Abstract
Relapsing fever (RF) is caused by tick- and louse-borne Borrelia spp., is characterized by recurrent fever, and is often misdiagnosed as malaria. Because of submicroscopic bacteremia, microscopy can be insensitive between febrile bouts. We designed a multiplex quantitative PCR (qPCR) assay to distinguish RF Borrelia from Plasmodium falciparum and P. vivax. The assay specifically (100%) amplified pathogenic RF Borrelia (1 copy/reaction). We then tested blood from participants within a Tanzanian cohort assessed at scheduled intervals and with fever. Among 8,617 blood samples from 2,057 participants surveyed routinely, 7 (0.08%) samples and 7 (0.3%) participants had RF DNA (median, 4.4 × 103 copies/ml). Of 382 samples from 310 febrile persons, 15 (3.9%) samples from 13 (4.2%) participants had RF DNA (median, 7.9 × 102 copies/ml). Five (1.3%) samples from 4 (1.3%) participants were found to harbor Borrelia by microscopy. We conclude that multiplex qPCR holds promise for improved clinical diagnosis and epidemiologic assessment of RF.
INTRODUCTION
Relapsing fever (RF) is an acute febrile illness caused by multiple Borrelia species, which differ by geographic location and are transmitted either by argasid (soft body) ticks or by human body lice. Fever recurs over weeks or months as Borrelia alters surface antigens, immune system escape occurs, and new waves of bacteremia with ≤106 bacteria/ml ensue (2, 7, 26). The sensitivity of Giemsa-stained peripheral blood smear analysis is limited for low-level bacteremia, and the technique is not useful during asymptomatic intervals. Microscopy is impractical for large studies and cannot accurately assess disease burden. Therefore, we developed a high-throughput, real-time multiplex quantitative PCR (qPCR) assay to detect RF Borrelia, Plasmodium falciparum, and P. vivax to support a large clinical trial in Tanzania. Herein we report diagnostic and epidemiological findings related to identification of RF Borrelia among trial participants.
MATERIALS AND METHODS
Participants. (i) Setting.
We sought to identify incidents of symptomatic and asymptomatic relapsing fever as an ancillary study to that of mass treatment with azithromycin for trachoma (Partnership for the Rapid Elimination of Trachoma [PRET+]). In the parent study, four villages in the Kongwa district in the central Dodoma region of Tanzania were selected for WHO-recommended single-dose mass treatment with azithromycin (20 mg/kg of body weight, up to 1 g) for highly prevalent (≥10%) active (trachomatous follicular or intense) trachoma (17, 21, 25). Within villages, 130 households per village (intervention group) were randomly selected. Within households, children <7 years of age, caregivers, and pregnant women were eligible; one child and one caregiver were randomly selected in addition to pregnant women. Four otherwise similar villages in the same geographic area with less-prevalent active trachoma (<10%) were chosen as controls. Enrollment was per the intervention group.
(ii) Routine surveillance.
Young children (0 months to 7 years of age) and adults (≥18 years of age) were assessed at baseline (before treatment in cases in which the participant was a member of an intervention group) and at 4, 12, 16, and 20 weeks. Children were also evaluated at 2, 6, or 8 weeks (1/3 at each interval). During routine surveillance visits, finger-prick capillary blood samples were spotted on Whatman 903 Protein Saver filter paper (Whatman Inc., Florham Park, NJ). In addition, blood smears were obtained.
(iii) Evaluation of fever.
Field workers visited participants twice weekly to obtain thick and thin blood smears from febrile (>101°F) participants to detect RF and malaria at the Amani Medical Research Centre in Muheza, Tanzania. Blood spots and duplicate smears were also obtained for qPCR and smear verification at the Johns Hopkins University (JHU).
Real-time quantitative PCR. (i) Samples for assay validation.
