Abstract
Virgibacillus pantothenticus has been shown to synthesize the compatible solute ectoine in response to high salinity or low growth temperature. We found that exogenously provided ectoine and hydroxyectoine also serve as protectants against these challenges. Transport studies with [14C]ectoine revealed that both types of stress induced a high-affinity ectoine uptake activity in V. pantothenticus. By using an Escherichia coli mutant defective in osmoprotectant uptake systems, a functional complementation approach for osmostress resistance in the presence of ectoine was employed to retrieve a gene encoding an ectoine transporter from V. pantothenticus. The cloned gene (ectT) encodes a protein (EctT) that is a member of the BCCT (betaine-choline-carnitine-transporter) family of carriers. Osmoprotection assays demonstrated that the EctT carrier mediates the preferential import of ectoine and hydroxyectoine but also possesses minor uptake activities for the compatible solutes proline and glycine betaine. Northern blot analysis with RNA isolated from V. pantothenticus revealed that a rise in the external osmolality or a reduction in growth temperature strongly increased the transcription of the ectT gene. Primer extension analysis demonstrated that ectT was transcribed under these conditions from a SigB-type promoter. SigB is the master regulator of the general stress regulon of bacilli and provides protection to cells against various challenges, including high salinity and low temperature. Both the synthesis of ectoine and the EctT-mediated uptake of ectoine and hydroxyectoine are triggered by the same environmental cues, high salinity and cold stress, and thereby provide, in a concerted fashion, the protection of V. pantothenticus against these challenges.
INTRODUCTION
To counteract the efflux of water and to promote a physiologically adequate level of cellular hydration and turgor, many microorganisms amass a selective class of organic osmolytes, the compatible solutes, for a sustained adjustment to high-osmolarity surroundings (6, 31, 63, 64). In addition to their well-studied function as osmoprotectants, compatible solutes also have protein-stabilizing properties that support the correct folding of polypeptides under denaturing conditions both in vitro and in vivo (4, 25, 56). They therefore also are referred to in the literature as chemical chaperones (13, 15). The stabilizing effects of compatible solutes on macromolecules and biosynthetic processes probably contributes to their physiological functions as protectants against heat (13, 23) and cold stress (3, 22).
The tetrahydropyrimidines ectoine and 5-hydroxyectoine are among the most widely used compatible solutes by members of the Bacteria and typically are synthesized in response to increases in environmental osmolarity (41). The three ectoine biosynthetic enzymes l-2,4-diaminobutyric acid transaminase (EctB), Nγ-acetyltransferase (EctA), and ectoine synthase (EctC) typically are encoded by a gene cluster, ectABC, whose transcription is upregulated in response to high salinity (41). A subset of ectoine producers also synthesizes a derivative of ectoine, 5-hydroxyectoine, through the stereospecific hydroxylation of ectoine via the EctD enzyme (47).
In addition to the osmoadaptive synthesis of ectoine and hydroxyectoine, many microorganisms also can take advantage of these compatible solutes as osmostress protectants when they are present in the growth medium. A number of ectoine uptake systems that belong to different transporter families have been identified in various microorganisms already (14, 16, 26–28, 36, 43, 55, 60). Most of these transporters function in the scavenging of ectoines as osmoprotectants (e.g., ProU and ProP) (27) or as recycling systems for endogenously synthesized and then excreted ectoines (e.g., TeaABC) (16), but some (e.g., EhuABCD and UehABC) serve primarily for the uptake of ectoines when they are used as nutrients (26, 36). Typically, the transcription of the genes for ectoine/hydroxyectoine transporters engaged in osmostress protection is induced in response to increases in the external osmolarity (11, 12, 33, 34, 38). Conversely, the transcription of those genes encoding transporters involved in the acquisition of ectoine/hydroxyectoine as nutrients is upregulated in response to the availability of these compounds in the growth medium (26, 36).
Virgibacillus pantothenticus (formerly Bacillus pantothenticus) (21) is a Gram-positive soil bacterium that requires pantothenic acid as a growth factor. Within its primary habitat, the upper layers of the soil, V. pantothenticus will experience frequent reductions in water availability and concomitant increases in salinity due to the drying of the soil (5). It also often will be faced with suboptimally high or low growth temperatures (22, 23). V. pantothenticus responds to increases in the external salinity by an osmotically induced synthesis of the compatible solutes proline and ectoine, but it does not produce hydroxyectoine (11, 33). Modest rises in the environmental salinity lead to moderate increases in the proline pool, whereas ectoine dominates the compatible solute pool of V. pantothenticus at higher salinities (34). This phenomenon has been termed “osmolyte switching” (50) and implies that ectoine is a more effective osmostress protectant than proline for severely salt-challenged V. pantothenticus cells.
The synthesis of ectoine in V. pantothenticus depends on an osmotically inducible ectABC operon (34). Interestingly, ectoine biosynthesis in V. pantothenticus also can be elicited by growth at low (15°C) but not at high (48°C) temperature (34), suggesting a protective function of ectoine against sustained cold stress. The triggering of ectoine production by low growth temperature occurs at the transcriptional level, and such a cold stress induction of ectABC expression has not been observed in any other ectoine-producing microorganism.
We now have asked whether exogenously provided ectoine and hydroxyectoine also would protect V. pantothenticus against osmotic and cold stress and found that this was indeed the case. We identified the structural gene (ectT) for an ectoine/hydroxyectoine transporter (EctT) from V. pantothenticus that belongs to the BCCT family (65) of uptake systems. We found that the transcription of the ectT gene can be induced both by a rise in salinity and by a drop in growth temperature, and that both transcriptional responses are dependent on SigB, the master regulator of the general stress response in bacilli (20, 44).
MATERIALS AND METHODS
Chemicals.
Ectoine and hydroxyectoine were kind gifts from T. Schwarz and G. Lentzen (Bitop AG, Witten, Germany). Glycine betaine, choline, and carnitine were obtained from Sigma-Aldrich (Steinheim, Germany); proline betaine (stachydrine-hydrochloride) was purchased from Extrasynthese (Genay, France); and choline-O-sulfate, dimethylsulfoniopropionate (DMSP), and dimethylsulfonioactetate (DMSA) were from laboratory stocks. [14C]ectoine (4.22 MBq mmol−1) was biologically prepared from l-[U-14C]glutamic acid in high-salinity-stressed Brevibacterium linens ATTC 9175 cells and purified by paper chromatography as described by Jebbar et al. (27). The antibiotics chloramphenicol, ampicillin, and kanamycin were purchased from Sigma-Aldrich (Steinheim, Germany).
Bacterial strains.
The Escherichia coli strain DH5α (Clontech, Heidelberg, Germany) was routinely used for the propagation of cloning vectors and recombinant plasmids. The MC1400-derived E. coli strain MKH13 [Δ(putPA)101 Δ(proP)2 Δ(proU)608 Δ(betTIBA)], which carries defects in the choline uptake system BetT, the glycine betaine biosynthetic genes, and the compatible uptake systems ProU and ProP, has been described (17). The type strain (DSM 26T) of V. pantothenticus was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ; Braunschweig, Germany). The wild-type B. subtilis laboratory strain JH642 (trpC2 pheA1; BGSC 1A96) was a kind gift from J. Hoch (The Scripps Research Institute). Its sigB mutant derivative, strain BLOB22[sigBΔ2::cat], has been described (61).
Media and growth conditions.
E. coli, B. subtilis, and V. pantothenticus strains were routinely grown and maintained on Luria-Bertani (LB) agar plates. E. coli strains were grown in minimal medium A (MMA) with 0.5% glucose as the carbon source (37). B. subtilis strains derived from the wild-type strain JH642 were grown in Spizizen's minimal medium (SMM) with 0.5% (wt/vol) glucose as the carbon source, l-tryptophane (20 mg liter−1), l-phenylalanine (18 mg liter−1), and a solution of trace elements (19). A chemically defined minimal medium containing 50 mg liter−1 pantothenic acid for the growth of V. pantothenticus with 0.5% glucose as the carbon source has been reported (33). Bacterial growth was monitored spectrophotometrically as the optical density of the cultures at 578 nm (OD578). The osmotic strength of minimal medium was increased by the addition of NaCl, sucrose, and glycerol from concentrated stock solutions, as required. The osmolality values of these media were determined with a vapor pressure osmometer (Vapor Pressure 5500; Wescor Inc., UT).
