∼40 kb of genomic DNA; eight of these exons are common to both the male and female messages.
A survey of mutants that had previously been mapped to 33D revealed one particularly interesting candidate, the aret locus, which is required for fertility in both sexes (Schüpbach and Wieschaus 1991; Castrillon et al. 1993). To ask whether bru and aret are the same gene, we used flanking intron sequences to design primers for PCR amplification of the female bru coding exons from genomic DNA. We amplified and sequenced the nine exons from three ethylmethane sulfonate (EMS)-induced alleles of aret: aretPD41, aretPA62, and aretQB72 (Schüpbach and Wieschaus 1991). These aret mutants do contain alterations of the bru coding sequence; two have missense mutations in the first RNA-binding domain, and the third has a nonsense mutation that is predicted to truncate the protein following the first two RNA-binding domains (Fig. 5A). As all three alleles were generated in the same screen (Schüpbach and Wieschaus 1991), comparison of the sequences to each other ruled out that the observed changes were strain-specific polymorphisms. Based on these data, we conclude that the aret locus encodes Bru.
Figure 5.
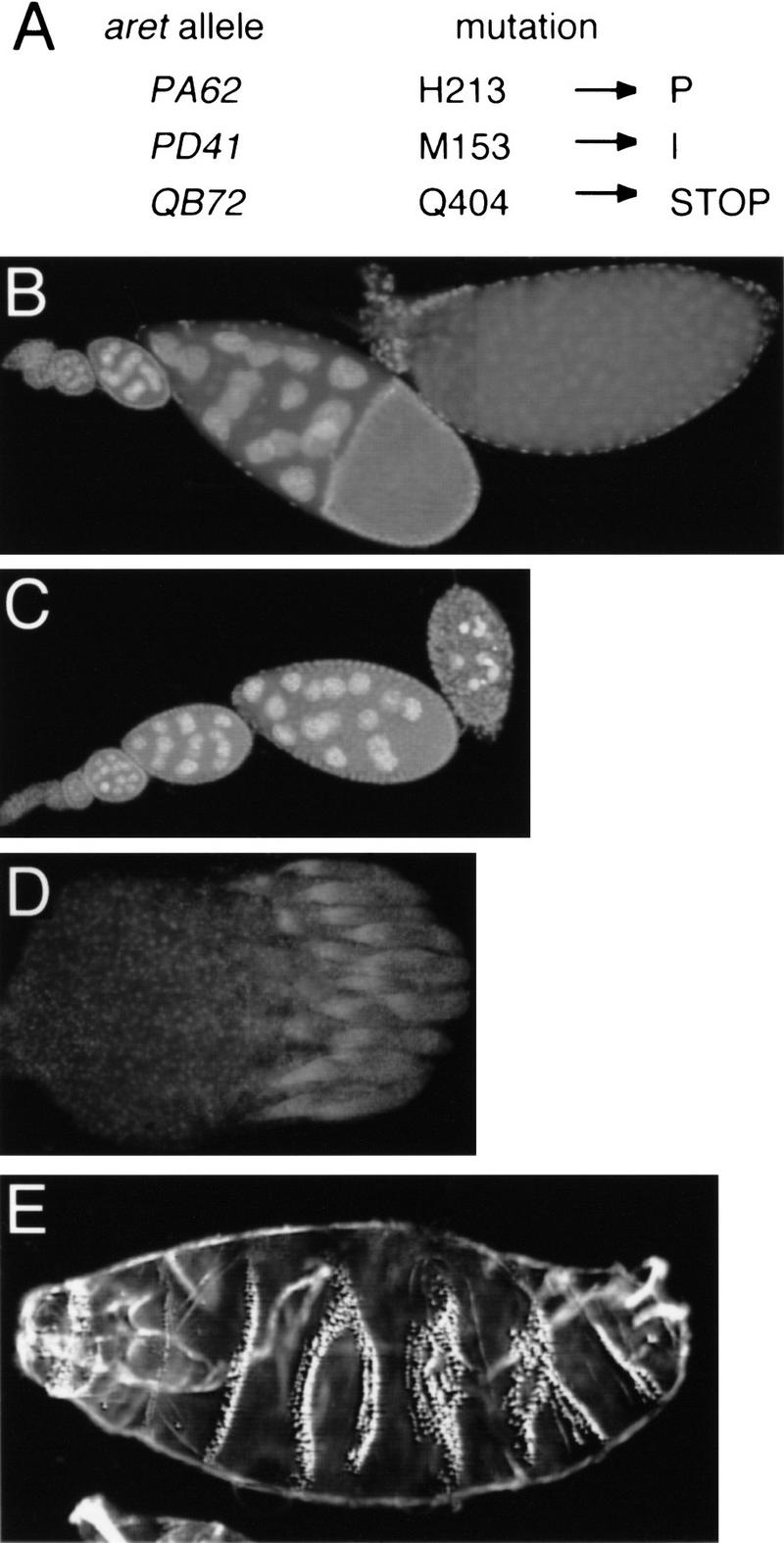
(A) Mutations in aret alleles. Amino acid positions refer to Fig. 3A. (B–D) Whole-mounted tissue stained with DAPI. (B) Wild-type ovariole composed of a string of progressively developing egg chambers, with the oldest egg chamber on the right. (C) Ovariole from an aretPA62/Df(2L)esc-P3-0 female. The progression of oogenesis appears normal until approximately stage 9, when the egg chambers deteriorate. No late-stage eggs are formed or laid. aretPD41/Df(2L)esc-P3-0 females have a similar phenotype. (D) A complete ovary, consisting of 15–20 ovarioles, from an aretQB72/Df(2L)esc-P3-0 female. All ovarioles are arrested in germarial stages. (E) Larval cuticle of an embryo from an aretPA62/aretPD41 mother showing complex defects in the pattern of segmentation (cf. Fig. 6A, wild-type cuticle). The severity of this phenotype is variable; the defects do not resemble the mutant phenotypes resulting from either over- or underexpression of osk.
