Abstract
During cytokinesis in Escherichia coli, the peptidoglycan (PG) layer produced by the divisome must be split to promote cell separation. Septal PG splitting is mediated by the amidases: AmiA, AmiB, and AmiC. To efficiently hydrolyze PG, the amidases must be activated by LytM domain factors. EnvC specifically activates AmiA and AmiB, while NlpD specifically activates AmiC. Here, we used an exportable, superfolding variant of green fluorescent protein (GFP) to demonstrate that AmiB, like its paralog AmiC, is recruited to the division site by an N-terminal targeting domain. The results of colocalization experiments indicate that EnvC is recruited to the division site well before its cognate amidase AmiB. Moreover, we show that EnvC and AmiB have differential FtsN requirements for their localization. EnvC accumulates at division sites independently of this essential division protein, whereas AmiB localization is FtsN dependent. Interestingly, we also report that AmiB and EnvC are recruited to division sites independently of one another. The same is also true for AmiC and NlpD. However, unlike EnvC, we find that NlpD shares an FtsN-dependent localization with its cognate amidase. Importantly, when septal PG synthesis is blocked by cephalexin, both EnvC and NlpD are recruited to septal rings, whereas the amidases fail to localize. Our results thus suggest that the order in which cell separation amidases and their activators localize to the septal ring relative to other components serves as a fail-safe mechanism to ensure that septal PG synthesis precedes the expected burst of PG hydrolysis at the division site, accompanied by amidase recruitment.
INTRODUCTION
Escherichia coli and other Gram-negative bacteria divide by coordinately constricting all three of their envelope layers, the inner and outer membranes along with the peptidoglycan (PG) layer sandwiched between them (17, 60). Envelope constriction is driven by a ring-shaped, multiprotein complex called the septal ring or divisome (17). The assembly of this machine is initiated by polymerization of the tubulin-like FtsZ protein into a ring-like structure, the Z-ring, just underneath the cytoplasmic membrane at the prospective site of fission (10). Several Z-ring associated proteins (FtsA, ZipA, ZapA, ZapB, and ZapC) play important roles in Z-ring formation and are thought to decorate and stabilize the structure as it forms (2, 23, 24, 26, 33, 48). Once assembled, the Z-ring is thought to serve as a scaffold for the recruitment of a large set of essential and auxiliary division proteins to the division site, together forming the trans-envelope septal ring machine. Studies in which the subcellular localization of one essential divisome component is observed in the absence of another have revealed a mostly linear dependency pathway for divisome assembly that starts with FtsZ and ends with FtsN (FtsZ [FtsA, ZipA], FtsK [FtsQLB], FtsW, FtsI, FtsN) (3, 11, 12, 28, 31, 32, 42, 52, 62, 65). The dependency pathway does not appear to reflect the temporal order of divisome assembly. Rather, analysis of septal ring assembly during the cell cycle suggests that maturation takes place in just two steps, with stable Z-rings forming and persisting for about 20% of the cell cycle before most of the remaining divisome components (from FtsQ to FtsN) are simultaneously recruited (1). This second “maturation” step is then closely followed by the initiation of cell constriction (1).
The contraction of the septal ring is associated with the highly localized production of new PG that is thought to be initially shared by the developing daughter cells (18, 50, 54, 66). The periplasmic PG amidases, AmiA, AmiB, and AmiC (Fig. 1), are required to split this shared septal PG to shape the new poles and allow constriction of the outer membrane to closely follow that of the inner membrane (14, 35, 49, 60). Amidases are PG hydrolases that break peptide cross-links in the PG meshwork by cleaving bonds that link stem peptides to the N-acetylmuramic acid component of the glycan strands. Mutants lacking amidase activity complete inner membrane constriction and fusion but fail to split septal PG. Consequently, they form long chains of cells connected by shared layers of PG and a partially constricted outer membrane layer (35, 49). A similar phenotype was observed for strains lacking the divisome-associated LytM (Pfam, peptidase_M23) factors, EnvC and NlpD (60). We recently demonstrated that these LytM proteins are potent and specific activators of PG hydrolysis by the amidases (59). In a purified system, EnvC was found to specifically activate AmiA and AmiB, while NlpD was found to specifically activate AmiC (59). The LytM factors are therefore key regulators controlling the activation of PG hydrolysis at the cytokinetic ring.
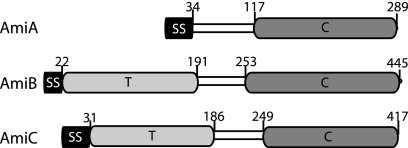
Fig. 1.
Domain organization of the E. coli LytC-type amidases. Shown are schematic diagrams of the predicted domain structures of AmiA (P36548), AmiB (P26365), and AmiC (P63883). Swiss-Prot accession numbers are given in parentheses. Amino acid numbers above each schematic indicate the predicted domain boundaries. SS; signal sequence; T, targeting domain; C, LytC-type amidase catalytic domain. Note that AmiA and AmiC are exported by the Tat system and AmiB via Sec (6).
Amidase activation must be tightly controlled to prevent the formation of breaches in the cell wall that can result in cell lysis. While the regulatory mechanisms governing amidase activation remain to be defined, strategies involving the control of amidase and/or LytM factor subcellular localization are likely to be employed. Because of difficulties with the export of functional green fluorescent protein (GFP) to the periplasm through the Sec translocon (25), we previously relied on the fact that AmiA and AmiC are substrates for Tat-mediated export to study their subcellular localization (6, 37). AmiA-GFP was found to be distributed throughout the periplasm at all stages of the cell cycle (6). AmiC-GFP, on the other hand, was found to accumulate at the division site of constricting cells, and its N-terminal domain was shown to be necessary and sufficient for septal targeting (6). In addition, the accumulation of AmiC at the division site was found to be dependent on the prior localization of FtsN (6), the final essential division protein in the divisome localization hierarchy (3).
In contrast to AmiA and AmiC, AmiB appears to be a substrate for Sec-mediated export (6, 37). We were therefore unable to study AmiB localization using the AmiB-GFP fusions constructed previously (6). In an accompanying note (21), we demonstrate the utility of a superfolding variant of GFP (sfGFP) (47) for localization studies of Sec-exported proteins. Here, we used sfGFP fusion proteins to demonstrate that AmiB, like its paralog AmiC, is recruited to the division site by an N-terminal targeting domain. We then used these fusion proteins to investigate the relative timing of amidase and LytM factor recruitment to the division site. Colocalization experiments indicate that EnvC is recruited to the division site well before its cognate amidase AmiB. Moreover, we show that EnvC and AmiB have differential FtsN requirements for their localization. EnvC accumulates at division sites independently of this essential division protein, whereas AmiB localization is FtsN dependent. Interestingly, we also report that AmiB and EnvC are recruited to division sites independently of one another. The same is also true for AmiC and NlpD. However, unlike EnvC, NlpD shares an FtsN-dependent localization with its cognate amidase. Importantly, when septal PG synthesis is blocked by cephalexin, both EnvC and NlpD are recruited to septal rings, whereas the amidases fail to localize. Our results thus suggest that the order in which cell separation amidases and their activators localize to the septal ring relative to other components serves as a failsafe mechanism to ensure that septal PG synthesis precedes the expected burst of PG hydrolysis at the division site, accompanied by amidase recruitment.
MATERIALS AND METHODS
Media, bacterial strains, and plasmids.
Cells were grown in LB (1% tryptone, 0.5% yeast extract, 0.5% NaCl) or minimal M9 medium (43) supplemented with 0.2% Casamino acids and 0.2% sugar (glucose, maltose, or arabinose as indicated in the figure legends). Unless otherwise indicated, antibiotics were used at 10, 15, 20, or 50 μg/ml for chloramphenicol (Cam) and tetracycline (Tet), ampicillin (Amp), kanamycin (Kan), or spectinomycin (Spec), respectively.
The bacterial strains used in this study are listed in Table 1. All strains used in the reported experiments are derivatives of MG1655 (29). Plasmids used in this study are listed in Table 2. Vectors with R6K origins are all derivatives of the CRIM plasmids developed by Haldimann and Wanner (30). They were either maintained in the cloning strain DH5α(λpir), where they replicate as plasmids, or they were integrated into phage attachment sites (HK022 or λ) using the helper vectors pTB102 (8) or pInt-ts (30), respectively, as described previously (30). Single-copy integrants were identified using diagnostic PCR (30). Integrated vectors were transferred between strains by P1-mediated transduction. All sfGFP and mCherry protein fusions used in this study possessed a short linker sequence (LEGPAGL) between the protein of interest and the C-terminal fluorescent reporter protein. The construction of plasmids is described in the supplemental material.
