Abstract
The RecA protein in its functional state is in complex with single-stranded DNA, i.e., in the form of a RecA filament. In SOS induction, the RecA filament functions as a coprotease, enabling the autodigestion of the LexA repressor. The RecA filament can be formed by different mechanisms, but all of them require three enzymatic activities essential for the processing of DNA double-stranded ends. These are helicase, 5′–3′ exonuclease, and RecA loading onto single-stranded DNA (ssDNA). In some mutants, the SOS response can be expressed constitutively during the process of normal DNA metabolism. The RecA730 mutant protein is able to form the RecA filament without the help of RecBCD and RecFOR mediators since it better competes with the single-strand binding (SSB) protein for ssDNA. As a consequence, the recA730 mutants show high constitutive SOS expression. In the study described in this paper, we studied the genetic requirements for constitutive SOS expression in recA730 mutants. Using a β-galactosidase assay, we showed that the constitutive SOS response in recA730 mutants exhibits different requirements in different backgrounds. In a wild-type background, the constitutive SOS response is partially dependent on RecBCD function. In a recB1080 background (the recB1080 mutation retains only helicase), constitutive SOS expression is partially dependent on RecBCD helicase function and is strongly dependent on RecJ nuclease. Finally, in a recB-null background, the constitutive SOS expression of the recA730 mutant is dependent on the RecJ nuclease. Our results emphasize the importance of the 5′–3′ exonuclease for high constitutive SOS expression in recA730 mutants and show that RecBCD function can further enhance the excellent intrinsic abilities of the RecA730 protein in vivo.
INTRODUCTION
The RecA protein is a central component of the recombination machinery which is required for double-strand break (DSB) repair and for producing genetic variation during conjugation in bacteria and meiosis in eukaryotes. An additional role of the RecA protein is in the induction of an SOS response. In order to exhibit its biological functions, the RecA protein must be in the form of a RecA-single-stranded DNA (ssDNA) filament (RecA filament). The RecA filament is formed after processing of DNA damage, i.e., DSBs and single-stranded gaps (SSGs). In wild-type (wt) Escherichia coli strains, DSBs are processed into RecA filaments by the RecBCD pathway of recombination, whereas SSGs utilize the RecF recombination pathway (16, 17). DSBs can also be processed into RecA filaments by the RecF recombination pathway when the RecBCD enzyme is missing or is inactive, as is the case in a mutant recBC sbcBC(D) strain with multiple mutations (17). Three essential enzymatic activities are required for the processing of DSBs and subsequent RecA filament formation: helicase, 5′–3′ exonuclease, and RecA loading onto ssDNA. After interaction with a Chi site (GCTGGTGG), the RecBCD enzyme exhibits all of these activities, whereas within the RecF recombination machinery, the particular enzymatic activity is connected to a particular protein, i.e., helicase to RecQ, 5′–3′ exonuclease to RecJ, and RecA loading to RecFOR. There is a specific mutation in the recB nuclease center (i.e., recB1080) which partially affects the RecBCD function, preserving helicase but eliminating nuclease and RecA loading activities (2, 41, 45). In a recB1080 mutant, the components of the two recombination machineries (RecBCD and RecF) are interchangeable and the processing of DSBs is dependent on RecB1080CD (helicase), RecJ (5′–3′ exonuclease), and RecFOR (RecA loading) (1, 13). The main role of the RecA filament in recombination and DSB repair is in the homology search and strand exchange process between two DNA molecules (6, 16).
The RecA filament has a different role in the induction of the SOS response, where it functions as a coprotease in autocleaving the LexA repressor. The cleaved LexA repressor has no affinity for cis regulatory regions (SOS boxes) upstream of the SOS genes, and as a consequence, more than 50 genes enhance their expression during the SOS response (20, 21, 25). The products of these genes are involved in DNA repair, homologous recombination, inhibition of cell division, mutagenesis, and other aspects of DNA metabolism (25, 29, 43). The SOS response is important because natural populations of bacteria often experience various environmental conditions which cause an increase in DNA damage. In the laboratory, the SOS response is often induced after the treatment of cells with various DNA-damaging agents. Some of these agents are responsible for the introduction of DSBs (X and gamma rays, bleomycin, etc.), and SOS induction after treatment with these agents is dependent on a functional RecBCD enzyme (4). However, after treatment with UV light, which primarily introduces pyrimidine dimers and subsequently SSGs, SOS induction is dependent on RecF pathway recombination genes (12, 36, 42). In addition to damage from the action of external agents, DNA can be damaged spontaneously, i.e., during the process of normal DNA metabolism, when ssDNA occurs as an intermediate. Collapsed replication forks are formed when the replication machinery passes through ssDNA containing a break or a gap. They are the most frequent and most dangerous spontaneous lesions (28). Collapsed replication forks have double-strand ends and are (similarly to induced DSBs) natural substrates for the RecBCD enzyme. The processing of these lesions leads to formation of RecA filaments and possibly to a spontaneous SOS response. Such an SOS response is called a constitutive (cSOS) response or basal SOS response and is a subject of this study. In wt cells the cSOS response is negligible, but in some mutants it can be much higher. The recA730 mutant is an extreme example of a mutant which exhibits very high cSOS expression (8, 35, 44).