Borrelia positive-control DNA samples from B. hermsii, B. parkeri, B. recurrentis, B. crocidurae, B. turicatae, and B. miyamotoi and heat-killed cultures of B. crocidurae were provided (courtesy Tom Schwann, NIAID, Rocky Mountain Laboratories, Hamilton, MT). DNA was prepared from B. burgdorferi, strain 297, propagated in vitro. Heat-killed RF spirochetes from culture were counted using dark field microscopy and spiked into EDTA-anticoagulated blood from a healthy volunteer at 105 bacteria/ml. Negative-control EDTA-anticoagulated blood samples were obtained from the Johns Hopkins Hospital laboratories. DNA was prepared using 200 μl of all blood samples and a Qiagen DNeasy blood and tissue kit (Qiagen Inc., Valencia, CA) and was resuspended in 200 μl of water.
(ii) Clinical samples.
Five 3-mm-diameter punches (∼25 μl of blood) from blood-impregnated Whatman 903 Protein Saver filter cards were used to prepare DNA with a Promega Wizard DNA purification (96-well) kit (Promega Corporation, Madison, WI). DNA was eluted into 200 μl of water, concentrated by precipitation with sodium acetate (pH 5), glycogen, and absolute ethanol, dried, and resuspended in 30 μl of water. Each 96-well DNA preparation from blood on filter paper included a negative control.
(iii) Development of multiplex qPCR assay.
By the use of AlleleID version 6.1 software (Premier Biosoft International, Palo Alto, CA), primers and probes targeting the RF Borrelia conserved glpQ gene, P. falciparum 18S rRNA gene, and P. vivax AMA1 (apical membrane antigen 1) gene were designed for use in a multiplex assay. To quantitate RF Borrelia, the amplicon product from B. crocidurae glpQ was cloned into a plasmid vector to enable a standard curve determined using dilutions (105, 103, 101, and 100 copies/μl) of plasmid B. crocidurae DNA.
The forward and reverse primers for glpQ were AAGCACCAACAGATTATGAATAC and ATTTGCCCATTAATAATGATTTGG, respectively, and the probe for glpQ was FAM-TCCAAGGTCCAATTCCGTCAGCAT-BHQ1, where FAM represents 6-carboxyfluorescein and BHQ1 represents Black Hole Quencher 1. Similarly, the forward and reverse primers for P. falciparum 18S rRNA were CCACATCTAAGGAAGGCAGCAG and CCTCCAATTGTTACTCTGGGAAGG, respectively, and the probe for P. falciparum 18S rRNA was Cy5-CCCACCATTCCAATTACAA-BHQ1. The forward and reverse primers for P. vivax AMA1 were ACGCCAAGTTCGGATTATGG and CCGTCATTTCTTCTTCATACTGAG, respectively, and the probe for P. vivax AMA1 was TET-TTGATCTGAGGCACTCGCTCCG-BHQ1.
We used a Bio-Rad CFX 384 real-time PCR detection system thermocycler (Bio-Rad, Hercules, CA) with 384-well plates. Each reaction included 2× iQ Multiplex Powermix (Bio-Rad) with 200 nM primers and probes, 1 μl of DNA for validation samples and controls, and 5 μl of DNA from filter paper blood (equivalent to DNA from 3.1 μl of blood).
Each PCR run included a standard curve, a positive genomic RF Borrelia control, and a no-template control sample in duplicate reactions. Standard two-step qPCR was performed with initial denaturation at 95° for 10 min followed by 40 cycles of denaturation at 95°C for 10 s and annealing/extension at 55°C for 30 s. Results were automatically analyzed using a single threshold for each probe-fluor pair and baseline-subtract curve fit to normalize each run. Results were manually inspected for quality, with baseline correction when needed. Endpoint analysis was conducted using Bio-Rad CFX 384 system software, where cutoffs were calculated as a fraction of the difference between maximum and minimum values of relative fluorescence for the fluor and plate and the average relative fluorescence for negatives over the last 5 cycles.
RF Borrelia in the Tanzanian cohort.
We used the qPCR to test control blood and blood from febrile and nonfebrile participants for RF Borrelia. Endpoints from duplicate samples were analyzed, and the samples were considered to contain Borrelia DNA only when both values were above the cutoff. In such cases, individual quantities were determined by standard curve comparisons and averaged to obtain a single quantification result for each sample (expressed as the number of glpQ copies per milliliter of blood DNA).
