Abstract
Streptococcus pyogenes Rgg is a regulatory protein that controls the transcription of 588 genes in strain NZ131 during the post-exponential phase of growth, including the virulence-associated genes encoding the extracellular SpeB protease, pullulanase A (PulA), and two extracellular nucleases (SdaB and Spd-3). Rgg binds to DNA proximally to the speB promoter (PspeB) to activate transcription; however, it is not known if Rgg binds to the promoters of other genes to influence expression, or if the perturbation of other global regulons accounts for the genome-wide changes in expression associated with the mutant. To address this issue, chromatin immunoprecipitation followed by DNA microarray analysis (ChIP-chip) was used to identify the DNA binding sites of Rgg. Rgg bound to 65 sites in the chromosome. Thirty-five were within noncoding DNA, and 43% of these were adjacent to genes previously identified as regulated by Rgg. Electrophoretic mobility shift assays were used to assess the binding of Rgg to a subset of sites bound in vivo, including the noncoding DNA upstream of speB, the genes encoding PulA, Spd-3, and a transcriptional regulator (SPY49_1113), and prophage-associated genes encoding a putative integrase (SPY49_0746) and a surface antigen (SPY49_0396). Rgg bound to all target DNAs in vitro, consistent with the in vivo results. Finally, analyses with a transcriptional reporter system showed that the DNA bound by Rgg contained an active promoter that was regulated by Rgg. Overall, the results indicate that Rgg binds specifically to multiple sites in the chromosome, including prophage DNA, to influence gene expression.
INTRODUCTION
The Gram-positive respiratory and skin pathogen Streptococcus pyogenes (group A streptococcus [GAS]) can be carried asymptomatically and is a common cause of uncomplicated pharyngitis, or “strep throat” (9). S. pyogenes also causes several life-threatening diseases, such as streptococcal toxic shock syndrome and necrotizing fasciitis, the so called “flesh-eating” disease (9). Prompt treatment of pharyngitis with antibiotics decreases the likelihood of dissemination and can prevent postinfection autoimmune sequelae such as acute rheumatic fever (ARF) (16). S. pyogenes also contributes to secondary bacterial pneumonia following influenza infections (2). During the recent H1N1 influenza A virus pandemic, 29% of deaths were attributed to secondary bacterial pneumonia, and of these, 27% were caused by S. pyogenes (2). Moreover, the incidence of invasive S. pyogenes disease increased significantly (26%) in December 2010 and January 2011 in England, due, at least in part, to widespread influenza infections (58). Overall, S. pyogenes causes more than 500,000 deaths each year worldwide, and no vaccine is available to prevent these deaths (9). Thus, the burden of the microbe on human health is significant.
The determination of the genome sequences of more than a dozen isolates shows that much, but not all, of the genetic variation among isolates is due to differences in the number and types of bacteriophages in the genome (4, 17). All isolates sequenced thus far are polylysogenized, with as many as eight bacteriophages in the chromosome, which can constitute as much as 10% of the genome (20). These lambda-like viruses belong to the family Siphoviridae and often encode important virulence factors, including superantigens, hyaluronidases, and secreted nucleases (8, 25, 41, 50). Although some of the bacteriophage-encoded virulence genes were previously shown to be expressed during the course of infection, relatively little is known about the molecular factors that control their expression (54).
Rgg, also known as RopB (11, 31), of S. pyogenes belongs to a family of transcriptional regulators (Rgg family; TIGR01716) that are encoded in the chromosomes of several species of low-G+C Gram-positive bacteria (11, 31, 40, 42, 44, 53). The proteins have a helix-turn-helix (HTH)-XRE domain in the amino terminus, which is presumably responsible for DNA binding, and various carboxyl-terminal domains, including a tetratricopeptide repeat (TPR), which may mediate binding to peptide pheromones (34). Rgg is similar to Cro/cI prophage regulators, which has led to speculation that they may be derived from an ancestral prophage (36). Typically, Rgg orthologues control the transcription of adjacent genes (40, 42, 44, 53), which encode secreted proteases (11), nucleases (19), bacteriocins (42, 43), and peptides involved in intraspecies communication (21, 34).
In S. pyogenes, Rgg activates the transcription of the adjacent gene encoding streptococcal pyrogenic exotoxin B (SpeB) in the post-exponential phase of growth (11). It also represses the transcription of the other adjacent gene, encoding streptodornase B (SdaB; also known as mitogenic factor-1 [MF-1]), which is a secreted nuclease (19). In the post-exponential phase of growth, inactivation of rgg in strain NZ131 alters the expression of more than 500 additional genes from that in the parental strain (19). Among these are several virulence and prophage-related genes, including secreted proteases, nucleases, and antigens localized to the cell surface (19). Inactivation of rgg in strain NZ131 also altered metabolism. Specifically, the mutant ferments arginine and degrades serine during the exponential phase of growth, in contrast to the wild-type (wt) strain, which is a homolactate fermenter (13). As a result, the pH of the growth medium of the mutant is more neutral due to less excretion of lactic acid and the production of ammonia, and the concentrations of metabolic precursors and end products in the medium differ between the two strains (13), which is likely to indirectly influence gene expression.
Neely et al. (40) showed that Rgg binds to the promoter region of speB (PspeB) to activate transcription. With the exception of speB, it is not known if Rgg directly controls the expression of the other Rgg-regulated genes, or if differences in metabolism or the perturbation of other regulatory networks is responsible for the genome-wide changes in expression associated with the mutant strain. The aim of this study was to identify the DNA binding sites of Rgg on a genome scale in vivo, as an additional step in defining the regulon. The results indicate that Rgg binds to both coding and noncoding DNA and that the noncoding DNA bound by Rgg is upstream of genes associated with virulence, DNA repair, and metabolism and of prophage-associated genes, suggesting that Rgg directly regulates these genes.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The bacterial strains and plasmids used in this study are described in Table 1. S. pyogenes was grown at 37°C under a 5% CO2 atmosphere without agitation in Todd-Hewitt broth (Becton Dickinson, Sparks, MD) containing 0.2% (wt/vol) yeast extract (THY broth). Escherichia coli strain DH5α was obtained from Gibco-BRL (Gaithersburg, MD) and was grown in Luria-Bertani medium at 37°C with agitation. Antibiotics were used at the following concentrations: carbenicillin at 100 μg/ml for E. coli, spectinomycin at 100 μg/ml for E. coli and S. pyogenes, erythromycin at 2.5 μg/ml for S. pyogenes, and kanamycin at 50 μg/ml for E. coli and 500 μg/ml for S. pyogenes.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | hsdR17 recA1 gyrA endA1 relA1 | Invitrogen |