Table 1.
Bacterial strains used in this study
| Strain | Genotypeb | Strain constructionc | Source or reference |
|---|---|---|---|
| DH5α | F−hsdR17 deoR recA1 endA1 phoA supE44 thi-1 gyrA96 relA1 Δ(lacZYA-argF)U169 φ80dlacZΔM15 | Gibco BRL | |
| BW25113 | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) rph-1 Δ(rhaD-rhaB)568 hsdR514 | 4 | |
| CH34a | TB28 ΔftsN::Kanr | 27 | |
| JW2712-2 | BW25113 ΔnlpD::Kanr | 4 | |
| MG1655 | rph-1 ilvG rfb-50 | 29 | |
| TB10 | rph-1 ilvG rfb-50 λΔcro-bio nad::Tn10 | 38 | |
| TB28 | MG1655 ΔlacIZYA::frt | 7 | |
| TB134 | TB28 ΔenvC::Kanr | 60 | |
| TB137 | TB28 ΔamiC::Kanr | 60 | |
| TB139 | TB28 ΔnlpD::Kanr | 60 | |
| TB140 | TB28 ΔenvC::frt | 60 | |
| TB143 | TB28 ΔamiC::frt | TB137/pCP20 | |
| TB145 | TB28 ΔnlpD::frt | TB139/pCP20 | |
| TB170 | TB28 ΔamiB::Kanr | 59 | |
| HC260 | TB10 zapA-GFP Camr | λRec | |
| HC261 | TB28 zapA-GFP Camr | P1(HC260) × TB28 | |
| HC262 | TB28 ΔenvC::frt zapA-GFP Camr | P1(HC261) × TB140 | |
| HC284 | TB28 zapA-mCherry frt | TU211/pCP20 | |
| TU211 | TB10 zapA-mCherry Camr | λRec | |
| TU217 | TB28 ΔamiC::frt ΔnlpD::Kanr | P1(JW2712-2) × TB143 | |
| NP1 | TB28 zapA-GFP frt | HC261/pCP20 | |
| NP6 | TB28 ΔenvC::frt ΔamiB::Kanr | P1(TB170) × TB140 | |
| NP8 | TB28 ΔamiB::KanrzapA-mCherry Camr | P1(TU211) × TB170 | |
| NP20 | TB28 ΔenvC::frt zapA-GFP frt | HC262/pCP20 | |
| NP22a | TB28 ΔftsN::Kanr ΔenvC::frt zapA-GFP frt | P1(CH34) × NP20/pMG20 | |
| NP32 | TB28 ΔenvC::frt zapA-GFP::frt | HC262/pCP20 | |
| NP49a | TB28 ΔftsN::KanrzapA-GFP frt | P1(CH34) × NP1/pMG20 | |
| NP57 | TB28 ΔnlpD::KanrzapA-GFP frt | P1(TB139) × NP1 | |
| NP63 | TB28 ΔnlpD::frt zapA-GFP frt | NP57/pCP20 | |
| NP64a | TB28 ΔftsN::Kanr ΔnlpD::frt zapA-GFP frt | P1(CH34) × NP63/pMG20 | |
| NP84 | TB28 ΔamiC::frt zapA-mCherry Camr | P1(TB143) × TU211 | |
| NP90 | TB28 ΔamiB::Kanr zapA-mCherry Camr | P1(TB170) × TU211 | |
| NP94 | TB28 ΔnlpD::frt zapA-GFP Camr | P1(TB145) × HC261 |
Strains require a plasmid expressing ftsN for viability.
The Kanr cassette is flanked by frt sites for removal by FLP recombinase. An frt scar remains following removal of the cassette using FLP expressed from pCP20.
Strain construction by P1 transduction is described using the shorthand “P1(donor) × recipient.” Transductants were selected on LB Kan or Cam plates where appropriate. Strains resulting from the removal of a drug resistance cassette using pCP20 are indicated as “Parental strain/pCP20.” “λRec” indicates strains that were constructed by recombineering (see Materials and Methods for details).
Table 2.
Plasmids used in this study
| Plasmid | Genotypea | Origin | Source or reference |
|---|---|---|---|
| pCB24 | attHK022 bla lacIq Plac::ycfM-cTAP | R6K | 46 |
| pCB28 | attHK022 bla lacIq Plac::ycfM-sfGFP | R6K | This study |
| pCH201 | attHK022 bla lacIq Plac::GFP-ftsN | R6K | 32 |
| pCP20 | bla cat cI875 repA(Ts) PR::flp | pSC101 | 16 |
| pDY71 | attHK022 tetA tetR lacIq Plac | R6K | This study |
| pDY75 | attHK022 tetA tetR araC Para::SSdsbA-amiB | R6K | This study |
| pEZ4 | attλλ cat araC Psyn135::GFP-zapA | R6K | 5 |
| pHC531 | attλλ tetA tetR lacIq Plac-m3::slmA | R6K | 13 |
| pHC583 | attHK022 tetA tetR lacIq Plac-m3::slmA | R6K | This study |
| pInt-ts | bla cI875 repA(Ts) PR::intλλ | pSC101 | 30 |
| pJE80 | cat araC Para::sulA(sfiA) | pACYC/p15A | 7 |
| pKP5 | attλλ cat araC Para ::GFP-ftsN | R6K | This study |
| pMG20 | cat araC Para::SStorA-bfp-EftsN | pACYC/p15A | 27 |
| pMM60 | attHK022 tetA tetR lacIq Plac::ycfM-sfGFP | R6K | This study |
| pNP3 | attHK022 bla lacIq Plac::SSdsbA-amiB-mCherry | R6K | This study |
| pNP16 | attHK022 tetA tetR lacIq Plac-m3::amiC-GFP | R6K | This study |
| pNP18 | attHK022 tetA tetR lacIq Plac::envC-mCherry | R6K | This study |
| pNP19 | attHK022 tetA tetR lacIq Plac:: SSdsbA-amiB-sfGFP | R6K | This study |
| pNP20 | attHK022 tetA tetR lacIq Plac::nlpD-mCherry | R6K | This study |
| pNP21 | attHK022 tetA tetR lacIq Plac::GFP-ftsN | R6K | This study |
| pTB28 | bla lacIq Plac::amiC-GFP | colE1/pBR | 6 |
| pTB102 | cat cI875 repA(Ts) PR::intHK022 | pSC101 | 8 |
| pTB265 | attHK022 bla lacIq Para | R6K | 60 |
| pTB282 | attHK022 bla lacIq Plac::sdsbA-sfGFP | R6K | This study |
| pTB285 | attλλ cat araC Para | R6K | 13 |
| pTB311 | attHK022 bla lacIq Plac::SSdsbA-FLamiB-sfGFP | R6K | 59 |
| pTB314 | attHK022 bla lacIq Plac::nlpD-mCherry | R6K | 60 |
| pTB316 | attHK022 bla lacIq Plac::envC-mCherry | R6K | 60 |
| pTD9 | attHK022 bla lacIq Plac::nlpD-sfGFP | R6K | 60 |
| pTD80 | attλλ cat lacIq Plac::envC-mCherry | R6K | This study |
| pTD82 | attHK022 bla lacIq Plac::SSdsbA-FLamiB-sfGFP | R6K | This study |
| pTD83 | attHK022 bla lacIq Plac::SSdsbA-FLamiB(E382Q)-sfGFP | R6K | 59 |
| pTU136 | attHK022 bla lacIq Plac::SSdsbA-mCherry | R6K | 59 |
| pTU175 | aadA Psyn135::SSdsbA-mCherry | pSC101 | 59 |
| pTU191 | attHK022 bla lacIq Psyn135::GFP-ftsN | R6K | This study |
| pTU194 | attHK022 bla lacIq Psyn135::mCherry-zapA | R6K | This study |
PR, Para, and Para indicate the phage λR, lactose, and arabinose promoters, respectively. Psyn135 is a synthetic lac promoter with a consensus −35 element and no operators. sfGFP is superfolding GFP. SSdsbA encodes the signal sequence of DsbA. ftsNE encodes the essential domain of FtsN. FLamiB encodes full-length AmiB (residues 23 to 445). TamiB encodes the targeting domain of AmiB (residues 23 to 191). pDY71, pTB265, and pTB285 do not have inserts downstream of the promoter.