Molecular confirmation and phylogenetic analysis of RF Borrelia.
Residual DNA from qPCR (usually <1 μl) was resuspended in 30 μl of PCR-grade water and amplified by targeting a larger fragment of glpQ as well as a second target, flaB, common to RF Borrelia and B. burgdorferi. B. hermsii, B. parkeri, B. recurrentis, B. crocidurae, B. turicatae, B. miyamotoi, and B. burgdorferi DNA from control samples and all clinical samples containing RF Borrelia DNA as determined by qPCR were separately amplified using a volume of 2 μl of the original or diluted DNA with primers targeting an ∼950-bp flaB fragment and a 1,500-bp glpQ fragment. The forward and reverse primers for flaB were AGAATTAATMGHGCWTCTGATGATG and TGCYACAAYHTCATCTGTCATT (24) and for glpQ were GGTATGCTTATTGGTCTTC and TTGTATCCTCTTGTAATTG (1), respectively. Samples were amplified at 94°C for 3 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 3 min. After the 35th cycle, an additional 7-min extension at 72°C was used. Agarose gel electrophoresis identified a single band of the appropriate molecular size for sequencing; when no band was observed initially, 1 μl of the amplicon was used as the template for a second round of amplification using the same primers and amplification conditions (24). The PCR products were used directly, derived sequences were aligned using ClustalX, version 1.81 (27), and clade separation was achieved using neighbor-joining trees and 1,000 bootstrap iterations, with visualization performed using TreeView version 1.6.1 software.
Correlation of qPCR with blood smears.
All blood smears with PCR-detected RF Borrelia and an equivalent number without were examined at JHU by 3 different microscopists. Results of smears obtained from patients with fever and read in Tanzania were reviewed at JHU, and a subset was reread. All smears were interpreted in a blinded manner with respect to qPCR results.
Ethics.
The study was approved by the ethics committees of the Johns Hopkins School of Medicine and School of Public Health (Bloomberg) and by the National Institute for Medical Research in Tanzania. Informed consent was obtained from all adult participants and from guardians of pediatric participants.
Nucleotide sequence accession numbers.
All flaB partial sequences were deposited in GenBank with the following accession numbers: for RF4, JF910155; for RF6, JF910154; for RF8, JF910156; for RF16, JF910153; for RF19, JF910152; for RF20, JF910150; and for RF23, JF910151.
RESULTS
Participants.
The age ranges of the participants were from 0 months to 7 years for children and from 18 to 98 years for adults. The median age of the participants in the treatment group was 4 years (intraquartile range [IQR], 25 years) and in the control group was 4 years (IQR, 26 years). We assessed samples from 1,049 persons in 554 households in the intervention group and 1,008 persons in 539 households in the control group for RF Borrelia by qPCR. We tested 1,751 samples from 912 young children, 829 adults, and 10 subjects for whom age was not recorded at baseline and 6,866 samples obtained subsequently during 20 weeks of follow-up. We also tested 382 blood samples from 310 participants evaluated for fever.
Real-time quantitative PCR assay. (i) Analytical sensitivity and specificity.
The glpQ assay for RF Borrelia detected 105 to 100 copies of B. crocidurae glpQ linearly and efficiently (>85% to <115% efficiency). The assay did not detect glpQ when DNA was used from B. miyamotoi, B. burgdorferi, Rickettsia parkeri, R. rickettsii, Neorickettsia helminthoeca, Anaplasma phagocytophilum, Ehrlichia chaffeensis, or cultured Trypanosoma brucei rhodesiense.
(ii) Clinical specificity.
glpQ was not detected by qPCR using blood from patients infected with P. falciparum (17 patients), P. vivax (7 patients), P. ovale (3 patients), or P. malariae (2 patients) or with bacteremia due to Escherichia coli (2 patients), Staphylococcus aureus (2 patients), Pseudomonas aeruginosa (1 patient), or Enterococcus species (1 patient).
RF Borrelia in the Tanzanian cohort (Table 1).
Table 1.