| Rosetta 2(DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3) pRARE2 (Camr) | Novagen |
| S. pyogenes | ||
| NZ131 | M49 serotype | D.R. Martin, New Zealand |
| rgg mutant | NZ131 rgg mutant; Emr | 11 |
| SA5 | NZ131 rgg mutant complemented with pSA3; Emr Kanr; expresses an Rgg-Myc fusion protein | This study |
| wt::luc strain | NZ131 transformed with pKSM720; Specr | This study |
| wt::PspeB-luc strain | NZ131 transformed with pSA11; Specr | This study |
| wt::Pspd3-luc strain | NZ131 transformed with pSA12; Specr | This study |
| wt::PpulA-luc strain | NZ131 transformed with pSA13; Specr | This study |
| wt::P396-luc strain | NZ131 transformed with pSA14; Specr | This study |
| wt::P746-luc strain | NZ131 transformed with pSA15; Specr | This study |
| wt::P1113-luc strain | NZ131 transformed with pSA16; Specr | This study |
| rgg::luc strain | NZ131 rgg mutant transformed with pKSM720; Specr | This study |
| rgg::PspeB-luc strain | NZ131 rgg mutant transformed with pSA11; Specr | This study |
| rgg::Pspd3-luc strain | NZ131 rgg mutant transformed with pSA12; Specr | This study |
| rgg::PpulA-luc strain | NZ131 rgg mutant transformed with pSA13; Specr | This study |
| rgg::P396-luc strain | NZ131 rgg mutant transformed with pSA14; Specr | This study |
| rgg::P746-luc strain | NZ131 rgg mutant transformed with pSA15; Specr | This study |
| rgg::P1113-luc strain | NZ131 rgg mutant transformed with pSA16; Specr | This study |
| Plasmids | ||
| pTrcHis2B | Cloning vector; Ampr | Invitrogen |
| pSA1 | rgg ORF cloned into pTrcHis2B; Ampr | This study |
| pKK1 | Shuttle vector defective for replication in S. pyogenes; Kanr | 26 |
| pSA3 | rgg ORF in frame with Myc and 6× histidine tags cloned into pKK; Kanr | This study |
| pMAL-c4X | Cloning vector; Ampr | New England BioLabs |
| pSA4 | rgg ORF cloned into pMAL-c4X; Ampr | This study |
| pSA5 | Promoter region of speB cloned into pGEM-T-vector; Ampr | This study |
| pSA6 | Promoter region of spd3 cloned into pGEM-T-vector; Ampr | This study |
| pSA7 | Promoter region of pulA cloned into pGEM-T-vector; Ampr | This study |
| pSA8 | Promoter region of Spy49_0396 cloned into pGEM-T-vector; Ampr | This study |
| pSA9 | Promoter region of Spy49_0746 cloned into pGEM-T-vector; Ampr | This study |
| pSA10 | Promoter region of Spy49_1113 cloned into pGEM-T-vector; Ampr | This study |
| pKSM720 | GAS replicating plasmid with firefly luciferase and RBS; Specr | 28 |
| pSA11 | Noncoding region upstream of speB cloned into pKSM720; Specr | This study |
| pSA12 | Noncoding region upstream of spd3 cloned into pKSM720; Specr | This study |
| pSA13 | Noncoding region upstream of pulA cloned into pKSM720; Specr | This study |
| pSA14 | Noncoding region upstream of Spy49_0396 cloned into pKSM720; Specr | This study |
| pSA15 | Noncoding region upstream of Spy49_0746 cloned into pKSM720; Specr | This study |
| pSA16 | Noncoding region upstream of Spy49_1113 cloned into pKSM720; Specr | This study |
RBS, ribosome binding site.
DNA manipulation.
Plasmid DNA was isolated from E. coli by alkaline lysis using either the QIAprep Spin Miniprep kit (Qiagen, Valencia, CA) or Maxi/Midi prep purification systems (Qiagen). DNA fragments were isolated from agarose gels using the SpinPrep Gel DNA kit (Novagen, Madison, WI). PCR was carried out with GoTaq DNA polymerase (Promega, Madison, WI). DNA sequencing was performed at the DNA facility at Iowa State University (Ames).
Construction of an S. pyogenes strain expressing a Myc-tagged Rgg fusion protein.
A series of plasmids were constructed to create an S. pyogenes strain expressing an Rgg-Myc fusion protein (Table 1). An 843-bp region containing the rgg open reading frame (ORF) was obtained by PCR using genomic DNA (gDNA) from strain NZ131 (Table 1) as the template DNA with primers MycBamHI and MycXbaI (Table 2). The DNA was digested with BamHI and XbaI and was ligated with similarly digested plasmid pTrcHis2B (Invitrogen, Carlsbad, CA), which encodes Myc and 6× histidine epitope tags 3′ of the inserted ORF. The construct was designated pSA1 and was verified by PCR and DNA sequence analysis (data not shown). The rgg fusion protein ORF was subsequently subcloned into the streptococcal suicide vector pKK1 to create pSA3 (Table 1). The construct was also confirmed by DNA sequencing (data not shown).
Table 2.
Primers used in this study
| Primer | Sequencea (5′-3′) | Reference or source |
|---|---|---|
| Cloning | ||
| MycBamHI | TAAACCATGGATCCGGAAATTGGTGAAACCG | This study |
| MycXbaI | GAGGATCTAGAAGGGACAGTTTATGTTTAATGGC | This study |
| MBP-BamHIfwd | TCCGGATCCATGGAAATTGGTGAAACCG | This study |
| Rgg-stop-PstI | AAAACTGCAGCCTCAGGACAGTTTATGT TTAATGG | 40 |
| RGG-4 | GGCTATTGACCTTATGCACC | This study |
| VaFwd | CCCCTGATTCTGTGGATAACCGT | This study |
| EMSA and reporter fusions | ||
| speBfwd | GCGGGCATAGTTTTATCAACTGTCATAT | This study |
| speBrev | GCGGGCATAGTTTTATCAACTGTCATA T | This study |
| groEL2fwd | GCTACTCGACGTAACATTGTG | This study |
| groEL2rev | GGAGCCTTCGTACCCAGCAT | This study |
| spd3fwd | GGCGTAGCATTTAAATAAACGGAA | This study |
| spd3rev | GGTGCCTGTAAAATACGAATAAATAAGT | This study |
| pulAfwd | GCGGATCCGATGCCTTTTGTCTCAAACTG | This study |
| pulArev | GCCTCGAGCAACTTGATGTGTTAAGATACTAG | This study |
| 42KDa_BamHIfwd | GCGGATCCTAGTCGGTATCTAGAAGA | This study |
| 42KDa_XhoIrev | GCCTCGAGGAGTTCAGACTTGATTTCTG | This study |
| 1113_BamHIfwd | GCGGATCCCGTATCTGGACTGAGCAAAAC | This study |
| 1113_XhoIrev | GCCTCGAGGCGGTTAATATTTTTGTCAC | This study |
| int_BamHIfwd | GCGGATCCCTACACACTATTACCAC | This study |
| int_XhoIrev | GCCTCGAGTGGTATTCGTGGCGTG | This study |
| pspeB_IRfwd | GCGGATCCATGTCAAGCCTTCCTAGTTGATG | This study |
| pspeB_IRrev | GCCTCGAGTTTTTTTATACCTCTTTCAAAATAAG | This study |
| pspd3_IRfwd | GCGGATCCGTCGGACTAGTCTATGACAAA | This study |
| pspd3_IRrev | GCCTCGAGATCCATGTCCTCCTTTTATTATTTAC | This study |
Underlining indicates restriction sites incorporated into the primer.
The NZ131 rgg mutant (11) was transformed with pSA3 (Table 1), and integration into the native rgg locus via homologous recombination was confirmed by PCR using primers RGG-4, which anneals to chromosomal DNA, and VaFwd, which anneals to pSA3 (data not shown) (Table 1). The transformant was designated SA5 and encodes an Rgg-Myc-6×His fusion protein expressed by the native promoter.
ChIP.
