Abstract
All Streptococcus bovis blood culture isolates recovered from January 2003 to January 2010 (n = 52) at the Hospital Universitario Ramón y Cajal were reidentified on the basis of their genetic traits using new taxonomic criteria. Initial identification was performed by the semiautomatic Wider system (Fco. Soria-Melguizo, Spain) and the API 20 Strep system (bioMérieux, France). All isolates were reidentified by PCR amplification and sequencing of both the 16S rRNA and sodA genes and by mass spectrometry using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS; Bruker, Germany). Results of 16S rRNA/sodA gene sequencing were as follows: Streptococcus gallolyticus subsp. gallolyticus, 14/14 (number of isolates identified by 16S rRNA/number of isolates identified by sodA gene sequencing); Streptococcus gallolyticus subsp. pasteurianus, 24/24; Streptococcus spp., 7/0; Streptococcus infantarius subsp. infantarius, 0/2; Streptococcus lutetiensis, 0/5; Leuconostoc mesenteroides, 4/0; and Lactococcus lactis, 3/3. MALDI-TOF MS identified 27 S. gallolyticus isolates but not at the subspecies level, 4 L. mesenteroides isolates, 3 L. lactis isolates, and 6 S. lutetiensis isolates, whereas 12 isolates rendered a nonreliable identification result. Pulsed-field gel electrophoresis grouped all S. gallolyticus subsp. gallolyticus isolates into 3 major clusters clearly different from those of the S. gallolyticus subsp. pasteurianus isolates, which, in turn, exhibited no clonal relationship. The percentages of resistance to the tested antimicrobials were 38% for erythromycin, 23% for fosfomycin, 10% for levofloxacin, 6% for tetracycline, and 4% for co-trimoxazole. The most frequent underlying diseases were hepatobiliary disorders (53%), endocarditis (17%), and malignancies (12%). We conclude that sequencing of the sodA gene was the most discriminatory method and that S. gallolyticus subsp. pasteurianus appears to have a higher genetic diversity than S. gallolyticus subsp. gallolyticus.
INTRODUCTION
Streptococcus bovis, a nonenterococcal group D Streptococcus, can be found as part of the human gastrointestinal microbiota in 5 to 16% of individuals (17). However, it causes bacteremia and endocarditis, particularly in men and in the elderly (7, 8). The association of S. bovis bacteremia and colon tumors was established in the late 1970s (14). Moreover, recent studies have described a frequent association between its isolation and chronic liver and biliary tract disorders (10).
Streptococcal taxonomy has progressively changed according to the description of new species originally grouped as S. bovis. During the late 1990s and at the beginning of the first decade of the 2000s, several authors renamed S. bovis biotype I as Streptococcus gallolyticus subsp. gallolyticus (21), biotype II/1 as Streptococcus lutetiensis (18), and finally, biotype II/2 as S. gallolyticus subsp. pasteurianus (20).
Clinicians still remain unfamiliar with the new taxonomy of S. bovis species, mostly due to the complexity of the current nomenclature and specific identification requirements based on molecular microbiology techniques not available in routine clinical laboratories. Nevertheless, due to their specific disease association and their microbiology features, a proper identification of the S. bovis isolates is needed. The aim of this study was to review all S. bovis bacteremic episodes documented over the last 7 years at the Hospital Universitario Ramón y Cajal, focusing on the new taxonomy and the probable association of different subspecies and pathologies.
MATERIALS AND METHODS
Bacterial identification.
All S. bovis isolates (n = 52, from 51 patients) causing bacteremia recovered from blood cultures between January 2003 and January 2010 were studied. Initially, S. bovis identification was routinely performed using the commercial API 20 Strep gallery (bioMérieux, Marcy l'Etoile, France) and the semiautomated Wider system (Fco. Soria Melguizo, Madrid, Spain) (5). The ability to grow on bile esculin agar (BD, NY) was determined after 24 h of incubation at 37°C. Isolates were tested for the presence of Lancefield streptococcal antigen D by agglutination using a Slidex Strepto Plus kit (bioMérieux, Marcy l'Etoile, France).
