Abstract
Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry is used for the determination of molecular weights of different chemical compounds. We describe here the use of MALDI-TOF mass spectrometry to detect a carbapenem antibiotic, meropenem, and its degradation products. Buffered meropenem solution (0.1 mM Tris-HCl, pH 6.8) was mixed with an overnight culture of bacteria. After 3-h incubation, the reaction mixture was centrifuged, and the supernatant was analyzed by MALDI-TOF mass spectrometry. The presence or absence of peaks representing meropenem and its sodium salts was crucial. The average turnaround time of this test, considering the use of overnight culture, is 4 h. We validated this method for the detection of resistance to carbapenems in Enterobacteriaceae and Pseudomonas aeruginosa mediated by carbapenemase production. A total of 124 strains, including 30 carbapenemase-producing strains, were used in the study. The sensitivity of this method is 96.67%, with a specificity of 97.87%. Our results demonstrate the ability of this method to routinely detect carbapenemases in Enterobacteriaceae and Pseudomonas spp. in laboratories. This assay is comparable with a labor-intensive imipenem-hydrolyzing spectrophotometric assay that is a reference method for the detection of carbapenemase. As demonstrated here, MALDI-TOF mass spectrometry may be used in microbiological laboratories not only for microbial identification but also for other applications, such as studies of mechanisms of antibiotic resistance.
INTRODUCTION
Antibiotic resistance in Gram-negative rods, especially Enterobacteriaceae, Pseudomonas spp., and Acinetobacter spp., has been an increasing problem all over the world (4, 7, 16, 19). Resistant bacteria can significantly complicate the development of medicine, especially in surgery, hemato-oncology, and intensive care. Infections by multidrug-resistant Gram-negative bacteria are usually treated with carbapenems. Resistance to these antibiotics has increased in the past few years. This resistance is caused by an alteration in the outer membrane of the cell wall and by the production of carbapenemases (7). Carbapenemases are commonly encoded by gene cassettes of integrons, together with other resistance mechanism determinants (e.g., cross-resistance to aminoglycosides) (3). Strains resistant to all antibiotics have been described.
Carbapenemases are enzymes that are able to hydrolyze the amide bond of the β-lactam ring of β-lactams. There is no standardized direct method for the detection of carbapenemases in routine microbiological laboratories (7). The reference method is based on the spectrophotometric analysis of imipenem degradation, which requires preparation of a bacterial extract by sonication and the Hodge test, which is very difficult to standardize and requires an experienced microbiologist to interpret (3, 7, 18). Nondirect methods, commonly used in clinical microbiological laboratories, are based on the ability of some compounds to inhibit carbapenemases; for example, ion-chelating agents (e.g., EDTA and dipicolinic acid) inhibit metallo-β-lactamases (MBLs), and phenyl-boronic acid inhibits Klebsiella pneumoniae carbapenemases (KPCs) (6). However, the specificity of this method is limited (7).
Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry has been introduced into routine microbiological laboratories for the identification of bacteria and fungi but may potentially be applied in the complex diagnostic process (15, 19). Here we describe a new method for the detection of the meropenem molecule and the direct detection of carbapenemase activity in enterobacteria and Pseudomonas aeruginosa that is based on a simple preparation of the sample followed by MALDI-TOF mass spectrometry.
MATERIALS AND METHODS
Bacterial isolates and carbapenemase detection.
Well-typed bacterial carbapenem-nonsusceptible isolates from the collection of the Department of Microbiology of the Faculty of Medicine and University Hospital in Plzen were used for the study (1, 2, 8, 9). Additionally, clinical isolates susceptible to carbapenems were included (Table 1). Species were identified using MALDI-TOF mass spectrometry (Bruker Daltonics, Bremen, Germany; MALDI Biotyper). Some species were identified by the ENTEROtest 24 (Pliva Lachema Diagnostika, Brno, Czech Republic) based on the 24 biochemical tests. Escherichia coli ATCC 14169, K. pneumonia ATCC 13883, and P. aeruginosa ATCC 27853 were used as standardized negative controls. The MICs for meropenem and imipenem were determined by the microdilution broth method and Etest, respectively, and were interpreted with the use of 2010 EUCAST criteria (5). For all strains, carbapenemase production was determined with the imipenem hydrolytic assay by the use of a crude bacterial sonicate as previously described (22). A synergy disk test with ceftazidime, imipenem, meropenem, and EDTA as the inhibitors was performed to detect MBLs (3, 14). The approximation disk test described by Giske et al. (6) was used to detect MBLs, KPCs, and AmpC enzymes in all of the tested strains.