Overnight cultures of the S. pyogenes rgg mutant strain and SA5 were inoculated into 40 ml of THY broth in 50-ml tubes to an A600 of 0.08. The cultures were grown to either the exponential (A600, ∼0.35) or the post-exponential (A600, ∼0.60) phase of growth in the absence of antibiotics. Formaldehyde (1%, wt/vol) was added and incubated at room temperature for 30 min. Glycine (125 mM) was added to quench cross-linking, and cells were washed twice with 40 ml of phosphate-buffered saline (pH 7.3) and were centrifuged at 2,000 rpm for 30 min. The bacterial pellet was resuspended in 1 ml of immunoprecipitation (IP) buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, and 0.5% Triton X-100) supplemented with a mixture of protease inhibitors (Calbiochem, San Diego, CA). The samples were transferred to FastPROTEIN Blue tubes (MP Biomedicals, Solon, OH) and were lysed using a FastPrep FP120 device (Qbiogene, Inc., Carlsbad, CA). The lysates were centrifuged at 4,000 rpm for 10 min at 4°C, and the pellet containing the cross-linked chromatin was suspended in 1 ml IP buffer. The DNA was sheared by sonication, resulting in fragment sizes averaging 0.5 to 1 kb, as determined by agarose gel electrophoresis. Following sonication, DNA bound to Rgg was immunoprecipitated with a monoclonal antibody to Myc (Invitrogen) overnight at 4°C. A 50-μl volume of a 50% protein G-Sepharose slurry was added to the samples and was incubated at 4°C for 3 h, after which the slurry was washed four times with 1 ml of IP buffer and was resuspended in 150 μl of elution buffer (50 mM Tris-HCl [pH 8.0], 10 mM EDTA, and 1% sodium dodecyl sulfate [SDS]). The protein-DNA complexes were first eluted from the slurry by incubation at 65°C for 10 min and then centrifuged at 4,000 rpm for 10 min at 4°C, and the supernatant was collected. The formaldehyde-induced cross-links were reversed by incubation at 65°C overnight. The proteins and RNAs in the samples were degraded by incubation with 150 μl of Tris-EDTA buffer (TE) containing RNase A (10 mg/ml), proteinase K (100 μg/ml), and glycogen (0.27 mg/ml). The DNA was purified by phenol-chloroform extraction, precipitated with isopropanol, and washed with 70% ethanol. Immunoprecipitated DNA was suspended in 25 μl of sterile H2O. The degree of enrichment of PspeB and a portion of the groEL coding sequence in chromatin immunoprecipitation (ChIP) samples obtained from the rgg mutant and SA5 strains was determined with ABsolute SYBR green ROX Mix (ABgene House, Surrey, United Kingdom) using primers for the PspeB region (speBfwd and speBrev) (Table 2) and the control region (groEL2fwd and groEL2rev) (Table 2). Enrichment of PspeB was normalized to the amount of nonspecific groEL DNA in precipitated samples.
Amplification and labeling of ChIP DNA fragments.
Immunoprecipitated DNA was amplified and labeled using the MessageAmp II-Bacteria kit (Ambion, Austin, TX) with some modifications. In brief, the immunoprecipitated DNA was incubated at 95°C for 10 min. The samples were polyadenylated by incubation with 0.5 μl of poly(A) tailing ATP and 0.5 μl of terminal transferase at 37°C for 15 min. First-strand cDNA was synthesized by incubation with 10 μl of reverse transcription master mix for 2 h at 42°C. Second-strand cDNA was synthesized by incubation with 80 μl of second-strand master mix for 2 h at 16°C. The cDNA was purified using a cDNA filter cartridge according to the manufacturer's instructions. To the cDNA, 7.5 μl each of biotin-11-CTP (10 mM) and biotin-11-UTP (10 mM) were added and concentrated to a final volume of 18 μl. Antisense RNA (aRNA) was synthesized by incubation with 22 μl of in vitro transcription master mix at 37°C for 14 h. aRNA was purified using an aRNA filter cartridge according to the manufacturer's instructions. The quality of aRNA was checked using Agilent RNA 6000 nanochips (Agilent Technologies, Santa Clara, CA) (data not shown). The concentration of aRNA was determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA).
DNA microarray hybridization and data analysis.
Custom-designed Gene Chips representing the entire NZ131 genome were made by Affymetrix (Santa Clara, CA). The Gene Chips consist of 3,312 qualifiers representing 1,822 predicted open reading frames and 785 noncoding regions. In addition, 54 control oligonucleotides were used for spike-ins.
Labeled aRNA (10 μg) was hybridized to Gene Chips and was detected according to the manufacturer's instructions for antisense prokaryotic arrays (Affymetrix). The data from three experiments were normalized and analyzed using the Probe Logarithmic Intensity Error (PLIER) estimation algorithm in ArrayStar (DNAStar, Inc., Madison, WI). The regions that showed ≥2-fold enrichment in strain SA5 compared to the rgg mutant in all three biological replicates were considered potential areas of the chromosome directly bound by Rgg.
Multiple Em for Motif Elicitation (MEME), version 4.3, was used to search for motifs in the coding and the noncoding regions bound by Rgg in vivo. Searches were conducted with either the coding or the noncoding regions, or both, as the input sequence. The program was allowed to identify 6- to 50-bp motifs that occurred between 1 and 10 times per fragment on either strand of DNA. As a control, a similar number of coding and noncoding regions not bound by Rgg was selected, and a similar search was conducted using MEME. In addition, a similar strategy was used with the Gapped Local Alignment of Motifs (GLAM2), version 4.6.
Expression and purification of MBP-tagged Rgg fusion protein.
An 843-bp region containing the rgg ORF was amplified by PCR from NZ131 gDNA (Table 1) using primers MBP-BamHIfwd and Rgg-stop-PstI (Table 2). The product was digested with BamHI and PstI and was ligated into similarly digested pMALc4x (New England BioLabs, Ipswich, MA) to produce pSA4 (Table 1). Following verification by PCR and DNA sequence analysis (data not shown), pSA4 was transformed into E. coli Rosetta 2(DE3) cells (Novagen). Expression of the maltose binding protein (MBP)-Rgg fusion protein was induced with 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at an A600 of 0.5, and the cells were incubated for 3 h. The cultures were centrifuged at 4°C for 30 min at 8,000 rpm. The pellet was resuspended in column buffer (20 mM Tris-HCl, 200 mM NaCl, 1 mM EDTA, and 1 mM dithiothreitol [DTT]) and was stored overnight at −20°C. Bacteria were subsequently lysed by sonication and were centrifuged at 7,500 rpm for 30 min. The supernatant containing the soluble MBP-Rgg fusion protein was collected and purified using amylose resin under nondenaturing conditions according to the manufacturer's instructions (New England BioLabs). The eluants containing MBP-Rgg were concentrated using an Amicon Ultracel 3K centrifuge concentrator (Millipore, Billerica, MA). Glycerol was added to the pure protein to a final concentration of 20%, and the mixture was frozen at −80°C. The protein concentrations were determined using a Bio-Rad protein assay kit, and the purity of the protein was checked by SDS-polyacrylamide gel electrophoresis (PAGE).
Electrophoretic mobility shift assays (EMSA).
The previously characterized promoter region of speB (40) and noncoding DNA upstream of spd-3, pulA, Spy49_0396, Spy49_0746, and Spy49_1113 were amplified by PCR using NZ131 gDNA as template DNA with the following primer sets: speBfwd and speBrev, spd3fwd and spd3rev, pulAfwd and pulArev, 42KDa_BamHIfwd and 42KDa_XhoIrev, int_BamHIfwd and int_XhoIrev, and 1113_BamHIfwd and 1113_XhoIrev. The fragments designated as PspeB, Pspd3, PpulA, P396, P746, and P1113 fragments were separated by gel electrophoresis, purified, and cloned into pGEM-T (Promega) to create pSA5, pSA6, pSA7, pSA8, pSA9, and pSA10 (Table 1), respectively. The putative promoter fragments were then excised by digesting the plasmids with BamHI and XhoI, gel purified, dephosphorylated, and end labeled with [γ-32P]ATP using polynucleotide kinase. A similarly sized fragment of the groEL ORF was amplified from genomic DNA using groEL2fwd and groEL2rev (Table 2) and was gel purified for use as a nonspecific competing DNA. Various amounts of MBP-Rgg were incubated with the labeled putative promoter fragments in 25 μl of binding buffer (25 mM Tris-Cl [pH 7.5], 0.1 mM EDTA, 75 mM NaCl, 1 mM dithiothreitol, 10% glycerol, and 0.5 μg/ml calf thymus DNA) at room temperature for 20 min. Competition experiments were conducted by including the cold probe prior to protein addition. The reaction mixtures were separated on 6 or 8% nondenaturing polyacrylamide gels. The gels were dried and exposed to an Amersham Biosciences storage phosphor screen, and images were obtained by using a Typhoon 9400 instrument (GE Healthcare, Piscataway, NJ).
