Abstract
This study investigated microbiological, clinical, and management issues and outcomes for Danish fungemia patients. Isolates and clinical information were collected at six centers. A total of 334 isolates, 316 episodes, and 305 patients were included, corresponding to 2/3 of the national episodes. Blood culture positivity varied by system, species, and procedure. Thus, cases with concomitant bacteremia were reported less commonly by BacT/Alert than by the Bactec system (9% [11/124 cases] versus 28% [53/192 cases]; P < 0.0001), and cultures with Candida glabrata or those drawn via arterial lines needed longer incubation. Species distribution varied by age, prior antifungal treatment (57% occurrence of C. glabrata, Saccharomyces cerevisiae, or C. krusei in patients with prior antifungal treatment versus 28% occurrence in those without it; P = 0.007), and clinical specialty (61% occurrence of C. glabrata or C. krusei in hematology wards versus 27% occurrence in other wards; P = 0.002). Colonization samples were not predictive for the invasive species in 11/100 cases. Fifty-six percent of the patients had undergone surgery, 51% were intensive care unit (ICU) patients, and 33% had malignant disease. Mortality increased by age (P = 0.009) and varied by species (36% for C. krusei, 25% for C. parapsilosis, and 14% for other Candida species), severity of underlying disease (47% for ICU patients versus 24% for others; P = 0.0001), and choice but not timing of initial therapy (12% versus 48% for patients with C. glabrata infection receiving caspofungin versus fluconazole; P = 0.023). The initial antifungal agent was deemed suboptimal upon species identification in 15% of the cases, which would have been 6.5% if current guidelines had been followed. A large proportion of Danish fungemia patients were severely ill and received suboptimal initial antifungal treatment. Optimization of diagnosis and therapy is possible.
INTRODUCTION
Surveillance of fungemia was initiated in Denmark in 2003 and has demonstrated a high incidence of this condition and an increasing proportion of isolates belonging to the not fully susceptible species Candida glabrata and C. krusei from a Nordic as well as a global perspective (4, 9–11, 16, 23, 27, 40, 41, 46, 52).
A number of recent surveys have provided important information on underlying diseases and host factors in patients with fungemia. The most important factors are (i) critical illness with a prolonged stay in the intensive care unit (ICU); (ii) abdominal surgery, especially if it is complicated or repeated; (iii) low birth weight; (iv) acute necrotizing pancreatitis; (v) malignant disease; (vi) organ transplantation, especially of the liver; (vii) Candida colonization; and (viii) use of antibiotics, central venous catheters, steroids, dialysis, and total parenteral nutrition.
The crude 30-day mortality was 30 to 40% in most population-based studies enrolling patients until the turn of the millennium (2, 4, 12, 14, 16, 29, 41, 42, 49, 52) but was lower in recent studies (16, 17, 36) and higher for ICU patients (7, 33), patients infected with C. glabrata (54), and patients with delayed initiation of appropriate antifungal treatment (20, 34). Because timing and diagnostic sensitivity are still major issues in the management of fungemia, an understanding of diagnostic features, risk groups, and epidemiology is of utmost importance for selection of patients who may benefit from early antifungal treatment and for selection of the most appropriate antifungal agent before species identification is available.
On this background, the Danish surveillance scheme was continued, and information on underlying host factors, diagnostic procedures, and antifungal treatment was collected prospectively over a 1-year period for patients admitted to six major centers participating in the national surveillance of fungemia in order to provide a better understanding of the epidemiology and possible areas for improved diagnostics or management. The six participating centers covered half of the Danish population and included two-thirds of the national fungemia cases, with a species distribution mirroring the national data, suggesting that this study provides a representative picture of the national situation.
MATERIALS AND METHODS
Surveillance and population.
During a 1-year period from January to December 2006, fungal blood isolates were collected at the six major Danish departments of clinical microbiology, at Rigshospitalet and Hvidovre Hospital (serving Copenhagen City hospitals and the island of Bornholm since 1 January 2005), Herlev Hospital (serving hospitals in the County of Copenhagen), Statens Serum Institut (serving hospitals in the County of Roskilde), Odense University Hospital (serving hospitals in the County of Funen), and Skejby Hospital (serving hospitals in the County of Aarhus). Together, the six departments served 11 university and 20 district hospitals in the municipality of Copenhagen and the respective counties. The total number of admissions was 609,850, or 53% of the total number of nonpsychiatric hospital beds in Denmark. Altogether, these hospitals served a total population of 2,636,027 patients (1,294,166 males [49.1%] and 1,341,861 females [50.9%]), or 49% of the Danish population. Besides serving the populations of their respective catchment areas, the university hospitals are secondary or tertiary care centers for all of Denmark. In particular, all solid organ transplantations and autologous bone marrow transplantations in Denmark are performed at the participating hospitals, and all allogeneic bone marrow transplantations and liver transplantations are performed at Rigshospitalet. However, it was not feasible to determine the contributions of referred cases from other hospitals in Denmark. Information on the number of nonpsychiatric admissions was retrieved at the Denmark National Board of Health website (www.sundhedsdata.sst.dk). During the study period, two departments used the BacT/Alert blood culture system (bioMérieux, Marcy l'Etoile, France), and four used the Bactec blood culture system (Becton Dickinson, Franklin Lakes, NJ). Surveillance cultures were performed systematically at only one department of intensive care (at Rigshospitalet).
Information on the total number of bloodstream isolates was retrieved from the departments' laboratory information systems. Successive blood cultures with fungi from a patient were considered to be distinct episodes if they occurred at least 21 days apart or were caused by different species. Three hundred of the 334 isolates were sent to the reference laboratory, the Unit of Mycology and Parasitology at Statens Serum Institut, Copenhagen, Denmark, for verification of species identification and susceptibility testing. The isolates not referred from the participating hospital to the reference laboratory comprised 34 isolates (7.2%).
A pro forma document was provided to collect data on each patient with a fungus-positive blood culture and on the time course of the mycological diagnosis. The clinical information requested included the patient's age, sex, and underlying conditions, the hospital department at the time of positive blood culture, the presence or absence of an indwelling catheter, and whether or not the patient was receiving total parenteral nutrition, corticosteroid therapy, or mechanical ventilation or had undergone surgery. Other documented infections were noted, as well as any antibacterial or antifungal therapy given in the 2 weeks before blood culture, the therapy given for the present episode of fungemia, and whether or not the intravenous (i.v.) catheter was removed as part of antifungal treatment. Day 30 mortality was recorded and, whenever possible, classified as either related to the fungemia or not, based on a clinical and microbiological evaluation. Information regarding the diagnosis of the fungemia included the number of positive blood culture bottles and the number of samples taken, whether the blood culture was drawn peripherally or via a catheter, time to positivity, time to species identification, and presence of Candida colonization within the week before blood culture. While full information was not available for all patients, the cooperation and enthusiasm of the participating physicians and laboratories were very high, and data were obtained for 314 of the 316 episodes.