Table 1.
Summary of the meropenem hydrolysis assay data
| Strain type | Resistance mechanism | Bacterial species (no. of isolates tested) | MALDI-TOF analysis |
MIC range (μg/ml) |
|||
|---|---|---|---|---|---|---|---|
| No. of isolates with lack of peak at m/z: |
No. of isolates detected as carbapenemase producer | Meropenem | Imipenem | ||||
| 384 | 405 | ||||||
| Carbapenemase-producing strains | |||||||
| VIM | Pseudomonas aeruginosa (8) | 7 | 7 | 7 | 32->32 | >32 | |
| Klebsiella pneumoniae (3) | 3 | 0 | 3 | >32 | >32 | ||
| Serratia marcescens (3) | 4 | 2 | 4 | >32 | >32 | ||
| Enterobacter cloacae (4) | 3 | 2 | 4 | >32 | >32 | ||
| IMPa | Pseudomonas aeruginosa (6) | 6 | 3 | 6 | >32 | >32 | |
| KPCb | Klebsiella pneumoniae (5) | 4 | 4 | 5 | 4->32 | 2->32 | |
| NDM-1 | Klebsiella pneumoniae (1) | 1 | 0 | 1 | >32 | >32 | |
| Total no. of carbapenemase-producing strains | 30 | 29 | |||||
| Non-carbapenemase-producing strains | Resistance due to structural cell wall changes and the production of ESBL (CTX-M, SHV) or AmpC (DHA-1) β-lactamasesc | Klebsiella pneumoniae (23) | 0 | 0 | 0 | 2->32 | 4->32 |
| Resistant isolates not producing any carbapenemase | Pseudomonas aeruginosa (25) | 2 | 1 | 2 | 8->32 | 16->32 | |
| Citrobacter freundii (3) | 0 | 0 | 0 | 8–16 | 16 | ||
| Enterobacter cloacae (4) | 0 | 0 | 0 | 4–32 | 8–>32 | ||
| Susceptible isolatesd | Pseudomonas aeruginosa (14) | 0 | 0 | 0 | 0.125–2 | 0.25–2 | |
| Escherichia coli (4) | 0 | 0 | 0 | ≤0.125 | ≤0.125 | ||
| Klebsiella pneumoniae (15) | 0 | 0 | 0 | ≤0.125–0.25 | ≤0.125–0.5 | ||
| Serratia marcescens (2) | 0 | 0 | 0 | ≤0.125–0.125 | 0.25–1 | ||
| Enterobacter cloacae (4) | 0 | 0 | 0 | ≤0.125 | ≤0.125–0.25 | ||
| Total no. of non-carbapenemase-producing strains | 94 | 2 | |||||
MALDI-TOF mass spectrometry analysis of meropenem.
Commercially available meropenem containing sodium carbonate (Meronem, 500 mg; AstraZeneca UK, Ltd., Macclesfield, United Kingdom) was used for the experiments. Different meropenem concentrations were diluted in 20 mM Tris-HCl buffer, pH 6.8 (Sigma-Aldrich, Ltd., Prague, Czech Republic). The meropenem solution was stored at −80°C, without multiple refreezing, for 1 month.