Transcription reporter assays.
To construct transcriptional fusions, pKSM720, which has a luc gene encoding firefly luciferase, was used (28) (Table 1). Noncoding DNAs upstream of speB, spd-3, pulA, Spy49_0396, Spy49_0746, and Spy49_1113 were amplified with PCR using NZ131 gDNA as template DNA with primer pairs pspeB_IRfwd and pspeB_IRrev, pspd3_IRfwd and pspd-3_IRrev, pulAfwd and pulArev, 42KDa_BamHIfwd and 42KDa_XhoIrev, int_BamHIfwd and int_XhoIrev, and 1113_BamHIfwd and 1113_XhoIrev, respectively (Table 2). The DNA fragments were gel purified, digested with BamHI and XhoI, and cloned into the BglII and XhoI sites of pKSM720. The PspeB, Pspd3, PpulA, P396, P746, and P1113 DNA fragments were cloned 5′ to luc between the BglII and XhoI sites of pKSM720 to create pSA11, pSA12, pSA13, pSA14, pSA15, and pSA16, respectively. NZ131 was transformed with pKSM720, pSA11, pSA12, pSA13, pSA14, pSA15, and pSA16 by electroporation to create the wt::luc, wt::PspeB-luc, wt::Pspd3-luc, wt::PpulA-luc, wt::P396-luc, wt::P746-luc, and wt::P1113-luc strains (Table 1). Similarly, the rgg mutant strain NZ131 was transformed with pKSM720, pSA11, pSA12, pSA13, pSA14, pSA15, and pSA16 to create the rgg::luc, rgg::PspeB-luc, rgg::Pspd3-luc, rgg::PpulA-luc, rgg::P396-luc, rgg::P746-luc, and rgg::P1113-luc strains (Table 1). PCR was used to confirm the presence of the noncoding DNAs and the luc gene. The S. pyogenes strains containing the transcriptional fusion plasmids were grown to the post-exponential (A600, ∼0.60) phase of growth in THY broth, and luciferase activity was measured according to the manufacturer's instructions (Promega).
RESULTS
ChIP.
To identify Rgg binding sites in the S. pyogenes genome, chromatin immunoprecipitation (ChIP) was used to enrich DNA bound by Rgg in vivo. For this purpose, an S. pyogenes strain expressing an Rgg-Myc fusion protein (SA5) was created by inserting DNA encoding the fusion protein into the chromosome of an rgg mutant strain. Thus, the fusion protein is expressed under the regulatory control of the native promoter. In contrast to the parental strain, which does not express speB (11), the SA5 strain expressed speB at wild-type levels, indicating that the fusion protein functions similarly to the unmodified protein (data not shown).
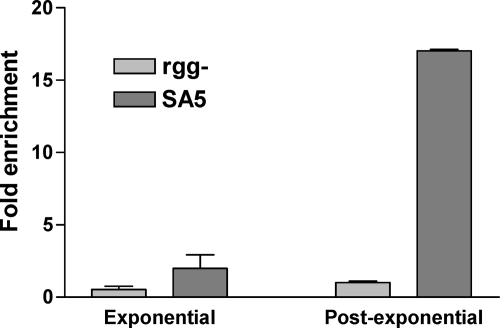
Strain SA5 was grown to the post-exponential phase of growth, and Rgg-bound DNA was immunoprecipitated with an antibody specific to the Myc epitope. As a negative control, the rgg mutant culture was treated similarly. To determine the specificity of the procedure, the enrichment of the speB promoter region (PspeB), which was previously shown to bind Rgg in vitro (40), was determined by using quantitative real-time PCR (qPCR), and the values were normalized to the amount of nonspecific DNA in the precipitate (as determined by measuring the amount of an internal portion of the groEL ORF). PspeB was 15-fold more abundant in samples obtained from strain SA5 than in those from the rgg mutant strain (Fig. 1), indicating that the ChIP procedure specifically enriched DNA known to bind Rgg.
Fig. 1.
Rgg binds to PspeB in vivo only in the post-exponential phase of growth. Quantitative PCR was used to measure the amounts of PspeB and, as a negative control, groEL in ChIP samples obtained from the rgg mutant and strain SA5 in either the exponential or the post-exponential phase of growth.
Rgg binds to PspeB in a growth phase-dependent manner.
Although Rgg is transcribed in both the exponential and post-exponential phases of growth (14), speB is expressed primarily during the post-exponential phase (11–12, 52). In the exponential phase of growth, Rgg interacts with LacD.1, a streptococcal aldolase that has evolved to control gene regulation, in a manner that prevents Rgg-dependent expression of speB (30). However, it is not clear if the Rgg-LacD.1 complex is associated with PspeB in a way that fails to activate expression or if the complex does not bind to PspeB. To address this issue, Rgg binding to PspeB was also determined in the exponential phase of growth using ChIP-qPCR. The results showed that Rgg does not bind PspeB in the exponential phase of growth (Fig. 1), which is consistent with the known pattern of speB expression.
Identification of Rgg binding sites in vivo.
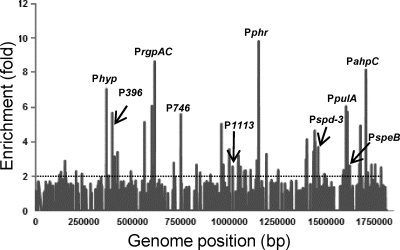
To determine if Rgg binds to chromosomal regions other than PspeB, DNA enriched by ChIP during the post-exponential phase of growth was hybridized to custom-designed Affymetrix Gene Chips representing the entire NZ131 chromosome. Three independent experiments were conducted using both SA5 and the rgg mutant strain. As predicted from the ChIP-qPCR results (Fig. 1), ChIP followed by DNA microarray analysis (ChIP-chip) showed enrichment of the PspeB DNA in samples obtained from the SA5 cultures but not in the rgg mutant controls (Table 3). Sixty-four additional regions of the chromosome were enriched at least 2-fold in all three biological replicates (Fig. 2 and Table 3; see also Table S1 in the supplemental material). Thirty-five (54%) binding sites were in noncoding DNA. Since only 14.7% of the NZ131 genome is noncoding, binding was biased toward noncoding DNA (Table 3; see also Table S1 in the supplemental material). In addition, 43% of the binding sites in noncoding DNA were adjacent to genes previously reported to be regulated by Rgg in the post-exponential phase of growth (Table 3), as determined by expression array analysis (19). Analysis of the Rgg binding regions using MEME and GLAM2 did not identify a unique motif.
Table 3.