Blood smear and qPCR testing of samples from participants evaluated for febrile illness and for routine surveillancea
| Patient category | No. of participants with indicated results/ total no. of participants (%) |
No. of blood smears with indicated results/ total no. of blood smears (%) | No. of qPCR-positive blood samples/ total no. of blood samples (%) | Median no. of copies/ml | |
|---|---|---|---|---|---|
| qPCR positive | Blood smear positive | ||||
| Febrile illness | 13/310 (4.2%) | 4/310 (1.3%) | 5/382 (1.1%) | 15/382 (3.9%) | 7.9 × 102 |
| Routine surveillance | 7/2,057 (0.3%) | 0/29b (0%) | 0/29b (0%) | 7/8,617 (0.008%) | 4.4 × 103 |
No participant with an RF Borrelia DNA- or Borrelia-positive blood smear result had Plasmodium DNA in blood or detected by blood smear analysis.
Data include results representing smears from 7 qPCR-positive and 22 qPCR-negative age- and sex-matched samples.
Of 8,617 blood samples obtained from 2,057 participants during routine surveillance, 7 (0.08%) samples from 7 (0.3%) participants gave qPCR-positive results for RF Borrelia. Six of the qPCR-positive samples were from participants in the control group (5 from village 8 and 1 from village 2); the 1 treated participant was from village 1. Six were <5 years of age; 3 were male. Four were obtained at baseline and 3 during subsequent routine surveillance (at 6 weeks, 8 weeks, and 4 months).
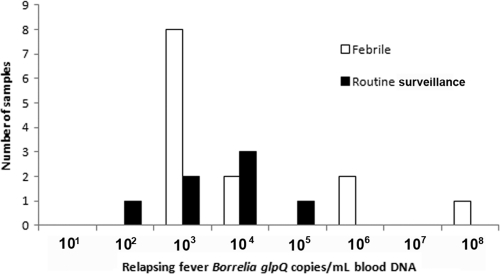
Among participants evaluated for fever, 15 (3.9%) of 382 samples from 13 (4.2%) of 310 subjects gave qPCR-positive results. Twelve of the qPCR-positive samples were from participants in the control group, and 3 were from treated participants (2 from village 4 and 1 from village 3). The age range of febrile qPCR-positive participants evaluated for fever was 0 to 4 years. qPCR results suggested that the level of RF spirochetemia was higher in patients with fever, but the difference was not statistically significant (P=0.37) (Fig. 1). Three participants had RF Borrelia detected by qPCR twice, including 2 participants febrile on dates separated by 27 and 70 days. For one of the two patients, the RF spirochetemia results were similar both times; for the other, the second sample had 105 more DNA copies than the first. For the third participant, 300-fold more RF spirochetal DNA was detected with fever than without 40 days later.
Fig. 1.
Quantitation of relapsing fever Borrelia by quantitative PCR among participants evaluated with febrile illness versus routine surveillance.
Molecular confirmation of RF Borrelia results and phylogenetic analysis.
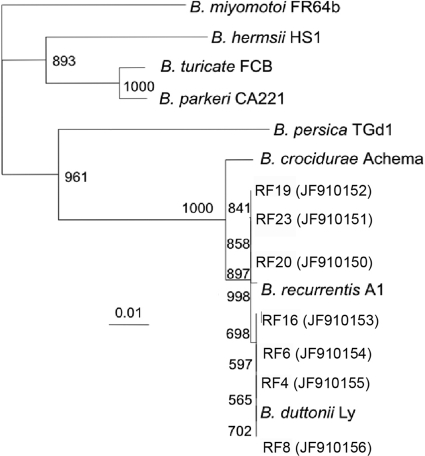
Resuspension and reamplification of DNA identified bands for glpQ of ∼1,500 bp in 4 of 25 samples and bands for flaB of ∼950 bp in 7 of 25 samples. For unknown reasons, none of the glpQ amplicons created for sequencing yielded good-quality sequence data. All 7 flaB amplicons yielded clear sequences in at least one direction for phylogenetic analysis. To ensure that the comparisons were of similar regions, the resulting assembled sequences were aligned, trimmed to compare homologous fragments, and realigned for dendrogram construction (Fig. 2). Between 579 and 607 bp of good-quality sequence data were used. Samples RF4 (JF910155), RF6 (JF910154), RF8 (JF910156), RF16 (JF910153), RF19 (JF910152), RF20 (JF910150), and RF23 (JF910151) all were at least 98.5% to 100% identical to the flaB sequences in the complete genomes of B. recurrentis A1 (GenBank accession no. CP000993.1∣) and B. duttonii Ly (GenBank accession no. CP000976.1).