Fluorescence microscopy.
Fluorescence microscopy was performed as described previously (60). See the figure legends for the specific growth conditions employed for each experiment. To generate a marker for the Z-ring, a zapA-GFP gene fusion was created at its native chromosomal locus by λ recombineering (68). A GFPmut2 (15) coding sequence and a linked cat cassette flanked by a zapA 3′-end sequence and a sequence downstream of zapA were amplified using pTB24 (5) as a template and the primers 5′-ACAAGGTCGCATCACCGAAAAAACTAACCAAAACTTTGAAGATCCCCCCGCTGAATTCATG-3′ and 5′-TTGTCTTCACGGTTACTCTACCACAGTAAACCGAAAAGTGGTGTA GGCTGGAGCTGCTTCG-3′. The resulting fragment was used for recombineering in strain TB10 as described previously (7). The zapA-GFP gene fusion linked to the cat cassette was transferred between strains by P1 transduction. When necessary, the cat cassette was evicted from recipient strains using FLP recombinase from pCP20 (16). A zapA-mCherry gene fusion at the native zapA locus was generated in a similar manner.
Other than ZapA-GFP, Tat-targeted AmiC-GFP was the only fusion construct employed in this study that utilized the GFPmut2 variant (15). All other constructs utilized sfGFP (47). The red fluorescent protein used for localization experiments was mCherry (55). For AmiB fusion proteins, the native AmiB signal sequence was replaced with the signal sequence peptide of DsbA (SSDsbA), which targets proteins through the cotranslational branch of the Sec pathway (51). All other periplasmic protein fusions except the essential domain (residues 71 to 105) of FtsN (EFtsN) were targeted using their native export signals. To deplete cellular FtsN activity, we took advantage of ΔftsN strains harboring plasmid pMG20 (cat araC Para::SStorA-bfp-EftsN) (27). This construct produces EFtsN fused to blue fluorescent protein (BFP). The fusion is targeted to the periplasm using the Tat-targeting sequence from TorA, and cells must overproduce SSTorA-BFP-EFtsN to support division (27). Depletion of cellular FtsN activity is therefore relatively rapid and efficient compared to the depletion of native FtsN.
RESULTS
AmiB is recruited to the division site via an N-terminal targeting domain.
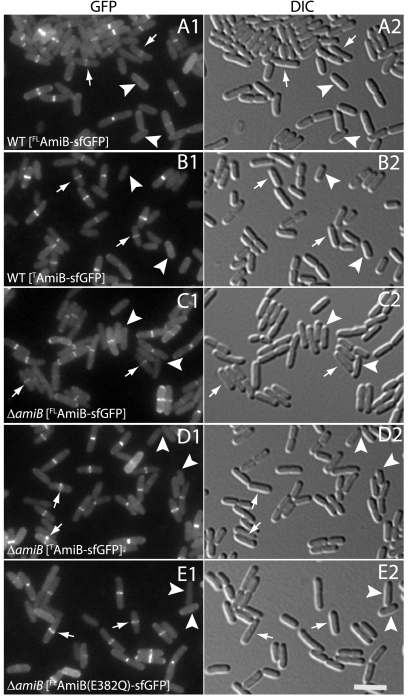
The subcellular localization of the PG amidases involved in cell division (AmiA, AmiB, and AmiC) was previously investigated using GFPmut2 fusions (6, 15). Unlike AmiA and AmiC, which are Tat substrates, an AmiB-GFPmut2 fusion protein was not fluorescent, suggesting that it was exported via Sec. Since we have demonstrated the ability of sfGFP to fold and fluoresce following Sec export (21), we constructed the fusion protein FLAmiB-sfGFP (fusion of SSDsbA, full-length AmiB [residues 23 to 445], and sfGFP) to study the subcellular localization of AmiB. Export was targeted through the cotranslational branch of the Sec export pathway by replacing the native AmiB signal sequence (residues 1 to 22) with SSDsbA (51). FLAmiB-sfGFP was functional, as indicated by its ability to correct the chaining defect of a strain lacking AmiA and AmiB (59; data not shown). Fluorescence microscopy revealed that the fusion protein accumulated at division sites in both AmiB+ and AmiB− cells (Fig. 2A and C). AmiB is thus positioned to directly participate in septal PG splitting. The recruitment of FLAmiB-sfGFP to division sites was correlated with the onset of envelope constriction (Fig. 2). Most cells lacking an observable constriction also lacked a clear AmiB-sfGFP band at midcell (Fig. 2A and C, arrowheads). However, a small subpopulation of unconstricted cells with a midcell accumulation of FLAmiB-sfGFP was consistently observed (Fig. 2A and C, arrows), suggesting that AmiB is likely to accumulate at midcell just as constriction is initiated (see below).
Fig. 2.
AmiB is recruited to the division site via an N-terminal targeting domain. (A to E) Cells of TB28 (WT) (A and B) or TB170 (ΔamiB) (C to E) harboring the integrated expression constructs attHKTB311 (Plac::SSdsbA-FLamiB-sfGFP) (A and C), attHKTD82 (Plac::SSdsbA-TamiB-sfGFP) (B and D), or attHKTD83 [Plac::SSdsbA- FLamiB(E382Q)-sfGFP] (E) were grown overnight in LB at 37°C. They were then diluted 1:100 in M9 maltose supplemented with 10 μM isopropyl-β-d-thiogalactopyranoside (IPTG), grown at 30°C to an optical density at 600 nm (OD600) of 0.5 to 0.7, and visualized on 2% agarose pads using GFP (panel 1) or differential interference contrast (DIC) (panel 2) optics. Arrows and arrowheads highlight unconstricted cells with or without a midcell band of fluorescence, respectively. The subcellular localization of other catalytically defective AmiB-sfGFP fusion proteins (H200A, E215A, or H269A) was identical to that of AmiB(E382Q)-sfGFP (E) (data not shown). Full-length AmiB (residues 23 to 445) is abbreviated as FLAmiB, and the targeting domain (residues 23 to 191) as TAmiB. Bar equals 4 μm.
In their primary structures, AmiB and AmiC have sizable N-terminal extensions preceding their amidase catalytic domains (Fig. 1). Interestingly, both of these factors localize to the division site, while AmiA, which lacks an equivalent extension (Fig. 1), displays a diffuse peripheral (periplasmic) localization pattern and does not specifically accumulate at the septum (6). Previous work indicated that the N-terminal domain of AmiC (targeting domain [TAmiC]) is responsible for targeting it to developing septa (6). Moreover, similar domains (AMIN domains) were found in a variety of exported proteins from other bacteria (19). By analogy with TAmiC, it has been proposed that these domains play an important role in determining the subcellular localization of other proteins as well (19). The N-terminal domain of AmiB is only weakly similar to that of AmiC. We therefore investigated whether or not this domain also functions as a localization domain. Indeed, cells expressing exported fusion protein TAmiB-sfGFP (fusion of SSDsbA, AmiB targeting domain [residues 23 to 191], and sfGFP) displayed prominent bands of fluorescence at midcell whether or not they also produced native AmiB (Fig. 2B and D). In all respects, the localization pattern of TAmiB-sfGFP was indistinguishable from that of the full-length fusion protein (compare Fig. 2A and C with B and D). We therefore conclude that TAmiB is sufficient for the recruitment of AmiB to the division site.
To determine if catalytic activity is required for the recruitment of AmiB to the division site, we studied the localization of AmiB-sfGFP fusion proteins with an H200A, E215A, H269A, or E382Q substitution. Each substitution was previously found to abolish EnvC-activated AmiB activity in vitro, and fusion proteins harboring these substitutions failed to correct the cell-chaining phenotype of a ΔamiA ΔamiB mutant (59). Nevertheless, all of the mutant proteins localized normally (Fig. 2E and data not shown), indicating that catalytic activity is dispensable for AmiB localization.