Noncoding DNA bound by Rgg
| Category and Spy49 no.a | Geneb | Description | ChIP-chip fold enrichmentc | Transcript fold change (rgg mutant/wt)d |
|---|---|---|---|---|
| Virulence associated | ||||
| 1626 | pulA | Pullulanase | 6.0 | 2 |
| 1692-1693 | mf1 | Streptodornase B/mitogenic factor 1 | 4.90 | 5 |
| 1455 | spd-3 | Streptodornase | 3.6 | 5 |
| 1690-1691 | speB | Streptococcal pyrogenic exotoxin B | 2.7 | −59 |
| Transcriptional regulator | ||||
| 1690-1691 | rgg | Rgg | 2.7 | 3 |
| 710-711 | cpsY | Putative transcriptional regulator | 2.8 | — |
| 1761-1762 | (—) | Putative arginine repressor | 2.7 | 5 |
| 1113 | (—) | Putative transcription factor | 2.4 | −2 |
| Prophage related | ||||
| 1720-1721 | (—) | Transposase | 8.1 | — |
| 355 | (—) | Hypothetical protein | 7.0 | ND |
| 396 | (—) | Surface antigen | 5.7 | 6 |
| 746 | (—) | Integrase | 5.6 | 11 |
| 565 | (—) | Transposase | 5.1 | 5 |
| 1499c | (—) | Phage endodeoxyribonuclease | 2.1 | — |
| Metabolism | ||||
| 612 | rgpAc | Rhamnosyltransferase | 8.7 | — |
| 1720-1721 | ahpC | Alkyl hydroperoxide reductase protein C | 8.1 | — |
| 1632c | relA | (p)ppGpp synthetase | 5.7 | — |
| 1429-1430 | mutY | A/G-specific adenine glycosylase | 3.4 | 2 |
| 1793-1794 | sdhB | l-Serine dehydratase, beta subunit | 2.5 | −3 |
| 850-851 | (—) | Polysaccharide deacetylase family protein | 2.2 | — |
| 850-851 | folC1 | Folylpolyglutamate synthase | 2.2 | −3 |
| Synthesis | ||||
| 967 | dpfB | Phosphopantothenate-cysteine ligase | 5.0 | — |
| 1436-1437 | (—) | RNase HIII | 4.6 | — |
| 143 | nusG | Transcription antitermination protein | 2.9 | — |
| 1047 | (—) | BigG family transcription antiterminator | 2.9 | 2 |
| 1761-1762 | argS | Arginyl-tRNA synthetase | 2.7 | 2 |
| 1793-1794 | mnmA | tRNA-specific 2-thiouridylase | 2.5 | — |
| 1091 | deaD2 | Putative RNA helicase | 2.3 | — |
| 1506 | dnaC | DNA replication protein C | 2.1 | — |
| Repair | ||||
| 1160 | phr | Deoxyribodipyrimidine photolyase | 9.8 | — |
| 603 | rexB | ATP-dependent exonuclease, subunit B | 6.0 | — |
| Hypothetical | ||||
| 1692-1693 | (—) | Hypothetical protein | 4.9 | — |
| 1436-1437 | (—) | Hypothetical protein | 4.6 | — |
| 1429-1430 | (—) | Hypothetical protein | 3.4 | — |
| 710-711 | (—) | Hypothetical protein | 2.8 | — |
Genes are categorized based on KEGG genome annotations. Spy49 numbers are the open reading frames based on the S. pyogenes strain NZ131 genome annotation (37). Contiguous genes that are likely to be cotranscribed are separated by a hyphen. Where a Spy49 number is underlined, the description is specific to the underlined Spy49 gene designation.
A dash within parentheses indicates that the gene is not named.
Calculated as a ratio of signal intensity between the SA5 and rgg mutant strains.
Determined previously (16). A dash indicates no change in the transcript level. ND, not determined.
Fig. 2.
Genomic Rgg-binding sites. The enrichment of DNA (y axis) in ChIP samples is plotted against the location of the DNA in the 1.8-Mb S. pyogenes genome (x axis).
Rgg binds to noncoding DNA upstream of several virulence-associated genes.
Depending on the isolate, the S. pyogenes chromosome can contain as many as four genes encoding extracellular DNases, some of which are associated with virulence (48, 56). For example, DNase Sda1 promotes pathogen dissemination by degrading neutrophil extracellular traps (NETs) (56). Strain NZ131 possesses two genes encoding secreted DNases: sdaB, encoding streptodornase B (SdaB), also known as mitogenic factor-1 (MF-1), which is adjacent to rgg in the chromosome, and spd-3, encoding streptodornase (also known as mitogenic factor-3 [MF-3]), which is carried by a temperate bacteriophage (37). Rgg bound to the noncoding DNA upstream of both sdaB and spd-3 and upstream of the gene encoding pullulanase (pulA), a surface-associated glycan-binding protein involved in virulence (Table 3) (22). Transcriptome and proteome analyses previously showed increases in sdaB, spd-3, and pulA expression in the rgg mutant over that in the wild-type strain (14, 19). Taken together, the results suggest that Rgg binds to DNA upstream of the virulence-associated genes sdaB, spd-3, and pulA to directly control expression.
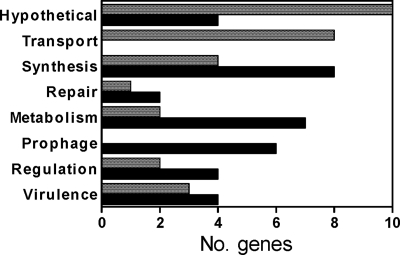
Rgg binds to noncoding DNA upstream of genes involved in metabolism and DNA repair.
The majority of ORFs adjacent to Rgg binding sites within noncoding DNA encode proteins either known or predicted to be involved in catabolism and in nucleic acid recombination and repair (Fig. 3; Table 3). For example, Rgg bound to the noncoding DNA upstream of sdhB (Table 3), encoding a component of the heterodimeric enzyme serine dehydratase, which converts serine to pyruvate. Previously, we showed that rgg inactivation increases both sdhB transcript and protein levels and is associated with an increase in the degradation of serine (10, 13, 19).
Fig. 3.
Functions of genes associated with Rgg binding sites. The x axis indicates the number of ORFs (shaded bars) or noncoding DNAs proximal and upstream (filled bars) of genes bound by Rgg and the respective functions (y axis).
In addition, Rgg bound adjacent to rexB, encoding ATP-dependent exonuclease, and adjacent to genes encoding a photolyase, which removes pyrimidine dimers created by UV radiation (57); RNase HIII, an endoribonuclease that digests double-stranded RNA and RNA/DNA hybrids (15) and regulates phage lysogeny by binding to the 5′ untranslated region (5′ UTR) of bacteriophage λ cIII (1); an RNA helicase; a transcription antiterminator in the BigG family; and NusG, a component of bacteriophage λ antitermination complexes (3, 29, 35, 47) (Table 3). In S. pyogenes, nusG can be cotranscribed with adjacent genes encoding NADase and streptolysin O, respectively (27), both of which are regulated by Rgg in strain NZ131 (19).
Rgg binds to noncoding DNA upstream of horizontally acquired genes.
Strain NZ131 has three prophages constituting 5.6% of the chromosome (37). Two of these (designated NZ131.2 and NZ131.3) are complete prophages, whereas NZ131.1 appears to be a remnant of a unique prophage (37). Rgg bound to six unique DNA sites within the three prophages. Binding to three sites was not associated with changes in the expression of proximal genes, as determined by comparing the transcript levels of the wild-type strain to those of the rgg mutant strain (Table 3); however, binding to the other three sites was associated with changes in the expression of the downstream ORFs (19). These binding sites were upstream of spd-3, which is carried by prophage NZ131.3; a gene encoding an integrase/excisionase (Spy49_0746) carried by prophage NZ131.2; and a gene encoding a surface antigen (Spy49_0396), which has a motif present in lysins (Table 3) (5, 6, 24, 38) and is carried by prophage NZ131.3. Differences in the expression of the prophage integrase between the rgg mutant and the wild-type strain were previously associated with changes in the frequency of prophage excision from the genome (19). Thus, 50% of the Rgg binding sites in prophage were upstream of known Rgg-regulated genes.