Subsequently, both 16S rRNA gene PCR with universal primers (primer 16-F [5′-AGGATTAGATACCCTGGTAGTCCA-3′] and primer 16-R [5′-AGGCCCGGGAACGTATTCAC-3′]) and sodA PCR with degenerate primers (primer dl [5′-CCITAYICITAYGAYGCIYTIGARCC-3′] and primer d2 [5′-ARRTARTAIGCRTGYTCCCAIACRTC-3′]) were performed (18). Amplicons (500 bp for 16S rRNA and 609 bp for sodA) were sequenced using an ABI Prism 377 automated sequencer (Perkin Elmer, Norwalk, CT) after purification with ExoSAP-IT (Amersham, Bucks, United Kingdom). Sequences were aligned using the ClustalW tool from the website www.ebi.ac.uk, and phylogenetic trees were constructed with TreeView software (Michael Eisen, Stanford University, Stanford, CA).
Mass spectrometry using a Bruker Biotyper MALDI78 TOF MS matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI TOF MS) system (Bruker Daltonics, Germany) was also performed as part of the bacterial reidentification scheme (3).
Antimicrobial susceptibility testing.
Susceptibility testing (microdilution method) was performed using Wider panels for Gram-positive organisms, and the results were interpreted according to Clinical and Laboratory Standards Institute criteria (5, 6).
Genetic diversity.
Clonal relatedness was determined by pulsed-field gel electrophoresis (PFGE) using a protocol initially described for Streptococcus suis serotype 2 (15). A dendrogram was constructed using the Phoretix (version 5.0) software (Nonlinear Dynamics Ltd., United Kingdom) on the basis of the Dice coefficient.
Patients.
Clinical charts of all patients were reviewed with the approval of the Ethics Committee of the Hospital Universitario Ramón y Cajal to assess both demographic and clinical data as well as diagnostics and treatments. All patients in whom colonic pathologies were suspected were submitted to examination by colonoscopy. A possible biliary source of bacteremia was assigned if there was a clinical or surgical diagnosis of acute cholecystitis or cholangitis, after the exclusion of other possible foci of infection.
RESULTS
Isolates previously identified as Streptococcus bovis were reidentified using the current nomenclature. All 52 isolates, including the nonstreptococcal ones, had a clear positive reaction with the latex agglutination test for group D Streptococcus. Initial data from the API 20 Strep system were not available, and contemporary profiles corresponded to 29 S. bovis II/2 isolates (which included 24 S. gallolyticus subsp. pasteurianus and 5 S. lutetiensis isolates, according to the sodA identification), 14 S. bovis I isolates, 2 S. bovis II/4 isolates, 3 Lactococcus lactis isolates, and 4 Leuconostoc sp. isolates.
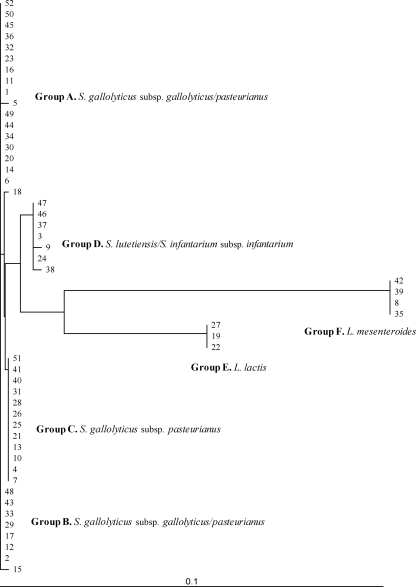
Nucleotide sequences of 16S rRNA amplicons (500 bp) allowed the classification of the isolates as Streptococcus gallolyticus subsp. gallolyticus (n = 14), Streptococcus gallolyticus subsp. pasteurianus (n = 24), Streptococcus spp. (n = 7), Lactococcus lactis (n = 3), and Leuconostoc mesenteroides (n = 4) (Fig. 1).