Because the background peaks, from the organic matrix in the spectra, with a low molecular mass of <500 Da can complicate MALDI-TOF analysis (17), we used 10 mg/ml of α-cyano-4-hydroxycinnamic acid (HCCA), 2,5-dihydroxybenzoic acid (DHB), and 2,5-dihydroxyacetophenone (DHAP) diluted in 50% ethanol or 50% acetonitrile in water as the matrix (all chemicals were obtained from Sigma-Aldrich, Prague, Czech Republic). One microliter of matrix solution was mixed with one microliter of the sample, applied onto a target (Bruker Daltonics GmbH, Bremen, Germany; MSP 96 Target, catalog no. 224989), and allowed to dry at room temperature. Mass spectra were acquired using a Microflex LT mass spectrometer with the flexControl 3.0 software (Bruker Daltonics GmbH, Bremen, Germany) operating in positive linear ion mode between m/z 360 and 600. The parameters were set as follows: ion source 1, 20 kV; ion source 2, 16.7 kV; lens, 7 kV; pulsed ion extraction, 170 ns; detection gain, 3.0×; electronic gain, regular; mode, low range; mass range selection, low range; laser frequency, 60 Hz; digitizer trigger level, 2,500 mV; laser attenuator, 24%; laser attenuator, 30%; and laser range, 70 to 90% (for the measurement of carbapenemase activity, strictly 90%). Spectra were measured manually in at least 5 positions.
Meropenem concentrations in the range of 0.01 mM to 1 mM and matrix concentrations in the range of 1 mg/ml to 10 mg/ml were tested. The degradation of meropenem, including the detection of amide bond hydrolysis, was analyzed by resuspending meropenem in 100 mM NaOH and 20 mM Tris to a final concentration of 10 mM, and neutralization with HCl to a pH of 8.0 followed.
Meropenem hydrolysis assay.
Cultures of the tested strains were incubated overnight on Mueller-Hinton agar (Bio-Rad Laboratories, Inc., Prague, Czech Republic). A heavy inoculum, 8 on the McFarland scale, was prepared in 3 ml of 20 mM Tris-HCl buffer (pH 6.8) with 150 mM NaCl. Then, 1 ml was immediately placed in an Eppendorf tube and centrifuged at 14,000 × g for 3 min. The supernatant was discarded, and 50 μl of 0.1 mM meropenem in 20 mM Tris-HCl buffer (pH 6.8) was added to the pellet and mixed by pipetting. The reaction mixture was incubated at 35°C for 3 h. Then, the reaction mixture was centrifuged, and 1 μl of the supernatant was used for MALDI-TOF mass spectrometry.
Spectrum analysis.
Spectra were analyzed using flexAnalysis 3.0 software (Bruker Daltonics GmbH, Bremen, Germany). Peaks were detected using the Centroid detection algorithm with a signal-to-noise threshold of 1, a relative intensity threshold of 0%, a minimum intensity threshold of 0, a peak width of 0.2 m/z, a height of 80%, a TopHat baseline subtraction, smoothing with the Savitzky-Golay algorithm (23), a width of 0.2 m/z, and a cycle of 1. The detected peaks were compared with the theoretical molecular masses of meropenem, its degradation products, and sodium salt variants, with a ±0.6 m/z tolerance.
Statistical analysis.
The sensitivity and specificity of the assay were calculated using McNemar's test.
Verification and internal calibration of the MALDI-TOF mass spectrometer.
The peaks of meropenem, its alkaline degradation products, and their sodium salts were used for internal calibration of the MALDI-TOF mass spectrometer.
RESULTS
MALDI-TOF mass spectrometry analysis of meropenem.
Three matrices were tested in different diluents. The spectra without additional background peaks in the interval of 375 to 475 m/z were obtained using 10 mg/ml of 2,5-dihydroxybenzoic acid (DHB) in 50% ethanol. In addition to the native meropenem molecule (m/z ca. 383.464), two variants of its sodium salt were also observed (m/z ca. 405.443 and 427.422) (Fig. 1 and 2). Using this matrix and these conditions, the lowest detection limit was found to be 50 μM (19.173 mg/liter). The modification of the meropenem molecule produced by β-lactamases is theoretically similar to that produced by hydrolysis of NaOH. Therefore, meropenem was hydrolyzed by NaOH, and the mass spectra were detected prior to the carbapenemase hydrolysis assay to avoid interference of the degradation products with other molecules in the sample (e.g., binding to proteins present in the β-lactamase extracts). After this test, peaks at m/z ca. 401.483 (native molecule with disrupted amide bond) as well as 423.462, 445.441, and 467.420 (three sodium salt variants) were observed. The results are shown in Fig. 2.