Assessing Rgg binding in vitro.
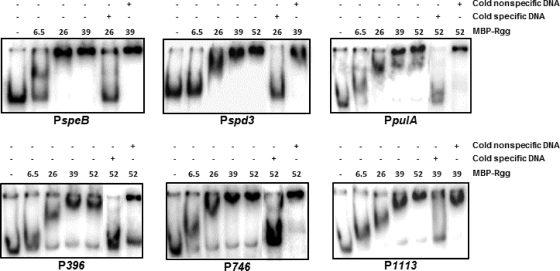
The Rgg-regulated promoter region of speB (40) and the noncoding DNAs upstream of spd-3, pulA, Spy49_0396, Spy49_0746, and Spy49_1113 that were bound by Rgg in vivo (Table 3) were selected for further in vitro analysis because of their roles in virulence, lateral gene transfer, and regulation. Moreover, the expression of each gene is altered in an rgg mutant strain, consistent with the idea that they are directly regulated by Rgg. To assess binding in vitro, the DNAs that bound Rgg in vivo were incubated with varying amounts of Rgg in gel shift assays. Rgg bound to all the target DNAs tested (Fig. 4).
Fig. 4.
Gel shift assays of selected target DNAs. The binding of Rgg to PspeB and the noncoding DNAs upstream of Spy49_1113, Spy49_0746, spd-3, pulA, and Spy49_0396 that bound to Rgg in vivo was assessed by incubation without Rgg or with 6.5, 26, 39, and 52 pmol of purified Rgg with radiolabeled DNA. Cold nonspecific DNA refers to unlabeled groEL, and the cold specific probe is unlabeled competing DNA.
Promoter activity assays.
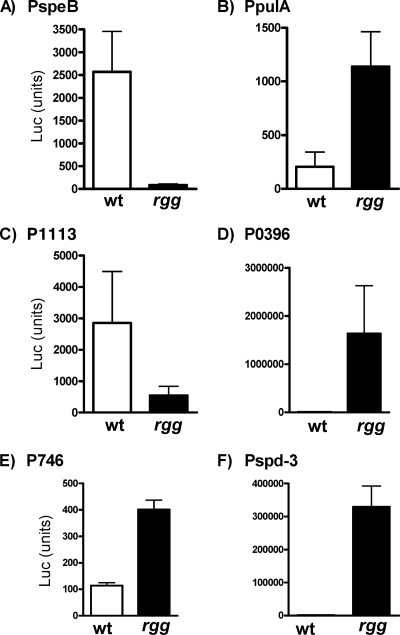
To determine whether the noncoding DNAs upstream of speB, spd-3, pulA, Spy49_0396, Spy49_0746, and Spy49_1113 that were bound by Rgg in vivo contained functional promoters regulated by Rgg, a transcriptional fusion reporter system was used. Each DNA region was cloned adjacent to a firefly luciferase reporter gene (luc), and the recombinant plasmids were used to transform both wild-type NZ131 and the rgg mutant strain. The transformants were grown to the post-exponential phase of growth, and promoter activity was measured by quantitating luciferase activity. Rgg activated the expression of the promoters associated with speB and Spy49_1113, whereas it repressed that of spd-3, Spy49_0396, and Spy49_0746, indicating that Rgg can both activate and repress gene expression (19) (Fig. 5). The results also showed that there is a functional promoter in each region of DNA bound by Rgg and that the promoter activity is regulated by Rgg, supporting the idea that Rgg regulates the expression of each of these genes by directly binding to their upstream noncoding regions.
Fig. 5.
Promoter activity of DNA bound by Rgg. Transcriptional fusions were constructed between the DNA upstream of speB (A), pulA (B), spy49_1113 (C), spy49_396 (D), spy49_746 (E), and spd-3 (F) and the firefly luciferase gene (luc). The plasmids were transformed into the wild type (wt) and the rgg mutant, which were then grown to the post-exponential phase of growth, and promoter activity was determined by measuring luciferase. The data shown are representative results from three independent biological replicates.
DISCUSSION
Rgg activates speB expression by direct binding to the promoter region in the post-exponential phase of growth. In addition to PspeB, Rgg binding sites were identified on a genome scale by using ChIP coupled to DNA microarrays. The results showed that Rgg bound to 65 regions in the chromosome, which included both coding and noncoding DNA. In many instances, but not all, Rgg bound to DNA upstream of ORFs whose expression is known to be influenced by Rgg, indicating that the genes are probably directly regulated by Rgg (19). Overall, the number of genes bound by Rgg is comparable to that for other global genome regulators, such as Bacillus subtilis CodY (a GTP-activated repressor) (39) and E. coli FNR (regulator of fumarate and nitrate reduction) (23).
Rgg binding to coding DNA.
Prokaryotic transcriptional regulators often bind to noncoding DNA in operator regions located upstream of the target ORF; however, intragenic promoters are also present among prokaryotes (51), and approximately 28% of the E. coli promoters are predicted to be within coding DNA (45). Similarly, recent characterization of CodY binding in Staphylococcus aureus showed that 42% of the binding sites were in coding DNA (33). More than half (55%) of the Rgg binding sites identified in this study were in coding DNA. Several explanations may account for this finding (32). For example, low-affinity interactions between Rgg and DNA may occur, which do not necessarily influence transcription, perhaps simply because the binding site is incidentally similar to the functional binding sequence. In this regard, the E. coli transcriptional repressor LexA binds to both transcriptionally inactive and active regions (55), and the authors speculated that sites in coding regions were lower-affinity sites that had not decayed over time (46, 55). In addition, Rgg binding within coding DNA may influence the expression of distal ORFs by mechanisms involving chromatin conformational changes, such as DNA looping. Another possibility is that a subset of Rgg binding sites may function as a cellular reservoir of the transcription factor. Binding to, and dissociation from, these sites may be responsive to changes in the environment such that the reservoir would function to fine-tune Rgg-mediated regulation. Thus, while many of the Rgg binding sites identified were in noncoding DNA, suggesting a direct role in controlling the expression of adjacent ORFs, the biological significance of the other Rgg binding sites, including those in coding DNA, remains to be determined.
Previously we showed that inactivation of rgg in NZ131 significantly (P < 0.05) altered the expression of 588 genes in the post-exponential phase of growth, including several regulatory proteins (19). ChIP-Chip analyses did not detect Rgg binding in vivo to DNA proximal to known global regulatory genes, which would have provided an explanation for the apparently large degree of indirect changes in gene expression associated with rgg inactivation in strain NZ131; however, Rgg did bind to the noncoding DNA regions upstream of three transcriptional regulators (cpsY, spy49_1761, and spy49_1113), the expression of two of which is changed following inactivation of rgg (Table 3). The regulators have not been characterized previously in S. pyogenes, and thus we cannot rule out the possibility that one or more are global regulators of gene expression and thus contribute to the significant number of indirect changes in expression associated with rgg inactivation in strain NZ131. Nonetheless, given the characterized changes in catabolism associated with the rgg mutant of strain NZ131 (13) and the information currently available, we favor a model whereby many indirect differences in gene transcription associated with the rgg mutant result from differences in metabolism (13). Analysis of Rgg binding sites in the chromosomes of isolates in which rgg inactivation is not associated with altered catabolic activity, such as MGAS5005 and SF370, would be useful in testing this model.