For growth experiments that involved E. coli, V. pantothenticus, or B. subtilis cultures, the cells were grown in 100-ml Erlenmeyer flasks with a culture volume of 20 ml. Growth curves of V. pantothenticus were recorded in 80-ml cultures in 500-ml Erlenmeyer flasks to ensure good aeration. All cultures were incubated in shaking water baths (set at 220 rpm) at the indicated temperatures. Typically, osmotically unstressed precultures were inoculated into hyperosmotic minimal media to an OD578 of 0.1; the cultures then were allowed to grow to the mid-logarithmic growth phase until the cells were used for further experiments. In experiments that involved continued cold stress, the cultures were inoculated from logarithmic precultures grown at 37°C to an OD578 of 0.1 and then were propagated in shaking water baths with a temperature set to 15°C. Osmotic up-shocks were performed with exponentially growing cultures (OD578 of about 0.5) by the addition of a prewarmed NaCl stock solution to reach the final NaCl concentrations indicated in the individual experiments. Ethanol shocks of V. pantothenticus cultures were performed by adding ethanol (from a 96% [vol/vol] solution to a final concentration of 4% [vol/vol] ethanol) to the growth medium. For acute cold stress experiments, exponentially growing cultures (OD578 of about 0.5) were rapidly shifted from 37 to 15°C.
To select for the presence of plasmid pHSG575 (57), the E. coli-Bacillus subtilis shuttle vector pRB373 (8), and its recombinant derivative plasmid pAK14 in E. coli host strains, we used chloramphenicol in the growth media at final concentrations of 30 and 100 μg ml−1 ampicillin, respectively. Five μg ml−1 of kanamycin was used for the selection of pRB373 and pAK14 when they were propagated in B. subtilis or in V. pantothenticus strains.
Cloning of the ectT gene from V. pantothenticus through functional complementation.
To clone ectoine transporter genes from V. pantothenticus, we prepared chromosomal DNA, partially cleaved it with the restriction enzyme Sau3A, ligated the resulting DNA fragments into the low-copy-number plasmid pHSG575 (Cmr) (57), and transformed the resulting gene library into the E. coli strain MKH13 [Δ(putPA)101 Δ(proP)2 Δ(proU)608 Δ(betTIBA)], which cannot grow in minimal medium containing 0.8 M NaCl. Recombinant strains were selected as chloramphenicol-resistant colonies on LB agar plates containing 30 μg ml−1 of the antibiotic. Osmotolerant MKH13 derivatives from this clone library were searched for by replica plating the colonies onto high-osmolarity minimal medium agar plates (MMA with 0.8 M NaCl and 30 μg ml−1 chloramphenicol) containing 1 mM ectoine.
Recombinant DNA techniques, construction of plasmids, and DNA sequence analysis.
Routine isolation and manipulations of plasmid DNA, the construction of recombinant DNA plasmids, the isolation of chromosomal DNA from V. pantothenticus, and the detection of homologous sequences by Southern hybridization using digoxigenin (DIG)-labeled DNA probes were carried out using standard procedures (49). The nucleotide sequence analysis of the cloned (pAK9) V. pantothenticus ectT gene and its flanking regions was determined by the chain termination method of Sanger using the Thermo Sequenase fluorescent-labeled primer cycle sequencing kit (Amersham Pharmacia Biotech, Freiburg, Germany) (deposited in GenBank under accession number AF421189). The DNA sequencing reactions were primed with synthetic oligonucleotides labeled at their 5′ end with the infrared dye IRD-800 (Eurofins MWG Operon, Ebersberg, Germany) and analyzed on a LI-COR DNA sequencer (model 4000; Eurofins MWG Operon, Ebersberg, Germany). The oligonucleotides used were purchased from Eurofins MWG Operon (Ebersberg, Germany). To construct a plasmid carrying the ectT regulatory region that would replicate in B. subtilis, we inserted a 787-bp fragment (amplified by PCR from genomic DNA of V. pantothenticus with the following primers: ectT5′BamHI, [AAGGATCCGGCATTTAGTTGGGCGC] and ectT3′EcoRI [AAGAATTCGACTCATAGGTTCAGC]) as a BamHI-EcoRI DNA fragment (for the nucleotide sequence of this DNA fragment, see GenBank accession number AF421189) into the E. coli-B. subtilis shuttle vector pRB373 (8). The inserted DNA fragment contains 450 bp upstream of the ectT ATG start codon and part (337 bp) of the ectT coding region; this plasmid was named pAK14.
Isolation of RNA and transcription analysis of the ectT gene.
To study the transcriptional regulation of the V. pantothenticus ectT gene in response to increases in the osmolarity of the growth medium or decreases in growth temperature, we performed Northern blot analysis. Aliquots equivalent to 2-ml culture volumes (OD578 of about 1) were withdrawn from growing V. pantothenticus cultures before and at the indicated time points after an osmotic up-shock with 700 mM NaCl or after a sudden reduction in growth temperature (from 37 to 15°C). Total RNA was isolated from these samples using the High Pure RNA isolation kit (Roche Diagnostics, Mannheim, Germany). We then used 15 μg of total RNAs isolated from these cells and separated the RNA according to size on a denaturing 1.4% agarose gel. ectT-specific mRNAs were detected using an ectT-specific DIG-labeled RNA probe covering an internal 589-bp ectT fragment in Northern blot analysis. The preparation of the single-stranded ectT-specific antisense RNA probe, hybridization conditions, and signal detection all were carried out as described by Holtmann and Bremer (23).
To determine the position of the ectT promoter, we used primer extension analysis to map the 5′ end of the ectT mRNA in V. pantotheticus. Total RNA was prepared from V. pantotheticus cells carrying plasmid pAK14 (ectT′) grown under osmotic stress conditions (with 700 mM NaCl), at low temperature (15°C), or from cultures that were exposed to 4% ethanol for a duration of 10 min. A DNA primer (ectTPE; 5′-ggcagagtagctccg-3′) complementary to the ectT mRNA and labeled at its 5′ end with the infrared dye IRD-800 was hybridized to the total RNA and extended with avian myeloblastosis virus reverse transcriptase (purchased from Promega GmbH, Mannheim, Germany). The formed cDNA was electrophoresed on a DNA sequencing gel alongside DNA sequencing reactions performed with plasmid pAK14 and primed with the same ectTPE oligonucleotide.
To study the influence of SigB on the expression of the V. pantothenticus ectT gene, we used the B. subtilis wild-type strain JH642 and its isogenic sigB mutant derivative strain BLOB22[sigBΔ2::cat] (61) carrying the ectT′ plasmid pAK14. These recombinant strains either were cultivated in SMM or were subjected to an osmotic up-shock (with 400 mM NaCl) and cultivated for 10 min at the elevated salinity to induce the SigB response of B. subtilis. Total RNA was isolated from these cells, and a primer extension reaction was performed with the above-described ectT-specific primer ectTPE.
Transport studies with [14C]ectoine.
Transport studies with [14C]ectoine (4.22 MBq mmol−1) were used to assess the influence of high salinity and low growth temperature on the ectoine uptake activity of V. pantotheticus cultures. V. pantothenticus cultures grown in SMM minimal medium (33) at 37°C to mid-exponential growth phase (OD578 of about 0.5) were subjected to sudden temperature or osmolarity shifts, as indicated. Sixty minutes after the shock, 2-ml samples were taken from each culture, and the initial [14C]ectoine uptake activity was monitored at a final substrate concentration of 14 μM. The minimal medium (SMM) used for cell growth and transport assays contained about 11 mM NaCl (kindly measured by Volker Müller, University of Frankfurt, Germany). The technical details of the transport assay followed previously described protocols (10, 23). The amount of [14C]ectoine taken up by the V. pantothenticus cells was determined by scintillation counting (LS6500; Beckmann Coulter, Brea, CA). To determine the kinetics of [14C]ectoine uptake in V. pantothenticus, transport rates were measured from cultures that were subjected to an osmotic up-shock with 0.7 M NaCl; the cells then were cultured for a further 60 min at 37°C, and subsequently 2-ml aliquots of the cultures were withdrawn and mixed with different [14C]ectoine concentrations (10 to 400 μM final concentrations). The measured uptake rates were used to calculate Km and Vmax values for [14C]ectoine uptake according to Michaelis-Menten kinetics.