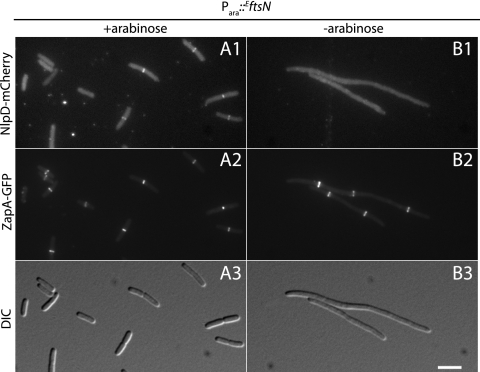
AmiB localization requires FtsZ and FtsN but is independent of EnvC.
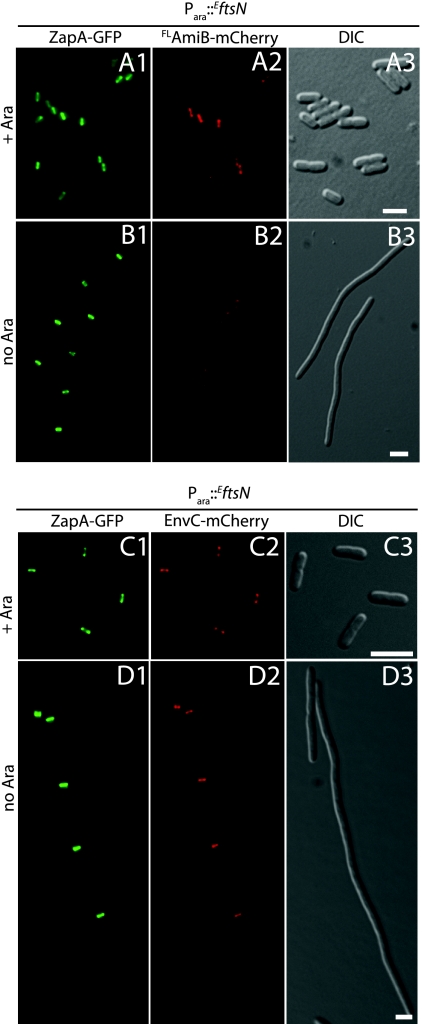
To begin investigating the requirements for AmiB recruitment to the division site, we visualized FLAmiB-sfGFP localization in FtsZ− and FtsN− filaments. As expected, FLAmiB-sfGFP assumed a peripheral, delocalized appearance when Z-ring formation was inhibited by the production of the division inhibitor SulA (SfiA) (9) (Fig. 3A and B). Thus, like all other divisome components studied to date, AmiB requires assembly of the Z-ring to accumulate at prospective division sites. To determine whether or not AmiB requires FtsN for septal localization, we constructed a ΔftsN strain (NP49) that harbored a plasmid (pMG20) encoding the small essential domain of FtsN (EftsN) (27) under the control of the arabinose promoter. This allowed for a more efficient depletion of cellular FtsN activity relative to that in a strain with full-length FtsN under inducible control. NP49 also encodes a zapA-GFP fusion at the native zapA locus so that proper Z-ring formation can be monitored upon depletion of EFtsN. To follow AmiB localization in NP49, we integrated an FLamiB-mCherry gene fusion under Plac control at the phage HK022 att site (attHKNP3). As expected, in the presence of arabinose, NP49(attHKNP3)/pMG20 cells appeared to grow and divide normally and displayed typical Z-ring and AmiB localization patterns (Fig. 4A). In the absence of arabinose, however, NP49(attHKNP3) cells failed to divide. Although Z-ring formation appeared normal in these filaments, FLAmiB-mCherry was not recruited to these structures (Fig. 4B). Thus, AmiB requires EFtsN for septal ring localization. Given the small size of EFtsN (35 residues) and its essentiality for division, it is unlikely to interact directly with the nonessential AmiB protein. Rather, we suspect that AmiB depends on EFtsN for its localization because cell constriction must be initiated before it can be recruited to the division site (see below).
Fig. 3.
AmiB recruitment to the division site is dependent on FtsZ. Cells of TB28(attHKTB311)/pJE80 [WT (Plac::SSdsbA-FLamiB- sfGFP)/Para::sulA] were grown overnight at 37°C in M9 glucose. They were then diluted 1:400 in M9 maltose with IPTG (10 μM) (A) or the same medium with 0.001% arabinose added (B). Cells were grown to an OD600 of 0.3 and were viewed on 2% agarose pads using GFP (panel 1) or DIC (panel 2) optics. Bar equals 4 μm.
Fig. 4.
Differential FtsN requirement for AmiB and EnvC recruitment to the division site. Cultures of NP49(attHKNP3)/pMG20 [ΔftsN zapA-GFP (Plac::SSdsbA-FLamiB-mCherry)/Para::EftsN] (A, B) or NP22(attHKTB316)/pMG20 [ΔftsN ΔenvC zapA-GFP (Plac:: envC-mCherry)/Para::EftsN] (C, D) were grown overnight in M9 maltose supplemented with 0.01% arabinose. Cells were washed twice with and resuspended in an equal volume of M9 medium without added sugar. They were then diluted 1:100 into M9 maltose and growth was continued at 30°C with the addition of either 0.01% arabinose (A and C) or 0.01% glucose (B and D). When the cultures reached an OD600 of 0.4 to 0.7, cells were visualized on 2% agarose pads using GFP (panels 1), mCherry (panels 2), or DIC (panels 3) optics. Bar equals 4 μm.
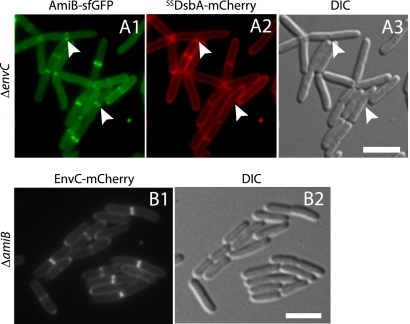
PG hydrolysis by AmiB is activated by EnvC (59). We therefore wondered whether AmiB localization is similarly dependent on EnvC. To test this, we monitored FLAmiB-sfGFP localization in a ΔenvC strain (Fig. 5A). Localization studies of periplasmic proteins in this background are complicated by the fact that unfused periplasmic GFP often appears to accumulate at the septa of EnvC− cells (7). To account for this, we coproduced exported mCherry and FLAmiB-sfGFP in a ΔenvC mutant. In these cells, we observed ring- or band-like accumulations of FLAmiB-sfGFP at nascent septa that lacked corresponding accumulations of periplasmic mCherry (Fig. 5A), indicating that AmiB does not require EnvC for its recruitment to the division site.
Fig. 5.
AmiB and EnvC are recruited independently to the division site. Cells of TB140(attHKTB311)/pTU175 [ΔenvC (Plac::SSdsbA-FLamiB-sfGFP)/Psyn135::SSdsbA-mCherry] (A) or NP6 (attHKTB316) [ΔenvC ΔamiB (Plac::envC-mCherry)] (B) were grown overnight in LB medium at 37°C. They were then diluted 1:100 in M9 maltose supplemented with 10 (A) or 0 (B) μM IPTG and grown at 30°C to an OD600 of 0.5 before they were visualized on 2% agarose pads with GFP (A1), mCherry (A2 and B1), or DIC (A3 and B2) optics. Arrowheads in panel A indicate sites of AmiB-sfGFP localization without corresponding accumulations of periplasmic mCherry. Bar equals 4 μm. A periplasmic marker was not required to assess EnvC-mCherry localization in AmiB− cells because the loss of AmiB function does not result in increased periplasmic volume at septa.
EnvC recruitment to the division site is FtsN and AmiB independent.
The results described above show that AmiB is dependent on FtsN for its recruitment to the division site. To determine if this is also true for EnvC, we monitored FLEnvC-mCherry localization in an EnvC− derivative of our FtsN depletion strain (NP22/pMG20). Unlike AmiB, FLEnvC-mCherry displayed a sharp band/ring localization pattern along the length of FtsN− filaments (Fig. 4C and D). These FLEnvC-mCherry bands colocalized perfectly with ZapA-GFP. Thus, unlike that of AmiB, EnvC recruitment to the divisome is independent of FtsN and does not require the initiation of cell constriction. As expected, FLEnvC-sfGFP also localized normally in ΔamiB cells, indicating that EnvC recruitment to the division site is correspondingly independent of AmiB (Fig. 5B).
AmiB is recruited to the division site after EnvC.
