Abstract
Candida auris is a newly described species whose clinical significance is not clear. Here, we describe the first three cases of nosocomial fungemia caused by C. auris, which confirms that it is a causative agent of bloodstream infections. All three patients presented persistent fungemia for 10 to 31 days. The isolates obtained from the three patients were misidentified as Candida haemulonii and Rhodotorula glutinis by the Vitek 2 and the API 20C systems, respectively. C. auris was confirmed by sequence analysis of the internal transcribed spacer region and D1/D2 regions of the 26S ribosomal DNA of the rRNA gene. The MIC ranges of amphotericin B (AMB), fluconazole (FLU), itraconazole, and voriconazole were 0.5 to 1, 2 to 128, 0.125 to 2, and 0.06 to 1 μg/ml, respectively. All isolates were susceptible to caspofungin (MIC = 0.06 μg/ml) and micafungin (MIC = 0.03 μg/ml). One patient developed breakthrough fungemia while receiving FLU therapy, and two patients who received FLU therapy followed by AMB showed therapeutic failure and fatal outcomes. Our cases show that C. auris fungemia can be persistent, despite FLU or AMB therapy, which emphasizes the importance of accurately identifying this species.
INTRODUCTION
Candida auris, an unusual species found in human ear specimens (4, 8), was first reported in Japan in 2009 when an isolate was recovered from the external ear canal of an inpatient (8). Subsequently, isolation of C. auris from ear specimens of 15 patients with chronic otitis media was reported in South Korea (4). Some of these isolates showed resistance to fluconazole (FLU), and pulsed-field gel electrophoresis (PFGE) analysis revealed that 15 isolates of C. auris from three South Korean hospitals shared seven PFGE patterns, which suggests that some of these isolates are related to clonal transmission (7). Therefore, C. auris is notable because of its resistance to azole antifungal agents and its potential for clonal transmission. However, because histopathological proof of fungal infection has not been obtained in any of these patients, the clinical significance of C. auris as a human pathogen has remained undetermined (4).
Here we report three cases of C. auris fungemia identified at three university hospitals in South Korea. Two of these cases were found during a multicenter surveillance study of candidemia conducted in South Korea in 2009. The other case was incidentally found by molecular identification of unidentified yeasts which were recovered in 1996 as bloodstream isolates. To the best of our knowledge, for the first time, we describe here the microbiological and clinical features of bloodstream isolates of C. auris, with an emphasis on their antifungal resistance and poor outcome due to persistent fungemia, despite therapy.
CASE REPORTS
Case 1.
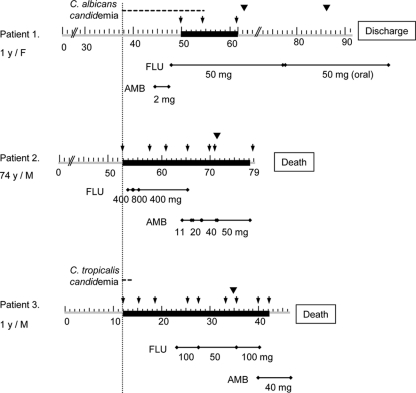
A 1-year-old girl visited Chonnam National University Hospital due to respiratory arrest caused by chicken bones in her throat. Upon arrival, she underwent an emergency operation for removal of the foreign bodies. After surgery, she was treated with antibiotics, central catheterization, and mechanical ventilation because of aspiration pneumonia and hypoxic encephalopathy. On hospital day 37, Candida albicans was isolated from the blood cultures, and it was repeatedly isolated for 18 days. On day 46, amphotericin B deoxycholate (AMB) was started, and on day 49, therapy was changed to intravenous FLU (50 mg/day) (Fig. 1). However, during FLU therapy, blood culture yielded C. albicans and C. auris persistently on days 51 and 54 and C. auris on day 60. After central venous catheter (CVC) removal on day 61, blood cultures became negative and FLU was continued for 48 days. No organism was found on central venous catheter tip culture. The patient completely recovered and was discharged on day 91.
Fig. 1.
Treatment regimens and outcomes for three patients with Candida auris fungemia. Therapeutic failure, which was defined as persistence of Candida in the bloodstream despite 3 days of antifungal therapy or development of breakthrough candidemia while receiving antifungal agents for 3 or more days, was observed in all three patients. Gray bars, period of hospital stay, with numbers denoting hospital days; black bars, period of blood culture positivity for C. auris; line with closed diamonds, period and dose (per day) of antifungal treatment; inverted closed triangles, time of removal of vascular catheter; arrows, dates of positive blood culture.
Case 2.