Database searches for EctT-related proteins.
Proteins that are homologous to the EctT protein from V. pantothenticus were searched via the Web server of the DOE Joint Genome Institute (http://www.jgi.doe.goc/) or that of the National Center for Biotechnology Information Institute (http://www.ncbi.nlm.nih.gov/) using the BLAST algorithm (1). Protein sequences related to the V. pantotheticus EctT protein were aligned and analyzed using ClustalW (58).
Nucleotide sequence accession number.
The DNA sequence of the V. pantothenticus (DSM 26T) ectT gene and its flanking regions has been deposited in GenBank under accession number AF421189.
RESULTS
Exogenously provided ectoine and hydroxyectoine function as osmotic and cold stress protectants.
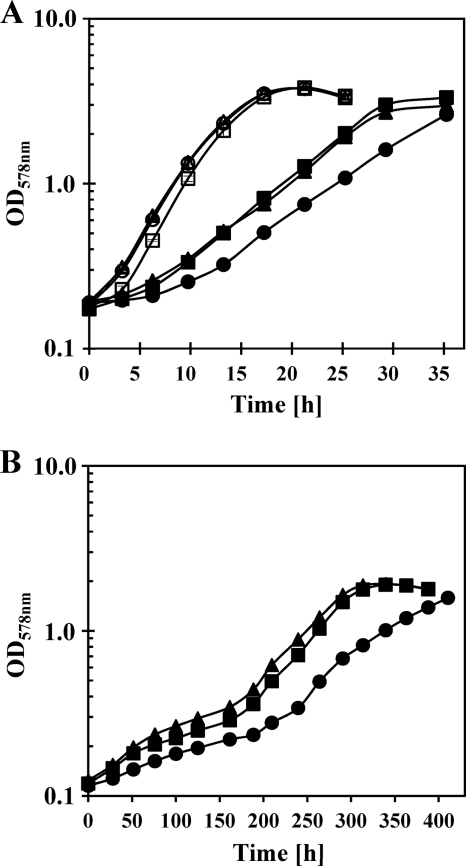
The synthesis of ectoine in V. pantothenticus is increased in response to high salinity and cold temperature (15°C) as a consequence of the induction of the transcription of the ectoine biosynthetic genes (34). We asked whether exogenously provided ectoine or hydroxyectoine would serve as protectants against sustained osmotic or cold stress as well. To this end, we grew V. pantothenticus cultures at 37°C in a minimal medium (SMM with supplements) with 1.2 M NaCl (2,700 mosmol kg−1) in the absence or presence of 1 mM ectoine or 1 mM hydroxyectoine. Both ectoine and hydroxyectoine afforded osmoprotection for V. pantothenticus, but their addition to the osmotically nonstressed culture cultivated in SMM (340 mosmol kg−1) had no beneficial effect (Fig. 1A). A possible cold-protective function of ectoine and hydroxyectoine was studied by growing V. pantothenticus at 15°C in SMM in the absence or presence of either 1 mM ectoine or 1 mM hydroxyectoine. Both ectoines significantly enhanced the growth of V. pantothenticus under cold stress conditions (Fig. 1B).
Fig. 1.
Protection of V. pantothenticus against salt and cold stress by ectoine and hydroxyectoine. (A) For salt stress protection assays, the cells were grown in SMM without (open symbols) or with (closed symbols) 1.2 M NaCl in the absence (open and closed circles) or presence of either 1 mM ectoine (open and closed triangles) or 1 mM hydroxyectoine (open and closed squares) at 37°C. (B) For cold stress protection assays, the V. pantothenticus cells were grown in SMM at 15°C in the absence (closed circles) or in the presence of either 1 mM ectoine (closed triangles) or 1 mM hydroxyectoine (closed squares).
V. pantothenticus possesses a high-affinity transport activity for ectoine that is stimulated by osmotic and cold stress.
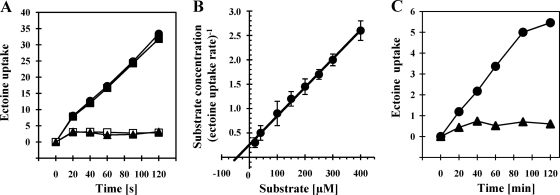
Osmotic and cold stress protection of V. pantothenticus by exogenously provided ectoine and hydroxyectoine (Fig. 1) suggests the presence of an uptake system for these compatible solutes. To measure ectoine uptake in V. pantothenticus, we performed transport experiments with radiolabeled [14C]ectoine (final substrate concentration, 14 μM). Ectoine uptake was strongly stimulated in cells that were grown in the presence of either equiosmolar (1,100 mosmol kg−1) concentrations of NaCl (0.4 M) or the nonionic osmolyte sucrose (Fig. 2A). These observations indicate that ectoine uptake by V. pantothenticus is triggered in response to a true osmotic stimulus and not solely as a response to salt stress. The stimulus triggering ectoine uptake requires the establishment of an osmotically active gradient across the cytoplasmic membrane, since a rise in the osmolality of the growth medium achieved by the addition of 0.68 M glycerol, a solute that can permeate at high concentrations readily through the membrane, did not cause an increase in [14C]ectoine uptake activity (Fig. 2A).
Fig. 2.
Uptake of radiolabeled [14C]ectoine under salt and cold stress conditions. (A) V. pantothenticus cells were grown in SMM (open squares), SMM with 0.4 M NaCl (closed squares), 0.67 M sucrose (closed circles), or 0.68 M glycerol (closed triangles) to early exponential growth phase (OD578 of about 0.4). The cells then were assayed for [14C]ectoine uptake in the respective growth medium at a final substrate concentration of 14 μM; [14C]ectoine uptake by the cells is given in nmol ectoine (mg protein)−1. The data shown are from representative transport experiments selected from three independent biological replicas. (B) [14C]ectoine uptake rates were determined in salt-shocked (with 0.7 M NaCl) cultures (60 min after the cold shock) at the indicated substrate concentrations of ectoine. The measured [14C]ectoine rates were plotted as substrate concentration (μM) per transport rate [nmol ectoine (mg protein min)−1] versus the substrate concentration (μM) according to Hanes-Woolf. The data shown represent three independent sets of experiments with errors given as standard deviations. (C) V. pantothenticus cells were grown in SMM either at 37°C (triangles) or at 15°C (circles) to early exponential growth phase (OD578 of about 0.4). The cells then were assayed in SMM for [14C]ectoine uptake at a final substrate concentration of 14 μM; [14C]ectoine uptake is given as nmol ectoine (mg protein)−1. The data shown are representative transport experiments selected from three biological replicas.
To determine the kinetic parameters of the osmotically induced ectoine uptake activity exhibited by V. pantothenticus, we grew cells at 37°C to mid-exponential phase in SMM and subjected them to a hyperosmotic shock by the addition of 0.7 M NaCl; after the further growth of the culture for 60 min to allow the acclimatization of the cells to the high-salinity growth condition, we measured [14C]ectoine transport rates at various ectoine concentrations (20 to 400 μM). The determined transport rates were plotted according to Hanes-Woolf, and from this graphic analysis (Fig. 2B) we determined a Vmax of ectoine transport in V. pantothenticus of 169 ± 29 nmol min−1 mg protein−1 and a Km of 44 ± 8 μM.
Since ectoine serves as a cold stress protectant for V. pantothenticus (Fig. 1B), we also measured initial [14C]ectoine uptake in cells that were cultivated at 15°C in SMM and compared their uptake activity to that of cells grown at 37°C. The data documented in Fig. 2C show that ectoine import in V. pantothenticus is strongly stimulated in cells cultivated at low temperature (15°C), whereas there is hardly any [14C]ectoine uptake activity measurable at 37°C in V. pantothenticus cells (Fig. 2C). However, the overall [14C]ectoine transport activity in cold-stressed cells was considerably lower than that present in salt-stressed cells.
We then asked if heat stress would induce ectoine uptake activity in V. pantothenticus. To test this, we grew V. pantothenticus at 48°C, a temperature close to the upper temperature limit of growth for this microorganism (34), but there was no increased [14C]ectoine uptake activity detectable (data not shown). Hence, the pattern of ectoine uptake by V. pantothenticus matches that of ectoine synthesis by this bacterium (34), in that both are inducible by osmotic and cold stress (Fig. 2A and C) but not by heat stress.