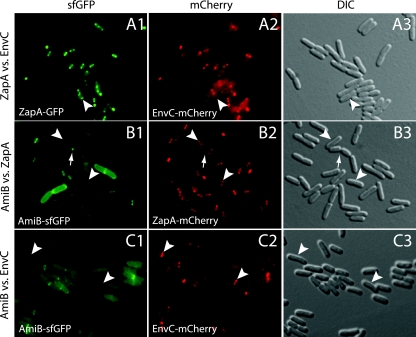
The differential FtsN requirements for AmiB and EnvC localization to the division site suggested that they may be recruited to the septal ring at different times during its assembly. To investigate this, we compared the colocalization frequency of EnvC or AmiB with that of ZapA and also directly monitored the colocalization of AmiB with EnvC (Fig. 6 and Table 3). Over 90% of cells producing both ZapA-GFP and FLEnvC-mCherry showed colocalization of ZapA and EnvC (Fig. 6A and Table 3). A relatively small percentage (5%) of these cells displayed a ZapA-GFP ring without a corresponding band of FLEnvC-mCherry. We did not observe any cells with a ring of FLEnvC-mCherry without a corresponding ZapA-GFP ring. The colocalization results therefore suggest that EnvC is recruited to the division site soon after the Z-ring is assembled. In contrast to the EnvC localization results, only 64% of cells producing FLAmiB-sfGFP and ZapA-mCherry showed colocalization of AmiB and ZapA (Fig. 6B and Table 3). A relatively high percentage of cells (33%) displayed a ZapA-mCherry ring without a corresponding band of FLAmiB-sfGFP. Thus, there appears to be a significant delay between Z-ring assembly and the recruitment of AmiB to the division site. Interestingly, we observed a significant number (12%) of deeply constricted cells with a focus of FLAmiB-sfGFP at the division site and ZapA-mCherry rings at midcell of the developing daughters (Fig. 6B, arrow, and Table 3). This is consistent with previous observations (7) indicating that the final stages of cell separation can occur independently of the Z-ring. The colocalization results with ZapA suggested that EnvC is recruited to the division site earlier than AmiB. This was confirmed when we directly compared their localization in cells producing both FLAmiB-sfGFP and FLEnvC-mCherry (Fig. 6C and Table 3). Over 30% of the cells analyzed showed a band of FLEnvC-mCherry without a corresponding band of FLAmiB-sfGFP, and the two proteins showed colocalization in only 52% of the cells analyzed. Overall, our results indicate that EnvC is a relatively early recruit to the division site, while AmiB arrives later in the cell cycle, presumably following further maturation of the septal ring into a constriction-competent machine.
Fig. 6.
Timing of EnvC recruitment to the septum relative to that of AmiB. Cells of HC262(attHKTB316) [zapA-GFP ΔenvC (Plac::envC-mCherry)] (A), NP8(attHKTB311) [ΔamiB zapA-mCherry (Plac::SSdsbA-FLamiB-sfGFP)] (B), or TB28(attλTD80)(attHKTB311) [(Plac::envC-mCherry)(Plac::SSdsbA-FLamiB-sfGFP)] (C) were grown overnight in LB medium at 37°C. They were then diluted 1:100 in M9 maltose supplemented with 10 μM IPTG and grown at 30°C to an OD600 of 0.4 to 0.6. Cells were visualized on 2% agarose pads with GFP (panel 1), mCherry (panel 2), or DIC (panel 3) optics. Arrows and arrowheads highlight constricted and unconstricted cells, respectively, where colocalization is not observed. Bar equals 4 μm.
Table 3.
Colocalization of fluorescent protein fusions at the division site
| Strain | Genotype | No. of cells | % of cells: |
||||||
|---|---|---|---|---|---|---|---|---|---|
| With indicated protein localized at division site |
With colocalized proteinsa | With only the indicated fusion protein localized at the division site |
|||||||
| ZapA ring | AmiB ring | EnvC ring | ZapA | AmiB | EnvC | ||||
| NP8 (attHKTB311) | zapA-mCherry ΔamiB (Plac::SSdsbA-amiB-sfGFP) | 153 | 99.3 | 64.4 | NAb | 63.7 | 33.1 | 0.6 | NA |
| HC262 (attHKTB316) | zapA-GFP ΔenvC (Plac::envC-mCherry) | 151 | 97.4 | NA | 92.8 | 92.8 | 4.6 | NA | 0 |
| TB28(attλTD80) (attHKTB311) | WT (Plac::envC-mCherry)(Plac::SSdsbA-amiB-sfGFP) | 157 | NA | 58.6 | 86.8 | 52.4 | NA | 2.4 | 34.4 |
“Colocalized proteins” refers to cells with both fusion proteins localized at the division site.
NA, not applicable.
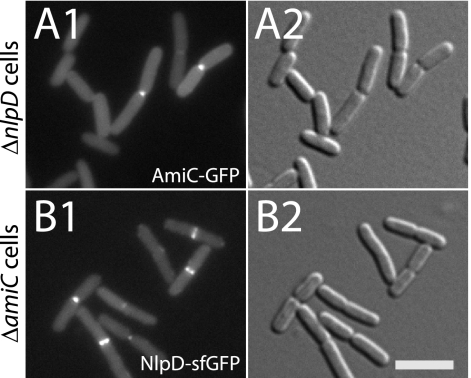
AmiC and NlpD localize to the division site independently of one another.
To determine if cell separation amidases are generally recruited to the division site independently of their activators and vice versa, we investigated AmiC and NlpD localization in the presence or absence of each other. As was the case for AmiB and its activator EnvC, AmiC-GFP localized to septa in both wild-type (WT) and ΔnlpD cells (Fig. 7A; also see Table S1 in the supplemental material) (6). Moreover, the frequency with which midcell bands of AmiC-GFP were observed did not change significantly in the presence or absence of NlpD (see Table S1). NlpD-sfGFP also localized to division sites in the absence of AmiC (Fig. 7B). NlpD localization also did not appear to be significantly affected by the loss of AmiC (see Table S1). Interestingly, we found that the frequency of cells with NlpD rings (43 to 64%) was considerably higher than the frequency with which we observed cells with AmiC rings (19 to 22%) (see Table S1). We therefore infer that, similar to EnvC, NlpD arrives at septa prior to its cognate amidase (see below).
Fig. 7.
AmiC and NlpD are independently recruited to the division site. Cells of TU217/pTB28/pTU175 [ΔamiC ΔnlpD/ Plac::amiC-GFP/Psyn135::SSdsbA-mCherry] (A) or TU217(attHKTD9) [ΔamiC ΔnlpD (Plac::nlpD-sfGFP)] (B) were grown overnight in LB at 37°C. They were then diluted 1:100 in M9 maltose supplemented with 25 (A) or 0 (B) μM IPTG and grown at 30°C to an OD600 of 0.4 to 0.5 before they were visualized on 2% agarose pads with GFP (panel 1) or DIC (panel 2) optics. Bar equals 4 μm.
Like AmiB, FtsN is required for the septal localization of AmiC (6). We therefore wondered if NlpD shares the FtsN requirement for localization with its cognate amidase or if, like EnvC, it localizes in an FtsN-independent manner. To this end, we monitored the localization of NlpD-mCherry in NP64(attHKTB314)/pMG20 [ΔnlpD ΔftsN zapA-GFP (Plac::nlpD-mCherry)/Para::EftsN] cells grown in the presence and absence of arabinose. As expected, when these cells were grown in medium containing arabinose, NlpD-mCherry localized to the division sites of constricting cells (Fig. 8A). When grown in the absence of arabinose, however, NlpD-mCherry failed to localize to Z-ring structures formed in the resulting FtsN− filaments (Fig. 8B). Thus, like AmiB and AmiC, NlpD requires EFtsN for its recruitment to the septal ring.
Fig. 8.
NlpD requires FtsN for recruitment to the division site. Cells of NP64(attHKTB314)/pMG20 [ΔftsN ΔnlpD zapA-GFP (Plac::nlpD-mCherry)/Para::EftsN] were grown in the presence (A) or absence (B) of arabinose as described in the legend to Fig. 4. Cells were visualized on 2% agarose pads with mCherry (panel 1), GFP (panel 2), or DIC (panel 3) optics. Bar equals 4 μm.
Septal PG assembly is required for amidase localization but not LytM factor recruitment.