Fig. 2.
Alignment and phylogenetic analysis of flaB amplified from the blood of 7 Tanzanian patients with relapsing fever caused by Borrelia, represented by RF4, RF6, RF8, RF16, RF19, RF20, and RF23, with GenBank accession numbers in parentheses. Borrelia miyamotoi is included to represent the outgroup. The data represent the numbers of bootstrap replicates out of 1,000 iterations for each branch. The inset scale represents the number of base pair substitutions per 1,000 nucleotides.
Correlation of qPCR with blood smear analysis.
Of 382 samples obtained from patients with fever, both PCR and smear analysis gave positive results for 4 (representing 3 participants, of whom 1 had positive results on two occasions 1 month apart), smear-positive and PCR-negative results for 1, smear-negative and PCR-positive results for 11, and smear-negative and PCR-negative results for 366. Hence, with qPCR as the gold standard, the sensitivity of smear analysis for detecting RF in samples from febrile participants was 26.7% and the specificity was 99.7%. None of 7 RF Borrelia PCR-positive or 22 Borrelia PCR-negative samples obtained at a routine surveillance visit gave smear-positive results for Borrelia.
Although 47 samples were qPCR positive for P. falciparum and/or P. vivax (41 for P. falciparum, 6 for P. vivax, and 1 for both), none was positive for both Borrelia and P. falciparum or P. vivax. Similarly, of the 20 samples that gave smear-positive results for P. falciparum, none was also positive for Borrelia. Hence, no participant sample that gave a qPCR- or smear-positive result for Borrelia was positive for malaria by qPCR or smear analysis.
DISCUSSION
East African tick-borne RF causes significant morbidity and mortality in pregnant women, infants, and young children. The estimated annual incidence of RF is 384/1,000 in children in the Mvumi district of Tanzania, including 163/1,000 in those <5 years of age (prevalence, 5%), and the perinatal mortality rate is 436/1,000 (prevalence, 7.5% in pregnant women) (19, 20). However, limitations in diagnosis have hampered assessment of the burden and distribution of RF in Tanzania and elsewhere.
Although historically considered the gold standard, blood smear analysis is insensitive, especially between relapses, for detection of infection with RF Borrelia. Evaluation of therapies and disease burden requires detection of both symptomatic and asymptomatic (subpatent) infection. Further, blood smear analysis is laborious and not practical for large studies. Given that RF mimics malaria, that both are endemic in Africa, and that misdiagnosis occurs (23), an ideal diagnostic tool would also differentiate RF from malaria.
This is the first report to describe a multiplex, quantitative, high-throughput real-time assay for RF and malaria and to apply it to assess a large cohort with possible RF Borrelia infection and/or malaria. We chose glpQ to broadly detect RF Borrelia but not B. burgdorferi (8); Halperin et al. showed that this target shares 98% identity with B. persica (11). We found that ∼0.08% of blood samples obtained for surveillance gave PCR-positive results for RF Borrelia and that 3.2% of the samples obtained from patients with fever gave positive results. We identified subclinical and clinical infections predominantly in young children, with no sex predomination. In contrast, Makwabe reported a male/female ratio of 1.4:1 among 94 smear-positive samples from children with RF (18).
We also documented the specific presence of B. duttonii and B. recurrentis RF Borrelia infection in central Tanzania. Flagellin gene-based nested PCR performed on argasid ticks and 3 persons suggested the presence of B. duttonii strain Ly in central Tanzania (9) Historically, East African RF is found only in humans and reservoir Ornithodoros ticks, but McCall and colleagues recently used PCR and flagellin-based sequencing to suggest that RF Borrelia infections occur in domestic animals (chicken and pigs) in central Tanzania (19, 20) Our high-throughput assay could support further epidemiologic study.