Enhanced ectoine transport in V. pantothenticus depends primarily on transcription stimulated by osmotic and cold stress.
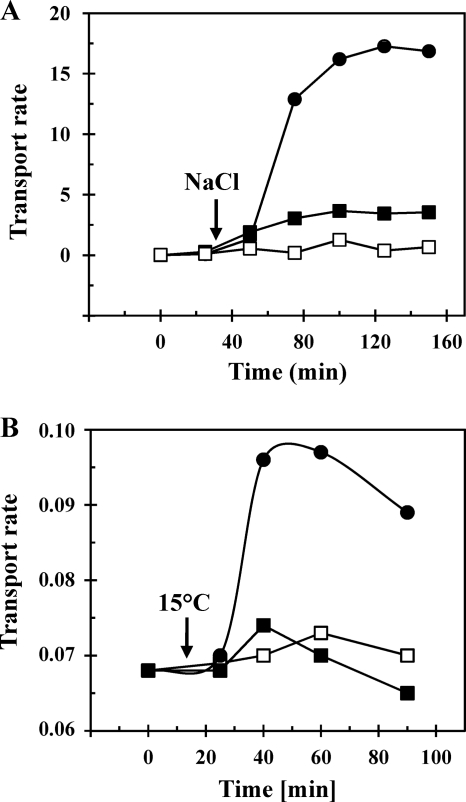
Several bacterial osmoprotectant uptake systems function as osmosensors and respond directly to osmotic pressure-derived cellular properties to enhance cellular compatible solute uptake activity (63). To test whether the observed osmotically triggered ectoine transport in V. pantothenticus was stimulated at the level of transport activity or was primarily dependent on osmotically stimulated transcription, we measured the [14C]ectoine transport rates in cells that were subjected to an osmotic up-shock with 0.4 M NaCl or in osmotically stressed cells in which de novo protein synthesis was blocked by the addition of 100 μg ml−1 chloramphenicol to the growth medium. We found that the strong increase in ectoine uptake activity of osmotically up-shifted cells was greatly reduced when the cells were pretreated with chloramphenicol (Fig. 3A). Hence, osmotically stimulated ectoine transport in V. pantothenticus depends primarily on de novo protein synthesis and consequently on osmotically stimulated transcription. However, the data documented in Fig. 3A also show that a minor activation of the ectoine transport activity present in V. pantothenticus must occur at the posttranscriptional level.
Fig. 3.
Increases in [14C]ectoine uptake in response to sudden increases in salinity or a drop in temperature. (A) V. pantothenticus cells were grown to early exponential growth phase (OD578 of about 0.4) at 37°C. The culture then was split into three subcultures for [14C]ectoine uptake assays at a final substrate concentration of 14 μM: (i) cells were grown in SMM (open squares), (ii) cells were subjected to an osmotic up-shock by the addition of 0.4 M NaCl (closed circles), and (iii) 100 μg ml−1 chloramphenicol was added to the culture prior to the osmotic up-shock elicited by the addition of 0.4 M NaCl (closed squares). (B) V. pantothenticus cells were grown to early exponential growth phase (OD578 of about 0.4) at 37°C. The culture then was split into three subcultures for [14C]ectoine uptake assays at a final substrate concentration of 14 μM: (i) cells were grown in SMM (open squares) at 37°C, (ii) cells were subjected to a sudden cold shock through a shift to 15°C (closed circles), and (iii) 100 μg ml−1 chloramphenicol was added to the culture prior to the temperature downshift to 15°C (closed squares). The transport assays were conducted at 37°C. [14C]ectoine uptake rates of the cells are given in nmol ectoine (mg protein min)−1.
We conducted a similar type of [14C]ectoine uptake experiment with V. pantothenticus cells subjected to a sudden temperate downshift (from 37 to 15°C), since it is know that systems for compatible solute uptake in Listeria monocytogenes (2) and Corynebacterium glutamicum (40) are stimulated at the level of transport activity by low temperature. We found that the observed increase in the ectoine uptake activity in cold-stressed V. pantothenticus cells (Fig. 2C) was dependent on de novo protein synthesis as well (Fig. 3B).
Cloning of a gene encoding an ectoine and hydroxyectoine uptake system from V. pantothenticus by functional complementation.
To identify ectoine transporters from V. pantothenticus at the molecular level, we used a functional complementation approach with an E. coli strain (MKH13) (17) that permits the selection of osmostress-resistant colonies in the presence of a chosen compatible solute (30). From a gene library of Sau3A chromosomal DNA segments inserted into the low-copy-number plasmid pHSG575 (Cmlr) (57) and transformed into strain MKH13, we identified, among approximately 20,000 to 30,000 Cmlr transformants, 20 colonies that grew under the selective conditions (MMA with 0.8 M NaCl and 1 mM ectoine). Each of these strains carried a plasmid with an approximately 4.4-kb insert, suggesting that these plasmids were identical. We therefore focused our further analysis on one of these plasmids, pAK9, and its retransformation into strain MKH13 yielded transformants that all were able to grow on SMM plates with 0.8 M NaCl in the presence of 1 mM ectoine but not on high-salinity agar plates lacking ectoine. Taken together, these observations suggested that plasmid pAK9 carries gene(s) from V. pantothenticus encoding an uptake system for ectoine.
Substrate specificity of the cloned ectoine transporter from V. pantothenticus.
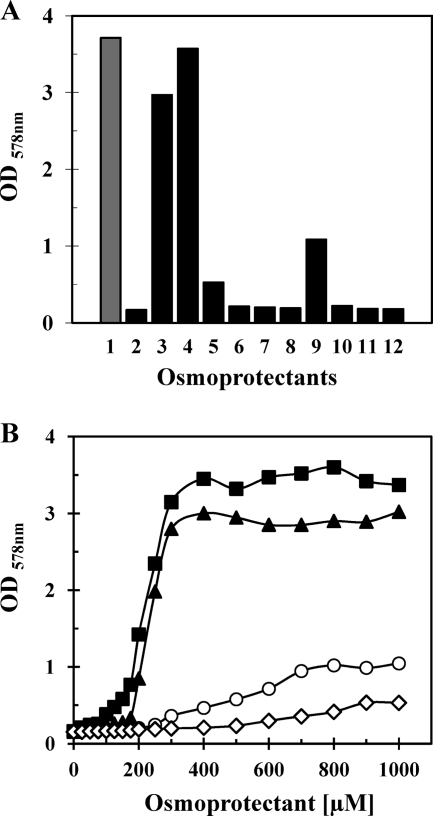
To determine the substrate specificity of the cloned V. pantothenticus ectoine transporter, we grew strain MKH13[pAK9] in shake flask experiments in high-osmolality minimal medium (MMA with 0.8 M NaCl) in the absence or presence (1 mM) of various compatible solutes for 16 h at 37°C. Both ectoine and hydroxyectoine exerted a strong osmoprotective effect (Fig. 4A). A minor degree of osmoprotection also was afforded to MKH13[pAK9] by the compatible solutes glycine betaine and proline (Fig. 4A), but no osmoprotection was conferred by the compatible solutes choline, carnitine, choline-O-sulfate, dimethylsulfonium acetate (DMSA), dimethylsulfonium propionate (DMSP), or proline betaine. The substrate profile of the cloned ectoine uptake system was corroborated in an experiment where we monitored the growth yield of osmotically stressed cultures of strain MKH13[pAK9] cultivated in the presence of various concentrations (0 to 1,000 μM) of ectoine or hydroxyectoine. Both ectoine and hydroxyectoine greatly stimulated the growth yield of strain MKH13[pAK9] in the high-salinity medium (Fig. 4B), whereas glycine betaine and proline afforded only moderate osmoprotective effects, even at higher substrate concentrations (Fig. 4B). We conclude from these growth experiments that plasmid pAK9 carries a gene(s) from V. pantothenticus that encodes a transport system with a preference for the compatible solutes ectoine and hydroxyectoine, but that this transport system also serves as a minor import route for both glycine betaine and proline.
Fig. 4.