Species identification.
Species identification at the reference laboratory was based on colony morphology on chromogenic agar (CHROMagar Co., Paris, France), microscopic morphology on corn meal agar and rice plus Tween agar (SSI Diagnostika, Hillerød, Denmark), growth at 35°C and 43°C, and use of a commercial system (ATB ID32C; bioMérieux, Marcy l'Etoile, France). In addition, commercial rapid tests for the identification of C. dubliniensis and C. glabrata were used increasingly over the study period (Bichro-Dubli and Glabrata RTT, respectively; Fumouze Diagnostics, Simoco, Denmark).
Susceptibility testing.
Susceptibility testing was performed according to the protocol in EUCAST definitive document EDef 7.1 (45). Manufacturers and stock concentrations of reagents were as follows: dimethyl sulfoxide (DMSO) (D8779), Sigma-Aldrich; fluconazole, Pfizer A/S, Ballerup, Denmark (10,000 μg/ml); amphotericin B (A2411), Sigma-Aldrich, Vallensbaek Strand, Denmark (5,000 μg/ml in DMSO); caspofungin, Merck, Sharp and Dohme, Glostrup, Denmark (5,000 μg/ml in DMSO); itraconazole, Janssen-Cilag, Birkerød, Denmark (5,000 μg/ml in DMSO); and voriconazole, Pfizer A/S, Ballerup, Denmark (5,000 μg/ml in DMSO). Twofold dilutions of drugs in RPMI medium supplemented to a final concentration of 2% glucose were prepared in microtiter plates and stored at −80°C until use. Microtiter plates were read spectrophotometrically at 490 nm. The MIC was defined as the lowest drug dilution giving 100% growth inhibition for amphotericin B and 50% growth inhibition for the other compounds. C. krusei ATCC 6862 was included as a control in each run, and the MIC determinations were accepted if they were within the published ranges for amphotericin B, fluconazole, itraconazole, and voriconazole (18) and within 0.25 to 2 μg/ml for caspofungin, for which no quality control range has yet been published.
Statistics.
Fisher's exact test and the chi-square test for independence were used. P values of <0.05 were considered statistically significant.
RESULTS
Epidemiological and microbiological findings. (i) Epidemiology.
During the 1-year study period, a total of 334 isolates from 316 episodes of fungemia were registered for 305 patients. Overall, the rates of fungemia were 0.5 case/1,000 admissions and 11.9 cases/100,000 somatic admissions (Table 1). The majority of patients were male (57.6%) and between 61 and 80 years of age (55.7%) (Table 1). Age-specific incidences (per 100,000 persons) were 25.1 cases among those aged <1 year, 1.7 cases among 1- to 9-year-olds, and the following for each 10-year age group between 10 and 89 years: 0.3, 2.2, 1.7, 7.8, 13.4, 35.6, 53.2, and 30.9 cases. Finally, the incidence was 28.8 cases/100,000 persons among those aged ≥90 years.
Table 1.
Characteristics of the six participating centers, rates of fungemia, and selected demographic factors for patients with fungemia
| Parameter | Value or description |
||||||
|---|---|---|---|---|---|---|---|
| Funen County | Roskilde County | Copenhagen County | Rigshospitalet | Aarhus County | Copenhagen City and Bornholm | Total | |
| Characteristics of participating centers | |||||||
| No. of hospitals (no. of university hospitals/no. of district hospitals) | 9 (1/8) | 3 (1/2) | 4 (3/1) | 1 (1/0) | 8 (3/5) | 6 (1/5) | 30 (10/20) |
| Population served in the catchment area in 2006 | 478,347 | 241,523 | 618,529 | NAa | 661,370 | 566,862 | 2,636,027a |
| No. of admissions in 2006 | 104,820 | 49,100 | 135,352 | 66,029 | 134,122 | 120,427 | 609,850 |
| Blood culture system | Bactec | Bactec | Bactecb | Bactec | BacT/Alert | BacT/Alert | |
| Fungemia data | |||||||
| No. of patients | 50 | 20 | 42 | 73 | 71 | 49 | 305 |
| No. of episodes | 50 | 20 | 42 | 80 | 72 | 52 | 316 |
| No. of isolates | 52 | 21 | 42 | 89 | 76 | 54 | 334 |
| No. (%) of polyfungal episodes | 2 (4) | 1 (5) | 0 | 6 (8) | 4 (6) | 1 (2) | 14 (4) |
| No. (%) of episodes including bacteria | 18 (36) | 5 (25) | 13 (31) | 17 (21) | 7 (10) | 4 (8) | 64 (20) |
| No. of episodes/1,000 admissions | 0.48 | 0.41 | 0.31 | 1.21 | 0.54 | 0.43 | 0.52 |
| No. of episodes/100,000 patient daysc | 11.3 | 9.7 | 7.9 | 23.5 | 12.5 | 9.5 | 11.9 |
| Demographic factors | |||||||
| No. (%) of females | 19 | 8 | 21 | 32 | 26 | 18 | 124 (40.7) |
| No. (%) of males | 31 | 12 | 21 | 41 | 45 | 31 | 181 (59.3) |
| No. (%) of patients at age (yr): | |||||||
| <1 | 1 | 6 | 1 | 8 (2.6) | |||
| 1-20 | 2 | 3 | 1 | 6 (2.0) | |||
| 21-40 | 1 | 2 | 4 | 5 | 3 | 15 (4.9) | |
| 41-60 | 14 | 6 | 7 | 22 | 15 | 17 | 81 (26.6) |
| 61-80 | 32 | 13 | 24 | 37 | 40 | 24 | 170 (55.7) |
| 81+ | 1 | 9 | 1 | 9 | 5 | 25 (8.2) | |
Rigshospitalet has a variable uptake area depending on specialty and additionally receives an extensive number of patients referred from other parts of Denmark due to the tertiary function of this hospital.
Use of a mycosis bottle in addition to aerobic and anaerobic bottles was introduced for high-risk patients, starting from November 2006.
Somatic patient days only.
