Abstract
Two 8-vinyl reductases, BciA and BciB, have been identified in chlorophototrophs. The bciA gene of Chlorobaculum tepidum was replaced with genes similar to bciB from other green sulfur bacteria. Pigment analyses of the complemented strains showed that the bciB homologs encode 8-vinyl reductases similar to those of cyanobacteria.
TEXT
Chlorophototrophic bacteria, algae, and plants synthesize chlorophylls (Chls) and/or bacteriochlorophylls (BChls) from the common substrate protoporphyrin IX (1, 4, 7, 12, 20). During the biosynthesis of most (B)Chls, the C-8 vinyl side chain on the tetrapyrrole B ring is reduced to an ethyl group, which may be further modified (7, 12). Exceptions include the 3,8-divinyl (DV) Chl a and b molecules that are synthesized by some Cyanobacteria (18).
Until AT5G18660 of Arabidopsis thaliana was shown to encode 8-vinyl reductase (14, 15), BchJ was mistakenly believed to be the enzyme responsible for the C-8 vinyl reduction in bacteria (6, 17). A homolog of the A. thaliana 8-vinyl reductase, designated bciA, is encoded by open reading frame (ORF) CT1063 in the green sulfur bacterium Chlorobaculum tepidum, and its product was also shown to have 8-vinyl reductase activity (6). However, many organisms which are known to synthesize monovinyl (MV) (B)Chls do not have BciA homologs (6); this means that another type of 8-vinyl reductase must occur in these organisms. In the cyanobacterium Synechococystis sp. strain PCC 6803, ORF slr1923 encodes a product essential for C-8 vinyl reduction (9, 10). Homologs of slr1923 are found in most but not all chlorophototrophic organisms without bciA homologs (10). However, heterologous expression of slr1923 in Escherichia coli was unsuccessful (10). Therefore, it was unclear whether the product of slr1923 was sufficient to catalyze the 8-vinyl reductase activity, required another protein(s) to form a multisubunit enzyme complex, or played some regulatory role. To acknowledge its role in (B)Chl biosynthesis, Bryant et al. designated slr1923 and its homologs as bciB (2).
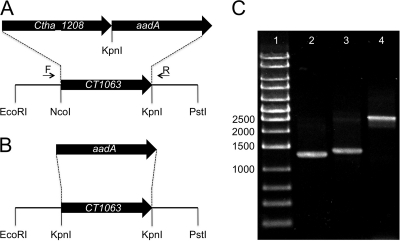
Heterologous expression of slr1923 in E. coli could have failed to reveal the expected 8-vinyl reductase activity for many reasons, including improper folding or conformation, inability to introduce a cofactor, or absence of required electron donors. These factors might be corrected by expressing appropriate genes from a different expression platform. This idea was tested by constructing a C. tepidum strain (ΔbciA::bciBCher-aadA) in which the native bciA gene (CT1063) was replaced by bciB (Ctha_1208) from another green sulfur bacterium (GSB), Chloroherpeton thalassium, together with a selectable aadA antibiotic resistance cartridge (5) (Fig. 1 A). A negative-control strain (ΔbciA::aadA), in which the bciA gene was simply replaced with aadA, was also constructed (Fig. 1B). These constructs were made in plasmid pUC19 as described previously (11) and were designed so that bciA was completely replaced from start to stop codons. The resulting plasmids were linearized with AhdI and used to transform C. tepidum to spectinomycin/streptomycin resistance (3). Complete segregation of alleles was verified by PCR (Fig. 1C) and DNA sequencing of PCR products. Pigments were extracted from cells using acetone-methanol (7:2, vol/vol) and analyzed by reversed-phase high-pressure liquid chromatography (HPLC) as described previously (6).
Fig. 1.
(A and B) Physical maps and agarose gel electrophoresis of PCR products showing construction and complete segregation of wild-type and mutant alleles for ΔbciA::bciBCher-aadA (A) and ΔbciA::aadA (B) mutant strains of C. tepidum. (C) Agarose gel electrophoresis of PCR products of bciA locus using primers “F” and “R” (as labeled in panel A). Lane 1, DNA size markers (sizes in bp indicated for selected markers); lane 2, amplicon produced using template DNA from C. tepidum wild type; lane 3, amplicon produced using template DNA from the ΔbciA::aadA strain; lane 4, amplicon produced using template DNA from the ΔbciA::bciBCher-aadA mutant.