Profiling of the substrate specificity of the EctT transporter. (A) The E. coli strain MKH13 (proU proP betT) carrying the ectT+ plasmid pAK9 was grown in MMA at 37°C in the absence (lane 1) or presence of 0.8 M NaCl (lane 2). Osmotically stressed cultures (with 0.8 M NaCl) were assayed for growth protection by the addition of 1 mM either ectoine (lane 3), hydroxyectoine (lane 4), glycine betaine (lane 5), choline (lane 6), carnitine (lane 7), choline-O-sulfate (lane 8), proline (lane 9), DMSA (lane 10), DMSP (lane 11), and proline betaine (lane 12); the optical densities of the cultures were measured after 16 h of incubation at 37°C. (B) Cultures of the E. coli strain MKH13 carrying the ectT+ plasmid pAK9 were grown in MMA with 0.8 M NaCl at 37°C with various concentrations of hydroxyectoine (squares), ectoine (triangles), proline (circles), or glycine betaine (diamonds). The optical densities of the cultures were measured after 16 h of incubation at 37°C. The data documented in panels A and B represent typical sets of experiments as judged from two independent biological replicas.
The cloned ectoine/hydroxyectoine uptake system (EctT) from V. pantothenticus is a member of the BCCT family of carriers.
To characterize the cloned ectoine/hydroxyectoine transporter from V. pantothenticus at the molecular level, we determined the nucleotide sequence of both DNA strands of part of the chromosomal segment present in plasmid pAK9. The DNA sequence of 2,199 bp was deposited in GenBank under accession number AF421189. The inspection of this DNA segment revealed a 1,503-bp open reading frame that codes for a 501-amino-acid-long protein with a calculated molecular mass of 55.1 kDa. This protein was identified through BLAST searches (1) as a member of the BCCT (betaine-choline-carnitine-transporter) family of secondary uptake systems (transporter classification system subgroup TC 2.A.15) (65). We refer here to the identified ectoine/hydroxyectoine transporter from V. pantothenticus as the EctT protein and have named its structural gene ectT (for ectoine transport).
The BCCT family of transporters comprises a large group of integral cytoplasmatic membrane proteins present in many Gram-negative and Gram-positive bacteria, most of which utilize electrochemical ion gradients (H+ or Na+) to fuel substrate import. The majority of the functionally characterized members of the BCCT family serve for the uptake of compatible solutes as protectants against osmotic or temperature stress or, more rarely (e.g., CaiT), as nutrients (65). The EctT protein from V. pantothenticus exhibits a degree of amino acid sequence identity to the 21 currently functionally characterized members of the BCCT family (65) that ranges between 49% identity to the EctM ectoine/hydroxyectoine transporter from Marinococcus halophilus and 24% identity to the CaiT proteins from E. coli and Proteus mirabilis.
A common denominator of BCCT-type carriers is the chemical structure of the transported substrates (29) that comprises the osmoprotectants choline, glycine betaine, carnitine, proline betaine, DMSA, and DMSP, compounds which feature positively charged trimethylammonium (e.g., glycine betaine), dimethylammonium (e.g., proline betaine), or dimethlysulfonium (e.g., DMSA and DMSP) head groups and a negatively charged carboxylate or alcohol function (65). More recently, some BCCT-type transporters (EctM and BetM from M. halophilus, EctP from C. glutamicum, and LcoP from C. glutamicum) have been shown to catalyze the uptake of ectoine and hydroxyectoine (43, 55, 60), substrates that also possess a negatively charged carboxylate and a positively charged nitrogen atom embedded in a ring structure. The EctT transporter from V. pantothenticus now joins the subgroup of the functionally studied ectoine/hydroxyectoine transporters within the BCCT family. It exhibits amino acid sequence identities of 49, 41, 34, and 32% to EctM, BetM, LcoP, and EctP, respectively.
A topology analysis of the EctT protein using the Transmembrane Protein Topology Prediction (TMHMM) (www.cbs.dtu.dk) program suggests that EctT should possess 12 membrane-spanning segments; both the N and the C termini are predicted to face the cytoplasm, a topological organization that has been found in the crystal structures of the BCCT-type transporters BetP and CaiT (46, 51). The predicted cytoplasmic N-terminal end of EctT comprises seven amino acids which are only weakly polar; the cytoplasmic C-terminal tail of EctT comprises 14 amino acids and is highly charged (eight acidic or basic residues). Amino acid sequence alignments of proteins belonging to the BCCT family previously have revealed a highly conserved region of 26 residues that are very rich in aromatic amino acids (29) and that can serve as a signature sequence motif of BCCT-type transporters in database searches (65). Not surprisingly, the EctT protein shares this signature sequence motif. As in other BCCT-type transporters, the corresponding segment of EctT is positioned along the lower part of the predicted TM8 and extends across the cytoplasm-facing connecting loop into the lower part of the predicted TM9.
Transcription of the ectT gene in V. pantothenticus is induced in response to osmotic and cold stress.
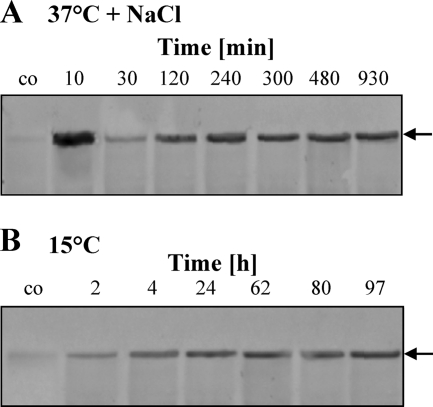
The transport assays (Fig. 2 and 3) conducted with V. pantothenticus suggest that the transcription of the ectT gene should be under osmotic and low-temperature control. To test whether this was indeed the case, we isolated total RNA from V. pantothenticus cells that were subjected to an osmotic up-shock with 0.7 M NaCl or a sudden drop in growth temperature (from 37 to 15°C) and then hybridized the isolated RNA to an ectT-specific single-stranded antisense RNA probe in a Northern blot analysis. Prior to the osmotic up-shock there was hardly any ectT mRNA detectable, whereas 10 min after such a shock strong ectT transcription was found, and elevated levels of the ectT transcript persisted for quite some time (930 min) (Fig. 5A). Likewise, ectT transcription also was stimulated by a sudden drop in growth temperature, and elevated levels of the ectT mRNA were present in cells grown for 97 h after the temperature downshift from 37 to 15°C (Fig. 5B). The data on the induction of ectT transcription in response to either salt or cold stress are fully consistent with our transport experiments that suggested that most of the [14C]ectoine uptake activity detectable in osmotic- or cold-stressed V. pantothenticus cells (Fig. 2) is dependent on de novo protein synthesis (Fig. 3) and consequently on prior gene transcription.
Fig. 5.
Northern blot analysis of ectT transcription in response to salt or cold stress. Cultures of V. pantothenticus were grown in SMM to mid-exponential growth phase (OD578 of about 1) at 37°C and then subjected to an osmotic up-shock with 0.7 M NaCl (A) and a sudden temperature downshift to 15°C (B). Cells were harvested at the indicated time points, and the ectT transcript was visualized through hybridization with a digoxigenin-labeled single-stranded antisense RNA probe.
Transcription of the ectT gene in V. pantothenticus is mediated by a SigB-type promoter.
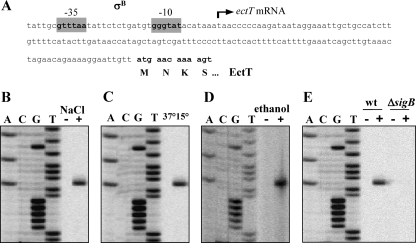
To pinpoint the transcriptional start point of the ectT mRNA and thereby to identify the ectT promoter, we performed a primer extension analysis with RNA isolated from V. pantothenticus cells harboring a plasmid (pAK14) with an ectT fragment carrying the regulatory region (450 bp) and part of the coding region (337 bp). The cells were grown in the absence of osmotic stress or were subjected to an osmotic up-shock with 0.7 M NaCl; total RNA was isolated and subjected to a primer extension reaction with an ectT-specific primer. A single reaction product was detected in the osmotically challenged V. pantothenticus cells (Fig. 6B) that corresponded to a T residue located 129 bp upstream of the predicted ATG start codon of the ectT reading frame (Fig. 6A). We detected sequences (Fig. 6A) preceding this transcription initiation site that closely resemble those for promoters recognized by the alternative transcription factor sigma B (SigB) (24), the master regulator of the general stress regulon in B. subtilis and other bacilli (20, 44). Transcription from the same SigB-type promoter also was induced in cells that were subjected to a temperature downshift from 37 to 15°C (Fig. 6C). Consistently with the Northern blot experiments (Fig. 5), the ectT promoter was not active in cells that were grown in the absence of either osmotic or chill stress, as assessed by primer extension analysis (Fig. 6B and C).