The mutant RecA730 (E38K) protein can be loaded onto ssDNA without the help of loading mediators: RecBCD or RecFOR proteins (7, 18). RecA loading by the RecBCD enzyme is well coordinated with the helicase and nuclease functions of the same enzyme and occurs immediately on nascent ssDNA. In contrast, RecA loading is not well coordinated with the helicase and nuclease in RecF and hybrid (RecB1080CD) recombination pathways since these functions are provided by different proteins. Under such conditions, single-strand binding (SSB) protein binds to ssDNA before the RecA protein since SSB has a higher affinity for ssDNA than RecA. Subsequently, RecA filament formation is achieved by the RecFOR system, which replaces SSB with RecA. Biochemically speaking, binding of RecA protein to ssDNA occurs in two steps (nucleation and filament extension), and the presence of SSB protein on ssDNA inhibits the nucleation step in RecA filament formation (32). The RecA730 mutant protein is able to achieve a nucleation step without the help of the RecA loading mediators RecFOR, since RecA730 competes with SSB protein for ssDNA better than wt RecA protein (7, 18, 19). This biochemical property of the mutant RecA730 protein is in agreement with the findings of genetic studies, i.e., the suppression of UV sensitivity of recFOR mutants by the recA730 allele (39, 40) and the fact that the recA730 mutant exhibits high cSOS expression (8, 35, 44). The recA730 (E38K) allele is also present in another constitutive SOS mutant, recA441, which consists of two point mutations: E38K and I298V. recA441 is a temperature-dependent allele which suppresses the UV sensitivity of recF mutants (37) at nonpermissive temperatures. The E38K (recA730) mutation is responsible for efficient competition with SSB protein (18, 19) and, consequently, constitutive SOS expression, whereas the I298V mutation is responsible for the temperature-dependent expression of the mutant phenotype.
It is estimated that under normal physiological conditions at any one time, about 15% of log-phase cells are involved in the process of homologous recombination (33), and this is in a good agreement with the observation that 15% of the log-phase wt cells have RecA filaments (28, 30). In contrast to this, only about 0.3% of wt cells express the SOS response (23), implying that the majority of RecA filaments formed in vivo are not connected with the expression of an SOS response. There are some differences in requirements between the RecA filaments involved in recombinational repair and those RecA filaments responsible for SOS induction. It has been demonstrated that the ATPase activity of RecA is essential for recombination but is not required for SOS induction after UV irradiation (10). Also, it has been proposed that the RecA filaments involved in SOS induction are longer (or have a different, i.e., extended, conformation) than the RecA filaments involved in recombination (10, 22).
Recently, the genetic requirements for cSOS expression in two recA mutants, i.e., recA4142 (F217Y) and recA730 mutants, were studied. It was shown that cSOS expression in a recA4142 mutant is dependent on recBCD, recFOR, recX, dinI, and xthA gene products (22). In contrast, it was shown that the cSOS response in a recA730 mutant is not dependent on any of these gene products (22). This conclusion was made by using a single mutation (in any of the above genes) in combination with the recA730 allele. In the study described in the present paper, combining multiple mutations in recombination genes together with recA730, we found that high cSOS expression in recA730 mutants has different requirements in different genetic backgrounds. Our genetic analysis was performed in three backgrounds (wt, recB1080, and recB null). We used the recB1080 allele since it switches recombination to a hybrid pathway. This provides an elegant way to dissect the biochemical activities required in vivo for the formation of a RecA filament which leads to cSOS expression. We found that in a wt background, the high cSOS expression of a recA730 mutant is partially dependent on RecBCD function. In a recB1080 background, the high cSOS expression of the recA730 mutant is partially dependent on the helicase activity of the RecB1080CD enzyme and is strongly dependent on the RecJ nuclease. Finally, the cSOS expression of a recA730 mutant in a recB-null background is dependent on the RecJ nuclease. These results emphasize the importance of the 5′–3′ exonuclease for high cSOS expression in recA730 mutants. In addition, they show that RecBCD function can moderately enhance the already excellent intrinsic abilities of the RecA730 enzyme in vivo.
MATERIALS AND METHODS
Bacterial strains and bacteriophages.
The E. coli strains used in this study are listed in Table 1. LMM1721 and LMM1934 were kindly provided by D. Zahradka from Ruđer Bošković Institute, Zagreb, Croatia. The P1 transduction was carried out as described by Miller (26). In some bacterial strains, recB1080 and recA730 mutations were additionally confirmed by sequencing. The recB21 mutation is a recB-null allele and was confirmed by PCR analysis.
Table 1.
E. coli K-12 strains used in this study