A 74-year-old man presenting with dyspnea and hoarseness was admitted to Ajou University Hospital. On hospital day 14, he underwent total laryngectomy due to laryngeal squamous cell carcinoma. After surgery, he developed complications of aspiration pneumonia and lower gastrointestinal bleeding. He was treated with antibiotics, central catheterization, and angiographic embolization. On day 53 of admission, a diagnosis of C. auris fungemia was made, and the fungemia persisted for 26 days. Antifungal therapy was initiated on day 54 with FLU (400 mg/day) and changed to AMB (50 mg/day) on day 64 (Fig. 1). The CVC was removed on day 72, and a culture of the CVC tip removed from the patient grew more than 15 colonies of C. auris. However, on day 79, the patient died due to septic shock and multiple-organ failure caused by persistent fungemia, despite antifungal therapy.
Case 3.
A 1-year-old boy with hemophagocytic lymphohistiocytosis and a history of colectomy due to perforated colon by pseudomembranous colitis was transferred to Wonju Christian Hospital for further chemotherapy. After admission, chemotherapy was continued, but fever developed on hospital day 10, and the white blood cell count decreased to 3,870/μl. On day 12, blood culture yielded Candida tropicalis and C. auris. After that, eight blood cultures performed on hospital days 15, 19, 25, 28, 33, 36, 40, and 42 yielded C. auris. Antifungal therapy was initiated with FLU (100 mg/day) on day 23 and changed to AMB on day 40. The peripheral vascular catheter was removed on day 35, and chemotherapy was stopped on day 39. However, fever persisted and the patient showed signs of disseminated intravascular coagulation and died due to septic shock caused by persistent fungemia on day 46 (Fig. 1).
MATERIALS AND METHODS
Six isolates from three patients (two isolates each) were available for this study (Table 1). All six isolates of Candida spp. were reidentified by standard laboratory procedures, including the use of API 20C (bioMérieux, Marcy l'Etoile, France) and Vitek 2 YST card (bioMérieux) systems and sequence analysis. In addition, the medical records of the patients were reviewed retrospectively. The demographic and clinical data included sex, age, diagnosis at admission, date of positive blood cultures, presence of central venous catheter, dates and dosages of antifungal drug administration, dates of catheter removal and culturing, and outcome of fungemia (4). Therapeutic failure was defined as either the persistence of Candida in the bloodstream, despite 3 days of antifungal therapy, or the development of breakthrough candidemia while receiving antifungal agents for 3 or more days (1, 4, 6).
Table 1.
Results of identification and antifungal susceptibility testing for 6 isolates from 3 patients with C. auris fungemiaa
| Patient | Isolate no. | Isolation date (mo/day/yr) | Identification resultb |
Antifungal MIC (μg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1/D2 domain and ITS sequence analysis | Vitek 2 YST | API 20C | AMB | FLU | ITR | VOR | Casp | Mica | |||
| 1 | 1 | 12/14/1996 | Candida auris (100) | Candida haemulonii (99) | Rhodotorula glutinis (99.9) | 0.5 | 128 | 2 | 1 | 0.06 | 0.03 |
| 2 | 12/20/1996 | C. auris (100) | C. haemulonii (99) | R. glutinis (99.9) | 0.5 | 128 | 2 | 1 | 0.06 | 0.03 | |
| 2 | 3 | 03/23/2009 | C. auris (100) | C. haemulonii (99) | R. glutinis (99.9) | 1 | 8 | 0.25 | 0.06 | 0.06 | 0.03 |
| 4 | 03/31/2009 | C. auris 100) | C. haemulonii (99) | R. glutinis (99.9) | 1 | 8 | 0.25 | 0.125 | 0.06 | 0.03 | |
| 3 | 5 | 08/03/2009 | C. auris (100) | C. haemulonii (99) | R. glutinis (99.9) | 1 | 2 | 0.125 | 0.03 | 0.06 | 0.03 |
| 6 | 08/09/2009 | C. auris (100) | C. haemulonii (99) | R. glutinis (99.9) | 0.5 | 2 | 0.125 | 0.03 | 0.06 | 0.03 | |
Abbreviations: Casp, caspofungin; Mica, micafungin.
Numbers in parentheses are the probability of correct identification (%).
The internal transcribed spacer (ITS) region (including the 5.8S rRNA gene) and the 26S rRNA gene D1/D2 domains were amplified with the primer pair of pITS-F (5′-GTCGTAACAAGGTTAACCTGCGG-3′) and pITS-R (5′-TCCTCCGCTTATTGATATGC-3′) and the primer pair of NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′), respectively (4). Sequence similarity searches were performed using the BLAST tool at the NCBI database (http://www.ncbi.nlm.nih.gov/blast).