Fig. 1.
Clustering of 16S rRNA nucleotide sequences obtained from all 52 isolates and constructed by TreeView software.
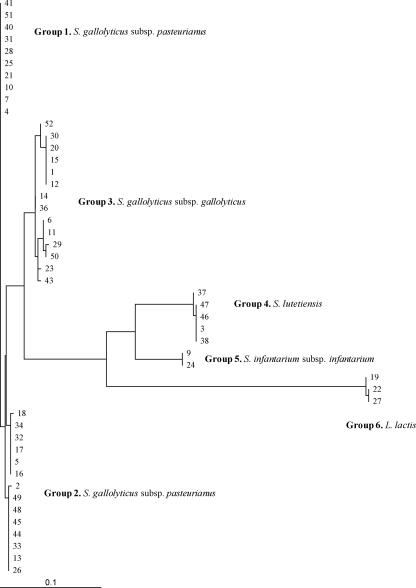
Isolates were also reclassified on the basis of the nucleotide sequence of an internal fragment of the sodA gene as S. gallolyticus subsp. gallolyticus (n = 14), S. gallolyticus subsp. pasteurianus (n = 24), Streptococcus infantarius subsp. infantarius (n = 2), Streptococcus lutetiensis (n = 5), and L. lactis (n = 3). The seven Streptococcus sp. isolates not identified at the species level by the 16S rRNA sequence analysis were characterized as S. infantarius subsp. infantarius and S. lutetiensis using sodA gene amplification and sequencing. Negative results for sodA amplification were consistently obtained in the 4 isolates previously identified as L. mesenteroides by 16S rRNA sequencing. The sodA nucleotide phylogenetic analysis discriminated the S. gallolyticus subsp. pasteurianus isolates into two clusters (Fig. 2), although the amino acid sequences remained identical (Fig. 3). However, several amino acid differences were detected in the S. gallolyticus subsp. gallolyticus group.
Fig. 2.
Clustering of sodA nucleotide sequences obtained from non-Leuconostoc isolates (n = 48) and constructed by TreeView software.
Fig. 3.
Comparison of the amino acid sequences for the different sodA alleles of the sodA protein detected.
Finally, MALDI-TOF MS identified 27 S. gallolyticus isolates but not subspecies, 4 L. mesenteroides isolates, 3 L. lactis isolates, and 6 S. lutetiensis isolates, whereas in 12 isolates a nonreliable identification result was obtained.
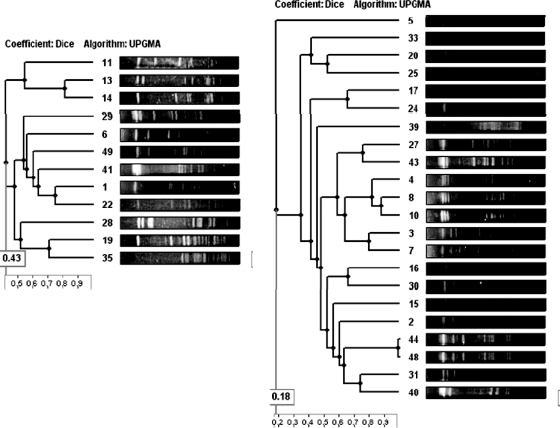
PFGE analysis with SmaI digestion grouped all S. gallolyticus subsp. gallolyticus isolates into 3 major clusters clearly different from those of the S. gallolyticus subsp. pasteurianus isolates, which, in turn, exhibited no related PFGE patterns among them (Fig. 4).
Fig. 4.
Dendrogram obtained for the 24 S. gallolyticus subsp. pasteurianus and 14 S. gallolyticus subsp. gallolyticus isolates using the unweighted-pair group method using average linkages method and Phoretix (version 5.0) software.