| Bacterial strain | Relevant genotype | Source or reference |
|---|---|---|
| V67 | recB21::IS186 argA21 hisG4 recF143 rpsL31 galK2 xyl-5 λ− F− | 31 |
| Bacterial strains related to AB1157 | ||
| AB1157 | F−thr-1 leuB6 Δ(gpt-proA)62 hisG4 thi-1 argE3 lacY1 galK2 ara-14 xyl-5 mtl-1 tsx-33 supE44 rpsL31 kdgK51 rfbD1 mgl-51 λ−rac negative | 3 |
| AM208 | AB1157 + recR256::Tn5 | R. G. Lloyd |
| N5170 | AB1157 + thr+ leu+ Δ(pro-lac) sfiA::Mud (Ap lac MuB::Tn9) | R. G. Lloyd |
| RIK174 | AB1157 + recBD1080A | 15 |
| IRB101 | AB1157 + recQ1803::Tn3 | 27 |
| IRB103 | AB1157 + recO1504::Tn5 | 27 |
| LMM1032 | AB1157 + recJ2052::Tn10 kan | D. Zahradka |
| LMM1721 | AB1157 + thyA748::Tn10 | D. Zahradka |
| LMM1934 | AB1157 + recA730 srl::Tn10 sfiA::Tn5 | D. Zahradka |
| WA576 | AB1157 + recF400::Tn5 | W. Wackernagel |
| IV252 | AB1157 + recF400::Tn5 | P1.WA576 × AB1157 |
| IV451 | AB1157 + recA730 srl::Tn10 | P1.LMM1934 × AB1157 |
| Bacterial strains related to MG1655 | ||
| MG1655 | F−rec+ (wt) | 3 |
| TRM452 | MG1655 + Δlac ΔattB::PBAD I-SceI | R. G. Lloyd |
| TRM387 | MG1655 + ΔargE::I-SceIcs::cat ΔattB::PBADI-SceI | 24 |
| IIB385 | MG1655 + Δlac ΔattB::PBAD I-SceI ΔargE::I-SceIcs::cat | P1.TRM387 × TRM452 |
| IIB386 | MG1655 + Δlac ΔattB::PBAD I-SceI ΔargE::I-SceIcs::cat sfiA::Mud (Ap lac MuB::Tn9) | P1.N5170 × IIB385 |
| IV462 | IIB386 + recA730 srl::Tn10 | P1.IV451 × IIB386 |
| IV468 | IIB386 + recA730 srl::Tn10 recO1504::Tn5 | P1.IRB103 × IV462 |
| IV469 | IIB386 + recA730 srl::Tn10 recJ2052::Tn10 kan | P1.LMM1032 × IV462 |
| IV471 | IIB386 + recA730 srl::Tn10 recQ1803::Tn3 | P1.IRB101 × IV462 |
| IV484 | IIB386 + thyA748::Tn10 | P1.LMM1721 × IIB386 |
| IV485 | IIB386 + recBD1080A | P1.RIK174 × IV484 |
| IV542 | IIB386 + recBD1080A recA730 srl::Tn10 | P1.IV451 × IV485 |
| IV543 | IIB386 + recBD1080A recO1504::Tn5 | P1.IRB103 × IV485 |
| IV544 | IIB386 + recBD1080A recJ2052::Tn10 kan | P1.LMM1032 × IV485 |
| IV545 | IIB386 + recBD1080A recQ1803::Tn3 | P1.IRB101 × IV485 |
| IV547 | IIB386 + recBD1080A recA730 srl::Tn10 recJ2052::Tn10 kan | P1.LMM1032 × IV542 |
| IV548 | IIB386 + recBD1080A recA730 srl::Tn10 recQ1803::Tn3 | P1.IRB101 × IV542 |
| IV570 | IIB386 + recB21::IS186 | P1.V67 × IV484 |
| IV571 | IIB386 + recB21::IS186 recA730 srl::Tn10 | P1.IV451 × IV570 |
| IV577 | IIB386 + recB21::IS186 recA730 srl::Tn10 recF400::Tn5 | P1.IV252 × IV571 |
| IV578 | IIB386 + recB21::IS186 recA730 srl::Tn10 recJ2052::Tn10 kan | P1.LMM1032 × IV571 |
| IV579 | IIB386 + recB21::IS186 recA730 srl::Tn10 recQ1803::Tn3 | P1.IRB101 × IV571 |
| IV585 | IIB386 + recF400::Tn5 | P1.IV252 × IIB386 |
| IV586 | IIB386 + recA730 srl::Tn10 recF400::Tn5 | P1.IV252 × IV462 |
| IV587 | IIB386 + recBD1080A recF400::Tn5 | P1.IV252 × IV485 |
| IV588 | IIB386 + recBD1080A recA730 srl::Tn10 recF400::Tn5 | P1.IV252 × IV542 |
| IV594 | IIB386 + recR256::Tn5 | P1.AM208 × IIB386 |
| IV595 | IIB386 + recA730 srl::Tn10 recR256::Tn5 | P1.AM208 × IV462 |
| IV596 | IIB386 + recBD1080A recR256::Tn5 | P1.AM208 × IV485 |
| IV597 | IIB386 + recBD1080A recA730 srl::Tn10 recR256::Tn5 | P1.AM208 × IV542 |
| IV598 | IIB386 + recB21::IS186 recA730 srl::Tn10 recR256::Tn5 | P1.AM208 × IV571 |
| IV599 | IIB386 + recB21::IS186 recA730 srl::Tn10 recO1504::Tn5 | P1.IRB103 × IV571 |
| IV600 | IIB386 + recBD1080A recA730 srl::Tn10 recO1504::Tn5 | P1.IRB103 × IV542 |
Medium and growth conditions.
Bacteria were grown in LB medium at 37°C with aeration to early log phase (optical density at 600 nm [OD600], ∼0.2) and then used for β-galactosidase assays.
Measurement of β-galactosidase activity.
All strains had an insertion of lacZ downstream of the regulatory region of sfiA, a gene which belongs to the SOS regulon. The levels of lacZ expression were assayed by determining β-galactosidase activity (Miller units), and this is proportional to the level of SOS response (26). After the strains reached early log phase (OD600, ∼0.2), at every 30 min during a 180-min incubation period (in a water bath at 37°C with aeration), aliquots (0.1 ml) of bacterial cells were taken for β-galactosidase activity assay. The β-galactosidase activity assay was performed in Eppendorf tubes containing 0.7 ml of Z buffer (60 mM Na2HPO4·2H2O, 40 mM NaH2PO4·H2O, 10 mM KCl, 1 mM MgSO4·7H2O), 0.001 ml of dithiothreitol (DTT), 0.02 ml of 0.1% sodium dodecyl sulfate (SDS), and 0.05 ml of chloroform. Eppendorf tubes were then placed at 28°C in an Eppendorf Thermomixer comfort, and after a 5-min incubation period at 28°C, ortho-nitrophenyl-β-d-galactopyranoside (ONPG; 0.2 ml) was added to start the reaction. When a yellow color appeared, the reaction was stopped by adding 0.5 ml of 1 M Na2CO3 and the optical density of the reaction mixture was measured at 420 nm on a NovaspecII visible spectrophotometer. β-Galactosidase activity (βgal; Miller units) was calculated with the equation (1,000 × OD420)/(V × tR × OD600), where V is the volume of culture used in the assay (0.1 ml), and tR is the reaction time (in minutes). The reaction time represents the time during which β-galactosidase catalyzes hydrolysis of ONPG (colorless solution) into ortho-nitrophenol (ONP; yellow solution) (26).