Our assay detected all but 1 blood smear-identified case of RF. This may have represented infection with a new RF Borrelia strain distinct from B. duttonii (9), although our assay broadly detects B. hermsii, the most closely related New World RF Borrelia species. Our assay was also 100% specific, differentiating RF Borrelia infection from malaria and bacteremia during validation and from malaria in the prospective clinical evaluation.
The assay detected RF Borrelia DNA in samples from smear-negative febrile and nonfebrile participants. Kisinza and colleagues similarly identified RF Borrelia infections by PCR more frequently in febrile children (6/54, or 11%) than in nonfebrile children (13/307, or 4%) (13) and found PCR to be more sensitive than microscopy; blood smears were positive for 3 (6%) febrile children and 7 (2%) nonfebrile children (13) We suggest that positive qPCR results for nonfebrile participants likely represent subpatent RF Borrelia infection. Biologic studies suggest that 105 to >106 spirochetes per ml are present with fever, but smears are negative between relapses (2, 26). However, species-level variations in spirochetemia and illness also occur; B. crocidurae is associated with lower levels of spirochetemia per milliliter and lower rates of mortality than B. duttonii (6). Repeated detection of RF Borrelia over time in one individual may represent relapsing infection or repeated infection due to ongoing exposure in tick-infested dwellings (29). We had limited power to detect an association between clinical illness and spirochetemia; however, infections of greater severity and with higher spirochetemia levels have been reported in cases of smear-defined RF (22). Although Halperin et al. found that PCR results were negative before the onset of fever for 52 tick-bitten participants (10 of whom developed RF) (11), RF DNA might be absent during the incubation period but present between relapses in infected individuals. Further longitudinal studies could confirm that positive qPCR results for smear-negative asymptomatic persons represent subpatent RF rather than false-positive results. We used a second Borrelia-specific target (flaB) and performed sequencing to confirm and further characterize these cases.
We found that a positive blood smear result was more likely for febrile participants, but most qPCR-positive cases did not give blood smear-positive results. van Dam and colleagues suggested that quantitative buffy coat analysis may be 100-fold more sensitive than thick-blood smear analysis (28). In Togo, Nordstrand and colleagues found that 10% of febrile participants with RF gave PCR-positive results and that 13% had corresponding antibodies despite negative blood smears (23). In contrast, Halperin et al. found of 19 of 21 participants with RF gave positive results by both PCR and blood smear analysis (11). Participants with positive PCR results and negative blood smear results could have low-concentration spirochetemia, but this would be unlikely if the estimate of 105 to >106 spirochetes per milliliter of blood during symptomatic disease were accurate. In our small febrile cohort, the median spirochetemia level was equivalent to only 800 spirochetes, much lower than anticipated. Participants could also have had another acute febrile illness and subpatent RF. We excluded malaria by smear and qPCR analysis, which is important since malaria-RF coinfections occur and may increase mortality rates due to RF (16, 23, 29). However, coinfection with another cause of fever is possible.
Despite enrolling a large cohort in an area of RT endemicity (12), we found that the baseline prevalence and incidence of subclinical and clinical RF were low. Optimally, we would have studied an untreated population. Azithromycin could have lowered the incidence of RF, since similar agents—doxycycline and erythromycin—are used to prevent (4) and treat (5) RF and azithromycin is an effective alternative treatment for treatment-susceptible early syphilis (10, 15). Participants may have had other undiagnosed febrile illnesses or may not have reported mild febrile illness. Therefore, RF Borrelia infection may be more common than our study results suggest. Our assay might be more sensitive if performed on the buffy coat (11), since Borrelia can be concentrated in this fraction of blood (3, 28). However, given the size of the clinical trial and the field conditions, we collected blood by finger prick onto filter paper for room-temperature storage and transport instead of collecting whole blood by venipuncture for transport on dry ice. Our collection methodology performed using the finger-prick technique supports proof of principle for our ultimate goal of point-of-care diagnosis.