Antimicrobial susceptibility results are shown in Table 1. All isolates remained susceptible to penicillin, ampicillin, amoxicillin-clavulanate, oxacillin, quinupristin-dalfopristin, linezolid, and rifampin. Resistance to the glycopeptides vancomycin and teicoplanin was observed only in L. mesenteroides isolates. As expected, all streptococcal isolates (n = 45) showed low-level resistance to aminoglycosides. Additionally, 16 isolates exhibited high-level resistance (MIC50, >1,000 mg/liter) to streptomycin, whereas high-level resistance to gentamicin was not observed. Resistance percentages for the other antimicrobials tested varied according to the bacterial species (Table 1).
Table 1.
Antibiotic susceptibility for the different species studied
| Species | % isolates antibiotic resistant (MIC50)a |
|||||
|---|---|---|---|---|---|---|
| Str | Lev | Ery | Cln | Fos | SxT | |
| S. gallolyticus subsp. gallolyticus (n = 14) | 42.8 (≤1,000) | 14.2 (2) | 35.7 (≤0.5) | 35.7 (≤0.5) | 35.7 (≤32) | 35.7 (≤1/19) |
| S. gallolyticus subsp. pasteurianus (n = 24) | 20.8 (≤1,000) | 8.3 (≤1) | 37.5 (≤0.5) | 25.0 (≤0.5) | 29.1 (≤32) | 25.0 (≤1/19) |
| S. infantarius subsp. infantarius (n = 2) | 0 (≤1,000) | 0 (≤1) | 0 (≤0.5) | 0 (≤0.5) | 0 (≤32) | 0 (≤1/19) |
| S. lutetiensis (n = 5) | 60.0 (>1,000) | 40.0 (2) | 60.0 (>2) | 60.0 (>2) | 20.0 (≤32) | 20.0 (≤1/19) |
| L. lactis (n = 3) | 66.6 (>1,000) | 0 (2) | 33.3 (≤0.5) | 33.3 (≤0.5) | 66.6 (>64) | 66.6 (>2/38) |
| L. mesenteroides (n = 4) | 0 (≤0.25) | 0 (2) | 50.0 (≤0.5) | 25.0 (≤0.5) | 25.0 (≤0.32) | 25.0 (≤1/19) |
High-level resistance, MIC50 > 1,000 mg/liter. Str, streptomycin; Lev, levofloxacin; Ery, erythromycin; Cln, clindamycin; Fos, fosfomycin; SxT, trimethoprim-sulfamethoxazole. The MIC50s, presented in parentheses, are in mg/liter.
When clinical data from all 51 patients with documented S. bovis bacteremia were considered, the patients' median age was 73.5 ± 15 years (range, 27 to 98 years; mode, 82 years). The gender distribution was 28 males (54.9%) and 23 females (45.1%). A mean of 6.5 episodes per year was documented, with the maximum (n = 10 episodes) occurring in 2005. An accumulation of cases between April and June (n = 21) was observed, with the incidence from October to December being lower (n = 7). Blood samples for culture were obtained in different wards, with the emergency unit being the most frequent (51%), followed by the general medicine unit (16%), intensive care unit (10%), gastroenterology unit (8%), and others (15%). The most frequent symptoms at hospital admission were fever and abdominal pain.
The patients' underlying diseases are shown in Table 2 . The most common feature detected was hepatobiliary disorder (53%), followed by endocarditis (17.6%), cardiovascular disease (11.7%), diverticulitis and/or colon polyps (11.7%), digestive tract carcinomas (8%), ferropenic anemia (6%), and other malignancies (4%). Only one patient died during the bacteremic episode. Interestingly, a female had two different bacteremic episodes separated by 3 years, and these were caused by genetically different S. gallolyticus subsp. pasteurianus isolates.
Table 2.