The most common species were C. albicans (53.1% [178/335 cases]), C. glabrata (21.8% [73/335 cases]), and C. krusei (7.8% [26/335 cases]). C. parapsilosis and C. tropicalis both accounted for 4.8% (16/335 cases) of cases, C. dubliniensis accounted for 3.3% (11/335 cases) of cases, and 2.4% (8/335 cases) of cases involved other Candida species, including two each involving C. pelliculosa and C. kefyr and one each involving C. guilliermondii, C. inconspicua/norvegensis, C. lusitaniae, and C. intermedia. Finally, 2.1% (7/335 cases) of the isolates were other fungi, including Saccharomyces cerevisiae (3/335 cases) and one each of Cryptococcus neoformans, Fusarium solani, and Rhodotorula sp. The species distribution varied by age and clinical specialty. For example, the C. glabrata proportion increased by age, from 0% (0/14 cases) in patients below 20 years of age to 42% in patients older than 80 years (11/26 cases), and the C. parapsilosis proportion decreased from 14% (2/14 cases) to 0% (0/26 cases) for the same age groups. Furthermore, C. albicans accounted for 92% (11/12 cases) of cases in neonatal and pediatric wards and 61.2% (93/152 cases) of cases in ICU departments, excluding hematological ICUs, but for only 6% (1/18 cases) of cases in hematological units (including hematological ICUs) (Table 2), whereas C. glabrata and C. krusei were significantly more common in the hematological wards (61% of episodes [11/18 cases]) than in other wards (29% of episodes [83/289 cases]) (P = 0.002) (Table 2). Polyfungal infections were found more often in patients in medical wards (9/93 cases [10%]) than in patients in surgical wards (4/159 cases [3%]) (P = 0.018) and most often (12/14 cases) involved at least one species with an inherently low susceptibility to fluconazole (C. glabrata, C. krusei, Saccharomyces cerevisiae, or Rhodotorula sp.) (Table 2).
Table 2.
Species distribution overall and by clinical specialty for 307 episodes of fungemiaa
| Species (n) | No. (%) of episodes |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mixed ICU | Surgical wards (159) |
Medical wards (93) |
Neonatal-pediatric wardsc | Other wards/no information on ward | Total | ||||||||
| ICU-S-GE | ICU-S | S-GE | S | Hematologyb | ICU-M | M | M-GE | Cardiology | |||||
| All species | 36 | 93 | 19 | 33 | 14 | 18 | 4 | 34 | 25 | 12 | 12 | 7 | 307 |
| C. albicans (161) | 25 (69) | 54 (58) | 10 (53) | 17 (52) | 7 (50) | 1 (6) | 4 (100) | 17 (50) | 5 (20) | 8 (67) | 11 (92) | 4 (57) | 163 (53) |
| C. dubliniensis (7) | 2 (2) | 1 (5) | 1 (3) | 1 (3) | 2 (17) | 7 (2) | |||||||
| C. glabrata (65) | 2 (6) | 24 (26) | 4 (21) | 6 (18) | 3 (21) | 5 (28) | 7 (21) | 13 (52) | 1 (14) | 65 (21) | |||
| C. krusei (18) | 3 (8) | 6 (6) | 1 (5) | 1 (3) | 6 (33) | 1 (3) | 18 (6) | ||||||
| C. parapsilosis (14) | 2 (6) | 1 (1) | 1 (5) | 4 (12) | 1 (3) | 3 (12) | 1 (8) | 1 (14) | 14 (5) | ||||
| C. tropicalis (13) | 2 (6) | 3 (3) | 1 (5) | 1 (3) | 1 (7) | 2 (11) | 1 (3) | 1 (4) | 1 (8) | 13 (4) | |||
| Polyfungal infections (15) | 2 (6) | 2 (2) | 2 (14) | 1 (6) | 4 (12) | 3 (12) | 1 (8) | 15 (5) | |||||
| Candida spp. (8) | 1 (5) | 3 (9) | 1 (7) | 2 (11) | 1 (14) | 8 (3) | |||||||
| Other fungi (4) | 1 (1) | 1 (6) | 2 (6) | 4 (1) | |||||||||
Data for dominating species are highlighted in bold. ICU, intensive care unit; S, surgical; GE, gastroenterology; M, medical.
Includes 5 patients in the ICU (2 C. glabrata, 1 C. krusei, and 1 polyfungal episode, as well as an episode with 1 other fungus).
Includes one patient in the ICU (with C. albicans).
(ii) Microbiological details for positive blood cultures.
Concomitant bacteremia was observed for 64 fungemia episodes (20%) and was reported significantly more often at centers using the Bactec blood culture system (53/192 episodes [28%]) than at those using the BacT/Alert system (11/124 episodes [9%]) (P < 0.0001 by Fisher's exact test) (Table 1). The episodes involved coagulase-negative staphylococci in 15 cases (14 of which were detected at centers using the Bactec system), Gram-negative rods in 14 cases (13 of which were detected at centers using the Bactec system), enterococci in 11 cases (8 of which were detected at centers using the Bactec system), Staphylococcus aureus in 5 cases (4 of which were detected at centers using the Bactec system), Lactobacillus or Bacillus spp. in 5 cases (all of which were detected at centers using the Bactec system), streptococci in 4 cases (2 of which were detected at centers using the Bactec system), and polybacterial bacteremia in 10 cases (7 of which were detected at centers using the Bactec system).
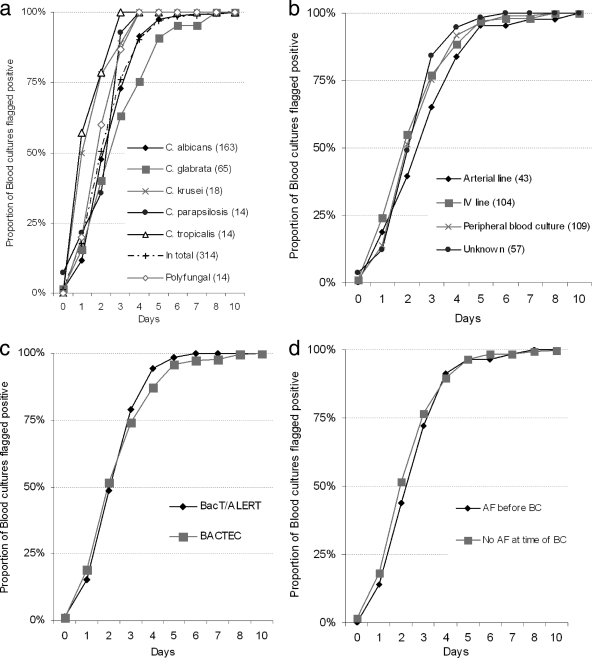
Information regarding the origin of blood culture and incubation time before positivity was available for 313 episodes. The median (range) incubation time before positivity was 2 days (0 to 10 days) but varied by species and by origin of blood culture (Fig. 1). Thus, blood cultures from episodes due to C. tropicalis or C. krusei were positive after a median incubation time of only 1 day (ranges of 1 to 3 days and 1 to 4 days, respectively), whereas blood cultures from episodes due to C. albicans, C. glabrata, or C. parapsilosis were positive after a median incubation time of 3 days, with 25% of the episodes due to C. glabrata being positive on day 4 or later (ranges of 0 to 10 days, 0 to 8 days, and 0 to 4 days, respectively) (Fig. 1a). Blood cultures obtained via an arterial line were positive later than blood cultures obtained via an i.v. line or by venipuncture (Fig. 1b). The necessary incubation times for the two blood culture systems were similar, i.e., 48.8% of samples turned positive by the BacT/Alert system and 51.6% did so by the Bactec system after 2 days of incubation (Fig. 1c). Finally, times to positivity were comparable for patients receiving antifungal agents and those not receiving these agents at the time of collection of blood culture (Fig. 1d).