In vitro antifungal susceptibility testing for AMB, FLU, itraconazole (ITR), voriconazole (VOR), caspofungin, and micafungin was performed by a broth microdilution assay according to the M27-A3 method of the Clinical and Laboratory Standards Institute (2). The MIC endpoints were read after 24 h of incubation in air at 35°C for caspofungin and micafungin and after 48 h of incubation for all the other drugs. The MIC of AMB was defined as complete inhibition of growth, while the MICs of FLU, ITR, VOR, caspofungin, and micafungin were defined as the lowest concentrations that produced a prominent decrease in turbidity (∼50%) relative to a drug-free control. Two reference strains, Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258, were included as quality control isolates in each test.
RESULTS AND DISCUSSION
On Sabouraud dextrose agar, the colonies of all the isolates were white to cream colored and smooth. Microscopic examination showed ovoid to globose budding yeast-like cells. No chlamydoconidia or pseudohyphae were formed on cornmeal agar. All the isolates grew well at 37 to 42°C but did not grow at 45°C. They failed to grow on cycloheximide-containing medium and were negative in the urease test and the nitrate assimilation test. The identification results and antifungal susceptibilities of six isolates obtained from three patients are shown in Table 1. DNA sequencing analysis of the ITS region and 26S rRNA gene D1/D2 domains showed 100% identity with C. auris (GenBank DNA database accession numbers JCM15448, EU884176, and EU881954), and all isolates were confirmed to be C. auris. However, the Vitek 2 system identified all of these isolates as Candida haemulonii (99% probability of identity). Our previous study showed that isolates of C. auris and those of C. haemulonii and Candida pseudohaemulonii were identified as C. haemulonii by the Vitek 2 system, which suggests that the Vitek 2 system cannot differentiate C. haemulonii and closely related Candida species (4). In addition, by the API 20C system, all 15 patient isolates of C. auris were identified as Rhodotorula glutinis, while isolates of C. haemulonii (1 patient isolate) and C. pseudohaemulonii (7 patient isolates) were identified as Kodamaea ohmeri (4). In the present study, three patient isolates yielded API 20C codes 6102073 (isolates from patients 1 and 2) and 6122073 (isolates from patient 3), all of which were identified as R. glutinis. The combined API 20C findings of this study and our previous study (4) showed that all 18 patient isolates of C. auris assimilated d-melezitose (MLZ); however, none of them assimilated N-acetylglucosamine (NAG), whereas all 7 patient isolates of C. pseudohaemulonii assimilated NAG but did not assimilate MLZ. Therefore, the assimilation patterns of NAG and MLZ may be useful for differentiating isolates of C. auris from those of C. pseudohaemulonii using the API 20C identification kit. These findings suggest that, in clinical microbiology laboratories, combined results of the Vitek 2 and the API 20C systems can be routinely applied to identify presumptive isolates of C. auris, although the sequence analysis should also be employed for accurate identification.
The MIC ranges of AMB, FLU, ITR, and VOR for six bloodstream isolates of C. auris were 0.5 to 1, 2 to 128, 0.125 to 2, and 0.06 to 1 μg/ml, respectively. They were susceptible to caspofungin (MIC, 0.06 μg/ml) and micafungin (MIC, 0.03 μg/ml). All of these antifungal susceptibility data were the same as or similar to those in our previous report (4). In the present study, two isolates from patient 1, who had been treated with azole antifungal agents, showed resistance to FLU (MIC, 128 μg/ml), and these isolates showed decreased susceptibility to ITR (MIC, 2 μg/ml) and VOR (MIC, 1 μg/ml). However, isolates from the other two patients were susceptible to FLU (MIC, 2 to 8 μg/ml), and their ITR and VOR MICs were 0.125 to 0.25 and 0.03 to 0.125 μg/ml, respectively. In our previous study, FLU MICs for all ear isolates of C. auris ranged from 2 to 128 μg/ml, and 47% (7/15 patients) of isolates showed FLU MICs of 64 μg/ml or more (4). These findings suggest that C. auris is innately less susceptible to FLU (MIC, ≥2 μg/ml) than most other species of Candida and that it may display the capacity to develop high-level resistance to FLU, similar to that of Candida glabrata (9).
Clinical features of the three patients with C. auris fungemia are summarized in Table 2. The three patients were either very young (1 year in two cases) or very old (74 years in one case). All had preexisting conditions and were receiving broad-spectrum antibiotics. Two had undergone surgery, two were using central venous catheters, and none had neutropenia. The clinical course and antifungal therapy for the three patients with fungemia are summarized in Fig. 1. Two patients (cases 1 and 3) had concomitant candidemia (C. albicans or C. tropicalis). In patient 1, breakthrough fungemia on antifungal therapy (AMB followed by FLU) caused by C. albicans developed. Her fungemia resolved completely after catheter removal and continued FLU therapy.
Table 2.