Patients' clinical features
| Clinical condition or finding | No. of patients |
|||||
|---|---|---|---|---|---|---|
| S. gallolyticus subsp. gallolyticus | S. gallolyticus subsp. pasteurianus | S. lutetiensis | S. infantarius subsp. infantarius | L. lactis | L. mesenteroides | |
| Cholecystitis/cholangitis | 3 | 5 | 1 | |||
| Cirrhosis | 2 | 10 | 1 | 1 | 1 | |
| HCVa | 5 | 1 | 1 | |||
| Acute appendicitis | 1 | |||||
| Colonic adenoma | 1 | 4 | 1 | |||
| Colonic polyps | 2 | 7 | 1 | |||
| Rectal carcinoma | 1 | |||||
| Diabetes | 2 | |||||
| Ischemic cardiopathy | 3 | 1 | 1 | 1 | ||
| Endocarditis | 1 | 6 | ||||
| Mitral stenosis | 1 | |||||
| Renal insufficiency | 1 | |||||
| Urinary tract infection | 1 | 1 | ||||
| Bladder cancer | 1 | |||||
| Prostate cancer | 1 | |||||
| Pulmonary cancer | 1 | |||||
| Mucosa-associated lymphoid tissue lymphoma | 1 | |||||
| Leukemia | 1 | |||||
HCV, hepatitis C virus.
DISCUSSION
The former S. bovis group is, at present, divided into four major subspecies (12), with two of them (S. gallolyticus subsp. gallolyticus, formerly S. bovis biotype I, and S. gallolyticus subsp. pasteurianus, formerly S. bovis biotype II/2) being genetically closely related and the other two (S. infantarius subsp. infantarius and S. lutetiensis, named S. infantarius subsp. coli by some authors [2] and formerly S. bovis biotype II/1) being more distantly related. Streptococcus gallolyticus subsp. macedonicus is also a member of the group, but it is generally considered nonpathogenic for humans. However, mainly due to discrepancies still remaining, a deeply genetics-based classification is awaited.
A clear association between S. gallolyticus subsp. gallolyticus causing bacteremia and/or endocarditis and the presence of colon cancer has been reported (1, 4, 11). Nevertheless, the basis of this association is still not completely understood. In this study, half of the patients had hepatobiliary disorders, whereas endocarditis or cardiovascular diseases were observed in only 17.6 and 11.7% of the patients, respectively. Colonic polyps and carcinomas of the gastrointestinal tract were diagnosed in 11.7 and 8% of the patients, respectively. When only those patients with S. gallolyticus subsp. gallolyticus bacteremia (n = 14) were considered, malignances had previously been diagnosed in 7 of them (50%), although some of them were not related to the gastrointestinal tract (lymphoma, leukemia, or prostate carcinoma), even though colonoscopy exploration was not performed in all patients.
Different authors suggest a positive association between S. gallolyticus subsp. gallolyticus isolation and male and elderly patients and endocarditis (7, 18). In our case, the gender distribution was balanced (55% of male individuals), the age of presentation was about 70 years, and endocarditis was detected in only 17% of patients. Our results are in concordance with those recently published by another Spanish group (9).
During the study period, 76,089 blood samples from patients with suspected bacteremia were processed in our laboratory. From these, 6,935 (9.1%) were positive, and 52 of them rendered an S. bovis identification (0.74%), although considering the data from our present work, only 45 isolates in fact corresponded to this species (0.64%). During the same period, 270 cases of infective endocarditis were diagnosed at the Hospital Universitario Ramón y Cajal, and these were mostly due to Staphylococcus aureus (30.7%), coagulase-negative staphylococci (17.4%), and Enterococcus (13.3%). S. bovis was identified in 7 cases (6 S. gallolyticus subsp. pasteurianus isolates and 1 S. gallolyticus subsp. gallolyticus isolate), representing 2.6% of all cases of endocarditis (E. Navas et al., unpublished results).