Bru has multiple roles, including translational repression of osk mRNA
The aret mutants allow for a genetic analysis of Bru function. Prior work has demonstrated that mutations in aret can affect gametogenesis in males and females, establishing roles for Bru in both spermatogenesis and oogenesis. Here we focus on the function of Bru in the ovary; thus far, we have limited our analysis to the three sequenced alleles of aret, as knowledge of the molecular defect can be useful in the interpretation of phenotypes.
aret alleles were tested as hemizygotes for ovarian phenotypes. In females hemizygous for either aretPA62 or aretPD41, alleles encoding missense mutations that alter the first of the three RNA-binding domains, oogenesis appears to proceed normally until approximately stage 9, at which time the egg chambers degenerate (Fig. 5C). In females hemizygous for aretQB72, which carries a nonsense codon upstream of the third RNA-binding domain, oogenesis is arrested at an extremely early stage (Fig. 5D). Very few germ cells appear to be present in these ovaries, and osk transcript is not detectable by in situ hybridization or by RT–PCR (data not shown). aretQB72 is among the most severe of the existing aret alleles. Our observations are essentially the same as those of Schüpbach and Wieschaus (1991) concerning females homozygous for various aret alleles, and support the conclusion that aret is required at an early step in oogenesis.
We also examined aretPA62/aretPD41 transheterozygotes and found that these females did complete oogenesis and lay eggs, some of which hatched into viable larvae. However, the majority of the embryos from these mothers displayed variable and complex cuticle defects involving partial or complete fusion of adjacent segments (Fig. 5E).
A major goal of our analysis has been to evaluate the conclusion, based on biochemical data, that Bru acts as a repressor of osk mRNA translation. In previous experiments, osk transcripts with mutated BREs that are not bound by Bru in vitro were found to be translated precociously in the oocyte during stages 5–7 of oogenesis, whereas wild-type osk mRNA is translated only after localization to the posterior pole of the oocyte in stage 9 (Kim-Ha et al. 1995). Because strong aret alleles, including aretQB72, arrest oogenesis well before stage 5 (Schüpbach and Wieschaus 1991) and may not even form an oocyte or express osk mRNA, we were unable to use such mutants to confirm the role of Bru. A common strategy to surmount such a difficulty involves the use of partial loss-of-function mutations: In this particular case, weaker aret alleles might still be strong enough to disrupt osk translational regulation but would allow oogenesis to proceed to a point where a role in osk translation could be tested. However, some of our data raise substantial concerns about using this approach with the existing aret alleles. In particular, the molecular analysis of two weak aret alleles, aretPA62 and aretPD41, demonstrates that both have mutations in the first of the three RNA-binding domains. This domain is not required for binding to BREs, as the original partial Bru cDNA isolated in our expression screen encoded a carboxy-terminal portion of Bru containing only the third of the three RNA-binding domains (see Fig. 3C). Taken together, these data predict that the mutant Bru proteins encoded by aretPA62 and aretPD41 should bind osk mRNA and therefore may not have any defect in osk regulation. Antibody staining of whole-mounted ovaries hemizygous for aretPA62 or aretPD41 supports this prediction; these ovaries do not show precocious expression of Osk protein during early stages of oogenesis (data not shown). In addition, embryos from aretPA62/aretPD41 transheterozygotes do not obviously display any of the head defects that result from ectopic Osk expression (Fig 5E).
As an alternate approach, we used flies sensitized to changes in the level of Osk protein and determined the consequences of reducing wild-type Bru protein levels. If Bru does act to repress osk translation, a reduction in Bru protein might lead to a partial derepression of translation and, subsequently, to elevated osk activity. For this analysis we used the P[A7] transgene, which encodes a form of osk mRNA that retains BRE sequences but is mislocalized to the anterior of the oocyte (see Materials and Methods). Flies bearing P[A7] produce embryos with modest head defects caused by the misexpressed osk (Fig. 6A,B). These limited anterior defects arise from a low level of mislocalized osk; this phenotype should be very sensitive to changes in the amount of osk activity. To reduce levels of wild-type Bru protein, we made the P[A7] flies heterozygous for aretQB72. Now the P[A7] phenotype is substantially enhanced; the embryos from these mothers display extensive anterior deletions, often accompanied by duplication of posterior pattern elements (Fig. 6C). Two independent P[A7] insertion lines were tested with consistent results. A similar result is obtained using P[A7] flies heterozygous for Df(2L)esc-P3-0, a deficiency that deletes aret. In contrast, P[A7] flies heterozygous for either of two different aret+ second chromosomes (kelchRF and spireRP; these chromosomes also serve as a general control for nonspecific effects on the P[A7] phenotype in flies heterozygous for recessive maternal-effect mutations involved in oogenesis or early body patterning) retained the P[A7] phenotype. As a further control we used flies carrying the P[A6] transgene, which encodes a mislocalized osk mRNA lacking BRE-containing 3′ UTR sequences (see Materials and Methods). Although the P[A6] and P[A7] phenotypes are similar, the P[A6] phenotype is not enhanced in aret− heterozygotes. Our results show that lowering wild-type bru dosage increases the amount of osk activity, supporting the identification of Bru as a translational repressor of osk mRNA.
Figure 6.
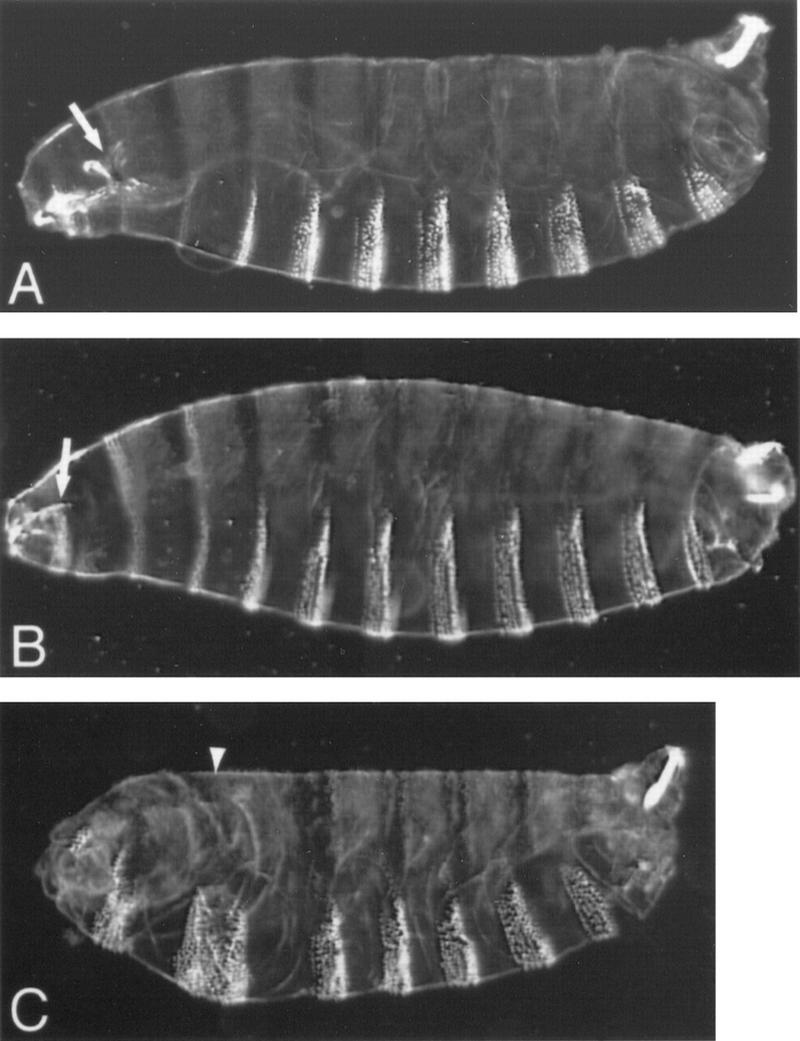
Bru regulates osk activity (A–C) Larval cuticles; anterior is to the left. (A) Wild type; the arrow indicates cephalopharyngeal skeleton. (B) Embryo from a mother carrying transgene P[A7]; anterior expression of transgenic Osk causes defects in the head structures, which can be seen here as a reduction in the cephalopharyngeal skeleton (arrow). (C) Embryo from a mother carrying P[A7] and additionally heterozygous for aretQB72; the P[A7] phenotype is enhanced such that head structures are deleted and replaced with a mirror-image duplication of several abdominal segments. The arrowhead marks the axis of mirror-image duplication. Note that embryos from mothers heterozygous for aretQB72 alone do not show a mutant phenotype (data not shown).