We conclude that the high-throughput, quantitative multiplex real-time PCR assay described shows promise for specific detection of RF Borrelia, quantitation of spirochetemia in symptomatic and asymptomatic infection, and assessment of the burden of RF versus other causes of fever. We affirm that RF causes febrile illness in Tanzania, especially in children, and suggest that qPCR may effectively support further epidemiologic studies to delineate risk factors for RF, including exposure to tick vectors, and to evaluate control measures. Another platform, such as loop-mediated isothermal amplification, may be more practical for clinical diagnosis of RF in resource-poor settings (14). Comprehensive, longitudinal studies of acute febrile illness are required for confirmation of the sensitivity and specificity of qPCR versus blood smear analysis for RF and of the ultimate utility of qPCR to better assess the global burden of RF Borrelia infection.
ACKNOWLEDGMENTS
We thank Tom Schwann (NIAID, Rocky Mountain Laboratories, Hamilton, MT) for Borrelia positive-control DNA and heat-killed Borrelia grown in culture. Lirong Shi and Amrita Raj processed the filter paper samples for DNA. We thank Edward Sambu and Fikirini Msuya (laboratory technologists, Amani Centre) for their assistance with training of local field staff and for reading peripheral blood smears. We thank Stephen Magesa (Director, Amani Centre) for technical support.
This work was supported in part by the Bill and Melinda Gates Foundation (National Institute of Medical Research, Supplemental Project to Partnership for the Rapid Elimination of Trachoma, PRET+ [48027], Sheila West PI) and by NIAID U01 AI068613 (HIV Prevention Trials Network—Laboratory Network). M.E.R. was supported by a Johns Hopkins Center for Global Health Junior Faculty grant, a Clinician Scientist Career Development Award from the Johns Hopkins School of Medicine, and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K23AIO83931).
Footnotes
Published ahead of print on 20 July 2011.
REFERENCES
- 1. Bacon R. M., Pilgard M. A., Johnson B. J., Raffel S. J., Schwan T. G. 2004. Glycerophosphodiester phosphodiesterase gene (glpQ) of Borrelia lonestari identified as a target for differentiating Borrelia species associated with hard ticks (Acari:Ixodidae). J. Clin. Microbiol. 42: 2326–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bryceson A. D., et al. 1970. Louse-borne relapsing fever. Q. J. Med. 39: 129–170 [PubMed] [Google Scholar]
- 3. Cobey F. C., Goldbarg S. H., Levine R. A., Patton C. L. 2001. Short report: detection of Borrelia (relapsing fever) in rural Ethiopia by means of the quantitative buffy coat technique. Am. J. Trop. Med. Hyg. 65: 164–165 [DOI] [PubMed] [Google Scholar]
- 4. Croft A. M., Jackson C. J., Darbyshire A. H. 2006. Doxycycline for the prevention of tick-borne relapsing fever. N. Engl. J. Med. 355: 1614; author's reply, 1614-1615 [DOI] [PubMed] [Google Scholar]
- 5. Cutler S. J. 2006. Possibilities for relapsing fever reemergence. Emerg. Infect. Dis. 12: 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cutler S. J., Abdissa A., Trape J. F. 2009. New concepts for the old challenge of African relapsing fever borreliosis. Clin. Microbiol. Infect. 15: 400–406 [DOI] [PubMed] [Google Scholar]
- 7. Dworkin M. S., Schwan T. G., Anderson D. E., Jr., Borchardt S. M. 2008. Tick-borne relapsing fever. Infect. Dis. Clin. North Am. 22: 449–468,viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fraser C. M., et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390: 580–586 [DOI] [PubMed] [Google Scholar]