Surprisingly, 4 Leuconostoc mesenteroides and 3 Lactococcus lactis isolates causing bacteremia were initially identified as S. bovis by the semiautomated system used in our laboratory. When reidentification was obtained by 16S rRNA gene sequencing, the bacteria were reidentified using the same system, and it was observed that the misleading identification was due to their slow biochemical reactivity; at 24 h of incubation the identification was again S. bovis, whereas at 48 h, the identification was correct. Moreover, vancomycin resistance in Leuconostoc isolates was observed only at 48 h. Another important factor to be considered is the positive agglutination of our Leuconostoc and Lactococcus isolates for Lancefield antigen D. We were unable to find similar references to this finding, and this fact has been demonstrated only for Pediococcus sp. isolates (16).
As it has been described by other authors (13, 19), we detected differences in isolate identification between the 16S rRNA and the sodA gene sequencing methods, with partial sequencing of the sodA gene being considered the most accurate method of identification. Recently, a high concordance between MALDI-TOF MS and sodA nucleotide sequencing has been described (12), although in our experience, the Bruker technology is not yet able to identify Streptococcus isolates, especially S. gallolyticus subsp. pasteurianus. The results obtained with the contemporary API 20 Strep system agree with the sodA sequencing classifications, although 5 S. lutetiensis isolates showed the same profile as the S. gallolyticus subsp. pasteurianus isolates. Nevertheless, the API 20 Strep system can be considered a useful method to discriminate the two main S. gallolyticus subspecies, but from clinical and epidemiological points of view, it could also be of interest to further discriminate S. infantarius subsp. infantarius and S. lutetiensis using the sodA analysis.
In spite of many efforts that have been made to improve S. bovis group identification, data from genetic studies remain unavailable. The multilocus sequence typing tool has not yet been developed, and there has been only one reference describing high genetic diversity among Italian S. bovis isolates, which was determined by PFGE (22). In our experience, high genetic diversity was also observed among the 45 streptococcal isolates.
Our study demonstrated that in a routine clinical microbiology laboratory, incorporation of a MALDI-TOF MS system will partially solve the problems with identification of members of the S. bovis group. Nevertheless, this system will indicate the presence of S. gallolyticus and further molecular testing can be performed if needed. The presence of data entries in the database for different S. gallolyticus subspecies should be a goal for future MALDI-TOF MS systems for testing of other species.
ACKNOWLEDGMENTS
R.D.C. has a Miguel Servet contract (CB05/137) from the Instituto de Salud Carlos III-FIS. M.R.-B. has a contract from the Instituto de Salud Carlos III-FIS, project AI07/90034. This work was partially funded by the European Project TROCAR (HEALTH-F3-2008-223031).
Footnotes
Published ahead of print on 13 July 2011.
REFERENCES
- 1. Abdulamir A. S., Hafidh R. R., Bakar F. A. 2011. The association of Streptococcus bovis/gallolyticus with colorectal tumors: the nature and the underlying mechanisms of its etiological role. J. Exp. Clin. Cancer Res. 30:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beck M., Frodl R., Funke G. 2008. Comprehensive study of strains previously designated Streptococcus bovis consecutively isolated from human blood cultures and emended description of Streptococcus gallolyticus and Streptococcus infantarius subsp. coli. J. Clin. Microbiol. 46:2966–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bizzini A., Durussel C., Bille J., Greub G., Prod'hom G. 2010. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J. Clin. Microbiol. 48:1549–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boleij A., Schaeps R. M., Tjalsma H. 2009. Association between Streptococcus bovis and colon cancer. J. Clin. Microbiol. 47:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cantón R., et al. 2000. Evaluation of the Wider system, a new computer-assisted image-processing device for bacterial identification and susceptibility testing. J. Clin. Microbiol. 38:1339–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2007. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. Document M100-S17 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Corredoira J., et al. 2008. Characteristics of Streptococcus bovis endocarditis and its differences with Streptococcus viridans endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 27:285–291 [DOI] [PubMed] [Google Scholar]
- 8. Durante-Mangoni E., et al. 2008. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch. Intern. Med. 168:2095–2103 [DOI] [PubMed] [Google Scholar]
- 9. Fernández-Ruiz M., et al. 2010. Streptococcus bovis bacteraemia revisited: clinical and microbiological correlates in a contemporary series of 59 patients. J. Infect. 61:307–313 [DOI] [PubMed] [Google Scholar]
- 10. González-Quintela A., Martínez-Rey C., Castroagudin J. C., Rajo-Iglesias M. C., Domínguez-Santalla M. J. 2001. Prevalence of liver disease in patients with Streptococcus bovis bacteraemia. J. Infect. 42:116–119 [DOI] [PubMed] [Google Scholar]
- 11. Gupta A., Madani R., Mukhtar H. 2010. Streptococcus bovis endocarditis, a silent sign for colonic tumour. Colorectal Dis. 12:164–171 [DOI] [PubMed] [Google Scholar]
- 12. Hinse D., et al. 2011. Differentiation of species of the Streptococcus bovis/equinus-complex by MALDI-TOF mass spectrometry in comparison to sodA sequence analyses. Syst. Appl. Microbiol. 34:52–57 [DOI] [PubMed] [Google Scholar]
- 13. Hoshino T., Fujiwara T., Kilian M. 2005. Use of phylogenetic and phenotypic analyses to identify nonhemolytic streptococci isolated from bacteremic patients. J. Clin. Microbiol. 43:6073–6085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klein R., et al. 1977. Association of Streptococcus bovis with carcinoma of the colon. N. Engl. J. Med. 297:800–802 [DOI] [PubMed] [Google Scholar]
- 15. Luey C. K., et al. 2007. Rapid pulsed-field gel electrophoresis protocol for subtyping of Streptococcus suis serotype 2. J. Microbiol. Methods 68:648–650 [DOI] [PubMed] [Google Scholar]
- 16. Murray P. R., Baron E. J., Jorgensen J. H., Pfaller M. A., Yolken R. H. (ed.). 2003. Manual of clinical microbiology, 8th ed American Society for Microbiology, Washington, DC [Google Scholar]
- 17. Noble C. J. 1978. Carriage of group D streptococci in the human bowel. J. Clin. Pathol. 31:1182–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poyart C., Quesne G., Trieu-Cuot P. 2002. Taxonomic dissection of the Streptococcus bovis group by analysis of manganese-dependent superoxide dismutase gene (sodA) sequences: reclassification of ‘Streptococcus infantarius subsp. coli ’ as Streptococcus lutetiensis sp. nov. and of Streptococcus bovis biotype II.2 as Streptococcus pasteurianus sp. nov. Int. J. Syst. Evol. Microbiol. 52:1247–1255 [DOI] [PubMed] [Google Scholar]
- 19. Sasaki E., Osawa R., Nishitani Y., Whiley R. A. 2004. Development of a diagnostic PCR assay targeting the Mn-dependent superoxide dismutase gene (sodA) for identification of Streptococcus gallolyticus. J. Clin. Microbiol. 42:1360–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schlegel L., et al. 2000. Streptococcus infantarius sp. nov., Streptococcus infantarius subsp. infantarius subsp. nov. and Streptococcus infantarius subsp. coli subsp. nov., isolated from humans and food. Int. J. Syst. Evol. Microbiol. 50:1425–1434 [DOI] [PubMed] [Google Scholar]
- 21. Schlegel L., Grimont F., Ageron E., Grimont P. A. D., Bouvet A. 2003. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int. J. Syst. Evol. Microbiol. 53:631–645 [DOI] [PubMed] [Google Scholar]
- 22. Tripodi M. F., Fortunato R., Utili R., Triassi M., Zarrilli R. 2005. Molecular epidemiology of Streptococcus bovis causing endocarditis and bacteraemia in Italian patients. Clin. Microbiol. Infect. 11:814–819 [DOI] [PubMed] [Google Scholar]