Fig. 6.
Primer extension analysis of the ectT transcript in V. pantothenticus. (A) DNA sequence of the ectT regulatory region; the arrow shows the 5′ end of the ectT transcript, and the position of the inferred SigB-dependent promoter is indicated. V. pantothenticus cells carrying plasmid pAK14 (ectT′) were grown in SMM to mid-exponential growth phase (OD578 of about 1) at 37°C and then subjected to an osmotic up-shift with 0.7 M NaCl (B), a sudden temperature downshift to 15°C (C), or an ethanol shock (4%, vol/vol) (D). The 5′ end of the ectT transcript was determined by a primer extension reaction. (E) Primer extension analysis of the ectT transcript in the heterologous host B. subtilis. The B. subtilis wild-type strain and its sigB mutant derivative strain BLOB22, carrying plasmid pAK14, were grown in SMM to mid-exponential growth phase (OD578 of about 1) at 37°C. One culture of each strain was left untreated (−) or subjected to an osmotic up-shift with 0.4 M NaCl (+), the cells were allowed to grow for 10 min, total RNA was isolated, and the 5′ end of the ectT transcript was determined by a primer extension reaction.
Transcription from SigB-type promoters in B. subtilis can be induced by a variety of environmental cues and metabolic stress conditions (20, 44). A characteristic and very strong inducer of the SigB-controlled general stress response in B. subtilis is the exposure of the cells to ethanol. We challenged V. pantothenticus cells with 0.4% ethanol and then determined the 5′ end of the ectT mRNA by primer extension analysis. Ethanol treatment induced ectT transcription effectively and led to the production of an mRNA species (Fig. 6D) with the same 5′ end as that detected in either osmotic- or chill-stressed cells (Fig. 6B and C). Taken together, these data leave little doubt that the transcription of ectT in V. pantothenticus is under the control of an RNA polymerase complexed with the alternative sigma factor SigB.
Heterologous expression of the ectT gene in B. subtilis is strictly dependent on SigB.
V. pantothenticus currently is not amenable to genetic manipulations, and consequently a sigB mutant of V. pantothenticus is not available. To provide further proof that the V. pantothenticus ectT gene is indeed under the control of a SigB-type promoter, we transformed an isogenic pair of sigB+ (strain JH642) or sigB mutant (strain BLOB22) (61) B. subtilis strains with the ectT′ plasmid pAK14. We then determined the 5′ end of the ectT transcript in the resulting strains by the primer extension analysis of RNA isolated from osmotically challenged cultures (with 400 mM NaCl for a duration of 10 min). The transcription of the V. pantothenticus ectT gene in the heterologous host B. subtilis was detected only in the SigB-proficient strain JH642 (Fig. 6E), and the 5′ end of the ectT mRNA in B. subtilis was the same as that determined by the primer extension experiment in V. pantothenticus (Fig. 6B, C, and D). These data therefore are consistent with our conclusion that the ectT gene in V. pantothenticus is transcribed from a SigB-type promoter.
Ectoine and hydroxyectoine are metabolically inert in V. pantothenticus.
Because a number of microorganisms can use ectoine and hydroxyectoine as carbon, nitrogen, and energy sources (26, 48, 52, 59), we questioned whether V. pantothenticus also uses these compounds as nutrients. To test this, we cultivated V. pantothenticus cells in a minimal medium with 30 mM ectoine or hydroxyectoine as the sole carbon or nitrogen source but found no growth in such cultures (data not shown). Consequently, ectoine and hydroxyectoine are accumulated by V. pantothenticus solely as metabolically inert protectants against osmotic and cold stress.
DISCUSSION
The transcription of the ectoine biosynthetic gene cluster (ectABC) in the soil-dwelling bacterium V. pantothenticus (21) is triggered by increases in salinity and by the continued cultivation of the cells at low temperature (15°C) (34). The low-temperature induction of an ectABC gene cluster is unusual and suggests a stress-adaptive value of ectoine production by V. pantothenticus in response to both of these environmental challenges. The data presented here reveal a new facet of the cold and salt stress adaptation of the soil bacterium and show that an exogenous supply of either ectoine or hydroxyectoine provides salt and cold stress protection as well (Fig. 1).
The expression of genes encoding various transporters for compatible solutes in B. subtilis (54) and Listeria monocytogenes (53) already have been shown to be under the control of the master regulator of the general stress response system, SigB. This stress response system operates in many Gram-positive bacteria and affords preemptive stress resistance against various environmental insults and metabolic imbalances (20, 44), including salt and sustained cold stress (7, 9, 24). Our finding that the transcription of the ectT gene from V. pantothenticus responses to well-established SigB-inducing cues (salt, cold, and ethanol stress) (Fig. 6B, C, and D) is fully in line with available data on the induction of genes belonging to the SigB regulon of B. subtilis (20, 44). However, we note that no molecular details are known currently about the SigB-dependent general stress responses system operating in V. pantothenticus. The DNA sequence of the V. pantothenticus ectT promoter (Fig. 6A) closely conforms to typical B. subtilis SigB-type promoters (20, 44), and its functioning in the heterologous host B. subtilis depends on the presence of the SigB transcription factor (Fig. 6E). Taken together, these data leave little doubt that the osmotic and chill stress control of ectT transcription is mediated by a V. pantothenticus SigB-dependent general response system. However, our data do not rigorously rule out that the transcription of ectT in V. pantothenticus is driven by another type of promoter that is located far upstream of the SigB-type promoter that we have mapped.
Since an exogenous supply of ectoine or hydroxyectoine provides osmotic and cold stress protection for V. pantothenticus (Fig. 1), the question as to the source of these compounds in natural ecosystems arises. In contrast to the synthesis of the compatible solutes glycine betaine and proline, there are no Eukarya known to produce ectoines, hence the presence of these compounds in the environment must stem from microorganisms. The ability to synthesize ectoine and hydroxyectoine is widespread in members of the domain Bacteria (41, 47, 52). Osmotically down-shocked or decomposed microbial ectoine/hydroxyectoine producers thus are likely the source of ectoines in natural settings, with estimated nM to low-μM ranges of concentration (62).
The constraints on the supply of ectoine/hydroxyectoine in natural environments require the presence of high-affinity uptake systems for their use as stress protectants or nutrients by microorganisms. Furthermore, the activity of these ectoine/hydroxyectoine transporters must be stimulated in response to environmental cues, and/or the transcription of their structural genes must be induced in a timely fashion to counteract the environmental challenge. The high-affinity ectoine uptake activity (Km of about 44 μM) present in V. pantothenticus (Fig. 2B) meets these criteria. Its induction by salt and cold stress points to the use of this compound as a stress protectant rather than as a nutrient. Indeed, in contrast to several other microorganisms (26, 36, 48), V. pantothenticus cannot use ectoine or hydroxyectoine as sole sources of carbon or nitrogen.
The EctT transporter from V. pantothenticus is a member of the BCCT family of carriers that currently has close to 3,000 members (http://www.ebi.ac.uk/interpro/; search query BCCT). Only a few of these proteins (22 representatives) have been functionally studied (65), and five of these, EctM (60), EctP (43), LcoP (55), BetM (60), and EctT (this study), have a demonstrated ectoine or ectoine/hydroxyectoine uptake activity. The amino acid sequence of the EctT protein is most closely related to that of the EctM transporter from M. halophilus (DSM 20408T) (identity of 49%) (60), but its substrate profile (ectoine, glycine betaine, and proline) corresponds most closely to that of the EctP transporter from C. glutamicum (43) (Fig. 4), although the amino acid sequence of EctP is only modestly related to that of EctT (identity of 32%).