- 9. Fukunaga M., Ushijima Y., Aoki L. Y., Talbert A. 2001. Detection of Borrelia duttonii, a tick-borne relapsing fever agent in central Tanzania, within ticks by flagellin gene-based nested PCR. Vector Borne Zoonotic Dis. 1: 331–338 [DOI] [PubMed] [Google Scholar]
- 10. Golden M. R., Marra C. M., Holmes K. K. 2003. Update on syphilis: resurgence of an old problem. JAMA 290: 1510–1514 [DOI] [PubMed] [Google Scholar]
- 11. Halperin T., et al. 2006. Detection of relapsing fever in human blood samples from Israel using PCR targeting the glycerophosphodiester phosphodiesterase (glpQ) gene. Acta Trop. 98: 189–195 [DOI] [PubMed] [Google Scholar]
- 12. Jongen V. H., van Roosmalen J., Tiems J., Van Holten J., Wetsteyn J. C. 1997. Tick-borne relapsing fever and pregnancy outcome in rural Tanzania. Acta Obstet. Gynecol. Scand. 76: 834–838 [DOI] [PubMed] [Google Scholar]
- 13. Kisinza W. N., McCall P. J., Mitani H., Talbert A., Fukunaga M. 2003. A newly identified tick-borne Borrelia species and relapsing fever in Tanzania. Lancet 362: 1283–1284 [DOI] [PubMed] [Google Scholar]
- 14. Lin X., Chen Y., Lu Y., Yan J., Yan J. 2009. Application of a loop-mediated isothermal amplification method for the detection of pathogenic Leptospira. Diagn. Microbiol. Infect. Dis. 63: 237–242 [DOI] [PubMed] [Google Scholar]
- 15. Lukehart S. A., et al. 2004. Macrolide resistance in Treponema pallidum in the United States and Ireland. N. Engl. J. Med. 351: 154–158 [DOI] [PubMed] [Google Scholar]
- 16. Lundqvist J., et al. 2010. Concomitant infection decreases the malaria burden but escalates relapsing fever borreliosis. Infect. Immun. 78: 1924–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mabey D., Solomon A. W. 2008. Mass antibiotic administration for eradication of ocular Chlamydia trachomatis. JAMA 299: 819–821 [DOI] [PubMed] [Google Scholar]
- 18. Makwabe C. M. 1984. Tick borne relapsing fever in Tanzanian children. Cent. Afr. J. Med. 30: 148, 150 [PubMed] [Google Scholar]
- 19. McCall P. J., et al. 2007. Does tick-borne relapsing fever have an animal reservoir in East Africa? Vector Borne Zoonotic Dis. 7: 659–666 [DOI] [PubMed] [Google Scholar]
- 20. McConnell J. 2003. Tick-borne relapsing fever under-reported. Lancet Infect. Dis. 3: 604. [DOI] [PubMed] [Google Scholar]
- 21. Melese M., et al. 2008. Comparison of annual and biannual mass antibiotic administration for elimination of infectious trachoma. JAMA 299: 778–784 [DOI] [PubMed] [Google Scholar]
- 22. Melkert P. W. 1991. Mortality in high risk patients with tick-borne relapsing fever analysed by the Borrelia-index. East. Afr. Med. J. 68: 875–879 [PubMed] [Google Scholar]
- 23. Nordstrand A., et al. 2007. Tickborne relapsing fever diagnosis obscured by malaria, Togo. Emerg. Infect. Dis. 13: 117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scoles G. A., Papero M., Beati L., Fish D. 2001. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 1: 21–34 [DOI] [PubMed] [Google Scholar]
- 25. Solomon A. W., et al. 2008. Two doses of azithromycin to eliminate trachoma in a Tanzanian community. N. Engl. J. Med. 358: 1870–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stoenner H. G., Dodd T., Larsen C. 1982. Antigenic variation of Borrelia hermsii. J. Exp. Med. 156: 1297–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Dam A. P., van Gool T., Wetsteyn J. C., Dankert J. 1999. Tick-borne relapsing fever imported from West Africa: diagnosis by quantitative buffy coat analysis and in vitro culture of Borrelia crocidurae. J. Clin. Microbiol. 37: 2027–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vial L., et al. 2006. Incidence of tick-borne relapsing fever in west Africa: longitudinal study. Lancet 368: 37–43 [DOI] [PubMed] [Google Scholar]