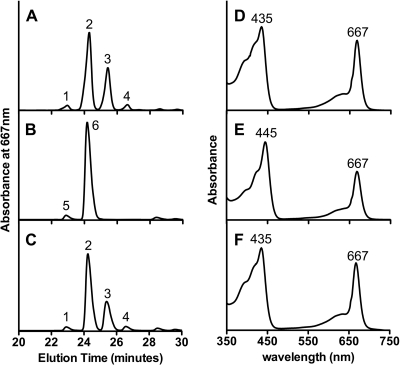
Consistent with results obtained previously when bciA was insertionally inactivated (6), the ΔbciA::aadA mutant synthesized only two homologs of BChl cF (BChl c esterified with farnesol), because the vinyl group at the C-82 position could no longer be methylated by BchQ (8). The Soret absorption peak of BChl cF of the ΔbciA::aadA mutant was redshifted 10 nm to 445 nm compared to that of BChl cF of wild-type cells (Fig. 2 A, B, D, and E). Thus, replacement of bciA by aadA resulted in the loss of 8-vinyl reductase activity and resulted in the synthesis of [8-vinyl]-BChl cF. However, the ΔbciA::bciBCher-aadA strain synthesized the same distribution of BChl cF homologs as did the wild-type cells. The number of homologs, their abundance, and their absorption spectra were identical to those of wild-type cells (Fig. 2A, C, D, and F). These results demonstrated that BciB functionally complemented the loss of 8-vinyl reductase activity caused by deletion of the bciA gene. If BciB were a subunit of multisubunit enzyme complex, it would be extremely unlikely that C. tepidum would possess genes encoding (all) the other subunit(s) of that complex except BciB. Therefore, it is likely that BciB is sufficient to produce 8-vinyl reductase activity (in the metabolic context of a GSB cell). These experiments do not address the identity of the electron donor to BciB, but they suggest that a donor, perhaps the abundant [8Fe-8S] ferredoxins that are present in these cells (21), is available and efficiently donates electrons to BciB.
Fig. 2.
HPLC elution profiles (A to C) and absorption spectra (D to F) of extracted BChl cF of C. tepidum wild-type cells (A and D), ΔbciA::aadA mutant (B and E), and ΔbciA::bciBCher-aadA mutant (C and F). BChl cF homologs in peaks 1 to 6 have been identified in previous studies (6, 8). Compound 1 is [8-ethyl-12-methyl]-BChl cF, compound 2 is [8-ethyl-12-ethyl]-BChl cF, compound 3 is [8-n-propyl-12-ethyl]-BChl cF, compound 4 is [8-iso-butyl-12-ethyl]-BChl cF, compound 5 is [8-vinyl-12-methyl]-BChl cF, and compound 6 is [8-vinyl-12-ethyl]-BChl cF. Absorption spectra are shown for the BChl cF homolog corresponding to the largest peak (peaks 2, 6, and 2 in panels A, B, and C, respectively), although spectra were identical for all BChl cF homologs from each cell type.
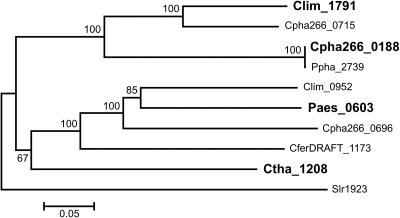
A phylogenetic analysis of BciB homologs was performed for all sequenced GSB genomes. Surprisingly, homologs of both bciA and bciB were found in several GSB strains (Table 1; Fig. 3). Additionally, some GSB strains possessed multiple copies of BciB that could produce redundant protein activities (Table 1). To determine if this was the case, three of the bciB homologs, Clim_1791, Cpha266_0188, and Paes_0603, representing different branches of the phylogenetic tree (Fig. 3), were tested for their ability to complement a bciA deletion mutation as described above. All three strains synthesized BChl c like the wild-type strain (data not shown). These results suggested that all of the bciB homologs in GSBs probably encode functional 8-vinyl reductases.
Table 1.
Distribution of bciA and bciB homologs in some GSB genomes
| Organism | No. of homologs of: |
|
|---|---|---|
| BciAa | BciB | |
| Chlorobium ferrooxidans DSM 13031b | 1 | 1 |
| Chlorobium limicola DSM 245 | 0 | 2 |
| Chlorobium phaeobacteroides DSM 266 | 0 | 3 |
| Chlorobium clathratiforme DSM 5477 | 1 | 1 |
| Prosthecochloris aestuarii DSM 271 | 1 | 1 |
| Chloroherpeton thalassium ATCC 35110 | 0 | 1 |
Only organisms with bciB homologs are listed here. Three GSBs have no bciA gene; all GSBs lacking BciB homologs have only one BciA homolog.
Genome incomplete.