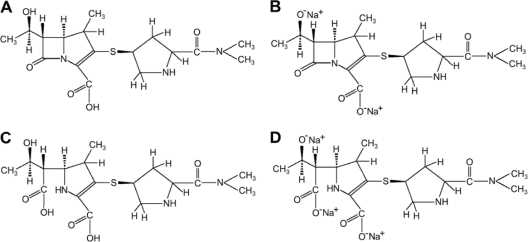
Fig. 1.
The chemical structures of meropenem, 383.464 Da (A), its disodium salt, 427.422 Da (B), a meropenem degradation product with a disrupted amide bond, 401.483 Da (C), and its trisodium salt, 467.420 Da (D).
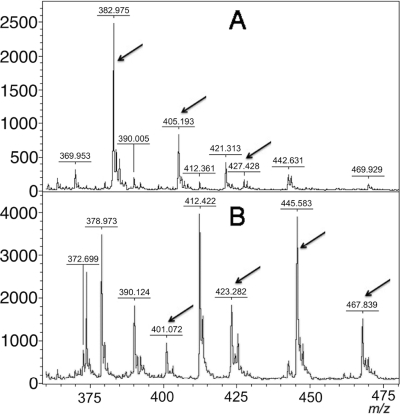
Fig. 2.
Mass spectra of meropenem (detected mass 382.975 m/z) and its sodium salts (405.193 m/z and 427.428 m/z) (A) and meropenem with a disrupted amide bond (401.072 m/z) and its sodium salts (423.282 m/z, 445.583 m/z, and 467.839 m/z) (B). Peaks corresponding to the tested molecules are indicated with arrows. Units on the y axes represent relative intensity.
Meropenem hydrolysis assay.
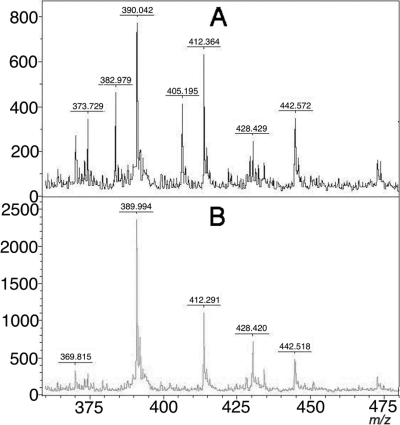
The assay was validated with 124 strains. Thirty isolates produced different carbapenemases (VIM, IMP, NDM-1, and KPC-2) and were identified as Pseudomonas aeruginosa and different species of Enterobacteriaceae. Ninety-four negative controls were divided into groups, including strains resistant (n = 55) and susceptible (n = 39) to carbapenems according to the EUCAST criteria (5). More detailed information is provided in Table 1. After the non-carbapenemase-producing bacteria were incubated for 3 h in buffered meropenem solution, peaks representing meropenem and its two sodium salts were detected (m/z 383, 405, and 427, respectively). Using software to detect the peaks, the peak corresponding to intact meropenem was not present in 29 of the 30 carbapenemase-producing strains (Fig. 3 and Table 1). In carbapenemase-producing isolates, the observed peak corresponded to m/z 427 for all except three isolates. For the three strains, the m/z 383 peak may have been interpreted as noise (Fig. 4), but no m/z 405 peaks (meropenem sodium salt) were detected in the spectra of these strains. Therefore, we defined a positive as a lack of the m/z 383 and/or m/z 405 peaks. There were no false-positive or false-negative results from the Enterobacteriaceae, but two false-positive results were detected from the 25 P. aeruginosa strains that did not produce carbapenemase. Both strains belong to the carbapenem-resistant group. In one VIM-2 metallo-β-lactamase-producing P. aeruginosa strain, all three peaks were detected. Therefore, this strain was classified as a non-carbapenemase-producing strain. No degradation products were detected from the strains that produced carbapenemase. The sensitivity of this method was 96.67%, and the specificity was 97.87%. The average turnaround time of this test is 4 h when the overnight culture of bacteria is available.
