Abstract
Rates of commitment to germinate and germination of Bacillus subtilis spores with mixtures of low concentrations of germinants acting on different germinant receptors (GRs) were much higher than the sums of the rates of commitment and germination with individual germinants alone. This synergism with mixtures of nutrient germinants was not seen with spores lacking GRs responsible for recognizing one or several components of the germinant mixtures and was not eliminated by either a gerD mutation or overexpression of one of the GRs involved in this synergism. This synergism was also not seen between the germinant l-valine, which acts via a GR, and the germinant dodecylamine, which does not act via any GR. These results indicate that spores not only integrate but can also amplify signals from multiple germinants and multiple GRs in determining rates of commitment and overall spore germination. This amplification can be explained by a simple mechanism in which a single signal integrator triggers germination above an accumulation threshold. Direct cooperative action between GRs may further add to the synergism seen in germination triggered by multiple GRs. Further experiments and modeling are required to determine the relative contributions of these different mechanisms.
INTRODUCTION
Spores of Bacillus species are metabolically dormant and can remain so for long periods (24). However, such spores can come back to life rapidly when exposed to appropriate nutrient germinants (23, 24). These nutrient germinants are recognized by germinant receptors (GRs) located in the spore's inner membrane, with this recognition somehow triggering subsequent germination events. In the best-studied spore former, Bacillus subtilis, there are three functional GRs, GerA, GerB, and GerK, each of which is composed of three subunits, A, B, and C. The GerA GR responds to l-alanine or l-valine alone, while the GerB and GerK GRs together respond to a mixture of l-asparagine, d-glucose, d-fructose, and K+ (AGFK), although neither the GerB nor the GerK GR alone triggers spore germination in response to any of the individual AGFK components (23). GerA GR function is also stimulated by d-glucose binding to the GerK GR, although d-glucose alone does not trigger B. subtilis spore germination (2).
The mechanism whereby individual GRs cooperate to allow or accelerate germination with germinant mixtures is not known. However, it has been suggested that either different individual GRs form complexes in the spore's inner membrane or there is some mechanism that integrates signals from different individual GRs or GR complexes and this integration determines the ultimate rate of germination (2, 8). One likely example of integration of signals from different GRs would be in spore germination with mixtures of germinants, for example, l-valine and AGFK. In particular, one might see synergism between such germinant mixtures in overall rates of spore germination (10, 26). In the current work, we have examined rates of both commitment to germinate and germination itself of B. subtilis spores with a range of concentrations of mixtures of nutrient germinants that alone can trigger germination via GRs and have compared these results with those for spores lacking various GRs or the GerD protein. We have also examined the ability of the germinant dodecylamine, which does not act via the GRs (21), to exhibit synergism with a germinant that does act via a GR. The results indicate that there is significant synergism between germinants that act via different GRs, especially at low concentrations of multiple germinants, in determining rates of both commitment of spores to germinate, as well as spore germination itself. However, dodecylamine did not exhibit synergism in germination with a germinant that acted via a GR.
MATERIALS AND METHODS
The strains used in this work were isogenic derivatives of a laboratory strain of B. subtilis 168 (PS832). They were PS533 (wild-type), carrying plasmid pUB110 encoding resistance to kanamycin (Kmr; 10 μg/ml) (22); FB87 (ΔgerB ΔgerK), lacking genes for the GerB and GerK GRs (16); FB20 (ΔgerA), lacking the genes for the GerA GR (17); FB10 (gerB*), with a mutation in the gerB operon, termed gerB*, that allows the GerB* GR to trigger spore germination in response to l-asparagine alone (16); FB62 (ΔgerD), lacking the gerD gene essential for normal GR-dependent spore germination (20); PS3501 (gerB* PsspD::gerA), carrying the gerB* allele and with the gerA operon under the control of the strong forespore-specific sspD promoter, giving elevated GerA levels in spores (6); PS3665 (gerB* ΔgerA ΔgerK), carrying the gerB* allele and lacking genes encoding the GerA and GerK GRs (2); and PS3929 (gerB* ΔgerD), carrying the gerB* allele and lacking GerD. Strain PS3929 was constructed by the transformation of strain FB10 with chromosomal DNA from strain FB62 and selection for the spectinomycin resistance cassette that has replaced most of the gerD coding sequence in strain FB62 (20). Spores of these strains were prepared at 37°C on 2× SG medium plates without antibiotics, and spores were harvested, cleaned and stored as described previously (15, 17, 18). Spores in which synergism was to be compared were prepared and cleaned together, and all of the spores used were free (≥98%) of growing or sporulating cells, germinated spores, and cell debris as determined by phase-contrast microscopy.