Clinical features of three cases with C. auris fungemia
| Clinical feature | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age/sexa | 1 yr/F | 74 yr/M | 1 yr 2 months/M |
| Underlying disease | Hypoxic encephalopathy, aspiration pneumonia | Laryngeal cancer | Hemophagocytic lymphohistiocytosis |
| Bloodstream isolate of C. auris | |||
| Date (mo/day/yr) of first isolation | 12/11/1996 | 03/23/2009 | 07/27/2009 |
| No. of positive blood cultures (dateb) | 3 (51, 54, 60) | 7 (53, 57, 61, 65, 70, 71, 78) | 9 (12, 15, 19, 25, 28, 33, 36, 40, 42) |
| Clinical status at time of positive culture | |||
| Immunosuppressive state | No | No | Yes |
| Neutropenia (<109/liter) | No | No | No |
| Presence of CVC | Yes | Yes | No |
| Broad-spectrum antibiotics | Yes | Yes | Yes |
| Parenteral nutrition | Yes | Yes | Yes |
| Surgery within 30 days | No | Yes | Yes |
| Intensive care | No | Yes | Yes |
| Previous antifungal agents within 30 days | Yes | No | No |
| Concomitant bacteremia | No | No | No |
| Concomitant candidemia | Yes (C. albicans) | No | Yes (C. tropicalis) |
| Indwelling urinary catheter | No | Yes | No |
| Therapy | |||
| CVC removal (dateb) | Yes (61) | Yes (71) | Not applicable |
| Antifungal therapy after culture | Yes | Yes | Yes |
| CVC tip culture result after removal | No growth | C. auris | Not applicable |
| Outcome of fungemia | Cleared | Not cleared | Not cleared |
| Outcome of patient | Improved | Expired | Expired |
F, female; M, male.
The numbers indicate the days from the beginning of hospitalization in each patient.
In two patients (cases 2 and 3), FLU was administered as an initial therapeutic agent because the rate of azole resistance in Candida species is low in South Korea (3) and species identification by molecular methods and antifungal susceptibility testing are not routinely performed in many institutions. As a result of persistent fungemia despite FLU therapy, FLU was replaced with intravenous AMB in both patients. However, both patients showed therapeutic failure and had a fatal outcome. Some possibility exists that azole as well as polyene therapy can be inadequate for infection caused by this species, although in vitro antifungal susceptibility tests show variable results. Further clinical data on antifungal therapy, including echinocandins, for C. auris are needed.
Isolation of C. auris from ear specimens was unrecognized prior to 2009 (4, 7, 8). Our cases demonstrate, possibly for the first time, that C. auris can be a human pathogen that causes nosocomial bloodstream infections. Note also that C. auris bloodstream isolates from case 1 were obtained in 1996, which suggests that the paucity of isolation of this species from clinical specimens may reflect, in part, the difficulty in identifying this species. Antifungal resistance is an important concern in managing invasive Candida infections (5), and therefore, our cases emphasize the importance of accurate species identification for Candida species.
ACKNOWLEDGMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of South Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0021556) and by a grant (CRI10041) from the Chonnam National University Hospital Research Institute of Clinical Medicine.
Footnotes
Published ahead of print on 29 June 2011.
REFERENCES
- 1. Clancy C. J., Yu V. L., Morris A. J., Snydman D. R., Nguyen M. H. 2005. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob. Agents Chemother. 49:3171–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed. M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. Jung S. I., et al. 2010. Multicenter surveillance of species distribution and antifungal susceptibilities of Candida bloodstream isolates in South Korea. Med. Mycol. 48:669–674 [DOI] [PubMed] [Google Scholar]
- 4. Kim M. N., et al. 2009. Candida haemulonii and closely related species at 5 university hospitals in Korea: identification, antifungal susceptibility, and clinical features. Clin. Infect. Dis. 48:e57–e61 [DOI] [PubMed] [Google Scholar]
- 5. Loeffler J., Stevens D. A. 2003. Antifungal drug resistance. Clin. Infect. Dis. 36:S31–S41 [DOI] [PubMed] [Google Scholar]
- 6. Nguyen M. H., et al. 1998. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J. Infect. Dis. 177:425–430 [DOI] [PubMed] [Google Scholar]
- 7. Oh B. J., et al. 2011. Biofilm formation and genotyping of Candida haemulonii, Candida pseudohaemulonii, and a proposed new species (Candida auris) isolates from Korea. Med. Mycol. 49:98–102 [DOI] [PubMed] [Google Scholar]
- 8. Satoh K., et al. 2009. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 53:41–44 [DOI] [PubMed] [Google Scholar]
- 9. Shin J. H., et al. 2007. Changes in karyotype and azole susceptibility of sequential bloodstream isolates from patients with Candida glabrata candidemia. J. Clin. Microbiol. 45:2385–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]