Bru interacts with Vas
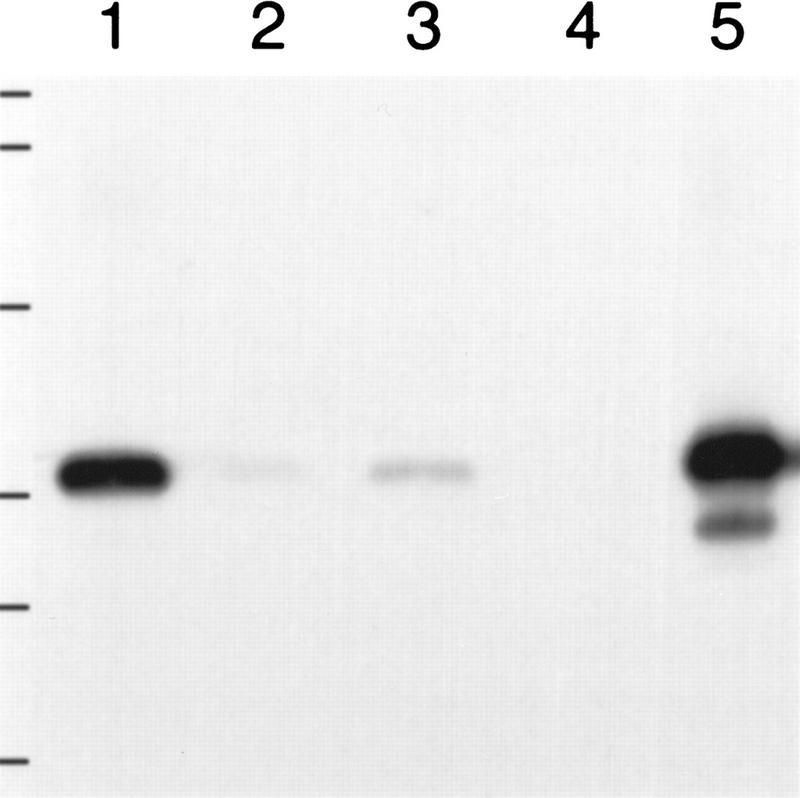
Repression of osk translation by Bru is alleviated upon localization of osk mRNA to the posterior pole of the oocyte (Kim-Ha et al. 1995; Markussen et al. 1995; Rongo et al. 1995; Wilson et al. 1996). The mechanism of this process is unknown; however, it seems likely that the RNA helicase Vas (Liang et al. 1994) is involved, as it is localized to the posterior pole of the oocyte (Hay et al. 1990; Lasko and Ashburner 1990) and is required for efficient activation of osk translation (Markussen et al. 1995; Rongo et al. 1995). Interestingly, we independently isolated a clone encoding a fragment of Bru (amino acids 1–417; Fig. 3A) in a far Western screen of a Drosophila cDNA expression library with radiolabeled Vas protein (see Materials and Methods). Using affinity chromatography, we have confirmed that Bru interacts physically with Vas in vitro (Fig. 7).
Figure 7.
Bru and Vas proteins interact in vitro. Western blot probed with α-BruA antiserum. (Lanes 1,3) Eluate from glutathione–Sepharose 4B beads bound by GST–Vas, challenged with lysate from bacteria expressing a fragment of Bru (BruA; see Materials and Methods), and eluted with SDS sample buffer (lane 1) or factor Xa (lane 3). (Lanes 2,4) Similar beads bound by GST alone, challenged with the BruA lysate, and eluted with SDS sample buffer (lane 2) or factor Xa (lane 4). (Lane 5) Crude BruA-expressing bacterial lysate. Positions of molecular mass markers are indicated at left and correspond to sizes of 138, 86.8, 47.8, 33.3, 28.6 and 20.7 kD.
Discussion
The Osk protein is specifically deployed at the posterior pole of the Drosophila oocyte, where it directs posterior patterning of the egg and embryo. If Osk protein is expressed in other regions of the oocyte, it directs ectopic posterior development in the embryo, which is lethal (Ephrussi and Lehmann 1992; Smith et al. 1992). Proper deployment of Osk protein involves at least three mechanisms: prelocalization of osk mRNA (Ephrussi et al. 1991; Kim-Ha et al. 1991), translational control to ensure that only localized osk mRNA is active (Kim-Ha et al. 1995; Markussen et al. 1995; Rongo et al. 1995; Wilson et al. 1996), and anchoring of Osk protein to the posterior cortex of the oocyte (Webster et al. 1994). The initial demonstration of translational control of osk came from the analysis of a protein, Bru, that binds to specific sequences, BREs, present in multiple copies in the osk mRNA 3′ UTR. When mutated, these sites are no longer bound by Bru and premature translation of unlocalized Osk transcripts ensues (Kim-Ha et al. 1995). The work presented here extends the analysis of Bru at the molecular level, through the cloning of cDNAs encoding Bru, and at the genetic level, through the demonstration that aret alleles have mutations in the bru gene. Our results further support the identification of Bru as a translational repressor of osk mRNA and indicate that Bru regulates not only osk but also other transcripts involved in gametogenesis and early development. We speculate that Bru-mediated regulation of transcripts other than Osk will also involve translational control.