Fig. 3.
Neighbor-joining phylogenetic tree of BciB homologs in green sulfur bacteria. The tree was based on 1,000 bootstrap samplings, and bootstrap support values per 100 samples are shown for each node. The slr1923 product of the cyanobacterium Synechocystis sp. PCC 6803 was used as outgroup. The bar denotes 0.05 changes per amino acid site. Homologs tested in C. tepidum in this study are indicated in bold. Prefixes to locus tags used in this figure are as follows: Clim for Chlorobium limicola DSM 245, Cpha266 for Chlorobium phaeobacteroides DSM 266, Ppha for Chlorobium clathratiforme DSM 5477, Paes for Prosthecochloris aestuarii DSM 271, CferDRAFT for Chlorobium ferrooxidans DSM 13031, and Ctha for Chloroherpeton thalassium ATCC 35110.
These observations raise two interesting questions: why do some organisms retain genes for two different 8-vinyl reductases, and why do some organisms have multiple copies of what appear to be functionally redundant genes? Similar observations have been made for bchE and acsF genes, whose products catalyze the isocyclic ring formation in (B)Chl biosynthesis (1, 4, 7, 12, 20). Many organisms have both bchE and acsF genes (16; A. M. Garcia Costas, Z. Liu, L. P. Tomsho, S. C. Schuster, D. M. Ward, and D. A. Bryant, submitted for publication), and some organisms have two copies of acsF (13). These gene products function under different oxygen concentrations, which allows organisms to synthesize (B)Chls under a range of environmental conditions (13, 16). It is possible that the BciA and BciB homologs have a similar complementary relationship, such that each is optimally active under different conditions. However, it is not yet clear what these conditions might be. BciA has no oxygen-sensitive redox cofactors and could be expressed in an active form in E. coli (6), but BciB is predicted to contain one or more Fe/S clusters, which might be oxygen sensitive. It is possible that Fe could be limiting in some environments, and the ability to switch to a protein lacking metal cofactors might be advantageous.
The occurrence of multiple divinyl reductases in some GSBs may also underscore the importance of reduced C-8 side chains for BChls in these organisms. In Cyanobacteria, mutant strains with DV-Chl a grew much slower than wild-type cells under high light (9, 10), because of rapid bleaching of DV-Chl a and dissociation of reaction center complexes (19). Disruption of bciA in C. tepidum caused significant differences in the suprastructure formed by BChl c in chlorosomes, which resulted in a 31-nm blueshift of the absorption maximum of the BChl c in chlorosomes (6). These changes resulted in less absorption per BChl c molecule (see reference 6), less-efficient energy transfer from BChl c to the BChl a associated with CsmA in the base plates because of the energy gap, and much greater spectral overlap with Chl a-containing organisms. All of these changes would lead to lower light-harvesting efficiency and lower growth rates. Although the ΔbciA::aadA mutant grew only slightly slower (13%) than the wild type under high-light conditions (∼100 μmol photons s−1 m−2), it grew at only 49% of the rate of the wild type under low-light conditions (∼20 μmol photons s−1 m−2; data not shown). Therefore, the ability to reduce the C-8 vinyl group represents a significant advantage for GSBs, which often live under low- and growth-limiting light conditions.
Acknowledgements
This research was supported by grant DE-FG02-94ER20137 from the U.S. Department of Energy to D.A.B.