RESULTS
It was recently shown that cSOS expression in a recA4142 mutant is dependent on recBCD, recFOR, recX, dinI, and xthA gene products (22). In contrast, in a recA730 mutant, cSOS expression is not dependent on these gene products (22). This conclusion was obtained using a recA730 allele in combination with particular single mutations in the aforementioned genes and using an SOS reporter system (sfiAp-gfp), which is based on fluorescence in single cells. The finding that cSOS expression in a recA730 mutant does not depend on essential recombination genes was surprising, and we wanted to test this using a different approach. In our genetic analysis, we combined the recA730 allele with multiple mutations in recombination genes. Our particular approach was to use strains with a recB1080 mutation, which are characterized by use of the hybrid (RecB1080CD) pathway for RecA filament formation (13). This approach allowed us better insight into the biochemical functions required in vivo for the formation of a RecA filament. All of our strains contained sfiA-β-galactosidase gene fusions, and SOS expression was measured as β-galactosidase activity (Miller units).
Requirement for helicase function.
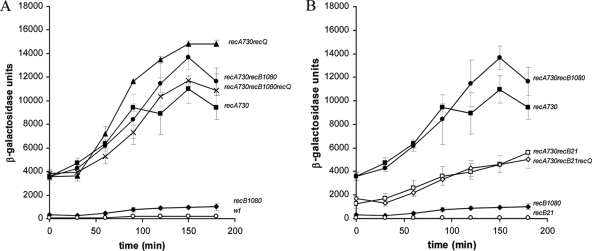
It is visible from Fig. 1 that there are three quantitative levels of cSOS expression. One is high cSOS expression, characterized by the recA730 mutant; the second is a moderate cSOS expression, characterized by the recB1080 mutant; and the third is a very low or negligible cSOS expression, which is a feature of wt cells and the recB-null mutants. The effect of inactivation of helicase function on high cSOS expression in recA730 mutants was first studied in the wt and recB1080 backgrounds. Since the wt RecBCD enzyme and mutant RecB1080CD enzyme have helicase activity, we expected that inactivation of RecQ (the main helicase of the RecF pathway) would not affect the level of cSOS expression. We found that both the recA730 single mutant and the recA730 recQ double mutant had high levels of cSOS expression (Fig. 2 A). Also, the recA730 recB1080 double mutant and the recA730 recB1080 recQ triple mutant had high levels of cSOS expression (Fig. 2A). These results indicate that the RecQ helicase is not required for cSOS expression by the recA730 mutant in the wt and recB1080 genetic backgrounds. In addition to this, we wanted to address a more important question, i.e., whether the inactivation of RecBCD helicase affects high cSOS expression in a recA730 mutant. To do this, we compared the cSOS expression in recA730 recB1080 and recA730 recB21 double mutants. The recB21 mutation is a null allele which eliminates all three activities (helicase, nuclease, and RecA loading) of the RecBCD enzyme, whereas the recB1080 mutation preserves helicase activity but eliminates nuclease and RecA loading activities. Figure 2B shows that the recA730 recB21 double mutant had a considerably lower level of cSOS expression (maximal value, close to 6,000 units) relative to the recA730 recB1080 double mutant (maximal value, almost 14,000 units) and the recA730 single mutant (maximal value, ∼11,000 units). This means that inactivation of the RecBCD helicase has a substantial effect on cSOS expression in a recA730 mutant. However, the level of cSOS expression in the recA730 recB21 double mutant (maximal value, ∼6,000 units) is still high. The conclusion from this result is that the RecBCD helicase is partially required for high cSOS expression in a recA730 mutant. Another question is whether the mutant RecA730 protein requires another helicase function (RecQ) in vivo to achieve this (still) high level of cSOS expression found in a recA730 recB21 double mutant. To test this, we compared cSOS expression in the recA730 recB21 double mutant and the recA730 recB21 recQ triple mutant. We found that the recA730 recB21 recQ triple mutant had the same level of cSOS expression as the recA730 recB21 double mutant (Fig. 2B). This suggests that the RecQ helicase function is not required for cSOS expression by a recA730 mutant in the recB21 background. The main conclusions from all these results are that the RecBCD helicase function is partially required for very high cSOS expression of a recA730 mutant (in wt and recB1080 backgrounds) and that the missing RecBCD helicase function (in the recB21 background) can somehow be rescued by the function of the mutant RecA730 protein.
Fig. 1.
Levels of constitutive SOS expression, shown as β-galactosidase units, of recA730 (bacterial strain IV462) (■), recB1080 (bacterial strain IV485) (♦), wt (bacterial strain IIB386) (×), and recB21 (bacterial strain IV570) (○) strains. The values are means of at least three independent experiments.
Fig. 2.
Effects of mutations in helicase functions on high cSOS expression in recA730 mutants. (A) Levels of SOS expression, shown as β-galactosidase units, of recA730 (bacterial strain IV462) (■), recA730 recQ (bacterial strain IV471) (▴), recA730 recB1080 (bacterial strain IV542) (•), recA730 recB1080 recQ (bacterial strain IV548) (×), recB1080 (bacterial strain IV485) (♦), and wt (bacterial strain IIB386) (○) strains; (B) levels of SOS expression, shown as β-galactosidase units, of recA730 (bacterial strain IV462) (■), recA730 recB1080 (bacterial strain IV542) (•), recA730 recB21 (bacterial strain IV571) (□), recA730 recB21 recQ (bacterial strain IV579) (⋄), recB1080 (bacterial strain IV485) (♦), and recB21 (bacterial strain IV570) (○) strains. The values are means of at least three independent experiments.
Requirement for nuclease function.