Specific translational control of gene expression appears to be widespread, particularly for maternal mRNAs and mRNAs acting in gametogenesis. However, extensive genetic screens for maternal-effect and female-sterile mutants did not immediately focus attention on this critical form of post-transcriptional control in Drosophila; the prevalence and importance of translational regulation has instead emerged primarily from the analysis of specific mRNAs and their patterns of protein expression. A current challenge is to characterize the translational regulatory factors that bind to these mRNAs; in the long term, however, it will be important to determine how to apply genetic techniques to identify other factors involved in translational control. The results we have obtained in characterizing Bru suggest potential approaches to both the molecular and the genetic analysis of translational regulation.
Expression cloning of RNA-binding proteins
Much of the current work on translational regulation of specific mRNAs focuses on cis-acting regulatory elements and the factors that bind them (Curtis et al. 1995). Isolation of these factors is a prerequisite for detailed studies of their function. Although many DNA-binding proteins and a number of double-stranded RNA-binding proteins (e.g., Bass et al. 1994; Lee et al. 1996) have been isolated by expression screening methods in which cloned proteins are bound to a solid substrate (Vinson et al. 1988), very few proteins that bind other RNA structures have been cloned successfully in this way. We were able to isolate Bru by developing an expression cloning method that relies on a solution-based biochemical assay (Webster and Macdonald 1997). The method described here can be used in combination with any solution-based assay, including gel shift and UV cross-linking. This protocol provides an alternate approach to expression screening that may prove to be of general use in isolating RNA-binding proteins.
The function of Bru
The function originally ascribed to Bru was that of a translational repressor of osk mRNA. Bru was shown to bind specifically to multiple sites in the osk 3′ UTR, and a multiply mutated form of the mRNA unable to bind Bru in vitro was translationally derepressed in vivo (Kim-Ha et al. 1995). These results strongly implicated Bru as the translational repressor; nevertheless, it remained possible that a protein that was not detected by our UV cross-linking assays also bound specifically to the same region and was the true repressor of osk translation. Our genetic data now lend further support to the conclusion that Bru is the translational repressor. Eliminating one copy of the wild-type aret/bru gene, and thereby reducing the amount of wild-type Bru protein, leads to a subtle but significant increase in the level of osk activity. We presume that this increase results from a modest derepression of osk translation.
How does Bru act to repress translation? It seems likely that although Bru binds osk mRNA downstream of the protein coding region, it affects either the initiation or the progression of translation. Here we will consider two general models for the mechanism of Bru-mediated repression. The first model involves the osk poly(A) tail. Changes in poly(A) tail length are associated with changes in translation: In general, mRNAs with long tails (or tails being actively extended) are efficiently translated, whereas mRNAs with short tails are not (for review, see Sachs and Wahle 1993). In this model Bru would either direct deadenylation or repress extension of the osk poly(A) tail, with a subsequent readenylation or activation event being required for translation of osk at the posterior. Two lines of evidence provide hints consistent with such a scheme. First, a 17-nucleotide element in the 3′ UTR of the Xenopus Eg2 maternal mRNA, which is required for deadenylation of the message (Bouvet et al. 1994), contains a consensus BRE sequence, UUAUAUGU. Two factors bind specifically to this element (Bouvet et al. 1994); both are similar in size to the Xenopus homolog of Bru, Etr-1 (Knecht et al. 1995). Given the similarities in binding site and size, one of these factors could be Etr-1, which would suggest that Bru could also be involved in deadenylation. Second, the Drosophila Orb protein, which is required for localization and translation of osk mRNA (Christerson and McKearin 1994; Lantz et al. 1994; Kim-Ha et al. 1995; Markussen et al. 1995), is the homolog of the Xenopus CPEB protein, which acts in cytoplasmic polyadenylation of certain maternal mRNAs (Hake and Richter 1994). If Orb activates osk translation by polyadenylation, a prior deadenylation step might be required.
This model predicts that an oskBRE− message, which is aberrantly translated early in oogenesis, will have a longer poly(A) tail than a wild-type osk transcript before stage 8 of oogenesis, when wild-type osk is first translated. However, experiments examining the poly(A) tail length of osk and oskBRE− messages in RNA prepared from young ovaries (in which the egg chambers had only developed as far as stage 7) show no obvious difference in the tail lengths of these messages (P.J. Webster and P.M. Macdonald, unpubl.); in addition, no difference is seen in the tail length of wild-type osk mRNA from young ovaries and ovaries that are enriched for late-stage egg chambers. In all cases, the tail length is heterogeneous, ranging from only a few adenine residues to ∼50, and no apparent change in the distribution within this range is seen in comparing either the osk and oskBRE− messages in the young egg chambers or the wild-type osk message before and during the time of active translation. For wild-type osk, it is possible that only a small fraction of the total pool of message is being actively polyadenylated and translated at any one time, and that we cannot visualize this small amount. Nevertheless, the oskBRE− data are inconsistent with a model in which the binding of Bru substantially influences polyadenylation, although we cannot rule out that a small change in the number of adenine residues below the resolution of our assay (<10 nucleotides) is occurring.
A second model for the mechanism of Bru function hypothesizes an interaction between the 3′ and 5′ ends of the osk message. It has been shown recently in yeast that a poly(A)-binding protein(PABP)/poly(A) tail complex can interact with the cap structure of an mRNA via the translation initiation factor eIF4G, supporting the idea that translational initiation may be positively influenced by contact between the 3′ and 5′ ends of a polyadenylated, capped message (Tarun and Sachs 1996). It is possible that Bru interferes with this interaction, potentially by disrupting either the binding of PABP to the osk poly(A) tail or the binding of eIF4G to the PABP/poly(A) tail complex, and thus represses efficient initiation of Osk translation. We do not currently have experimental support for or against this model.