Fig. 3.
Mass spectra of the meropenem hydrolysis assay of a non-carbapenemase-producing isolate (A) and a carbapenemase (KPC-2)-producing isolate (B). Units on the y axes represent relative intensity.
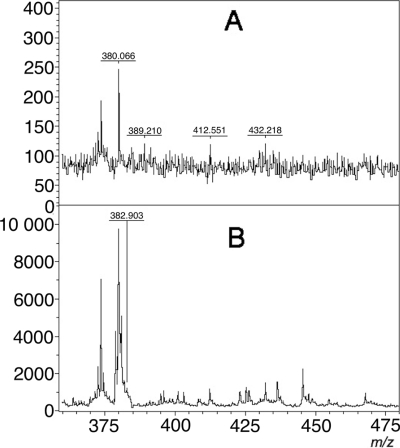
Fig. 4.
Mass spectra of special interest: unreliable spectrum (A) and spectrum obtained after meropenem digestion by some carbapenemase-producing strains (KPC-2) (B). Analysis represented by the spectrum shown in panel A had to be repeated; using flexAnalysis 3.0 software with a signal-to-noise threshold of 1, the peak at ca. 383 m/z in the spectrum shown in panel B was detected. This peak may be interpreted as noise. No peak at 405 m/z was detected. Therefore, this strain was correctly interpreted as carbapenemase producer.
DISCUSSION
MALDI-TOF mass spectrometry has been introduced into clinical microbiological laboratories for routine diagnostics (15, 19). This method is a quick, cheap tool that is used to identify bacteria and fungi (10, 15). This method can also be used for other biochemical analyses of microbial cells, e.g., lipopolysaccharide analysis of colistin-resistant isolates, as well as for research at the molecular level (21). Other applications in clinical laboratory diagnosis, e.g., in immunology and biochemistry, may be available soon (11, 19). After a proper validation, some of these applications will be easily introduced into diagnostic practice.
Mass spectrometry in conjunction with an electrospray ion source was used for the analysis of meropenem (13). To our knowledge, MALDI-TOF mass spectrometry has not been used to determine resistance mechanisms, except for resistance to colistin (10) in Gram-negative bacteria, nor has it been used for the analysis of antibiotic molecule modifications.
Our results show that a DHB matrix diluted in 50% ethanol should be used to visualize the meropenem mass spectra. When other matrices and solvents were tested, the spectra had significant backgrounds, and interpretation of the results was difficult. The buffer solution used for the assay also seems to be important. We tested many buffers with the optimal pH of 6.8 (e.g., MOPS [3-N-(morpholino)propanesulfonic acid] and HEPES [data not shown]), but spectra without excess background were obtained only when Tris-HCl buffer was used. Many β-lactamases have optimal activity at pH 6.8, which is slightly lower than the proper interval of Tris-HCl buffering. When a higher pH was used, we observed rapid conversion of meropenem molecules to sodium and disodium salts. For the analysis of other antibiotic molecules, optimization of the experiment conditions (matrix and buffer) has to be done.
As described above, before the reaction for carbapenemase detection was performed, spectra were internally calibrated with meropenem and the meropenem degradation products obtained by NaOH hydrolysis. This calibration seems to be useful for mass spectrum analysis and the detection of molecules after carbapenemase hydrolysis. However, we did not observe any degradation product in most strains using our assay. We expect that these products are nonspecifically bound to protein debris present in the reaction mixture or are further decomposed to smaller molecules.
For the peak detection, we tried to analyze the spectra manually, but this approach was too subjective. Therefore, we decided to detect the peaks using special flexAnalysis 3.0 software with the configuration described in Materials and Methods.