In order to determine whether 5′–3′ exonuclease activity is required for cSOS expression in the recA730 mutants, we studied cSOS expression in wt, recB1080, and recB21 backgrounds. It was shown that RecJ nuclease is crucial for recombination (13), SOS induction after introduction of DSBs (38), and cSOS expression in a recB1080 mutant (14). Figure 3A shows that the recA730 recJ double mutant had high cSOS expression, even higher than that of the recA730 single mutant. This means that in a wt background, the requirement for 5′–3′ exonuclease is not dependent on a functional recJ gene product. This activity is most likely provided by the wt RecBCD enzyme, which has its own 5′–3′ exonuclease activity. Figure 3A also compares cSOS expression in the recA730 recB1080 double mutant and in the recA730 recB1080 recJ triple mutant. It is clear that cSOS expression in the recA730 recB1080 recJ triple mutant was almost abolished (it was below the level of moderate cSOS expression). From this result, it can be concluded that the RecJ nuclease is essential for high cSOS expression in a recA730 recB1080 double mutant. Finally, we tested the effect of inactivation of the RecJ nuclease in a recB21 background. cSOS expression in the recA730 recB21 recJ triple mutant was much lower than that in the recA730 recB21 double mutant (Fig. 3B). However, the effect of recJ mutation in a recB21 background was not as strong as in the recB1080 background since the recA730 recB21 recJ triple mutant showed a moderate level of cSOS expression, similar to that of the recB1080 mutant. The general conclusion based on Fig. 3 is that 5′–3′ exonuclease function is essential for high cSOS expression in recA730 mutants.
Fig. 3.
Effects of mutation in the RecJ nuclease on cSOS expression in recA730 mutants. (A) Levels of SOS expression, shown as β-galactosidase units, of recA730 (bacterial strain IV462) (■), recA730 recJ (bacterial strain IV469) (▴), recA730 recB1080 (bacterial strain IV542) (•), recA730 recB1080 recJ (bacterial strain IV547) (×), and recB1080 (bacterial strain IV485) (♦) strains; (B) levels of SOS expression, shown as β-galactosidase units, of recA730 (bacterial strain IV462) (■), recA730 recB21 (bacterial strain IV571) (□), recA730 recB21 recJ (bacterial strain IV578) (⋄), recB1080 (bacterial strain IV485) (♦), and recB21 (bacterial strain IV570) (○) strains. The values are means of at least three independent experiments.
Requirement for RecA loading function.
We studied the effect of inactivation of RecA loading function on cSOS expression in recA730 mutants. When only one RecA loading function was inactivated, as was the case in recA730 recB1080 and recA730 recO double mutants, there was no decrease in the level of cSOS expression compared with that in the recA730 single mutant (Fig. 4 A). The same result was obtained when both RecA loading mechanisms (RecBCD and RecFOR) were inactivated. This is visible from the comparison of cSOS expression in the recA730 recB1080 recO triple mutant and the recA730 single mutant (Fig. 4A). The same results were obtained by using recF and recR mutations instead of the recO mutation, i.e., recA730 recF and recA730 recR double mutants, as well as recA730 recB1080 recF and recA730 recB1080 recR triple mutants (data not shown). These results show that RecA loading by mediator proteins is not important for high cSOS expression in wt and recB1080 backgrounds. We also tested the requirements for RecA loading function in a recB21 (recB-null) background, where the level of cSOS expression is lower than that in wt and recB1080 backgrounds. This level of cSOS expression can also be characterized as high level since it is considerably higher than the moderate level in a recB1080 mutant. cSOS expression in recA730 recB21 recFOR triple mutants was comparable to cSOS expression in a recA730 recB21 double mutant (Fig. 4B). The conclusion from these experiments is similar, i.e., that cSOS expression by a recA730 mutant in a recB21 background does not require mediator proteins for RecA loading in vivo.
Fig. 4.
Effects of mutations in RecA loading functions on cSOS expression in recA730 mutants. (A) Levels of SOS expression, shown as β-galactosidase units, of recA730 (bacterial strain IV462) (■), recA730 recO (bacterial strain IV468) (▴), recA730 recB1080 (bacterial strain IV542) (•), recA730 recB1080 recO (bacterial strain IV600) (×), recB1080 (bacterial strain IV485) (♦), and wt (bacterial strain IIB386) (○) strains; (B) levels of SOS, expression, shown as β-galactosidase units, of recA730 (bacterial strain IV462) (■), recA730 recB21 (bacterial strain IV571) (□), recA730 recB21 recF (bacterial strain IV577) (×), recA730 recB21 recO (bacterial strain IV599) (•), recA730 recB21 recR (bacterial strain IV598) (⋄), recB1080 (bacterial strain IV485) (♦), and recB21 (bacterial strain IV570) (○) strains. The values are means of at least three independent experiments.
DISCUSSION
We studied the genetic requirements for high cSOS expression in recA730 mutants in three different genetic backgrounds. Using multiple mutations in recombination genes together with the recA730 allele, we found that some of the essential recombination genes are required for cSOS expression in the recA730 mutant. Our particular approach was to use the recB1080 genetic background in order to dissect biochemical functions essential for cSOS expression in the recA730 mutant. The requirements in a recB1080 background were compared with the requirements in wt and recB-null mutant backgrounds, and the results were presented from the aspect of the appropriate biochemical functions of particular recombination gene products in different situations.