Fig. 1.
Time to blood culture positivity overall and by species. Numbers of episodes per species are indicated in parentheses. AF, antifungal; BC, blood culture.
One center (Copenhagen County) introduced the use of a Bactec mycosis blood culture bottle for selected high-risk patients in the last two study months. In five episodes (none with ongoing antifungal treatment), a mycosis bottle was used simultaneously with aerobic and anaerobic bottles. In three of these episodes, the mycosis bottle was the only one that was positive (positive for C. albicans, C. glabrata, or C. parapsilosis), whereas in two episodes, the mycosis bottle and at least one of the two to four traditional bottles were positive (for C. albicans or C. glabrata).
(iii) Yeast colonization.
Colonization with yeast within the last 7 days before the day of blood culture was documented in 149/316 episodes (47.1%), and the isolate(s) was identified in 100 episodes. For the remaining episodes, samples either were not taken or were culture negative for yeast. The species causing the fungemia was different from the colonizing flora in 11/100 cases. These included 9/70 cases with C. albicans as the only colonizing fungal species but with fungemia with C. dubliniensis (2 cases), C. tropicalis (1 case), C. glabrata (3 cases), C. albicans plus S. cerevisiae (1 case), C. krusei (1 case), or S. cerevisiae (1 case). Furthermore, in 2/10 cases, C. glabrata was the only colonizing fungus detected, whereas the invasive disease was polyfungal, i.e., C. glabrata was present in combination with C. albicans or C. dubliniensis. The invasive organism was part of the colonizing flora in all other cases, including the 14 cases with polyfungal colonization, and infection was monofungal in all of these cases.
Clinical and outcome characteristics. (i) Underlying host factors.
More than half of the patients had undergone surgery (177 episodes [56%]), the majority of which were abdominal surgeries (81%), and half of the patients were in the ICU (Table 3). One-third of the patients had underlying malignant disease, including solid cancer (24%) and hematological disease (9%), and only 5% were neutropenic (Table 3). Solid organ transplantation had been performed in seven patients (2%; 3 kidney, 3 liver, and 1 combined lung and kidney transplantation), five of whom survived, and 3 patients were autologous stem cell transplant recipients, all of whom survived. Thirty-five patients had underlying diabetes mellitus (12%), three were premature infants (1%), and six were burn patients (2%). Ninety-two percent of the patients had a central venous catheter in place (288/304 patients), 67/294 (23%) patients were on renal replacement therapy, and 73/277 (26%) patients had received corticosteroid therapy in the preceding 2 weeks. The majority of the patients had received antibiotic treatment within the preceding 2 weeks (85.7% [269/314 patients]), and cephalosporins, penicillins, metronidazole, and quinolones were the most commonly prescribed drug classes (data not shown). In a univariate analysis, mortality was significantly higher in patients in the ICU and in patients with leukocytosis, whereas no difference in mortality was observed for the other underlying diseases and host factors (Table 3).
Table 3.
Underlying conditions, host factors, and mortality for 314 patients with fungemia
| Condition (no. of patients with information) | No. of patients | % of patients | No. of deaths | % Mortality | P value for mortalityf |
|---|---|---|---|---|---|
| Surgery (314) | |||||
| Abdomen | 144 | 46 | 58 | 40 | NSb |
| Thorax | 15 | 5 | 4 | 27 | NSb |
| Othera | 13 | 4 | 6 | 46 | |
| Multitraumatized | 5 | 2 | 0 | 0 | |
| No | 137 | 44 | 47 | 34 | |
| ICU (294) | |||||
| Yes | 161 | 51 | 76 | 47 | 0.0001c |
| No | 133 | 42 | 32 | 24 | |
| Mechanical ventilation (290) | |||||
| Yes | 135 | 43 | 64 | 47 | 0.0001c |
| No | 155 | 49 | 40 | 26 | |
| Steroidal therapy (282) | |||||
| Yes | 75 | 24 | 30 | 40 | NSc |
| No | 207 | 66 | 68 | 33 | |
| Parenteral nutrition (288) | |||||
| Yes | 119 | 38 | 38 | 32 | NSc |
| No | 169 | 54 | 63 | 37 | |
| Dialysis (299) | |||||
| Yes | 78 | 25 | 34 | 44 | NSc |
| No | 221 | 70 | 75 | 34 | |
| Solid cancer (314) | |||||
| Yese | 76 | 24 | 30 | 39 | NSc |
| No | 153 | 76 | 85 | 36 | |
| Hematologic disease (300) | |||||
| Yese | 28 | 9 | 8 | 29 | NSc |
| No | 272 | 87 | 102 | 38 | |
| Leukocyte count (286) | |||||
| <0.5 | 17 | 5 | 4 | 24 | |
| 0.5-1.0 | 3 | 1 | 1 | 33 | |
| 2.0-5.0 | 24 | 8 | 7 | 29 | |
| 5.0-7.5 | 37 | 12 | 9 | 24 | |
| 7.6-10 | 36 | 11 | 11 | 31 | |
| >10 | 169 | 54 | 73 | 43 | 0.0086d |
| Total | 314 | 100 | 115 | 37 |
Other surgeries included 6 orthopedic surgeries, 5 neurosurgeries, 1 urologic surgery, and 1 percutaneous coronary angiography.
Comparing this surgery with no surgery.
Comparing “yes” and “no” groups.
Comparing patients with leukocyte counts of >10 with patients with leukocyte counts of ≤10.
Solid cancers involved the gastrointestinal tract in 38 cases, were of urogenital origin in 19 cases, and were other cancers in 19 cases. Hematological disease included 19 cases of acute leukemia, 2 cases of chronic leukemia, 3 cases of lymphoma, and 4 other hematological diseases.
NS, not significant.
(ii) Antifungal treatment.