Our results thus far indicate that NlpD, AmiB, and AmiC require FtsN for their recruitment to the septal ring. We wondered whether this requirement was due to the lack of FtsN per se or if it reflects a requirement for septal PG biogenesis, which FtsN is thought to stimulate (27). To investigate this, we monitored EnvC, NlpD, AmiB, and AmiC localization in cells treated with the FtsI(PBP3) inhibitor cephalexin. Cell division is blocked by relatively low doses of cephalexin (10 μg/ml) or other penicillin-binding protein 3 (PBP3)-specific β-lactams (56). However, this treatment does not appear to significantly disrupt the recruitment of FtsI or other essential division proteins to septal rings (65), including FtsN (14, 64) (see Fig. S1 in the supplemental material), although this has not been universally observed (63). Filamentous cells resulting from cephalexin treatment therefore contain septal rings with the full complement of essential proteins. They just cannot initiate septal PG assembly due to FtsI inhibition. As expected from the results described above, EnvC remained capable of recruiting to septal rings in the presence of cephalexin (Fig. 9A). Interestingly, NlpD was also recruited to septal rings in cephalexin-treated cells, while both AmiB and AmiC failed to localize (Fig. 9B to D). Thus, septal rings recruit EnvC and NlpD prior to the start of septal PG assembly and are therefore primed for amidase activation. However, our results indicate that the septal PG assembly process must be initiated before the amidases concentrate at the division site.
Fig. 9.
Localization of LytM factors and amidases in the absence of septal PG assembly. Cells of NP32(attHKNP18) [ΔenvC zapA-GFP (Plac::envC-mCherry)] (A), NP94(attHKTB314) [ΔnlpD zapA-GFP (Plac::nlpD-mCherry)] (B), NP90(attHKNP19) [ΔamiB zapA-mCherry (Plac::SSdsbA-amiB-sfGFP)] (C), or NP84(attHKNP16) [ΔamiC zapA-mCherry (Plac-m3::amiC-GFP)] (D) were grown overnight in LB at 37°C. They were then diluted 1:100 in M9 maltose supplemented with 10 μM IPTG. Upon reaching an OD600 of 0.05, cephalexin (10 μg/ml) was added. Cells were then grown for an additional 3 to 4 doublings and then imaged on 2% agarose pads made with cephalexin (10 μg/ml). EnvC-mCherry (A) and NlpD-mCherry (B) colocalized with ZapA-GFP rings, with 84.1% (n = 164 rings) and 59.2% (n = 130 rings) colocalization, respectively. In contrast, AmiB-sfGFP (C) and AmiC-GFP (D) showed only minimal colocalization with ZapA-mCherry, with 3.5% (n = 171 rings) and 1.5% (n = 134 rings), respectively. Treatment of cells with other PBP3-specific β-lactams (furazlocillin, piperacillin, and aztreonam) yielded similar results (data not shown). Images for untreated control cells are presented in Fig. S2 in the supplemental material. DIC images are shown in panels 1, mCherry in panels 2, and GFP in panels 3. Bar equals 4 μm.
DISCUSSION
To better understand the regulation of septal PG splitting, we studied the recruitment of the cell separation amidase AmiB to the septal ring. We showed that, like its paralog AmiC (6), AmiB is recruited to the septal ring by an N-terminal targeting domain. Surprisingly, we found that both of these amidases localized to the division site independently of their cognate LytM activator. The activators, EnvC and NlpD, were also found to localize normally in the absence of their target amidases. Colocalization studies indicated that EnvC is an early septal ring recruit that localizes to the Z-ring shortly after it is formed. AmiB and NlpD, on the other hand, do not appear to be recruited until about the time constriction is initiated, while AmiC appears to only be recruited to the septum of deeply constricted cells. Accordingly, EnvC displayed a subcellular localization pattern with a different requirement for FtsN than the other separation factors. NlpD and the amidases require FtsN for recruitment to the septal ring, while EnvC does not. Importantly, we also showed that when septal PG synthesis was blocked by cephalexin, both EnvC and NlpD were still recruited to septal rings, whereas AmiB and AmiC failed to localize.
Coordination of septal PG synthesis and hydrolysis.
Overall, our results are consistent with the recent proposal that FtsN and the amidases promote a self-enhancing reaction sequence at the septal ring that helps initiate and drive cell constriction (27) (Fig. 10). This positive feedback loop is thought to result from the interplay between EFtsN and its PG-binding SPOR domain (SFtsN) according to the following scenario (27). (i) Weak association of FtsN with the septal ring localizes a small amount of EFtsN at the division site to directly or indirectly activate a low level of PG synthesis by the penicillin-binding proteins (PBPs), most likely FtsI (PBP3) and/or PBP1b (20, 39, 44, 67). (ii) This generates septal PG for splitting by the amidases, and their activity, in turn, leads to the production of PG strands lacking peptides. (iii) These denuded glycans are bound by SFtsN (61), leading to the recruitment of more EFtsN to the division site and the further stimulation of septal PG synthesis. The cycle is thought to repeat, strengthening with each repetition to promote and sustain cell constriction.
Fig. 10.
Model for the coordination of septal PG assembly and hydrolysis. Shown is a model for the coordination of septal PG synthesis and hydrolysis based on the self-enhancing FtsN recruitment model proposed previously (27). When FtsN begins to recruit to the septum, it is thought to stimulate PG synthesis by the PBPs and may also activate (directly or indirectly) the activity of the LytM factors, which in turn activate the amidases. Septal PG serves as a localization signal for amidase (AmiB and AmiC) recruitment to midcell. It is also hydrolyzed by the amidases to form the cell poles and generate denuded glycan strands that serve as recruitment signals for the PG-binding SPOR domain of FtsN. With each progression of the cycle, more FtsN is recruited to further enhance the process of septal synthesis and splitting (cell constriction). See text for details.
The combination of our current findings with the FtsN accumulation model provides a framework for understanding how PG synthesis and hydrolysis might be coordinated during division (Fig. 10). An important feature of the framework is that AmiB and AmiC require septal PG synthesis to be recruited to the septal ring (Fig. 10). Thus, the septal ring assembly pathway appears to include a built-in fail-safe mechanism to ensure that septal PG biogenesis precedes the high level of PG hydrolysis that is likely to accompany amidase localization. While at this point it is unclear how amidase recruitment is triggered by the production of septal PG, an attractive possibility is that the N-terminal targeting domains of AmiB and AmiC recognize a structural feature unique to this newly synthesized material.
Curiously, an AmiB− AmiC− double mutant does not have a strong cell separation phenotype (14), suggesting that amidase localization is dispensable for cell separation. This indicates that AmiA can largely facilitate cell separation on its own even though it does not specifically accumulate at the division site. Other factors in addition to septal PG-dependent amidase localization must therefore also play a role in coordinating amidase activation at the septum with septal PG biogenesis. Experiments with β-lactams provide clues to how this might be accomplished. While treatment with relatively low levels of the FtsI-specific β-lactam cephalexin (10 μg/ml) inhibits division, treatment with higher levels (50 μg/ml) or with less-specific β-lactams like ampicillin results in rapid cell lysis from lesions that emanate from septa (14, 22, 40, 41, 53, 57, 60). Previous studies indicate that this rapid lysis is dependent upon all of the essential cell division proteins, including FtsN, as well as the LytM factors and the amidases (14, 60). AmiA appeared to be the most important amidase for rapid lysis (14), suggesting that miscoordinated AmiA activation at the septum by EnvC may be the primary lytic determinant. The finding that rapid β-lactam-induced lysis also requires FtsN (14) further suggests that amidase activation by EnvC at the septum may be coupled to the recruitment of FtsN, perhaps indirectly through an effect of FtsN on the synthases (Fig. 10). We therefore infer that FtsN is probably directly or indirectly modulating amidase activity at the septum in at least three ways: (i) by stimulating EnvC activity, (ii) by promoting NlpD recruitment to the septal ring, and (iii) by stimulating the production of septal PG, which in turn concentrates AmiB and AmiC at the septum. Thus, while the coordination of the degradative activity of the amidases with the synthetic activity of the PBPs is clearly more complicated than proposed previously (14), our results support the idea that the sequential and interdependent recruitment of FtsN and the amidases to the septal ring plays an important role.
A potential role for EnvC in FtsN accumulation at the division site.