Previously, we discovered inter- and intraserotypic variation in the Rgg regulon following inactivation of the rgg gene in serotype M49 strains CS101 and NZ131 and in serotype M1 strains 5005 and SF370 (18). The results showed that Rgg has a core regulon consisting of speB and the adjacent hypothetical protein gene spy49_1689c but that aside from these genes, the regulon differs significantly depending on the isolate under study (18). Rgg bound to noncoding, conserved DNA upstream of at least one gene whose expression is regulated in strain NZ131 but not in the other isolates examined. In strain NZ131, Rgg bound to DNA upstream of the ORF designated Spy49_0396 both in vivo (Table 3) and in vitro (Fig. 4). Rgg represses transcription of Spy49_0396 in the post-exponential phase of growth, as determined both by promoter assays (Fig. 5D) and by DNA microarrays (19). The nucleotide sequence of the Rgg binding site in NZ131 is identical to those in isolates SF370 and MGAS5005; however, inactivation of rgg in these strains does not appear to influence expression. It will be of interest to determine if Rgg binds to this site in vivo in strains SF370 and MGAS5005, or if strain-specific cofactors influence the specificity of Rgg binding. Such information is likely to provide insight into the molecular basis of the variation of the regulon among isolates of S. pyogenes.
Rgg binds directly and specifically to prophage DNA.
The genome sequences of more than 13 isolates of S. pyogenes, representing 7 serotypes, have been determined. All isolates are polylysogenized (49). Temperate bacteriophages typically encode one or more integrases/excisionases that facilitate phage integration and excision. Coculture of S. pyogenes with pharyngeal epithelial cells and the addition of hydrogen peroxide or DNA-damaging agents, such as mitomycin C and UV light, are some of the factors that cause prophage induction (4, 7). Our previous studies showed that rgg inactivation decreases the frequency of prophage NZ131.2 excision from the chromosome (previously referred to as NZ131.1) (19, 37). The ChIP-chip results indicate that Rgg binds to at least six sites within prophage DNA, including noncoding DNA adjacent to a gene encoding a putative integrase/excisionase (Spy49_746), and binding was recapitulated in vitro with gel shift assays (Fig. 3). The expression of Spy49_746 is 11-fold greater in the rgg mutant strain than in the wild-type strain (Table 3). Together, the results suggest that Rgg alters the frequency of prophage excision/insertion by binding directly to prophage DNA to alter the expression of the enzyme.
Significant variation in the Rgg regulon occurs among different isolates of S. pyogenes (18). One possible explanation is that Rgg-mediated changes in prophage gene expression may cause secondary, or indirect, changes in chromosomal gene expression. This would seem to be particularly likely if the changes in expression alter the frequency of prophage induction. Thus, we speculate that the variation in the type and number of bacteriophages present in the genomes of various S. pyogenes isolates may contribute to strain-associated variation in the Rgg regulon. In this context, a prophage could generate phenotypic diversity in the species by altering chromosomal gene expression. In other words, the coevolution of bacteriophage and S. pyogenes regulatory circuits may enhance the ability of the bacterial species to adapt, through natural selection, to various ecological niches, including adaptation to the human host immune response. From the standpoint of the bacteriophage, participation in a global regulatory circuit of the bacterial host, such as Rgg, may increase the likelihood that the prophage will be maintained in the species, since perturbations in regulatory networks associated with the loss of the prophage could be detrimental to the host cell.
In summary, this study identified the genome-wide DNA binding characteristics of Rgg in the post-exponential phase of growth. The results indicate that Rgg binds to both coding and noncoding DNA, although binding is biased toward noncoding DNA and in several instances was shown to be in the promoter regions of Rgg-mediated genes. Of particular importance was the discovery that Rgg binds directly to specific prophage DNA and influences prophage gene expression, which may reflect the ongoing coevolution of the pathogen and temperate bacteriophage.
Supplementary Material
ACKNOWLEDGMENTS
We thank Adhar Manna, Alex Erkine, and Kelsi Anderson for technical assistance.
Funding was provided by the Mid-American Consortium on Gram-Positive Pathogens, the Sanford School of Medicine of the University of South Dakota, and the University of Nebraska Medical Center.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Altuvia S., Locker-Giladi H., Koby S., Ben-Nun O., Oppenheim A. B. 1987. RNase III stimulates the translation of the cIII gene of bacteriophage lambda. Proc. Natl. Acad. Sci. U. S. A. 84:6511–6515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anonymous. 2009. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May-August 2009. MMWR Morb. Mortal. Wkly. Rep. 58:1071–1074 [PubMed] [Google Scholar]
- 3. Artsimovitch I., Landick R. 2000. Pausing by bacterial RNA polymerase is mediated by mechanistically distinct classes of signals. Proc. Natl. Acad. Sci. U. S. A. 97:7090–7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banks D. J., Lei B., Musser J. M. 2003. Prophage induction and expression of prophage-encoded virulence factors in group A Streptococcus serotype M3 strain MGAS315. Infect. Immun. 71:7079–7086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bateman A., Bycroft M. 2000. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 299:1113–1119 [DOI] [PubMed] [Google Scholar]
- 6. Birkeland N. K. 1994. Cloning, molecular characterization, and expression of the genes encoding the lytic functions of lactococcal bacteriophage φLC3: a dual lysis system of modular design. Can. J. Microbiol. 40:658–665 [DOI] [PubMed] [Google Scholar]
- 7. Broudy T. B., Pancholi V., Fischetti V. A. 2001. Induction of lysogenic bacteriophage and phage-associated toxin from group A streptococci during coculture with human pharyngeal cells. Infect. Immun. 69:1440–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brussow H., Canchaya C., Hardt W. D. 2004. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68:560–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carapetis J. R., Steer A. C., Mulholland E. K., Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5:685–694 [DOI] [PubMed] [Google Scholar]
- 10. Chaussee M. A., Callegari E. A., Chaussee M. S. 2004. Rgg regulates growth phase-dependent expression of proteins associated with secondary metabolism and stress in Streptococcus pyogenes. J. Bacteriol. 186:7091–7099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaussee M. S., Ajdic D., Ferretti J. J. 1999. The rgg gene of Streptococcus pyogenes NZ131 positively influences extracellular SPEB production. Infect. Immun. 67:1715–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaussee M. S., Phillips D., Ferretti J. J. 1997. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect. Immun. 65:1956–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaussee M. S., Somerville G. A., Reitzer L., Musser J. M. 2003. Rgg coordinates virulence factor synthesis and metabolism in Streptococcus pyogenes. J. Bacteriol. 185:6016–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaussee M. S., Watson R. O., Smoot J. C., Musser J. M. 2001. Identification of Rgg-regulated exoproteins of Streptococcus pyogenes. Infect. Immun. 69:822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Condon C. 2003. RNA processing and degradation in Bacillus subtilis. Microbiol. Mol. Biol. Rev. 67:157–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cunningham M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desiere F., McShan W. M., Van Sinderen D., Ferretti D., Brussow H. 2001. Comparative genomics reveals close genetic relationships between phages from dairy bacteria and pathogenic streptococci: evolutionary implications for prophage-host interactions. Virology 288:325–341 [DOI] [PubMed] [Google Scholar]