The glycine betaine-specific BetP carrier from C. glutamicum is, without any doubt, the functionally best-understood member of the BCCT family (32, 46, 65). The transport activity of BetP is activated in less than a second in response to osmotic stress (32). BetP activation is elicited by an osmotic stress-derived cellular signal (possibly increases in the intracellular K+ pool) that modulates the intersubunit interaction of the extended carboxy terminus of BetP within the BetP trimer assembly (39, 42). The EctT transporter from V. pantothenticus lacks such an extended carboxy terminus, and our finding that the transport activity of EctT is not strongly activated by an osmotic stimulus (Fig. 3A) thus can be rationally understood. Hence, in contrast to BetP, enhanced ectoine uptake by the BCCT-type EctT carrier from V. pantothenticus under osmotic stress conditions depends primarily on enhanced ectT transcription (Fig. 3 and 5).
The BetP and EctT transporters also differ in their responses to cold stress. The transport activity of BetP is stimulated by low temperature (40), but surprisingly the cold activation of BetP activity is not transformed into an adaptive cellular response of C. glutamicum, since glycine betaine does not serve as a cold stress protectant for this bacterium (40). In contrast to BetP, EctT is not activated at the level of transporter activity by cold stress (Fig. 3B), but EctT-mediated ectoine uptake does provide cold stress protection for V. pantothenticus (Fig. 1B) owing to the enhanced transcription of the ectT gene (Fig. 5 and 6B and C).
The beneficial effect of compatible solutes as water-attracting osmolytes is quite well understood in terms of cellular physiology (6, 31, 63–65). However, the ways in which compatible solutes function as temperature stress protectants is far from clear. One possible explanation is that the increase in the intracellular compatible solute pool enhances the volume of free cytoplasmic water (45). However, it is equally possible that the cold stress protective effects of compatible solutes are unrelated to their effects on cellular hydration. Instead, they might stem from their ability to act as chemical chaperones to influence the biological performance of macromolecules and robustness of biosynthetic processes under stress conditions (13, 15, 22, 23, 25, 56). Ectoine and hydroxyectoine have known effects on the stability and functioning of proteins, on nucleic acids, on the properties of membranes, on biosynthetic processes, and on the viability of whole cells (18, 35, 41).
Whatever the precise molecular, biochemical, and biophysical mechanisms might be that underpin the physiological function of ectoine and hydroxyectoine as chill and osmostress protectants, the common pattern of the induction of the transcription of the ectABC biosynthetic (34) and of the ectT transporter genes (this study) permits V. pantothenticus to mount a well-integrated cellular defense to growth-restricting osmotic and low-temperature challenges by the accumulation of compatible solutes.
ACKNOWLEDGMENTS
We are very grateful to Jutta Gade for her expert technical assistance during part of this study, and we greatly appreciate the help of Vickie Koogle in editing the manuscript. We thank T. Schwarz and G. Lentzen (Bitop AG, Witten, Germany) for a generous gift of ectoines. We are grateful to V. Müller (University of Frankfurt, Germany) for measuring the Na+ content of growth media. We profited from discussions with C. Ziegler (Max Planck Institute of Biophysics, Frankfurt, Germany) on the topological orientation of BCCT-type carriers with the membrane and their ligand binding properties.
Financial support for this study was provided by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie and by a grant from the LOEWE program of the State of Hessen through the Centre for Synthetic Microbiology (SynMicro, Marburg, Germany).
Footnotes
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angelidis A. S., Smith G. M. 2003. Role of the glycine betaine and carnitine transporters in adaptation of Listeria monocytogenes to chill stress in defined medium. Appl. Environ. Microbiol. 69:7492–7498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bayles D. O., Wilkinson B. J. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23–27 [DOI] [PubMed] [Google Scholar]
- 4. Bourot S., et al. 2000. Glycine betaine-assisted protein folding in a lysA mutant of Escherichia coli. J. Biol. Chem. 275:1050–1056 [DOI] [PubMed] [Google Scholar]
- 5. Bremer E. 2002. Adaptation to changing osmolarity, p. 385–391 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and its closest relatives. ASM Press, Washington, DC [Google Scholar]
- 6. Bremer E., Krämer R. 2000. Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes in bacteria, p. 79–97 In Storz G., Hengge-Aronis R. (ed.), Bacterial stress responses. ASM, Washington, DC [Google Scholar]
- 7. Brigulla M., et al. 2003. Chill induction of the SigB-dependent general stress response in Bacillus subtilis and its contribution to low-temperature adaptation. J. Bacteriol. 185:4305–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brückner R. 1992. A series of shuttle vectors for Bacillus subtilis and Escherichia coli. Gene 122:187–192 [DOI] [PubMed] [Google Scholar]
- 9. Budde I., Steil L., Scharf C., Völker U., Bremer E. 2006. Adaptation of Bacillus subtilis to growth at low temperature: a combined transcriptomic and proteomic appraisal. Microbiology 152:831–853 [DOI] [PubMed] [Google Scholar]
- 10. Bursy J., et al. 2008. Synthesis and uptake of the compatible solutes ectoine and 5-hydroxyectoine by Streptomyces coelicolor A3(2) in response to salt and heat stresses. Appl. Environ. Microbiol. 74:7286–7296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bursy J., Pierik A. J., Pica N., Bremer E. 2007. Osmotically induced synthesis of the compatible solute hydroxyectoine is mediated by an evolutionarily conserved ectoine hydroxylase. J. Biol. Chem. 282:31147–31155 [DOI] [PubMed] [Google Scholar]
- 12. Calderón M. I., et al. 2004. Complex regulation of the synthesis of the compatible solute ectoine in the halophilic bacterium Chromohalobacter salexigens DSM 3043T. Microbiology 150:3051–3063 [DOI] [PubMed] [Google Scholar]
- 13. Chattopadhyay M. K., et al. 2004. The chemical chaperone proline relieves the thermosensitivity of a dnaK deletion mutant at 42°C. J. Bacteriol. 186:8149–8152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choquet G., Jehan N., Pissavin C., Blanco C., Jebbar M. 2005. OusB, a broad-specificity ABC-type transporter from Erwinia chrysanthemi, mediates uptake of glycine betaine and choline with a high affinity. Appl. Environ. Microbiol. 71:3389–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diamant S., Eliahu N., Rosenthal D., Goloubinoff P. 2001. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J. Biol. Chem. 276:39586–39591 [DOI] [PubMed] [Google Scholar]
- 16. Grammann K., Volke A., Kunte H. J. 2002. New type of osmoregulated solute transporter identified in halophilic members of the bacteria domain: TRAP transporter TeaABC mediates uptake of ectoine and hydroxyectoine in Halomonas elongata DSM 2581(T). J. Bacteriol. 184:3078–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haardt M., Kempf B., Faatz E., Bremer E. 1995. The osmoprotectant proline betaine is a major substrate for the binding-protein-dependent transport system ProU of Escherichia coli K-12. Mol. Gen. Genetics 246:783–786 [DOI] [PubMed] [Google Scholar]
- 18. Harishchandra R. K., Wulff S., Lentzen G., Neuhaus T., Galla H. J. 2010. The effect of compatible solute ectoines on the structural organization of lipid monolayer and bilayer membranes. Biophys. Chem. 150:37–46 [DOI] [PubMed] [Google Scholar]
- 19. Harwood C. R., Archibald A. R. 1990. Growth, maintenance and general techniques, p. 1–26 In Harwood C. R., Cutting S. M. (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Inc., Chichester, United Kingdom [Google Scholar]
- 20. Hecker M., Pane-Farre J., Völker U. 2007. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 61:215–236 [DOI] [PubMed] [Google Scholar]
- 21. Heyndrickx M., et al. 1999. Proposal of Virgibacillus proomii sp. nov. and emended description of Virgibacillus pantothenticus (Proom and Knight 1950) Heyndrickx et al. 1998. Int. J. Syst. Bacteriol. 49:1083–1090 [DOI] [PubMed] [Google Scholar]