Finally, what does the in vitro interaction between Bru and Vas proteins signify? The fact that in vivo both colocalize with osk mRNA to the posterior of the oocyte and that both act in the translation of osk mRNA, Bru in repression and Vas in activation, suggests that their interaction may be functionally significant. However, the requirement for Vas in osk translation is independent of the osk 3′ UTR (Rongo et al. 1995), and therefore of the BREs, suggesting that Vas does not activate osk translation simply by relieving Bru-mediated repression. There are a number of other potential explanations for the Bru/Vas interaction that have not been investigated yet. For instance, as Vas is related to the translation initiation factor eIF4A, it may activate translation through interaction with the osk 5′ UTR. An interaction between Vas and Bru bound to the osk 3′ UTR might restrict the ability of Vas to associate with the 5′ UTR until after the osk transcript is appropriately localized.
Further characterization of the mode of Bru action would be facilitated by reconstitution of osk translational repression in a simplified system. Attempts to establish Bru-dependent repression in transfected Drosophila Schneider cells have not been successful (P.J. Webster and P.M. Macdonald, unpubl.), suggesting that ovarian factors other than Bru are required.
Bru regulates multiple mRNAs
The aret phenotype indicates that osk cannot be the only target of Bru regulation, as osk is not required for the early stages of oogenesis (Lehmann and Nüsslein-Volhard 1986) and the misregulation of osk by mutation of the BREs does not cause early defects in gametogenesis (Kim-Ha et al. 1995). In addition, although osk is expressed only in females, many aret alleles are also male-sterile because of reduced numbers of sperm bundles and a lack of motile sperm (Schüpbach and Wieschaus 1991; Castrillon et al. 1993). Finally, the disrupted segmentation seen in embryos from aretPA62/aretPD41 transheterozygotes is not a phenotype that can be attributed to the misregulation of osk. Thus, Bru has multiple roles in development; given its role in repression of osk mRNA translation, we expect that Bru regulates the translation of multiple transcripts.
It may be possible to identify other regulated transcripts by the presence of BRE sequences. One such candidate is the grk mRNA. UV cross-linking experiments have shown that Bru binds to the grk 3′ UTR, which contains at least one and possibly several BREs (Kim-Ha et al. 1995); we report here that Bru colocalizes with grk mRNA in vivo. Expression of the Grk protein occurs throughout the early stages of oogenesis (Roth et al. 1995); thus, if Bru does regulate grk mRNA, it does not appear to restrict translation as tightly as for osk. Nevertheless, Bru could influence the level of grk translation more subtly, a role consistent with the differing concentrations of BREs in the two mRNAs: osk has many copies; grk has few.
Some targets of Bru action may not have BREs. Our expression cloning of bru revealed that a portion of the protein containing only one of the three RNA-binding domains retained the property of binding specifically to BREs. Whether the other RNA-binding domains have similar or different binding specificities remains to be established. However, if they can bind to other sequences, Bru could also regulate mRNAs lacking BREs.
We have established that Bru acts at multiple stages of oogenesis, leading to a complex mutant phenotype that masks its role in the control of osk expression. This observation explains why the function of Bru in anteroposterior body patterning was not revealed by the isolation and characterization of a large number of maternal-effect mutants affecting embryonic body pattern (Tearle and Nüsslein-Volhard 1987; Schüpbach and Wieschaus 1989, 1991), a strategy that has been extremely informative about other aspects of pattern formation. Given the pleiotropic phenotypes resulting from lack of Bru, we expect that other genes acting together with Bru, or in similar yet distinct processes of translational control, may also have phenotypes that obscure their role in the regulation of a particular gene. The preferred approaches for future genetic analysis of translational control may therefore involve screens that do not require mutants to be examined as homozygotes. A number of such screens have been described recently; one has identified aubergine, a gene initially defined by female-sterile mutations, as an activator of osk translation (Wilson et al. 1996). Collections of Drosophila mutants with early defects in gametogenesis (Tearle and Nüsslein-Volhard 1987; Schüpbach and Wieschaus 1991; Castrillon et al. 1993) may prove to be rich sources of translational control factors.
The evolutionary conservation of bru indicates that it has a function of general importance in a variety of organisms. Our analysis of bru suggests that this function may involve the translational control of specific genes; such control is likely to be important in the biology of higher eukaryotes in a broad range of situations that call for the precise expression of particular proteins.
Materials and methods
Standard protocols were used for all nucleic acid and protein manipulations unless otherwise noted.
Library construction and expression screening
A detailed protocol for the procedure used to clone Bru can be found in Webster and Macdonald (1997). Poly(A)-primed ovarian cDNAs were cloned into the Lambda ZAP vector (Stratagene) in an oriented fashion such that one-third of the clones would be predicted to form in-frame protein fusions with upstream lacZ sequences. The λ library was converted into a plasmid library by in vivo excision following Stratagene’s protocols and transformed into Escherichia coli strain SOLR (Stratagene). For screening, the bacterial culture was titered, and aliquots containing 250 bacteria were grown to saturation in 2 ml of rich liquid medium. Protein expression was induced with 10 mm IPTG. The amount of 0.5 ml of each culture was stored at 4°C; the remaining 1.5 ml was used to prepare protein extracts by pelleting the bacteria and incubating the pellet on ice for 15 min in 30 μl of 150 mm NaCl, 50 mm Tris-Cl (pH 8.0), 1 mm EDTA, 1% NP-40, 1 mm DTT, 1 mm PMSF, and 2 mg/ml of freshly prepared lysozyme. The lysate was centrifuged for 10 min at 4°C, and the supernatant diluted 1:1 with ice-cold 40% glycerol and either assayed immediately or stored at −70°C. Each extract was tested in a UV cross-linking assay (see below); once a positive pool was identified, the stored culture from that pool was titered and used to inoculate cultures of less complex pools (∼15 bacteria each), and the entire procedure was repeated. For the final round, a positive pool of 15 was plated out, and individual colonies were picked, grown in liquid cultures, and assayed as above.
Far Western screening
To generate radiolabeled Vas protein, bacteria expressing a glutathione S-transferase (GST)–Vas fusion protein (Liang et al. 1994) were grown to log phase in 100 ml of M9 minimal medium, pelleted, and resuspended in M9 minimal medium lacking sulfate, but supplemented with 5 mCi of [35S]sodium sulfate and 0.1 mm IPTG. Cells were labeled at 37°C for 3 hr, and pelleted, lysed, and sonicated as described in Liang et al. (1994). GST–Vas protein was purified by glutathione–Sepharose column chromatography, and the GST sequences were removed by cleavage with factor Xa.