This method was validated on 124 strains. Thirty of them produced carbapenemases (VIM, IMP, KPC, and NDM), and 52 were resistant to carbapenems due to another mechanism (e.g., porin loss or efflux). The MICs of meropenem and imipenem varied among the tested isolates as well as among the same strain. For example, KPC-producing K. pneumoniae belonged to the same sequence type (ST), ST258, and the MICs ranged in the interval of 2 to >32 μg/ml for imipenem and 4 to >32 μg/ml for meropenem. A similar situation was described for carbapenem-resistant isolates of K. pneumoniae producing no carbapenemase. Most of them belonged to the international clone ST11, and the MICs ranged within a broad interval (Table 1). For Enterobacteriaceae, our assay was able to detect the carbapenemase independently of the MICs to carbapenems.
False-positive and false-negative results were found with P. aeruginosa only. False-positive results could be explained by other resistance mechanisms, e.g., the interaction of meropenem with a component of the cell wall and extended-spectrum or AmpC β-lactamases (12). False-negative results may be explained by low expression of carbapenemase. The background peaks can also complicate the interpretation of this assay. In the future, these problems, as well as the use of carbapenemase inhibitors and better stabilization of carbapenem molecules, should be investigated.
The time of the cultivation of the isolate used for the meropenem hydrolysis assay should be studied as well. When using an old culture (48 h), especially of Pseudomonas aeruginosa, the activity of carbapenemase may be affected by the production of proteolytic enzymes, and false-negative results may be obtained (data not shown). We strongly recommend using a culture in the exponential phase of growth (18 h).
As described in the introduction, there is no direct assay for the detection of carbapenemase in routine diagnostic laboratories, except the modified Hodge test (18). However, this test is very difficult to standardize, and false-positive results are observed, especially in CTX-M extended-spectrum β-lactamase (ESBL)-producing bacteria that are resistant to carbapenems (18). Detection of carbapenemase activity by MALDI-TOF analysis is similar to the reference method, the detection of imipenem hydrolysis using UV spectrometry, but the assay described here does not require the preparation of a cell-free bacterial extract. The average turnaround time of this test is comparable to the turnaround time of the reference method and significantly faster than times achieved using other inhibitor-based cultivation techniques.
The method should also be validated for other bacterial species possessing any carbapenemase, e.g., Acinetobacter baumannii, which may produce a low level of this enzyme, and also for other carbapenemase types (e.g., GES, OXA-48, GIM, SIM, SPM, etc.). A low expression level of the β-lactamase and the thickness of the cell wall may complicate the detection of carbapenemase activity.
MALDI-TOF mass spectrometry may be used for analysis of other antibiotic molecules, including other β-lactams. The stability of the molecule tested, appropriate buffer, and matrix would have to be optimized.
MALDI-TOF mass spectrometry can be used for general applications in microbiological laboratories in addition to the identification of bacteria and fungi. We believe that many bacterial mechanisms of antibiotic resistance (e.g., enzymatic modification of other β-lactams and aminoglycosides and efflux analysis) can be detected using this technology not only in research but also in routine microbiological diagnosis.
ACKNOWLEDGMENTS
We thank Marek Gniadkowski and Helena Zemlickova for providing some of the MBL/KPC-producing strains used in this study.
This work was supported by the research project grants NT11032-6/2010 and NS9717-4/2008 from the Ministry of Health of the Czech Republic and MSM0021620819 from the Ministry of Education of the Czech Republic.
We declare no competing financial interest.
Footnotes
Published ahead of print on 20 July 2011.