- 22. Hoffmann T., Bremer E. 2011. Protection of Bacillus subtilis against cold stress via compatible-solute acquisition. J. Bacteriol. 193:1552–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holtmann G., Bremer E. 2004. Thermoprotection of Bacillus subtilis by exogenously provided glycine betaine and structurally related compatible solutes: involvement of Opu transporters. J. Bacteriol. 186:1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Höper D., Völker U., Hecker M. 2005. Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis. J. Bacteriol. 187:2810–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ignatova Z., Gierasch L. M. 2006. Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proc. Natl. Acad. Sci. U. S. A. 103:13357–13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jebbar M., Sohn-Bösser L., Bremer E., Bernard T., Blanco C. 2005. Ectoine-induced proteins in Sinorhizobium meliloti include an ectoine ABC-type transporter involved in osmoprotection and ectoine catabolism. J. Bacteriol. 187:1293–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jebbar M., Talibart R., Gloux K., Bernard T., Blanco C. 1992. Osmoprotection of Escherichia coli by ectoine: uptake and accumulation characteristics. J. Bacteriol. 174:5027–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jebbar M., von Blohn C., Bremer E. 1997. Ectoine functions as an osmoprotectant in Bacillus subtilis and is accumulated via the ABC-transport system OpuC. FEMS Microbiol. Lett. 154:325–330 [Google Scholar]
- 29. Kappes R. M., Kempf B., Bremer E. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J. Bacteriol. 178:5071–5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kempf B., Bremer E. 1995. OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis. J. Biol. Chem. 270:16701–16713 [DOI] [PubMed] [Google Scholar]
- 31. Kempf B., Bremer E. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high osmolality environments. Arch. Microbiol. 170:319–330 [DOI] [PubMed] [Google Scholar]
- 32. Krämer R., Ziegler C. 2009. Regulative interactions of the osmosensing C-terminal domain in the trimeric glycine betaine transporter BetP from Corynebacterium glutamicum. Biol. Chem. 390:685–691 [DOI] [PubMed] [Google Scholar]
- 33. Kuhlmann A. U., Bremer E. 2002. Osmotically regulated synthesis of the compatible solute ectoine in Bacillus pasteurii and related Bacillus spp. Appl. Environ. Microbiol. 68:772–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuhlmann A. U., Bursy J., Gimpel S., Hoffmann T., Bremer E. 2008. Synthesis of the compatible solute ectoine in Virgibacillus pantothenticus is triggered by high salinity and low growth temperature. Appl. Environ. Microbiol. 74:4560–4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kurz M. 2008. Compatible solute influence on nucleic acids: many questions but few answers. Sal. Syst. 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lecher J., et al. 2009. The crystal structure of UehA in complex with ectoine-A comparison with other TRAP-T binding proteins. J. Mol. Biol. 389:58–73 [DOI] [PubMed] [Google Scholar]
- 37. Miller J. H. 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 38. Mustakhimov I. I., et al. 2010. Identification and characterization of EctR1, a new transcriptional regulator of the ectoine biosynthesis genes in the halotolerant methanotroph Methylomicrobium alcaliphilum 20Z. J. Bacteriol. 192:410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ott V., Koch J., Spate K., Morbach S., Krämer R. 2008. Regulatory properties and interaction of the C- and N-terminal domains of BetP, an osmoregulated betaine transporter from Corynebacterium glutamicum. Biochemistry 47:12208–12218 [DOI] [PubMed] [Google Scholar]
- 40. Ozcan N., Krämer R., Morbach S. 2005. Chill activation of compatible solute transporters in Corynebacterium glutamicum at the level of transport activity. J. Bacteriol. 187:4752–4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pastor J. M., et al. 2011. Ectoines in cell stress protection: uses and biotechnological production. Biotechnol. Adv. 28:782–801 [DOI] [PubMed] [Google Scholar]
- 42. Perez C., Khafizov K., Forrest L. R., Krämer R., Ziegler C. 17 June 2011. The role of trimerization in the osmoregulated betaine transporter BetP. EMBO Rep. [Epub ahead of print.] doi:10.1038/embor.2011.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peter H., Weil B., Burkovski A., Krämer R., Morbach S. 1998. Corynebacterium glutamicum is equipped with four secondary carriers for compatible solutes: identification, sequencing, and characterization of the proline/ectoine uptake system, ProP, and the ectoine/proline/glycine betaine carrier, EctP. J. Bacteriol. 180:6005–6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Price C. W. 2011. General stress responses in Bacillus subtilis and related Gram-positive bacteria, p. 301–318 In Storz G., Hengge R. (ed.), Bacterial stress responses, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 45. Record M. T., Jr., Courtenay E. S., Cayley D. S., Guttman H. J. 1998. Responses of E. coli to osmotic stress: large changes in amounts of cytoplasmic solutes and water. Trends Biochem. Sci. 23:143–148 [DOI] [PubMed] [Google Scholar]
- 46. Ressl S., Terwisscha van Scheltinga A. C., Vonrhein C., Ott V., Ziegler C. 2009. Molecular basis of transport and regulation in the Na(+)/betaine symporter BetP. Nature 458:47–52 [DOI] [PubMed] [Google Scholar]
- 47. Reuter K., et al. 2010. Synthesis of 5-hydroxyectoine from ectoine: crystal structure of the non-heme iron(II) and 2-oxoglutarate-dependent dioxygenase EctD. PLoS One 5:e10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rodríguez-Moya J., et al. 2010. Involvement of EupR, a response regulator of the NarL/FixJ family, in the control of the uptake of the compatible solutes ectoines by the halophilic bacterium Chromohalobacter salexigens. BMC Microbiol. 10:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sambrook J., Fritsch E. F., Maniatis T. E. 1989. Molecular cloning. A laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 50. Saum S. H., Müller V. 2007. Salinity-dependent switching of osmolyte strategies in a moderately halophilic bacterium: glutamate induces proline biosynthesis in Halobacillus halophilus. J. Bacteriol. 189:6968–6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schulze S., Koster S., Geldmacher U., Terwisscha van Scheltinga A. C., Kühlbrandt W. 2010. Structural basis of Na(+)-independent and cooperative substrate/product antiport in CaiT. Nature 467:233–236 [DOI] [PubMed] [Google Scholar]
- 52. Schwibbert K. 16 September 2010. A blueprint of ectoine metabolism from the genome of the industrial producer Halomonas elongata DSM 2581(T). Environ. Microbiol. [Epub ahead of print.] doi:10.1111/j.1462-2920.2010.02336.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sleator R. D., Hill C. 2010. Compatible solutes: a listerial passe-partout? Gut Microbes 1:77–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spiegelhalter F., Bremer E. 1998. Osmoregulation of the opuE proline transport gene from Bacillus subtilis-contributions of the σA- and σB-dependent stress-responsive promoters. Mol. Microbiol. 29:285–296 [DOI] [PubMed] [Google Scholar]
- 55. Steger R., Weinand M., Krämer R., Morbach S. 2004. LcoP, an osmoregulated betaine/ectoine uptake system from Corynebacterium glutamicum. FEBS Lett. 573:155–160 [DOI] [PubMed] [Google Scholar]
- 56. Street T. O., Bolen D. W., Rose G. D. 2006. A molecular mechanism for osmolyte-induced protein stability. Proc. Natl. Acad. Sci. U. S. A. 103:13997–14002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gothoh T. 1987. High-copy-number and low-copy-number plasmid vectors for lacZ α-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63–74 [DOI] [PubMed] [Google Scholar]
- 58. Thompson J. D., Higgins D. G., Gibson T. J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vargas C., et al. 2006. Ectoines as compatible solutes and carbon and energy sources for the halophilic bacterium Chromohalobacter salexigens. J. Appl. Microbiol. 100:98–107 [DOI] [PubMed] [Google Scholar]
- 60. Vermeulen V., Kunte H. J. 2004. Marinococcus halophilus DSM 20408T encodes two transporters for compatible solutes belonging to the betaine-carnitine-choline transporter family: identification and characterization of ectoine transporter EctM and glycine betaine transporter BetM. Extremophiles 8:175–184 [DOI] [PubMed] [Google Scholar]
- 61. von Blohn C., Kempf B., Kappes R. M., Bremer E. 1997. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol. Microbiol. 25:175–187 [DOI] [PubMed] [Google Scholar]
- 62. Welsh D. T. 2000. Ecological significance of compatible solute accumulation by micro-organisms: from single cells to global climate. FEMS Microbiol. Rev. 24:263–290 [DOI] [PubMed] [Google Scholar]
- 63. Wood J. M. 2011. Osmotic stress, p. 133–156 In Storz G., Hengge R., (ed.), Bacterial stress responses, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 64. Wood J. M., et al. 2001. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130:437–460 [DOI] [PubMed] [Google Scholar]
- 65. Ziegler C., Bremer E., Krämer R. 2010. The BCCT family of carriers: from physiology to crystal structure. Mol. Microbiol. 78:13–34 [DOI] [PubMed] [Google Scholar]