A D. melanogaster ovarian cDNA expression library in λgt22 (a gift from P. Tolias, Public Health Research Institute, New York, NY) was plated and induced with IPTG. Plaques were transferred onto GeneScreen Plus (NEN), and the membranes were then treated to the following incubations, all at 4°C: hydration in buffer 1 (25 mm HEPES–KOH (pH 7.7), 25 mm NaCl, 5 mm MgCl2, 1 mm DTT), denaturation for 2 × 10 min in 6 m guanidine in buffer 1, neutralization in decreasing concentrations of guanidine (10 min each in 3 m, 1.5 m, 0.75 m, 0.38 m, 0.19 m guanidine in buffer 1), and finally 2 × 10 min in buffer 1. Filters were then blocked in 5% dry milk and 0.05% NP-40 in buffer 1 for 1 hr, and then in 1% dry milk and 0.05% NP-40 in buffer 1 for 30 min. Filters were probed with 100 μg of radiolabeled Vas protein per five to eight 137-mm circular filters in ∼25 ml of binding buffer [20 mm HEPES-KOH (pH 7.7), 2.5 mm MgCl2, 1 mm DTT, 0.05% NP-40, 1% dry milk] overnight at 4°C. Membranes were washed 3 × 10 min in binding buffer, air-dried, and exposed to film. Positive plaques were purified and rescreened as above.
UV cross-linking and supershift assay
Radiolabeled RNA probes were made by in vitro transcription of cloned templates consisting of eight tandem copies of the sequences GATCCAATGTATGTTAATTGTATGTATTA [+ probe, containing a fragment of the Osk 3′ UTR corresponding to bases 3475–3498 of Kim-Ha et al. (1991)]. The BREs are underlined (note that the second underlining includes two potential overlapping BREs), and GATCCAATaTgaGTTAATTtgAgt- TATTA (− probe; mutated nucleotides shown in lowercase) and probe concentration adjusted to 1 × 106 cpm per μl. Protein extracts were prepared from hand-dissected ovaries and testes by homogenization in ice-cold 150 mm NaCl, 50 mm Tris-Cl (pH 8.0), 1% NP-40, and 1 mm PMSF, followed by centrifugation at 4°C for 10 min. An equal volume of ice-cold 40% glycerol was added to the supernatant, and the extract was stored at −70°C.
For the UV cross-linking assay, a reaction mix containing 5 μl protein extract from either flies or bacteria, 1 μl of 10× binding buffer (20 mm MgCl2, 60 mm HEPES at pH 7.9, 300 mm KCl), 1 μl of 10 mg/ml yeast tRNA, 1 μl of 10 mg/ml heparin, and 2 μl of water was incubated at room temperature for 5 min, followed by addition of 1 × 106 cpm probe and further incubation for 5 min. Samples were irradiated on ice with 1 J of UV light in a Stratagene UV cross linker. Excess probe was digested by the addition of 30 μg of RNase A at room temperature for 15 min. Following the addition of 2× protein sample loading buffer, samples were heated to 95°C and resolved on 10% gels by SDS-PAGE followed by autoradiography. The UV cross-linking/supershift assay contained the following modifications: after the cross-linked products were RNased, 1 μl of rabbit polyclonal serum (α-BruA, α-Knirps, α-Bicoid or α-Exuperantia) was added to each sample and incubated at room temperature for 10 min. The samples were adjusted to a final concentration of 125 mm Tris-Cl (pH 6.8), 0.1% SDS, and 10% glycerol, loaded directly onto an SDS–polyacrylamide denaturing gel, and the gel maintained at room temperature by electrophoresis at low voltage.
Nucleic acid analysis
cDNAs were isolated from ovary libraries (our own and a gift of L. Kalfayan) and a testis library (gift of T. Hazelrigg; Hazelrigg and Tu 1994) by hybridization with the original bru clone under standard conditions, and were sequenced on both strands with Sequenase (U.S. Biochemical) using the dideoxy method. bru cDNA sequences have been submited to GenBank under accession numbers U58976 (female) and U73846 (male). Sequence manipulation was performed using the GCG and BLAST programs. For Northern blot analysis, 20 μg of total RNA was loaded per lane, and quantitation of the samples was verified after transfer to nitrocellulose by hybridization with rp49 (O’Connell and Rosbash 1984).
An ovarian bru cDNA was mapped to genomic P1 clones DSO7537 and DSO8114 (Berkeley Drosophila Genome Project, distributed by M. Scott, Stanford University, CA) by Southern hybridization. Restriction fragments of the P1s were subcloned, and oligonucleotide primers were used to sequence the bru coding regions of the genomic DNA and identify exon boundaries. The sequence of an additional 50–300 nucleotides was determined at the boundaries for each intron, and intronic primers were designed for PCR of the nine exons encompassing the coding region of the female transcript; sequences of these primer pairs as well as an approximate genomic map of the region can be found on our web site (http://www-leland.stanford.edu/~pmac/new/pwprimers.html) and are also available upon request. Exons were amplified from genomic DNA of aret/Df(2L)esc-P3-0 flies, and the PCR products sequenced directly with Sequenase (U.S. Biochemical) using the dideoxy method.
Antibodies and protein analysis
Polyclonal antibodies were raised in rabbits (α-BruA) and rats (α-BruB) to bacterially expressed Bru protein fragments containing amino acids 141–417 (Fig. 3A; α-BruA) or 416–604 (Fig. 3A; α-BruB). For Western blot analysis, ovaries and testes from well-fed 4- to 5-day-old w1118 flies were dissected and homogenized in 2× protein sample buffer plus 1 mm PMSF. w1118 embryos were collected 0–2 hr after egg laying and dechorionated in bleach for 1 min, washed with 0.1% Triton X-100, and homogenized as above. Samples were heated to 100°C for 5 min, and the proteins were separated by SDS-PAGE on 10% gels. Staining with Coomassie blue was used for gross quantitation of protein samples, and approximately equal amounts were loaded in each lane for Western blots. Bru proteins were detected on Western blots using α-BruA antiserum at a dilution of 1:1000 or α-BruB at a dilution of 1:10,000.