REFERENCES
- 1. Baraniak A., et al. 2009. Emergence of Klebsiella pneumoniae ST258 with KPC-2 in Poland. Antimicrob. Agent Chemother. 53:4565–4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chudáčková E., et al. 2010. Carbapenem-non-susceptible strains of Klebsiella pneumoniae producing SHV-5 and/or DHA-1 β-lactamases in a Czech hospital. FEMS Microbiol. Lett. 309:62–70 [DOI] [PubMed] [Google Scholar]
- 3. Cornaglia G., et al. for the ESCMID Study Group for Antimicrobial Resistance Surveillance (ESGARS) 2007. Metallo-β-lactamases as emerging resistance determinants in Gram-negative pathogens: open issues. Int. J. Antimicrob. Agents 29:380–388 [DOI] [PubMed] [Google Scholar]
- 4. Dijkshoorn L., Nemec A., Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5:939–951 [DOI] [PubMed] [Google Scholar]
- 5. European Committee on Antimicrobial Susceptibility Testing 2010. Breakpoint tables for interpretation of MICs and zone diameters. Version 1.1, April 2010. European Committee for Antimicrobial Susceptibility Testing, Düsseldorf, Germany: http://www.eucast.org [Google Scholar]
- 6. Giske C. G., et al. 2011. A sensitive and specific phenotypic assay for detection of metallo-beta-lactamases and KPC in Klebsiella pneumoniae with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin. Microbiol. Infect. 17:552–556 [DOI] [PubMed] [Google Scholar]
- 7. Grundmann H., et al. for the CNSE Working Group 2010. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusion from a meeting of national experts. Euro Surveill. 15:pii=19711. [DOI] [PubMed] [Google Scholar]
- 8. Hrabák J., et al. 2011. Regional spread of Pseudomonas aeruginosa ST357 producing the IMP-7 metallo-β-lactamase in Central Europe. J. Clin. Microbiol. 49:474–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hrabák J., et al. KPC-2-producing Klebsiella pneumoniae isolated from a Czech patient previously hospitalized in Greece and in vivo selection of colistin resistance. Folia Microbiol., in press. doi:10.1007/s12223-011-0057-6 [DOI] [PubMed]
- 10. Lin P.-C., Tsai P. J., Weng M. F., Chen Y. C. 2005. Affinity capture using vancomycin-bound magnetic nanoparticles for the MALDI-MS analysis of bacteria. Anal. Chem. 77:1753–1760 [DOI] [PubMed] [Google Scholar]
- 11. Lin P.-C., Tseng M. C., Su A. K., Chen Y. J., Lin C. C. 2007. Functionalized magnetic nanoparticles for small-molecule isolation, identification, and quantification. Anal. Chem. 79:3401–3408 [DOI] [PubMed] [Google Scholar]
- 12. Livermore D. M., Woodford N. 2006. The β-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 14:413–419 [DOI] [PubMed] [Google Scholar]
- 13. Mendez A., Chagastelles P., Palma E., Nardi N., Schapoval E. 2008. Thermal and alkaline stability of meropenem: degradation products and cytotoxicity. Int. J. Pharm. 350:95–102 [DOI] [PubMed] [Google Scholar]
- 14. Miriagou V., et al. 2010. Acquired carbapenemases in Gram-negative bacterial pathogens: detection and surveillance issues. Clin. Microbiol. Infect. 16:112–122 [DOI] [PubMed] [Google Scholar]
- 15. Murray P. R. 2010. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry: usefulness for taxonomy and epidemiology. Clin. Microbiol. Infect. 16:1626–1630 [DOI] [PubMed] [Google Scholar]
- 16. Nordmann P., Cuzon G., Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase producing bacteria. Lancet Infect. Dis. 9:228–236 [DOI] [PubMed] [Google Scholar]
- 17. Pan C., et al. 2007. Recent developments in methods and technology for analysis of biological samples by MALDI-TOF-MS. Anal. Bioanal. Chem. 387:193–204 [DOI] [PubMed] [Google Scholar]
- 18. Pasteran F., Mendez T., Guerriero L., Rapoport M., Corso A. 2009. Sensitive screening tests for suspected class A carbapenemase production in species of Enterobacteriaceae. J. Clin. Microbiol. 47:1631–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah H. N., Gharbia S. E. (ed.). 2011. Mass spectrometry for microbial proteomics. Wiley, Chichester, United Kingdom [Google Scholar]
- 20.Reference deleted.
- 21. Tran A. X., et al. 2005. Resistance to the antimicrobial peptide polymyxin requires myristoylation of Escherichia coli and Salmonella typhimurium lipid A. J. Biol. Chem. 280:28186–28194 [DOI] [PubMed] [Google Scholar]
- 22. Woodford N., et al. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A β-lactamase, KPC-3, in a New York medical center. Antimicrob. Agents Chemother. 48:4793–4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang C., He Z., Yu W. 2009. Comparison of public peak detection algorithms for MALDI mass spectrometry data analysis. BMC Bioinformatics 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]