We found that RecBCD enzyme function is partially required for high cSOS expression in wt and recB1080 backgrounds (Fig. 2B; compare the cSOS expression in the recA730 recB21 double mutant with that in the recA730 single mutant and the cSOS expression in the recA730 recB21 double mutant with that in the recA730 recB1080 double mutant). It is obvious for the recB1080 background that this specific function is the RecB1080CD-dependent helicase. The reason is in the fact that recB1080 mutation retains the helicase but eliminates nuclease and RecA loading, whereas recB21 mutation abolishes all three activities of the RecBCD enzyme. Therefore, the difference between them is that the recB1080 mutant has RecBCD-dependent helicase function. We propose that helicase and 5′–3′ exonuclease are the critical RecBCD functions required for the higher level of cSOS expression by the recA730 mutant in a wt background compared with the recB21 background (we will argue this later). This result is not in agreement with a previous report (22), where no difference is found for cSOS expression between the recA730 mutant and recA730 recB double mutant. The explanation for this discrepancy is in the type of media used for bacterial growth. In the previous study (22) the authors used minimal medium, whereas our strains were grown in rich medium. We also tested cSOS expression in minimal medium and found that the recA730 mutant and recA730 recB21 double mutant have similar levels of cSOS expression (data not shown), supporting the above explanation. The cSOS expression in a recA730 recB21 double mutant does not require RecBCD or RecQ helicase function since the introduction of a mutation in the RecQ helicase does not decrease cSOS expression (Fig. 2B; compare the cSOS expression in the recA730 recB21 recQ triple mutant with that in the recA730 recB21 double mutant). This means that another helicase (excluding RecBCD and RecQ) could be used, or alternatively, the RecA730 mutant protein can overcome the missing helicases, most probably by its own weak helicase/translocase function. The wt bacterial RecA protein possesses an apparent motor function (5). It is possible that mutant RecA730 protein has a stronger motor function which, coupled with ATP hydrolysis, could unwind a limited stretch of double-stranded DNA, allowing its self-loading onto ssDNA. This is in agreement with biochemical data showing that the RecA730 protein can produce a joint molecule without the presence of helicase in recombination reactions in vitro (11). The final result on the requirement of helicase activity is that the recQ mutation has no effect on cSOS expression in wt and recB1080 backgrounds (Fig. 2A), which is in accordance with the presence of the RecBCD helicase function in these backgrounds.
Our most straightforward result is the indispensability of 5′–3′ exonuclease function for high cSOS expression in recA730 mutants. In a wt background, the 5′–3′ exonuclease is provided by the RecBCD enzyme (Fig. 3A), whereas in recB1080 and recB21 backgrounds, it is dependent on the RecJ nuclease (Fig. 3A and B, respectively). On the basis of this, one can conclude that the RecBCD enzyme helicase function is also required for high cSOS expression of the recA730 mutant in a wt background. The reason is obvious since the RecBCD enzyme cannot exhibit nuclease activity without the ability to unwind DNA (16, 34). The importance of 5′–3′ exonuclease for high cSOS expression could be explained by its efficient coordination (or interaction) with the weak RecA730 helicase. It is known that the ability of the recA730 mutation to suppress recombination deficiency in recFOR mutants depends on a functional RecJ nuclease (37, 39, 40). The ability to produce a joint molecule in recombination reactions in vitro also requires the RecJ nuclease (11). In addition to this, it is proposed that the RecA filament required for the SOS response has different properties compared to the RecA filament involved in recombination. One specific feature of the RecA filament required for SOS expression is that it is longer or that it has an extended conformation (10, 22). The efficient 5′–3′ exonuclease activity in recA730 mutants could be connected with the efficient production of long stretches of ssDNA which are subsequently coated by RecA protein. Such longer RecA filaments could be appropriate for high cSOS expression in recA730 mutants.
We also found that mediators of RecA loading are not required for high cSOS expression in a recA730 mutant. There are two alternative mechanisms of RecA loading within the cell, which are dependent on RecBCD and RecFOR proteins, respectively (16). RecBCD-dependent RecA loading occurs normally in wt cells and is essential for recombination and DNA repair, as well as for SOS induction after gamma irradiation or after introduction of DSBs by I-SceI endonuclease. However, RecFOR-dependent RecA filament formation occurs during the processing of double-strand ends in a recB1080 mutant (13). Neither of them is required for high cSOS expression in recA730 mutants (Fig. 4A; compare the cSOS expression in recA730 recB1080 and recA730 recO double mutants with that in the recA730 single mutant and the expression in the recA730 recB1080 recO triple mutant with that in the recA730 single mutant). Also, the RecFOR mediator proteins are not required for cSOS expression by a recA730 mutant in a recB21 background (Fig. 4B; compare the cSOS expression of recA730 recB21 double mutant with that of the recA730 recB21 recFOR triple mutant). These findings are in agreement with the biochemical property of mutant RecA730 protein, which is able to achieve the nucleation step of RecA filament formation without the help of RecFOR mediators (7, 18). This is a consequence of its efficient competition with SSB protein for ssDNA, a property not shared with wt RecA protein (19).
In all experiments, cSOS expression was increasing over time, implying that it is phase dependent. The common feature of an induced SOS response is that it also increases over time (if DNA lesions are not repaired). This is correlated with the increase in the amount of RecA protein, which self-regulates its own transcription from the earliest stages of the SOS response (25). When the SOS response is highly induced, the level of RecA protein is high enough to initiate transcription from the latest SOS gene promoters (which contain two SOS boxes). A similar reasoning could be applied to cSOS expression in recA730 mutants, where the RecA730 superprotein efficiently initiates SOS expression after processing of a small amount of spontaneously formed DNA lesions. This could be just a partial explanation for the increase of cSOS expression in the recA730 mutants. The additional explanation could be connected with the changes in environmental conditions during bacterial growth (highly crowded bacterial population, the presence of excreted material, hypoxia, and the limited food source). The capacity for recombinational repair in stationary-phase cells is lower than that in exponential-phase cells, at least due to the lower chromosomal copy number per stationary-phase cell. If DNA lesions are not repaired, some of the existing RecA730 filaments could be responsible for higher cSOS expression. This higher cSOS expression in stationary phase could also be independent of SOS regulation, as is the case in the expression of colicin E1, the product of the cea gene (one of the latest SOS genes with two SOS boxes). It was shown that spontaneous production of colicin E1 is highly increased in stationary phase due to depletion of nutrients from the medium (9).