Antifungal agents had been given as prophylactic, empirical, or preemptive therapy at least 1 day before the blood culture was drawn in 49 episodes. Fluconazole was prescribed in 33 cases (67.3%), an amphotericin B formulation was used in 6 cases (12.2%), caspofungin was used in 3 cases (6.1%), voriconazole was used in 2 cases (4.1%), and several antifungal compounds were prescribed in 5 cases (10.2%). Subsequent candidemia due to C. glabrata, C. krusei, or S. cerevisiae was significantly more common in patients who had received antifungal agents for at least 1 week before becoming blood culture positive (57.1% [16/28 patients]) than in patients with no or shorter exposure (28.3% [73/258 patients] and 28.6% [6/21 patients], respectively) (P = 0.007 by the chi-square test for independence).
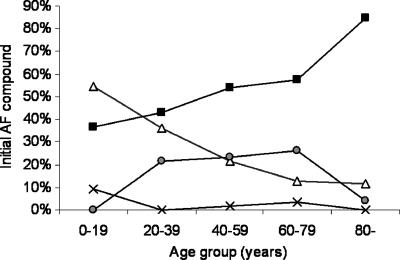
In 278 episodes, antifungal treatment was given after the blood culture was obtained. Fluconazole was prescribed most often (Table 4). There was a noticeable difference in choice of antifungal agent by age group. Thus, the proportion of episodes initially treated with fluconazole increased by age, from 36% in the pediatric setting to 85% for the elderly population, whereas the opposite was true for amphotericin (Fig. 2). The initial antifungal treatment was considered suboptimal in 15% of cases, involving fluconazole for episodes with C. glabrata, C. krusei, S. cerevisiae, or Trichosporon spp., caspofungin for those with C. parapsilosis or Fusarium spp., and voriconazole for those with C. glabrata or C. krusei (Table 4). Overall, antifungal therapy was changed in 99 cases (35%). Seventy-four changes involved fluconazole, i.e., a step-down to fluconazole in 9 cases and a change from initial fluconazole treatment to a broad-spectrum agent in 65 cases. For C. albicans episodes specifically, a step-down to fluconazole therapy was performed in 19% (9/48 cases) of the cases initially treated with a broader agent. For patients with C. glabrata episodes who initially received fluconazole, this was changed to amphotericin (4 episodes) or caspofungin (11 episodes) in 56% of cases (Table 4).
Table 4.
Antifungal treatment of patients with fungemia
| Treatment parameter | No. (%) of cases or % of cases (no. of cases with change/total no. of cases)b |
||||
|---|---|---|---|---|---|
| Amphotericin B formulation | Caspofungin | Fluconazole | Voriconazole | Total | |
| First choice | 48 (17) | 63 (23) | 159 (57) | 8 (3) | 278 |
| No change | 35 (73) | 48 (76) | 90 (57) | 6/8 | 179 (64) |
| Suboptimal initial therapya | 0 (0) | 3 (5) | 33 (21) | 6/8 | 42 (15) |
| Antifungal modifications upon species identification | |||||
| Step-down for C. albicans infection | 20 (4/20) | 15 (4/26) | NA | 1/2 | 19 (9/48) |
| Change of fluconazole for C. glabrata infection | NA | NA | 56 (15/27) | NA | 56 (15/27) |
Initial antifungal therapy was considered suboptimal for the following drug-bug combinations: fluconazole and C. glabrata (27), C. krusei (3), S. cerevisiae (1), or Trichosporon spp. (1); caspofungin and C. parapsilosis (2) or Fusarium spp. (1); and voriconazole and C. glabrata (1) or C. krusei (5).
NA, not applicable.
Fig. 2.
Initial antifungal compound by age group. Solid squares, fluconazole; open triangles, amphotericin B formulation; gray circles, caspofungin; ×, voriconazole.
(iii) Outcomes.
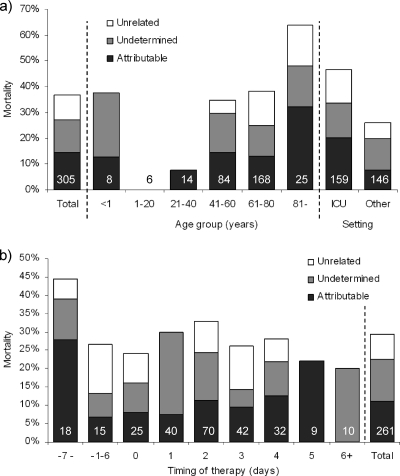
The 30-day mortality was 37% (113/305 patients) overall, 37% (18/49 patients) for patients who received at least 1 day of antifungal treatment before blood culture, and 28% (80/278 patients) among patients who received antifungal treatment after the blood culture was obtained. In 44/113 fatal cases, the death was related primarily or in part to the fungemia (38.9% of deaths; 14.4% of all patients) (Fig. 3a). In 30/113 cases (26.5% of deaths; 9.8% of all patients), it was classified as unrelated to the fungemia, while in 39/113 cases the cause of death was not available (34.5%; 12.8% of all patients) (Fig. 3a). Hence, the attributable mortality was at least 14.4% and was 22.0% if unclassified cases were excluded. The overall as well as attributable mortality was highest for patients older than 80 years of age and lowest for 1- to 20-year-old patients (Fig. 3a), with a significant trend for an increase with age (P = 0.009). Finally, the overall, attributable, and unrelated mortality rates (47%, 20%, and 13%, respectively) were all higher in the ICU setting than for patients in other wards (26%, 8%, and 6%, respectively) (Fig. 3a). For 261 episodes, information was available regarding the timing of therapy and outcome (Fig. 3b). Overall mortality rates ranged from 20 to 44%, with no difference depending on timing of treatment for either overall mortality or the cases classified as attributable to the fungemia. Numerically, mortality was highest for cases involving C. krusei (36%) and lowest for cases involving C. parapsilosis (25%) or other Candida spp. (14%) (P = 0.183) (Table 5). For episodes involving C. glabrata, the mortality was significantly lower for patients receiving caspofungin than for those receiving fluconazole as initial antifungal therapy (12% versus 48%; P = 0.023). For the other species, no significant difference depending on initial antifungal agent was observed (Table 5).
Fig. 3.
Thirty-day mortality according to age (a) and according to timing of initiation of therapy for 261 patients receiving at least 1 day of antifungal treatment (b). Numbers on bars indicate the number of patients in each group. We calculated the days to the start of therapy by subtracting the start date of antifungal therapy from the culture date of the first blood sample that was positive for yeast growth. Negative values indicate the number of days the patient had been on antifungal treatment at the time the blood culture was drawn.
Table 5.