Histochemical staining
Ovaries were fixed for 20 min in 200 μl PP (4% paraformaldehyde in PBS), 0.2% Tween 20, 20 μl of DMSO, and 600 μl of heptane, then washed in five changes of PBS + 0.2% Tween 20 (PBST). Ovaries were treated for 3–5 min in PBST supplemented with 50 μg/ml of proteinase K, followed by incubation for 2 × 1 min in 0.2% glycine in PBST. (Proteinase K digestion was found to be essential for visualizing posteriorly localized Bru.) Ovaries were postfixed for 20 min in PP. Washing was as above, blocking was in four changes (1 hr each) of PBST + 0.2% Triton X-100 + 1% BSA (PBSBT) + 2% normal goat serum. α-BruA antiserum was used at a 1:1000 or 1:2000 dilution in PBSBT and incubated overnight, washes (six changes in 1 hr total) were in PBSBT, incubation with secondary antibody (biotinylated goat anti-rabbit IgG; Vectastain; 1:400 dilution; preadsorbed with ovaries) was in PBSBT overnight at 4°C followed by 2 hr at room temperature. Final washes were in PBST, and staining was detected as described (Lasko and Ashburner 1990). All manipulations were at room temperature, except for the secondary antibody incubation. For DAPI staining, ovaries were fixed in PP for 15 min, washed in PBST, stained in 1 μg/ml of DAPI in PBST for 5 min, and washed again in PBST.
Transgenic flies
Transgene P[A7] (A.N. Harris and P.M. Macdonald, unpubl.) contains the osk promoter and complete coding region and the first 535 bases of the osk 3′ UTR (a region containing multiple BRE consensus sequences), followed by the region of the bicoid 3′ UTR containing anterior localization signals (Macdonald and Struhl 1988). Transgene P[A6] is identical to P[A7] except for the replacement of the 535 bases of osk 3′ UTR with a 357-base region of the osk 3′ UTR that does not contain any BREs (bases 3082–3438 of Kim-Ha et al. 1991). Cuticle preparations were as described (Wieschaus and Nüsslein-Volhard 1986).
Bru–Vas affinity chromatography
A fragment of bru encoding amino acids 157–418 (Fig. 3A; BruA) was subcloned in-frame into the 6× His-tagged expression vector pQE31 in the E. coli strain XL1-Blue (Stratagene). Protein expression was induced with IPTG for 2–3 hr at 37°C. GST–Vas expression in pGEX-3X was as described previously (Liang et al. 1994). Bacteria expressing BruA were lysed by sonication in 0.5 × PBS in the presence of 50 μg/ml of aprotinin, 25 μg/ml of pepstatin A, 100 μg/ml of TPCK, 100 μg/ml of TLCK, and 1 mm EDTA. GST–Vas and GST-expressing lysates were loaded onto glutathione–Sepharose 4B columns (Pharmacia) that were prewashed with five bed volumes of PBS. The columns were washed with 50 ml of wash buffer (50 mm Tris-Cl at pH 7.5, 150 mm NaCl) and 20 ml of PBS, and the BruA-expressing lysate was passed through the column three times. The columns were washed extensively as described with wash buffer and then with five bed volumes of cleavage buffer (50 mm Tris-Cl at pH 8.0, 100 mm NaCl, 1 mm CaCl2). A portion of the beads was removed, and proteins were eluted with one volume of 2× SDS sample buffer; the remainder of the beads was incubated with factor Xa (1% wt/vol).
Supernatants were collected from all elutions and brought to 1× SDS sample buffer, and the proteins were separated by SDS-PAGE. The Western blot was performed using α-BruA antibodies at a dilution of 1:1000.
Acknowledgments
We thank T. Schüpbach, A. Harris, and the Bloomington Stock Center for fly stocks; J. Hiebert for the developmental Northern blot, M. Scott and the Berkeley Drosophila Genome Project for P1 clones; T. Hazelrigg and P. Tolias for cDNA libraries; S. Driscoll-Plump for advice on chromosome in situ hybridization; and P. Good for discussions concerning Xenopus etr-1. We thank S. Jackson, Y. Lie, J. Mulligan, J. Schnorr, and C. Smibert for discussions and comments on the manuscript. This work was supported by a Helen Hay Whitney Foundation fellowship (to P.J.W.), operating grants from National Cancer Institute of Canada (NCIC) and Natural Sciences and Engineering Research Council (to P.L.), and a David and Lucile Packard fellowship and National Institutes of Health grant GM54409 (to P.M.M.) P.J.W. is an American Fellow of the American Association of University Women; P.L. is a Research Scientist of the NCIC, supported by funds from the Canadian Cancer Society; and P.M.M. is a Coyote Point Fellow.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL pmac@leland.stanford.edu; FAX (650) 725-9668.
References
- Bandziulis RJ, Swanson MS, Dreyfuss G. RNA-binding proteins as developmental regulators. Genes & Dev. 1989;3:431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Bass BL, Hurst SR, Singer JD. Binding properties of newly identified Xenopus proteins containing dsRNA-binding motifs. Curr Biol. 1994;4:301–314. doi: 10.1016/s0960-9822(00)00069-5. [DOI] [PubMed] [Google Scholar]
- Bouvet P, Omilli F, Arlot-Bonnemains Y, Legagneux V, Roghi C, Bassez T, Osborne HB. The deadenylation conferred by the 3′ untranslated region of a developmentally controlled mRNA in Xenopus embryos is switched to polyadenylation by deletion of a short sequence element. Mol Cell Biol. 1994;14:1893–1900. doi: 10.1128/mcb.14.3.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Gönczy P, Alexander S, Rawson R, Eberhart CG, Viswanathan S, DiNardo S, Wasserman SA. Toward a molecular genetic analyis of spermatogenesis in Drosophila melanogaster: Characterization of male-sterile mutants generated by single P element mutagenesis. Genetics. 1993;135:489–505. doi: 10.1093/genetics/135.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christerson LB, McKearin DM. orb is required for anteroposterior and dorsoventral patterning during Drosophila oogenesis. Genes & Dev. 1994;8:614–628. doi: 10.1101/gad.8.5.614. [DOI] [PubMed] [Google Scholar]
- Curtis D, Lehmann R, Zamore PD. Translational regulation in development. Cell. 1995;81:171–178. doi: 10.1016/0092-8674(95)90325-9. [DOI] [PubMed] [Google Scholar]
- Davidson EH. Gene activity in early development. Orlando, FL: Academic Press; 1986. [Google Scholar]
- Ephrussi A, Lehmann R. Induction of germ cell formation by oskar. Nature. 1992;358:387–392. doi: 10.1038/358387a0. [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Dickinson LK, Lehmann R. oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 1991;66:37–50. doi: 10.1016/0092-8674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- Gavis ER, Lehmann R. Translational regulation of nanos by RNA localization. Nature. 1994;369:315–318. doi: 10.1038/369315a0. [DOI] [PubMed] [Google Scholar]