According to our results, we propose a model for the formation of RecA filaments which are responsible for high cSOS expression in recA730 mutants (Fig. 5). This model considers three essential enzymatic activities (helicase, 5′–3′ exonuclease, and RecA loading) and connects them to the appropriate gene functions in wt, recB1080, and recB21 genetic backgrounds. In a wt background, helicase and 5′–3′ exonuclease functions are provided by the RecBCD enzyme. The RecA loading function can be provided either by RecA730 itself (autoloading) or by the RecBCD enzyme (this possibility still exists, since RecBCD already provides helicase and nuclease functions and has an unaffected RecA loading function). From our results, it is clear that both possibilities for RecA loading could be equally efficient in producing cSOS expression. In a recB1080 background, the helicase function is provided by the RecB1080CD enzyme, the 5′–3′ exonuclease function is provided by the RecJ nuclease, and the RecA loading function is provided by RecA730 itself (autoloading). Finally, in a recB21 background, another helicase (excluding RecQ) or a weak helicase/translocase function of the RecA730 protein can be used instead of RecBCD helicase. This other helicase or weak helicase/translocase of mutant RecA730 protein is well coordinated or enhanced by the action of efficient RecJ nuclease, which provides a 5′–3′ exonuclease function. The RecA loading function is again the result of the RecA730 self-loading. On the basis of our results that cSOS expression is higher in the presence of a wt or partially defective RecBCD enzyme, one can conclude that RecBCD enzyme functions can further improve the already excellent intrinsic performance of mutant RecA730 protein in vivo.
Fig. 5.
Model of RecA filament formation required for cSOS expression by a recA730 mutant in wt, recB1080, and recB21 genetic backgrounds. For details, see the text.
In conclusion, we have shown that high cSOS expression of recA730 mutants requires different gene functions in different genetic backgrounds. The RecJ nuclease is critical for high cSOS expression in recB1080 and recB-null backgrounds, whereas the RecBCD functions are required for the maximal level of cSOS expression in recA730 mutants.
ACKNOWLEDGMENTS
We are grateful to Mirjana Filipović and Mirela Kosinjski for technical assistance.
This work was supported by the Croatian Ministry of Science (grant 098-0982913-2867).
Footnotes
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Amundsen S. K., Smith G. R. 2003. Interchangeable parts of the Escherichia coli recombination machinery. Cell 112:741–744 [DOI] [PubMed] [Google Scholar]
- 2. Anderson D. G., Churchill J. J., Kowalczykowski S. C. 1999. A single mutation recBD1080A eliminates RecA protein loading but not chi recognition by RecBCD enzyme. J. Biol. Chem. 274:27139–27144 [DOI] [PubMed] [Google Scholar]
- 3. Bachmann B. J. 1972. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol. Rev. 36:525–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaudhury A. M., Smith G. R. 1985. Role of Escherichia coli RecBC enzyme in SOS induction. Mol. Gen. Genet. 201:525–528 [DOI] [PubMed] [Google Scholar]
- 5. Cox M. M. 2003. The bacterial RecA protein as a motor protein. Annu. Rev. Microbiol. 57:551–577 [DOI] [PubMed] [Google Scholar]
- 6. Dillingham M. S., Kowalczykowski S. C. 2008. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol. Mol. Biol. Rev. 72:642–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eggler A. L., Lusetti S. L., Cox M. M. 2003. The C terminus of the Escherichia coli RecA protein modulates the DNA binding competition with single-stranded DNA binding protein. J. Biol. Chem. 278:16389–16396 [DOI] [PubMed] [Google Scholar]
- 8. Ennis D. G., Levine A. S., Koch W. H., Woodgate R. 1995. Analysis of recA mutants with altered SOS functions. Mutat. Res. 336:39–48 [DOI] [PubMed] [Google Scholar]
- 9. Eraso J. M., Chidambaram M., Weinstock G. M. 1996. Increased production of colicin E1 in stationary phase. J. Bacteriol. 178:1928–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gruenig M. C., et al. 2008. RecA-mediated SOS induction requires an extended filament conformation but no ATP hydrolysis. Mol. Microbiol. 69:1165–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Handa N., Morimatsu K., Lovett S. T., Kowalczykowski S. C. 2009. Reconstitution of initial steps of dsDNA break repair by the RecF pathway of E. coli. Genes Dev. 23:1234–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hegde S., Sandler J. S., Clark A. J., Madiraju M. V. 1995. recO and recR mutations delay induction of the SOS response in Escherichia coli. Mol. Gen. Genet. 246:254–258 [DOI] [PubMed] [Google Scholar]
- 13. Ivančić-Baće I., et al. 2003. RecFOR function is required for DNA repair and recombination in a RecA loading-deficient recB mutant of Escherichia coli. Genetics 163:485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ivančić-Baće I., Vlašić I., Salaj-Šmic E., Brčić-Kostić K. 2006. Genetic evidence for the requirement of RecA loading activity in SOS induction after UV irradiation in Escherichia coli. J. Bacteriol. 188:5024–5032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jockovich M. E., Myers R. S. 2001. Nuclease activity is essential for RecBCD recombination in Escherichia coli. Mol. Microbiol. 41:949–962 [DOI] [PubMed] [Google Scholar]
- 16. Kowalczykowski S. C. 2000. Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci. 25:156–165 [DOI] [PubMed] [Google Scholar]