Mortality by species and initial antifungal treatment for the 278 patients receiving antifungal treatment
| Species (n) | Overall % mortality | No. of deaths/no. of treated patients (% mortality) with initial antifungal compound |
P value | |||
|---|---|---|---|---|---|---|
| Amphotericin deoxycholate or lipid formulation | Caspofungin | Fluconazole | Voriconazole | |||
| C. krusei (22) | 36 | 1/4 | 5/10 (50) | 0/3 | 2/5 | |
| C. tropicalis (15) | 33 | 1/5 | 1/3 | 3/7 | 0/0 | |
| C. glabrata (58) | 32 | 4/13 (31) | 2/17 (12) | 13/27 (48) | 0/1 | 0.023a |
| C. albicansb (154) | 28 | 7/20 (35) | 6/28 (21) | 30/104 (29) | 0/2 | 0.34c |
| C. parapsilosis (15) | 25 | 0/4 | 0/2 | 3/9 (33) | 0/0 | |
| Candida spp. (9) | 14 | 0/2 | 0/1 | 1/6 | 0/0 | |
| Other fungi (5) | 17 | 0/0 | 1/2 | 0/3 | 0/0 | |
| Total (278) | 29 | 13/48 (27) | 15/63 (24) | 50/159 (31) | 2/8 | 0.327d |
Comparing mortality rates for patients with C. glabrata receiving caspofungin or fluconazole as the first antifungal compound.
Figures are for C. albicans (148 isolates) and C. dubliniensis (6 isolates).
Comparing mortality rates for patients with C. albicans receiving caspofungin or amphotericin B as the first antifungal compound.
Comparing mortality rates for all patients receiving caspofungin or fluconazole as the first antifungal compound.
DISCUSSION
This study has revealed several important issues concerning the epidemiology, diagnostics, and treatment of fungemia, with implications for a better understanding and improved management of this disease.
The species distribution varied by age, as previously described (4, 11, 23, 46), but also by specialty. Thus, C. glabrata and C. krusei together accounted for half of the cases in patients with underlying hematological disease and in patients at departments of medical gastroenterology. The association between hematology and fungal species has been addressed by others (1, 32, 56). Thus, C. albicans and C. krusei were involved in 14 to 35% and 12 to 24% of cases, respectively, in hematology patients in Australia, Europe, and the United States (22, 47, 48, 51). On this background, our low rates of C. albicans and high rates of C. krusei infection were expected. However, to our knowledge, the large proportion of C. glabrata infections among medical gastroenterology patients has not been reported previously. The median age of the medical gastroenterology patients with fungemia was 61 years, which is lower than that of the total patient population (66 years), and only three of these patients had received azoles before the blood culture was taken. Thus, apparently, neither older age nor azole prophylaxis explains the high C. glabrata rate in this setting.
Polyfungal episodes were significantly more common in medical than in surgical patients. Nace et al. also reported a trend toward more underlying medical diseases among 40 cases of poly-Candida sp. candidemia, but the difference did not reach statistical significance in that study (35).
Yeast colonization was registered in half of the patients but was identified to the species level in only two-thirds of these. The invasive species had been detected in the colonizing flora in all cases with either polyfungal colonization or colonization including less common species. In contrast, the blood isolate belonged to a species different from the colonizing one in 13% of the cases with either C. albicans or C. glabrata monofungal colonization. In half of these cases, treatment guided by the colonizing flora would have been suboptimal. Several studies have previously shown a strong genetic association between the colonizing flora and invasive isolates (15, 19, 28) and have reported that invasive infection rarely arises without prior colonization (30, 39). Our findings suggest that results for colonization samples should be evaluated with caution unless such samples are given high priority (including systematic surveillance culture sampling, the use of chromogenic agars, and species identification of all isolates) in the decision process regarding choice of antifungal compound.
We previously demonstrated a differential performance of the two major automated blood culture systems for the detection of C. glabrata and therefore recommended that a mycosis bottle be included when the Bactec blood culture system is used (11). This was done at one of the centers during the last two study months, with promising results, though the numbers are too small for us to draw any firm conclusions. Moreover, in the present study, we found a difference in the detection rates of episodes with concomitant bacteremia. Thus, significantly fewer such cases were detected at centers using the BacT/Alert system than at those using the Bactec system. Whereas the sensitivity issue for detection of C. glabrata by the Bactec system has also been shown in vitro (26) and the use of fungal selective agars is recommended in order not to miss detection of fungi in polymicrobial specimens (8), we are not aware of studies systematically evaluating the performance of detection of fungi and bacteria simultaneously in blood culture systems. However, a mycosis bottle taken at the same time may increase the diagnostic sensitivity of both systems due to a possible negative influence of concomitant bacteremia in the BacT/Alert system or low sensitivity for C. glabrata infections in the Bactec system. This should be explored further.
More patients in our study had undergone surgery or were in the ICU than was the case in similar studies (16, 17, 23, 36, 52). In the most recent studies, which included patients from Australia and Spain who were enrolled after the millennium, only a quarter of the patients were in the ICU, whereas this was the case for half of the patients in our cohort (16, 17, 36). ICU stay was significantly associated with mortality in our study, as expected (33). Hence, the overall mortality of 38%, which may seem high compared to that in postmillennium reports (22 to 25% mortality), was likely due to this larger proportion of patients with severe underlying disease (16, 17, 36, 38). In agreement with this, the attributable mortality was between 14.4 and 22% and thus in the lower half of the values reported in the literature (10%, 14.5%, 21.5%, 34.7%, and 49%) (21, 24, 57), and the attributable as well as unrelated mortality was higher in the ICU setting in our study.
Antifungal compounds were used prophylactically/empirically in a subset of patients, without an apparent impact on survival or on diagnostics as evaluated by time to blood culture positivity. A larger proportion of infections due to species with intrinsically reduced susceptibility to fluconazole was seen in these patients, in accordance with previous observations (13, 31, 48, 53). However, previous studies have suggested that this reflects a decrease in the number of fluconazole-susceptible infections rather than an increase in the number of fluconazole-resistant infections (25, 31, 53).
Several studies have shown that early treatment improves overall survival (20, 34, 50). To our surprise, we were not able to detect any difference in outcome dependent on the timing of therapy. Either timing had a lesser impact on overall mortality due to the patient population being more severely ill or mortality was driven to a large extent by the underlying disease (7, 44). However, we also observed that attributable mortality was not affected by the timing of therapy. Alternatively, our observations may reflect the fact that the patients most likely to receive early treatment were those with the highest a priori risk of poor outcomes due to being those with the most risk factors and severe underlying disease or those with the highest fungal loads, leading to an earlier blood culture positivity.