It was initially proposed that the self-enhancing accumulation of FtsN at the division site begins with its weak association with the septal ring mediated by its N-terminal transmembrane domain (27). The observation that EnvC is an early septal ring recruit that localizes independently of FtsN suggests an alternative or parallel scenario in which EnvC participates in the initiation of the FtsN accumulation cycle. In this case, EnvC may possess some basal level of activity at the septal ring that is not dependent on the prior recruitment of FtsN. Thus, low-level PG hydrolysis at the prospective division site may occur early on in its development as AmiA and AmiB diffusing in the periplasm encounter EnvC at the septum. Recent results using a new PG-labeling reagent suggest that this is likely the case (45). Because at this stage AmiB has yet to specifically accumulate at the septal ring and the proposed activation of EnvC by FtsN would not yet be in effect, the overall level of PG hydrolysis would probably be low enough so as not to cause cell lysis. This low-level amidase activity would generate sites of denuded glycans to recruit FtsN via its SPOR domain, thus allowing EFtsN to stimulate septal PG synthesis and promote the self-enhancing cascade that is thought to follow. Since NlpD is expected to begin accumulating at the septal ring with FtsN, it may also provide low-level amidase activity at the division site to reinforce the positive localization feedback loop.
Why are there two overlapping PG-splitting systems?
Unlike EnvC and AmiB, the activator NlpD and its cognate amidase AmiC are both late recruits to the septum that require FtsN for their localization. This suggests that NlpD-activated AmiC activity may be optimized for the final stages of the septal PG-splitting process. Such a late-acting system is probably important in rapidly growing cells because Z-rings sometimes form at midcell of the new daughters while cell separation is being completed (58) (Fig. 6B, arrows). Since EnvC is recruited to these new Z-rings shortly after their formation, late-stage septa may be devoid of EnvC-activated amidase activity. In these cases, NlpD and AmiC are probably needed to efficiently complete the separation process.
AmiB and NlpD both appeared to accumulate at the division site around the time of constriction initiation. We find that AmiC, on the other hand, only appears to localize to septa in deeply constricted cells (Fig. 7; also see Table S1 in the supplemental material). This is slightly different from our previous results indicating that AmiC recruits to the septum of all constricting cells (6). The reason for this discrepancy is not known, but it implies that the targeting domains of AmiB and AmiC might recognize distinct but related features of the septum to control when their corresponding amidase domains are concentrated at the division site. Interestingly, this also indicates that, like EnvC and AmiB, NlpD and AmiC accumulate at the septum at different times, with the activator preceding its cognate amidase. Accordingly, NlpD and AmiC show a differential requirement for FtsI activity for their septal localization. The significance of this is not currently clear, but it suggests the attractive possibility that the timing of AmiC localization might govern a potential switch from EnvC-AmiA/-B to NlpD-AmiC splitting systems during the latter stages of division.
Other roles for EnvC?
Although a major function of EnvC at the septum is the regulation of amidase activity, this does not appear to be its only one. In addition to cell separation defects, loss of EnvC function also causes FtsZ ring assembly defects at high temperatures and is synthetically lethal with defects in the Min system (7, 34, 36). This suggests a role for EnvC in Z-ring stabilization, as well as septal PG splitting. The observation that EnvC is recruited to the Z-ring shortly after its assembly is consistent with this additional function. Exactly how EnvC might stabilize the Z-ring remains unclear, but this may involve anchoring components of the Z-ring to the PG layer.
sfGFP and localization studies in the envelope.
From a technical standpoint, this report highlights the utility of sfGFP fusion proteins for periplasmic protein localization studies. Unlike Tat-targeted GFP, the ability to route sfGFP through Sec is likely to result in the generation of a wider variety of functional periplasmic fusion proteins. In addition, its use in combination with Sec-exported mCherry fusion proteins opens the door for colocalization studies in the envelope of live cells like those performed in this report for EnvC and AmiB.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the laboratory, especially Tsuyoshi Uehara, for discussions, support, and critical reading of the manuscript. We also thank Tsuyoshi Uehara, Hongbaek Cho, Desiree Yang, Monica Markovski, Felipe Bendezu, Catherine Paradis-Bleau, Katie Parzych, Piet de Boer, Cynthia Hale, and Matt Gerding for plasmids and strains. We are also grateful for the kind gift of piperacillin and furazlocillin from Ted Park.
This work was supported by the Massachusetts Life Science Center, the Burroughs Wellcome Fund, and the National Institutes of Health (grant R01 AI083365-01A1). T.G.B. holds a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Aarsman M. E. G., et al. 2005. Maturation of the Escherichia coli divisome occurs in two steps. Mol. Microbiol. 55:1631–1645 [DOI] [PubMed] [Google Scholar]
- 2. Adams D. W., Errington J. 2009. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat. Rev. Microbiol. 7:642–653 [DOI] [PubMed] [Google Scholar]
- 3. Addinall S. G., Cao C., Lutkenhaus J. 1997. FtsN, a late recruit to the septum in Escherichia coli. Mol. Microbiol. 25:303–309 [DOI] [PubMed] [Google Scholar]
- 4. Baba T., et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bendezú F., Hale C., Bernhardt T., de Boer P. 2009. RodZ (YfgA) is required for proper assembly of the MreB actin cytoskeleton and cell shape in E. coli. EMBO J. 28:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernhardt T. G., de Boer P. A. J. 2003. The Escherichia coli amidase AmiC is a periplasmic septal ring component exported via the twin-arginine transport pathway. Mol. Microbiol. 48:1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernhardt T. G., de Boer P. A. J. 2004. Screening for synthetic lethal mutants in Escherichia coli and identification of EnvC (YibP) as a periplasmic septal ring factor with murein hydrolase activity. Mol. Microbiol. 52:1255–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernhardt T. G., de Boer P. A. J. 2005. SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol. Cell 18:555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bi E., Lutkenhaus J. 1993. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J. Bacteriol. 175:1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bi E. F., Lutkenhaus J. 1991. FtsZ ring structure associated with division in Escherichia coli. Nature 354:161–164 [DOI] [PubMed] [Google Scholar]
- 11. Buddelmeijer N., Judson N., Boyd D., Mekalanos J. J., Beckwith J. 2002. YgbQ, a cell division protein in Escherichia coli and Vibrio cholerae, localizes in codependent fashion with FtsL to the division site. Proc. Natl. Acad. Sci. U. S. A. 99:6316–6321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen J. C., Beckwith J. 2001. FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division. Mol. Microbiol. 42:395–413 [DOI] [PubMed] [Google Scholar]
- 13. Cho H., McManus H. R., Dove S. L., Bernhardt T. G. 2011. Nucleoid occlusion factor SlmA is a DNA-activated FtsZ polymerization antagonist. Proc. Natl. Acad. Sci. U. S. A. 108:3773–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chung H. S., et al. 2009. Rapid {beta}-lactam-induced lysis requires successful assembly of the cell division machinery. Proc. Natl. Acad. Sci. U. S. A. 106:21872–21877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cormack B. P., Valdivia R. H., Falkow S. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33–38 [DOI] [PubMed] [Google Scholar]
- 16. Datsenko K. A., Wanner B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Boer P. A. J. 2010. Advances in understanding E. coli cell fission. Curr. Opin. Microbiol. 13:730–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Pedro M. A., Quintela J. C., Höltje J. V., Schwarz H. 1997. Murein segregation in Escherichia coli. J. Bacteriol. 179:2823–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Souza R. F., Anantharaman V., de Souza S. J., Aravind L., Gueiros-Filho F. J. 2008. AMIN domains have a predicted role in localization of diverse periplasmic protein complexes. Bioinformatics 24:2423–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Lallo G., Fagioli M., Barionovi D., Ghelardini P., Paolozzi L. 2003. Use of a two-hybrid assay to study the assembly of a complex multicomponent protein machinery: bacterial septosome differentiation. Microbiology 149:3353–3359 [DOI] [PubMed] [Google Scholar]