- 18. Dmitriev A. V., McDowell E. J., Chaussee M. S. 2008. Inter- and intraserotypic variation in the Streptococcus pyogenes Rgg regulon. FEMS Microbiol. Lett. 284:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dmitriev A. V., et al. 2006. The Rgg regulator of Streptococcus pyogenes influences utilization of nonglucose carbohydrates, prophage induction, and expression of the NAD-glycohydrolase virulence operon. J. Bacteriol. 188:7230–7241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferretti J. J., et al. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. U. S. A. 98:4658–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fontaine L., et al. 2010. A novel pheromone quorum-sensing system controls the development of natural competence in Streptococcus thermophilus and Streptococcus salivarius. J. Bacteriol. 192:1444–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gourlay L. J., et al. 2009. Group B streptococcus pullulanase crystal structures in the context of a novel strategy for vaccine development. J. Bacteriol. 191:3544–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grainger D. C., Aiba H., Hurd D., Browning D. F., Busby S. J. 2007. Transcription factor distribution in Escherichia coli: studies with FNR protein. Nucleic Acids Res. 35:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu S., Kong J., Kong W., Guo J., Ji M. 2010. Characterization of a novel LysM domain from Lactobacillus fermentum bacteriophage endolysin and its use as an anchor to display heterologous proteins on the surfaces of lactic acid bacteria. Appl. Environ. Microbiol. 76:2410–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hynes W. L., Hancock L., Ferretti J. J. 1995. Analysis of a second bacteriophage hyaluronidase gene from Streptococcus pyogenes: evidence for a third hyaluronidase involved in extracellular enzymatic activity. Infect. Immun. 63:3015–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kappeler K. V., et al. 2009. A naturally occurring Rgg variant in serotype M3 Streptococcus pyogenes does not activate speB expression due to altered specificity of DNA binding. Infect. Immun. 77:5411–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kimoto H., Fujii Y., Yokota Y., Taketo A. 2005. Molecular characterization of NADase-streptolysin O operon of hemolytic streptococci. Biochim. Biophys. Acta 1681:134–149 [DOI] [PubMed] [Google Scholar]
- 28. Kinkel T. L., McIver K. S. 2008. CcpA-mediated repression of streptolysin S expression and virulence in the group A streptococcus. Infect. Immun. 76:3451–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li J., Horwitz R., McCracken S., Greenblatt J. 1992. NusG, a new Escherichia coli elongation factor involved in transcriptional antitermination by the N protein of phage lambda. J. Biol. Chem. 267:6012–6019 [PubMed] [Google Scholar]
- 30. Loughman J. A., Caparon M. G. 2006. A novel adaptation of aldolase regulates virulence in Streptococcus pyogenes. EMBO J. 25:5414–5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lyon W. R., Gibson C. M., Caparon M. G. 1998. A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 17:6263–6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Macquarrie K. L., Fong A. P., Morse R. H., Tapscott S. J. 2011. Genome-wide transcription factor binding: beyond direct target regulation. Trends Genet. 27:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Majerczyk C. D., et al. 2010. Direct targets of CodY in Staphylococcus aureus. J. Bacteriol. 192:2861–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mashburn-Warren L., Morrison D. A., Federle M. J. 2010. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol. Microbiol. 78:589–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mason S. W., Li J., Greenblatt J. 1992. Host factor requirements for processive antitermination of transcription and suppression of pausing by the N protein of bacteriophage lambda. J. Biol. Chem. 267:19418–19426 [PubMed] [Google Scholar]
- 36. McDonnell G. E., Wood H., Devine K. M., McConnell D. J. 1994. Genetic control of bacterial suicide: regulation of the induction of PBSX in Bacillus subtilis. J. Bacteriol. 176:5820–5830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McShan W. M., et al. 2008. Genome sequence of a nephritogenic and highly transformable M49 strain of Streptococcus pyogenes. J. Bacteriol. 190:7773–7785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mesnage S., Chau F., Dubost L., Arthur M. 2008. Role of N-acetylglucosaminidase and N-acetylmuramidase activities in Enterococcus faecalis peptidoglycan metabolism. J. Biol. Chem. 283:19845–19853 [DOI] [PubMed] [Google Scholar]
- 39. Molle V., et al. 2003. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J. Bacteriol. 185:1911–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neely M. N., Lyon W. R., Runft D. L., Caparon M. 2003. Role of RopB in growth phase expression of the SpeB cysteine protease of Streptococcus pyogenes. J. Bacteriol. 185:5166–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Niemann H., Birch-Andersen A., Kjems E., Mansa B., Stirm S. 1976. Streptococcal bacteriophage 12/12-borne hyaluronidase and its characterization as a lyase (EC 4.2.99.1) by means of streptococcal hyaluronic acid and purified bacteriophage suspensions. Acta Pathol. Microbiol. Scand. B 84:145–153 [DOI] [PubMed] [Google Scholar]
- 42. Rawlinson E. L., Nes I. F., Skaugen M. 2005. Identification of the DNA-binding site of the Rgg-like regulator LasX within the lactocin S promoter region. Microbiology 151:813–823 [DOI] [PubMed] [Google Scholar]
- 43. Rawlinson E. L., Nes I. F., Skaugen M. 2002. LasX, a transcriptional regulator of the lactocin S biosynthetic genes in Lactobacillus sakei L45, acts both as an activator and a repressor. Biochimie 84:559–567 [DOI] [PubMed] [Google Scholar]
- 44. Sanders J. W., Venema G., Kok J., Leenhout K. 1998. Identification of a sodium chloride-regulated promoter in Lactococcus lactis by single-copy chromosomal fusion with a reporter gene. Mol. Gen. Genet. 257:681–685 [DOI] [PubMed] [Google Scholar]
- 45. Shavkunov K. S., Masulis I. S., Tutukina M. N., Deev A. A., Ozoline O. N. 2009. Gains and unexpected lessons from genome-scale promoter mapping. Nucleic Acids Res. 3:4919–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shimada T., Ishihama A., Busby S. J., Grainger D. C. 2008. The Escherichia coli RutR transcription factor binds at targets within genes as well as intergenic regions. Nucleic Acids Res. 36:3950–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sullivan S. L., Gottesman M. E. 1992. Requirement for E. coli NusG protein in factor-dependent transcription termination. Cell 68:989–994 [DOI] [PubMed] [Google Scholar]
- 48. Sumby P., et al. 2005. Extracellular DNase made by group A streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc. Natl. Acad. Sci. U. S. A. 102:1679–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sumby P., Whitney A. R., Graviss E. A., DeLeo F. R., Musser J. M. 2006. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suvorov A. N., Polyakova E. M., McShan W. M., Ferretti J. J. 2009. Bacteriophage content of M49 strains of Streptococcus pyogenes. FEMS Microbiol. Lett. 294:9–15 [DOI] [PubMed] [Google Scholar]
- 51. Tutukina M. N., Shavkunov K. S., Masulis I. S., Ozoline O. N. 2007. Intragenic promoter-like sites in the genome of Escherichia coli: discovery and functional implication. J. Bioinform. Comput. Biol. 5:549–560 [DOI] [PubMed] [Google Scholar]
- 52. Unnikrishnan M., Cohen J., Sriskandan S. 1999. Growth-phase-dependent expression of virulence factors in an M1T1 clinical isolate of Streptococcus pyogenes. Infect. Immun. 67:5495–5499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vickerman M. M., Sulavik M. C., Clewell D. B. 1995. Oral streptococci with genetic determinants similar to the glucosyltransferase regulatory gene, rgg. Infect. Immun. 63:4524–4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Virtaneva K., et al. 2005. Longitudinal analysis of the group A streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc. Natl. Acad. Sci. U. S. A. 102:9014–9019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wade J. T., Reppas N. B., Church G. M., Struhl K. 2005. Genomic analysis of LexA binding reveals the permissive nature of the Escherichia coli genome and identifies unconventional target sites. Genes Dev. 19:2619–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Walker M. J., et al. 2007. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 13:981–985 [DOI] [PubMed] [Google Scholar]
- 57. Weber S. 2005. Light-driven enzymatic catalysis of DNA repair: a review of recent biophysical studies on photolyase. Biochim. Biophys. Acta 1707:1–23 [DOI] [PubMed] [Google Scholar]
- 58. Zakikhany K., et al. 2011. Increase in invasive Streptococcus pyogenes and Streptococcus pneumoniae infections in England, December 2010 to January 2011. Euro Surveill. 16:pii:19785. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.