The mortality was significantly lower for patients with C. glabrata candidemia receiving caspofungin than for those receiving fluconazole as the first antifungal agent. Several reports have suggested that echinocandins are superior to fluconazole for candidemia not due to C. parapsilosis. Thus, a significantly better outcome was seen overall, and specifically for infections due to C. albicans or C. tropicalis, in a clinical trial comparing anidulafungin and fluconazole (43). Similarly, a significantly better outcome was demonstrated for patients receiving echinocandin in a laboratory-based candidemia surveillance program, and a numerically better response was seen for echinocandin monotherapy for each species except C. parapsilosis (36). In agreement with this, the Infectious Diseases Society of America (IDSA) recommends an echinocandin as first-line therapy, with a subsequent step-down to fluconazole if the organism is susceptible (37). Due to the very low prevalence of C. glabrata and C. krusei but the higher prevalence of C. parapsilosis in the neonatal and pediatric setting in Denmark, the Danish recommendations suggest the use of fluconazole as a first-line agent only in the neonatal and pediatric setting for patients not previously exposed to fluconazole but the use of an echinocandin in the adult population before species identification is obtained. This recommendation was not followed, as the proportion of patients receiving fluconazole as a first-line agent increased by age, leading to as many as 15% of patients initially receiving suboptimal treatment. This number would have been 6.5%, the vast majority of which would have been cases of C. parapsilosis in adults treated with an echinocandin, if the recommendations had been followed. C. parapsilosis is lowly virulent and, in general, associated with a higher survival rate (6). Thus, not only would fewer patients have received suboptimal treatment, but these cases might also have been less grave (3, 6, 20, 23, 38, 54, 55).
In conclusion, the take-home messages in this study are as follows. First, the outcomes for patients with C. glabrata infections were significantly better if the patients received caspofungin, not fluconazole, treatment. Second, not only is Denmark a high-incidence area, but the overall mortality is also high in comparison with that in other surveys conducted after the millennium. The high mortality may be explained in part by a larger proportion of patients with severe underlying diseases but might have been reduced if an echinocandin was given more often as first-line treatment in the adult population, as recommended in official guidelines (5, 37, 43). Third, we have shown that significantly fewer cases with concomitant bacteremia are diagnosed with the BacT/Alert system, suggesting a low sensitivity for fungemia in this important setting. This finding may suggest that a mycosis medium is also recommendable for this blood culture system to ensure maximal sensitivity for diagnosing fungemia.
ACKNOWLEDGMENTS
M.C.A. has received research support grants and honorariums for talks from Astellas, Gilead, Merck, and Pfizer and has received travel grants from Astellas, Merck, Pfizer, and Schering-Plough. S.S. has received a travel grant from Merck. L.N. has received travel grants from Astellas, Merck, and Gilead. J.D.K. has received funds for speaking, consultancy, advisory board membership, or travel from Gilead, Merck Sharp & Dohme, Pfizer, and Swedish Orphan.
Footnotes
Published ahead of print on 29 June 2011.
REFERENCES
- 1. Abi-Said D., et al. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24: 1122–1128 [DOI] [PubMed] [Google Scholar]
- 2. Ahlquist A., et al. 2009. Epidemiology of candidemia in metropolitan Atlanta and Baltimore City and County: preliminary results of population-based active, laboratory surveillance—2008-2009, abstr. M-1241. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 3. Almirante B., et al. 2006. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 44: 1681–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Almirante B., et al. 2005. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 43: 1829–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andes D., et al. 2010. Impact of therapy on mortality across Candida spp. in patients with invasive candidiasis from randomized clinical trials: a patient-level analysis, abstr. M-1312. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 6. Arendrup M., Horn T., Frimodt-Moller N. 2002. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection 30: 286–291 [DOI] [PubMed] [Google Scholar]
- 7. Arendrup M. C. 2010. Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care 16: 445–452 [DOI] [PubMed] [Google Scholar]
- 8. Arendrup M. C., et al. 2007. Diagnostics of fungal infections in the Nordic countries: we still need to improve! Scand. J. Infect. Dis. 39: 337–343 [DOI] [PubMed] [Google Scholar]
- 9. Arendrup M. C., et al. 2005. Seminational surveillance of fungemia in Denmark: notably high rates of fungemia and numbers of isolates with reduced azole susceptibility. J. Clin. Microbiol. 43: 4434–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arendrup M. C., et al. 2008. Semi-national surveillance of fungaemia in Denmark 2004-2006: increasing incidence of fungaemia and numbers of isolates with reduced azole susceptibility. Clin. Microbiol. Infect. 14: 487–494 [DOI] [PubMed] [Google Scholar]
- 11. Arendrup M. C., et al. 2010. National surveillance of fungemia in Denmark (2004 to 2009). J. Clin. Microbiol. 49: 325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Asmundsdottir L. R., et al. 2008. Molecular epidemiology of candidemia: evidence of clusters of smoldering nosocomial infections. Clin. Infect. Dis. 47: e17–e24 [DOI] [PubMed] [Google Scholar]
- 13. Bassetti M., et al. 2009. Incidence of candidaemia and relationship with fluconazole use in an intensive care unit. J. Antimicrob. Chemother. 64: 625–629 [DOI] [PubMed] [Google Scholar]
- 14. Boo T. W., O'Reilly B., O'Leary J., Cryan B. 2005. Candidaemia in an Irish tertiary referral hospital: epidemiology and prognostic factors. Mycoses 48: 251–259 [DOI] [PubMed] [Google Scholar]
- 15. Brillowska-Dabrowska A., Bergmann O., Jensen I. M., Jarlov J. O., Arendrup M. C. 2010. Typing of Candida isolates from patients with invasive infection and concomitant colonization. Scand. J. Infect. Dis. 42: 109–113 [DOI] [PubMed] [Google Scholar]
- 16. Chen S., et al. 2006. Active surveillance for candidemia, Australia. Emerg. Infect. Dis. 12: 1508–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cisterna R., et al. 2010. Nationwide sentinel surveillance of bloodstream Candida infections in 40 tertiary care hospitals in Spain. J. Clin. Microbiol. 48: 4200–4206 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Cuenca-Estrella M., et al. 2007. Multicentre determination of quality control strains and quality control ranges for antifungal susceptibility testing of yeasts and filamentous fungi using the methods of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin. Microbiol. Infect. 13: 1018–1022 [DOI] [PubMed] [Google Scholar]