- Gebauer F, Xu W, Cooper GM, Richter JD. Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J. 1994;13:5712–5720. doi: 10.1002/j.1460-2075.1994.tb06909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hake LE, Richter JD. CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell. 1994;79:617–627. doi: 10.1016/0092-8674(94)90547-9. [DOI] [PubMed] [Google Scholar]
- Hay B, Jan LY, Jan YN. Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development. 1990;109:425–433. doi: 10.1242/dev.109.2.425. [DOI] [PubMed] [Google Scholar]
- Hazelrigg T, Tu C. Sex-specific processing of the Drosophila exuperantia transcript is regulated in male germ cells by the tra-2 gene. Proc Natl Acad Sci. 1994;91:10752–10756. doi: 10.1073/pnas.91.22.10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Ha J, Smith JL, Macdonald PM. oskar mRNA is localized to the posterior pole of the Drosophila ooctye. Cell. 1991;66:23–35. doi: 10.1016/0092-8674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J, Kerr K, Macdonald PM. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell. 1995;81:403–412. doi: 10.1016/0092-8674(95)90393-3. [DOI] [PubMed] [Google Scholar]
- Knecht AK, Good PJ, Dawid IB, Harland RM. Dorsal-ventral patterning and differentiation of noggin-induced neural tissue in the absence of mesoderm. Development. 1995;121:1927–1936. doi: 10.1242/dev.121.6.1927. [DOI] [PubMed] [Google Scholar]
- Lantz V, Chang JS, Horabin JI, Bopp D, Schedl P. The Drosophila orb RNA-binding protein is required for the formation of the egg chamber and establishment of polarity. Genes & Dev. 1994;8:598–613. doi: 10.1101/gad.8.5.598. [DOI] [PubMed] [Google Scholar]
- Lasko PF, Ashburner M. Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes & Dev. 1990;4:905–921. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- Lee K, Fajardo MA, Braun RE. A testis cytoplasmic RNA-binding protein that has the properties of a translational repressor. Mol Cell Biol. 1996;16:3023–3034. doi: 10.1128/mcb.16.6.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R, Nüsslein-Volhard C. Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell. 1986;47:141–152. doi: 10.1016/0092-8674(86)90375-2. [DOI] [PubMed] [Google Scholar]
- Liang L, Diehl-Jones W, Lasko P. Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development. 1994;120:1201–1211. doi: 10.1242/dev.120.5.1201. [DOI] [PubMed] [Google Scholar]
- Macdonald PM, Struhl G. Cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 1988;336:595–598. doi: 10.1038/336595a0. [DOI] [PubMed] [Google Scholar]
- Markussen F-H, Michon A-M, Breitwieser W, Ephrussi A. Translational control of oskar generates Short OSK, the isoform that induces pole plasm assembly. Development. 1995;121:3723–3732. doi: 10.1242/dev.121.11.3723. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schüpbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGFα-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- O’Connell P, Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984;12:5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongo C, Gavis ER, Lehmann R. Localization of oskar RNA regulates oskar translation and requires Oskar protein. Development. 1995;121:2737–2746. doi: 10.1242/dev.121.9.2737. [DOI] [PubMed] [Google Scholar]
- Roth S, Neuman-Silberberg FS, Barcelo G, Schüpbach T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Sachs A, Wahle E. Poly(A) tail metabolism and function in eucaryotes. J Biol Chem. 1993;268:22955–22958. [PubMed] [Google Scholar]
- Sallés FJ, Lieberfarb ME, Wreden C, Gergen JP, Strickland S. Coordinate initiation of Drosophila development by regulated polyadenylation of maternal messenger RNAs. Science. 1994;266:1996–1999. doi: 10.1126/science.7801127. [DOI] [PubMed] [Google Scholar]
- Schüpbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics. 1989;121:101–117. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics. 1991;129:1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets MD, Wu M, Wickens M. Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature. 1995;374:511–516. doi: 10.1038/374511a0. [DOI] [PubMed] [Google Scholar]
- Smith JL, Wilson JE, Macdonald PM. Overexpression of oskar directs ectopic activaton of nanos and presumptive pole cell formation in Drosophila embryos. Cell. 1992;70:849–859. doi: 10.1016/0092-8674(92)90318-7. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992;68:201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Tarun SZ, Jr, Sachs AB. Association of the yeast poly(A) tail binding protein with translation initation factor elF-4G. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- Tearle R, Nüsslein-Volhard C. Tubingen mutants and stocklist. Dros Inf Serv. 1987;66:209–269. [Google Scholar]
- Timchenko LT, Miller JW, Timchenko NA, DeVore DR, Datar KV, Lin L, Roberts R, Caskey CT, Swanson MS. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 1996;24:4407–4414. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson CR, LaMarco KL, Johnson PF, Landschulz WH, McKnight SL. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes & Dev. 1988;2:801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- Webster PJ, Macdonald PM. Screening an expression library for RNA-binding proteins using a solution-based assay. In: Haynes SR, editor. Methods in molecular biology: RNA-protein interaction protocols. Totowa, NJ: The Humana Press; 1997. . (In press.) [Google Scholar]
- Webster PJ, Suen J, Macdonald PM. Drosophila virilis oskar transgenes direct body patterning but not pole cell formation or maintenance of mRNA localization in D. melanogaster. Development. 1994;120:2027–2037. doi: 10.1242/dev.120.7.2027. [DOI] [PubMed] [Google Scholar]
- Wharton RP, Struhl G. RNA regulatory elements mediate control of Drosophila body pattern by the posterior morphogen nanos. Cell. 1991;67:955–967. doi: 10.1016/0092-8674(91)90368-9. [DOI] [PubMed] [Google Scholar]
- Wickens M, Kimble J, Strickland S. Translational control of developmental decisions. In: Hershey JWB, Mathews MB, Sonenberg N, editors. Translational control. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1996. pp. 411–450. [Google Scholar]
- Wieschaus E, Nüsslein-Volhard C. Looking at embryos. In: Roberts DB, editor. Drosophila. A practical approach. Washington, D.C.: IRL Press; 1986. pp. 199–227. [Google Scholar]
- Wilson JE, Connell JE, Macdonald PM. aubergine enhances oskar translation in the Drosophila ovary. Development. 1996;122:1631–1639. doi: 10.1242/dev.122.5.1631. [DOI] [PubMed] [Google Scholar]