- 17. Kuzminov A. 1999. Recombinational repair of DNA damage in Escherichia coli and bacteriophage λ. Microbiol. Mol. Biol. Rev. 63:751–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lavery P. E., Kowalczykowski S. C. 1990. Properties of recA441 protein-catalyzed DNA strand exchange can be attributed to an enhanced ability to compete with SSB protein. J. Biol. Chem. 265:4004–4010 [PubMed] [Google Scholar]
- 19. Lavery P. E., Kowalczykowski S. C. 1992. Biochemical basis of the constitutive repressor cleavage activity of RecA730 protein. J. Biol. Chem. 267:20648–20658 [PubMed] [Google Scholar]
- 20. Lewis L. K., Harlow G. R., Greg-Jolly L. A., Mount D. W. 1994. Identification of high affinity binding sites for LexA which define new DNA damage-inducible genes in Escherichia coli. J. Mol. Biol. 241:507–523 [DOI] [PubMed] [Google Scholar]
- 21. Little J. W., Mount D. W. 1982. The SOS regulatory system of Escherichia coli. Cell 29:11–22 [DOI] [PubMed] [Google Scholar]
- 22. Long J. E., Renzette N., Centore R. C., Sandler S. J. 2008. Differential requirements of two recA mutants for constitutive SOS expression in Escherichia coli K-12. PLoS One 3:e4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCool J. D., et al. 2004. Measurement of SOS expression in individual Escherichia coli K-12 cells using fluorescence microscopy. Mol. Microbiol. 53:1343–1357 [DOI] [PubMed] [Google Scholar]
- 24. Meddows T. R., Savory A. P., Lloyd R. G. 2004. RecG helicase promotes DNA double-strand break repair. Mol. Microbiol. 52:119–132 [DOI] [PubMed] [Google Scholar]
- 25. Michel B. 2005. After 30 years of study, the bacterial SOS response still surprises us. PLoS Biol. 3:e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller J. H. 1992. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 27. Paškvan I., et al. 2001. The genetic dependence of RecBCD-Gam mediated double strand end repair in Escherichia coli. FEMS Microbiol. Lett. 205:299–303 [DOI] [PubMed] [Google Scholar]
- 28. Pennington J. M., Rosenberg S. M. 2007. Spontaneous DNA breakage in single living Escherichia coli cells. Nat. Genet. 39:797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Radman M. 1975. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 5A:355–367 [DOI] [PubMed] [Google Scholar]
- 30. Renzette N., et al. 2005. Localization of RecA in Escherichia coli K-12 using RecA-GFP. Mol. Microbiol. 57:1074–1085 [DOI] [PubMed] [Google Scholar]
- 31. Schultz D. W., Taylor A. F., Smith G. R. 1983. Escherichia coli RecBC pseudorevertants lacking chi recombinational hotspot activity. J. Bacteriol. 155:664–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shereda R. D., Kozlov A. G., Lohman T. M., Cox M. M., Keck J. L. 2008. SSB as an organizer/mobilizer of genome maintenance complexes. Crit. Rev. Biochem. Mol. Biol. 43:289–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steiner W. W., Kuempel P. L. 1998. Sister chromatid exchange frequencies in Escherichia coli analyzed by recombination at the dif resolvase site. J. Bacteriol. 180:6269–6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Telander Muskavitch K. M., Linn S. 1982. A unified mechanism for the nuclease and unwinding activities of the RecBC enzyme of Escherichia coli. J. Biol. Chem. 257:2641–2648 [PubMed] [Google Scholar]
- 35. Tessman E. S., Peterson P. 1985. Plaque color method for rapid isolation of novel recA mutants of Escherichia coli K-12: new classes of protease-constitutive recA mutants. J. Bacteriol. 163:677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thoms B., Wackernagel W. 1987. Regulatory role of recF in the SOS response of Escherichia coli: impaired induction of SOS genes by UV irradiation and nalidixic acid in a recF mutant. J. Bacteriol. 169:1731–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thoms B., Wackernagel W. 1988. Suppression of the UV-sensitive phenotype of Escherichia coli recF mutants by recA (Srf) and recA (Tif) mutations requires recJ+. J. Bacteriol. 170:3675–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vlašić I., Ivančić-Baće I., Imešek M., Mihaljević B., Brčić-Kostić K. 2008. RecJ nuclease is required for SOS induction after introduction of a double-strand break in a RecA loading deficient recB mutant of Escherichia coli. Biochimie 90:1347–1355 [DOI] [PubMed] [Google Scholar]
- 39. Vlašić I., Šimatović A., Brčić-Kostić K. 2011. recA730-dependent suppression of recombination deficiency in RecA loading mutants of Escherichia coli. Res. Microbiol. 162:262–269 [DOI] [PubMed] [Google Scholar]
- 40. Wang T. C. V., Chang H. Y., Hung J. L. 1993. Cosuppression of recF, recR and recO mutations by mutant recA alleles in Escherichia coli cells. Mutat. Res. 294:157–166 [DOI] [PubMed] [Google Scholar]
- 41. Wang J., Chen R., Julin D. A. 2000. A single nuclease active site of the Escherichia coli RecBCD enzyme catalyzes single-stranded DNA degradation in both directions. J. Biol. Chem. 275:507–513 [DOI] [PubMed] [Google Scholar]
- 42. Whitby M. C., Lloyd R. G. 1995. Altered SOS induction associated with mutations in recF, recO and recR. Mol. Gen. Genet. 246:174–179 [DOI] [PubMed] [Google Scholar]
- 43. Witkin E. M. 1976. UV mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol. Rev. 40:867–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Witkin E. M., McCall J. O., Volkert M. R., Werdmundsen I. E. 1982. Constitutive expression of SOS functions and modulation of mutagenesis resulting from resolution of genetic instability at or near the recA locus of Escherichia coli. Mol. Gen. Genet. 185:43–50 [DOI] [PubMed] [Google Scholar]
- 45. Yu M., Souaya J., Julin D. A. 1998. Identification of the nuclease active site in the multifunctional RecBCD enzyme by creation of a chimeric enzyme. J. Mol. Biol. 283:797–808 [DOI] [PubMed] [Google Scholar]