- 19. Dalle F., et al. 2000. Comparative genotyping of Candida albicans bloodstream and nonbloodstream isolates at a polymorphic microsatellite locus. J. Clin. Microbiol. 38: 4554–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garey K. W., et al. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43: 25–31 [DOI] [PubMed] [Google Scholar]
- 21. Gudlaugsson O., et al. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37: 1172–1177 [DOI] [PubMed] [Google Scholar]
- 22. Hachem R., Hanna H., Kontoyiannis D., Jiang Y., Raad I. 2008. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 112: 2493–2499 [DOI] [PubMed] [Google Scholar]
- 23. Hajjeh R. A., et al. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42: 1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hassan I., Powell G., Sidhu M., Hart W. M., Denning D. W. 2009. Excess mortality, length of stay and cost attributable to candidaemia. J. Infect. 59: 360–365 [DOI] [PubMed] [Google Scholar]
- 25. Holzknecht B. J., et al. 13 November 2010. Decreasing candidemia rate in abdominal surgery patients after introduction of fluconazole prophylaxis. Clin. Microbiol. Infect. [Epub ahead of print.] doi:10.1111/j.1469-0691.2010.03422.x [DOI] [PubMed] [Google Scholar]
- 26. Horvath L. L., George B. J., Murray C. K., Harrison L. S., Hospenthal D. R. 2004. Direct comparison of the BACTEC 9240 and BacT/ALERT 3D automated blood culture systems for Candida growth detection. J. Clin. Microbiol. 42: 115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kibbler C. C., et al. 2003. Management and outcome of bloodstream infections due to Candida species in England and Wales. J. Hosp. Infect. 54: 18–24 [DOI] [PubMed] [Google Scholar]
- 28. Lass-Florl C., et al. 2003. Fungal colonization in neutropenic patients: a randomized study comparing itraconazole solution and amphotericin B solution. Ann. Hematol. 82: 565–569 [DOI] [PubMed] [Google Scholar]
- 29. Laupland K. B., Gregson D. B., Church D. L., Ross T., Elsayed S. 2005. Invasive Candida species infections: a 5 year population-based assessment. J. Antimicrob. Chemother. 56: 532–537 [DOI] [PubMed] [Google Scholar]
- 30. Leon C. M., et al. 2009. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit. Care Med. 37: 1624–1633 [DOI] [PubMed] [Google Scholar]
- 31. Manzoni P., et al. 2008. Routine use of fluconazole prophylaxis in a neonatal intensive care unit does not select natively fluconazole-resistant Candida subspecies. Pediatr. Infect. Dis. J. 27: 731–737 [DOI] [PubMed] [Google Scholar]
- 32. Marr K. A., Seidel K., White T. C., Bowden R. A. 2000. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J. Infect. Dis. 181: 309–316 [DOI] [PubMed] [Google Scholar]
- 33. Marriott D. J., et al. 2009. Determinants of mortality in non-neutropenic ICU patients with candidaemia. Crit. Care 13: R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morrell M., Fraser V. J., Kollef M. H. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49: 3640–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nace H. L., Horn D., Neofytos D. 2009. Epidemiology and outcome of multiple-species candidemia at a tertiary care center between 2004 and 2007. Diagn. Microbiol. Infect. Dis. 64: 289–294 [DOI] [PubMed] [Google Scholar]
- 36. Ortega M., et al. 2010. Candida spp. bloodstream infection: influence of antifungal treatment on outcome. J. Antimicrob. Chemother. 65: 562–568 [DOI] [PubMed] [Google Scholar]
- 37. Pappas P. G., et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48: 503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pappas P. G., et al. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37: 634–643 [DOI] [PubMed] [Google Scholar]
- 39. Pittet D., Monod M., Suter P. M., Frenk E., Auckenthaler R. 1994. Candida colonization and subsequent infections in critically ill surgical patients. Ann. Surg. 220: 751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Playford E. G., Nimmo G. R., Tilse M., Sorrell T. C. 2010. Increasing incidence of candidaemia: long-term epidemiological trends, Queensland, Australia, 1999-2008. J. Hosp. Infect. 76: 46–51 [DOI] [PubMed] [Google Scholar]
- 41. Poikonen E., Lyytikainen O., Anttila V. J., Ruutu P. 2003. Candidemia in Finland, 1995-1999. Emerg. Infect. Dis. 9: 985–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poikonen E., Lyytikainen O., Ruutu P. 2009 Candidaemia in Finland, 1995-1999 versus 2004-2007, abstr. P1961. Abstr. Eur. Conf. Clin. Microbiol. Infect. Dis. [Google Scholar]
- 43. Reboli A. C., et al. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N. Engl. J. Med. 356: 2472–2482 [DOI] [PubMed] [Google Scholar]
- 44. Rex J. H., et al. 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 36: 1221–1228 [DOI] [PubMed] [Google Scholar]
- 45. Rodriguez-Tudela J. L., et al. 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14: 398–405 [DOI] [PubMed] [Google Scholar]
- 46. Sandven P., et al. 2006. Candidemia in Norway (1991 to 2003): results from a nationwide study. J. Clin. Microbiol. 44: 1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sipsas N. V., et al. 2009. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001-2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 115: 4745–4752 [DOI] [PubMed] [Google Scholar]
- 48. Slavin M. A., et al. 2010. Candidaemia in adult cancer patients: risks for fluconazole-resistant isolates and death. J. Antimicrob. Chemother. 65: 1042–1051 [DOI] [PubMed] [Google Scholar]
- 49. St.-Germain G., et al. 2008. Epidemiology and antifungal susceptibility of bloodstream Candida isolates in Quebec: report on 453 cases between 2003 and 2005. Can. J. Infect. Dis. Med. Microbiol. 19: 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taur Y., Cohen N., Dubnow S., Paskovaty A., Seo S. K. 2010. Effect of antifungal therapy timing on mortality in cancer patients with candidemia. Antimicrob. Agents Chemother. 54: 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tortorano A. M., et al. 2002. European Confederation of Medical Mycology (ECMM) prospective survey of candidaemia: report from one Italian region. J. Hosp. Infect. 51: 297–304 [DOI] [PubMed] [Google Scholar]
- 52. Tortorano A. M., et al. 2004. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur. J. Clin. Microbiol. Infect. Dis. 23: 317–322 [DOI] [PubMed] [Google Scholar]
- 53. Trick W. E., Fridkin S. K., Edwards J. R., Hajjeh R. A., Gaynes R. P. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35: 627–630 [DOI] [PubMed] [Google Scholar]
- 54. Viscoli C., et al. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28: 1071–1079 [DOI] [PubMed] [Google Scholar]
- 55. Weinberger M., et al. 2005. Characteristics of candidaemia with Candida-albicans compared with non-albicans Candida species and predictors of mortality. J. Hosp. Infect. 61: 146–154 [DOI] [PubMed] [Google Scholar]
- 56. Wingard J. R., et al. 1991. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N. Engl. J. Med. 325: 1274–1277 [DOI] [PubMed] [Google Scholar]
- 57. Zaoutis T. E., et al. 2005. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin. Infect. Dis. 41: 1232–1239 [DOI] [PubMed] [Google Scholar]