- 21. Dinh T., Bernhardt T. G. 2011. Using superfolder GFP for periplasmic protein localization studies. J. Bacteriol. 193:4984–4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donachie W. D., Begg K. J. 1970. Growth of the bacterial cell. Nature 227:1220–1224 [DOI] [PubMed] [Google Scholar]
- 23. Durand-Heredia J. M., Yu H. H., De Carlo S., Lesser C. F., Janakiraman A. 2011. Identification and characterization of ZapC, a stabilizer of the FtsZ ring in Escherichia coli. J. Bacteriol. 193:1405–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ebersbach G., Galli E., Møller-Jensen J., Löwe J., Gerdes K. 2008. Novel coiled-coil cell division factor ZapB stimulates Z ring assembly and cell division. Mol. Microbiol. 68:720–735 [DOI] [PubMed] [Google Scholar]
- 25. Feilmeier B. J., Iseminger G., Schroeder D., Webber H., Phillips G. J. 2000. Green fluorescent protein functions as a reporter for protein localization in Escherichia coli. J. Bacteriol. 182:4068–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galli E., Gerdes K. 2010. Spatial resolution of two bacterial cell division proteins: ZapA recruits ZapB to the inner face of the Z-ring. Mol. Microbiol. 76:1514–1526 [DOI] [PubMed] [Google Scholar]
- 27. Gerding M., et al. 2009. Self-enhanced accumulation of FtsN at division sites, and roles for other proteins with a SPOR domain (DamX, DedD, and RlpA) in Escherichia coli cell constriction. J. Bacteriol. 191:7383–7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goehring N. W., Beckwith J. 2005. Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr. Biol. 15:R514–R526 [DOI] [PubMed] [Google Scholar]
- 29. Guyer M. S., Reed R. R., Steitz J. A., Low K. B. 1981. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harbor Symp. Quant. Biol. 45:135–140 [DOI] [PubMed] [Google Scholar]
- 30. Haldimann A., Wanner B. L. 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J. Bacteriol. 183:6384–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hale C. A., de Boer P. A. 1999. Recruitment of ZipA to the septal ring of Escherichia coli is dependent on FtsZ and independent of FtsA. J. Bacteriol. 181:167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hale C. A., de Boer P. A. J. 2002. ZipA is required for recruitment of FtsK, FtsQ, FtsL, and FtsN to the septal ring in Escherichia coli. J. Bacteriol. 184:2552–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hale C. A., et al. 2011. Identification of Escherichia coli ZapC (YcbW) as a component of the division apparatus that binds and bundles FtsZ polymers. J. Bacteriol. 193:1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hara H., et al. 2002. Identification and characterization of the Escherichia coli envC gene encoding a periplasmic coiled-coil protein with putative peptidase activity. FEMS Microbiol. Lett. 212:229–236 [DOI] [PubMed] [Google Scholar]
- 35. Heidrich C., et al. 2001. Involvement of N-acetylmuramyl-L-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol. Microbiol. 41:167–178 [DOI] [PubMed] [Google Scholar]
- 36. Ichimura T., Yamazoe M., Maeda M., Wada C., Hiraga S. 2002. Proteolytic activity of YibP protein in Escherichia coli. J. Bacteriol. 184:2595–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ize B., Stanley N. R., Buchanan G., Palmer T. 2003. Role of the Escherichia coli Tat pathway in outer membrane integrity. Mol. Microbiol. 48:1183–1193 [DOI] [PubMed] [Google Scholar]
- 38. Johnson J. E., Lackner L. L., Hale C. A., de Boer P. A. J. 2004. ZipA is required for targeting of DMinC/DicB, but not DMinC/MinD, complexes to septal ring assemblies in Escherichia coli. J. Bacteriol. 186:2418–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karimova G., Dautin N., Ladant D. 2005. Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J. Bacteriol. 187:2233–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lederberg J. 1956. Bacterial protoplasts induced by penicillin. Proc. Natl. Acad. Sci. U. S. A. 42:574–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lederberg J. 1957. Mechanism of action of penicillin. J. Bacteriol. 73:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mercer K. L. N., Weiss D. S. 2002. The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site. J. Bacteriol. 184:904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 44. Müller P., et al. 2007. The essential cell division protein FtsN interacts with the murein (peptidoglycan) synthase PBP1B in Escherichia coli. J. Biol. Chem. 282:36394–36402 [DOI] [PubMed] [Google Scholar]
- 45. Olrichs N. K., et al. 2011. A novel in vivo cell-wall labeling approach sheds new light on peptidoglycan synthesis in Escherichia coli. Chembiochem 12:1124–1133 [DOI] [PubMed] [Google Scholar]
- 46. Paradis-Bleau C., et al. 2010. Lipoprotein cofactors located in the outer membrane activate bacterial cell wall polymerases. Cell 143:1110–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pédelacq J.-D., Cabantous S., Tran T., Terwilliger T. C., Waldo G. S. 2006. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 24:79–88 [DOI] [PubMed] [Google Scholar]
- 48. Pichoff S., Lutkenhaus J. 2002. Unique and overlapping roles for ZipA and FtsA in septal ring assembly in Escherichia coli. EMBO J. 21:685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Priyadarshini R., de Pedro M. A., Young K. D. 2007. Role of peptidoglycan amidases in the development and morphology of the division septum in Escherichia coli. J. Bacteriol. 189:5334–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ryter A., Hirota Y., Schwarz U. 1973. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J. Mol. Biol. 78:185–195 [DOI] [PubMed] [Google Scholar]
- 51. Schierle C. F., et al. 2003. The DsbA signal sequence directs efficient, cotranslational export of passenger proteins to the Escherichia coli periplasm via the signal recognition particle pathway. J. Bacteriol. 185:5706–5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmidt K. L., et al. 2004. A predicted ABC transporter, FtsEX, is needed for cell division in Escherichia coli. J. Bacteriol. 186:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schwarz U., Asmus A., Frank H. 1969. Autolytic enzymes and cell division of Escherichia coli. J. Mol. Biol. 41:419–429 [DOI] [PubMed] [Google Scholar]
- 54. Schwarz U., Ryter A., Rambach A., Hellio R., Hirota Y. 1975. Process of cellular division in Escherichia coli: differention of growth zones in the Sacculus. J. Mol. Biol. 98:749–759 [DOI] [PubMed] [Google Scholar]
- 55. Shaner N. C., et al. 2008. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat. Methods 5:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spratt B. G. 1975. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc. Natl. Acad. Sci. U. S. A. 72:2999–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Staugaard P., M. van den Berg F., Woldringh C. L., Nanninga N. 1976. Localization of ampicillin-sensitive sites in Escherichia coli by electron microscopy. J. Bacteriol. 127:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun Q., Margolin W. 1998. FtsZ dynamics during the division cycle of live Escherichia coli cells. J. Bacteriol. 180:2050–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Uehara T., Parzych K. R., Dinh T., Bernhardt T. G. 2010. Daughter cell separation is controlled by cytokinetic ring-activated cell wall hydrolysis. EMBO J. 29:1412–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Uehara T., Dinh T., Bernhardt T. G. 2009. LytM-domain factors are required for daughter cell separation and rapid ampicillin-induced lysis in Escherichia coli. J. Bacteriol. 191:5094–5107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ursinus A., et al. 2004. Murein (peptidoglycan) binding property of the essential cell division protein FtsN from Escherichia coli. J. Bacteriol. 186:6728–6737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang L., Lutkenhaus J. 1998. FtsK is an essential cell division protein that is localized to the septum and induced as part of the SOS response. Mol. Microbiol. 29:731–740 [DOI] [PubMed] [Google Scholar]
- 63. Wang L., Khattar M. K., Donachie W. D., Lutkenhaus J. 1998. FtsI and FtsW are localized to the septum in Escherichia coli. J. Bacteriol. 180:2810–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang S., Arends S. J., Weiss D. S., Newman E. B. 2005. A deficiency in S-adenosylmethionine synthetase interrupts assembly of the septal ring in Escherichia coli K-12. Mol. Microbiol. 58:791–799 [DOI] [PubMed] [Google Scholar]
- 65. Weiss D. S., Chen J. C., Ghigo J. M., Boyd D., Beckwith J. 1999. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J. Bacteriol. 181:508–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wientjes F. B., Nanninga N. 1989. Rate and topography of peptidoglycan synthesis during cell division in Escherichia coli: concept of a leading edge. J. Bacteriol. 171:3412–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wissel M. C., Weiss D. S. 2004. Genetic analysis of the cell division protein FtsI (PBP3): amino acid substitutions that impair septal localization of FtsI and recruitment of FtsN. J. Bacteriol. 186:490–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yu D., et al. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 97:5978–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.