Footnotes
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Bollivar D. W. 2006. Recent advances in chlorophyll biosynthesis. Photosynth. Res. 90:173–194 [DOI] [PubMed] [Google Scholar]
- 2. Bryant D. A., et al. 2011. Comparative and functional genomics of anoxygenic green bacteria from the taxa Chlorobi, Chloroflexi, and Acidobacteria. In R. L. Burnap and W. Vermaas (ed.), Advances in photosynthesis and respiration, vol. 33. Functional genomics and evolution of photosynthetic systems, p. 47–102Springer, Dordrecht, The Netherlands. [Google Scholar]
- 3. Frigaard N. U., Bryant D. A. 2001. Chromosomal gene inactivation in the green sulfur bacterium Chlorobium tepidum by natural transformation. Appl. Environ. Microbiol. 67:2538–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frigaard N. U., Gomez Maqueo Chew A., Maresca J. A., Bryant D. A. 2006. Bacteriochlorophyll biosynthesis in green bacteria, p. 201–221 In Grimm B., Porra R. J., Rüdiger W., Scheer H. Advances in photosynthesis and respiration, vol. 25 Springer, Dordrecht, The Netherlands [Google Scholar]
- 5. Frigaard N. U., Voigt G. D., Bryant D. A. 2002. Chlorobium tepidum mutant lacking bacteriochlorophyll c made by inactivation of the bchK gene, encoding bacteriochlorophyll c synthase. J. Bacteriol. 184:3368–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gomez Maqueo Chew A., Bryant D. A. 2007. Characterization of a plant-like protochlorophyllide a divinyl reductase in green sulfur bacteria. J. Biol. Chem. 282:2967–2975 [DOI] [PubMed] [Google Scholar]
- 7. Gomez Maqueo Chew A., Bryant D. A. 2007. Chlorophyll biosynthesis in bacteria: the origins of structural and functional diversity. Annu. Rev. Microbiol. 61:113–129 [DOI] [PubMed] [Google Scholar]
- 8. Gomez Maqueo Chew A., Frigaard N.-U., Bryant D. A. 2007. Bacteriochlorophyllide c C-82 and C-121 methyltransferases are essential for adaptation to low light in Chlorobaculum tepidum. J. Bacteriol. 189:6176–6184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Islam M. R., et al. 2008. slr1923 of Synechocystis sp. PCC6803 is essential for conversion of 3,8-divinyl(proto)chlorophyll(ide) to 3-monovinyl(proto) chlorophyll(ide). Plant Physiol. 148:1068–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito H., Yokono M., Tanaka R., Tanaka A. 2008. Identification of a novel vinyl reductase gene essential for the biosynthesis of monovinyl chlorophyll in Synechocystis sp. PCC6803. J. Biol. Chem. 283:9002–9011 [DOI] [PubMed] [Google Scholar]
- 11. Liu Z., Bryant D. A. 2011. Identification of a gene essential for the first committed step in the biosynthesis of bacteriochlorophyll c. J. Biol. Chem. 286:22393–22402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Z., Bryant D. A. Biosynthesis and assembly of bacteriochlorophyll c in green sulfur bacteria: theme and variations. In Kadish K. M., Smith K. M., Guilard R. Handbook of porphyrin sciencein press. World Scientific Publishers, Hackensack, NJ [Google Scholar]
- 13. Minamizaki K., Mizoguchi T., Goto T., Tamiaki H., Fujita Y. 2008. Identification of two homologous genes, chlAI and chlAII, that are differentially involved in isocyclic ring formation of chlorophyll a in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 283:2684–2692 [DOI] [PubMed] [Google Scholar]
- 14. Nagata N., Tanaka R., Satoh S., Tanaka A. 2005. Identification of a vinyl reductase gene for chlorophyll synthesis in Arabidopsis thaliana and implications for the evolution of Prochlorococcus species. Plant Cell 17:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakanishi H., et al. 2005. Characterization of the Arabidopsis thaliana mutant pcb2 which accumulates divinyl chlorophylls. Plant Cell Physiol. 46:467–473 [DOI] [PubMed] [Google Scholar]
- 16. Ouchane S., Steunou A. S., Picaud M., Astier C. 2004. Aerobic and anaerobic Mg-protoporphyrin monomethyl ester cyclases in purple bacteria: a strategy adopted to bypass the repressive oxygen control system. J. Biol. Chem. 279:6385–6394 [DOI] [PubMed] [Google Scholar]
- 17. Suzuki J. Y., Bauer C. E. 1995. Altered monovinyl and divinyl protochlorophyllide pools in bchJ mutants of Rhodobacter capsulatus. Possible monovinyl substrate discrimination of light-independent protochlorophyllide reductase. J. Biol. Chem. 270:3732–3740 [PubMed] [Google Scholar]
- 18. Tanaka R., Tanaka A. 2007. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 58:321–346 [DOI] [PubMed] [Google Scholar]
- 19. Tomo T., et al. 2009. Replacement of chlorophyll with di-vinyl chlorophyll in the antenna and reaction center complexes of the cyanobacterium Synechocystis sp. PCC 6803: characterization of spectral and photochemical properties. Biochim. Biophys. Acta 1787:191–200 [DOI] [PubMed] [Google Scholar]
- 20. Willows R. D. 2003. Biosynthesis of chlorophylls from protoporphyrin IX. Nat. Prod. Rep. 20:327–341 [DOI] [PubMed] [Google Scholar]
- 21. Yoon K. S., Bobst C., Hemann C. F., Hille R., Tabita F. R. 2001. Spectroscopic and functional properties of novel 2[4Fe-4S] cluster-containing ferredoxins from the green sulfur bacterium Chlorobium tepidum. J. Biol. Chem. 276:44027–44036 [DOI] [PubMed] [Google Scholar]