Prior to germination experiments, spores at an optical density at 600 nm (OD600) of 10 in water were heat activated for 30 min at 75°C and cooled on ice for ≤1 h before use. Unless otherwise noted, spores were routinely germinated at an OD600 of 0.5 at 37°C in a 96-well plate in 200 μl of 25 mM HEPES buffer (pH 7.4) with 50 μM terbium chloride as described previously (27) plus germinants as noted in individual experiments. Spore germination was measured as the release of the spore's large depot (∼10% of spore dry weight) of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) over a period of 120 min. In this time, ≥90% of the spores of the strains incubated with maximum levels of germinants that act on single GRs had germinated, as determined by the examination of ≥100 spores in a phase-contrast microscope (data not shown). DPA release was measured in a fluorescence plate reader by the formation of the highly fluorescent terbium-DPA complex that was quantified in relative fluorescence units (RFU) as described previously (27). Maximum rates of DPA release in arbitrary units were calculated from linear portions of DPA release curves as ΔRFU/min but are given in the figures as arbitrary units. The scales for rates of DPA release are identical in all of the figures, and all of the germination experiments were repeated at least twice with similar results.
Germination incubations in which spores' commitment to germinate was measured were as described above, and commitment was halted by acetic acid addition to germination incubations to lower the pH to ∼3.6 (27). Commitment is halted at this low pH, but committed spores still undergo germination events, in particular, DPA release, relatively normally, and DPA release was measured as described above. Percent commitment was calculated as the sum of spores that had germinated at the time of acetic acid addition plus those spores that released DPA following acetic acid addition divided by all of the spores that could germinate in 2 h times 100 (27).
To obtain a quantitative measure of synergy, we define the degree of synergy, ds, between two germinants as the ratio of the observed response to a germinant combination (g12) to the sum of the observed responses to single germinants (g1 + g2) as follows: ds = g12/(g1 + g2). The response, g, could be the rate of DPA release or the level of DPA release (see the supplemental material). The response is synergistic when ds is >1, additive when ds equals 1, and subadditive when ds is <1.
RESULTS
Synergism in spore germination between the GerA and GerB or GerB* GRs.
Wild-type B. subtilis spores germinate either with l-valine via the GerA GR or with AGFK through cooperative action of the GerB and GerK GRs (24) (Fig. 1A and B). Germination of these spores, as measured by rates of DPA release with mixtures of various l-valine or AGFK concentrations, was faster than with either l-valine or AGFK alone, which was not surprising. However, it was notable that rates of spore germination with valine-AGFK mixtures were significantly faster than the sums of the germination rates obtained with the same l-valine or AGFK concentrations individually (Fig. 1A and B). This synergism between l-valine and AGFK was particularly notable at low germinant concentrations, but the degree of synergy drops to below 1 at high germinant concentrations (Fig. 1C). Note that when AGFK was used as a germinant with wild-type spores, there was a constant amount of GFK present and only l-asparagine concentrations were varied. This was done to eliminate effects of alterations of d-glucose concentrations, since d-glucose can stimulate l-valine germination via the GerK GR and this stimulation is maximal at 10 mM glucose (2).
Fig. 1.
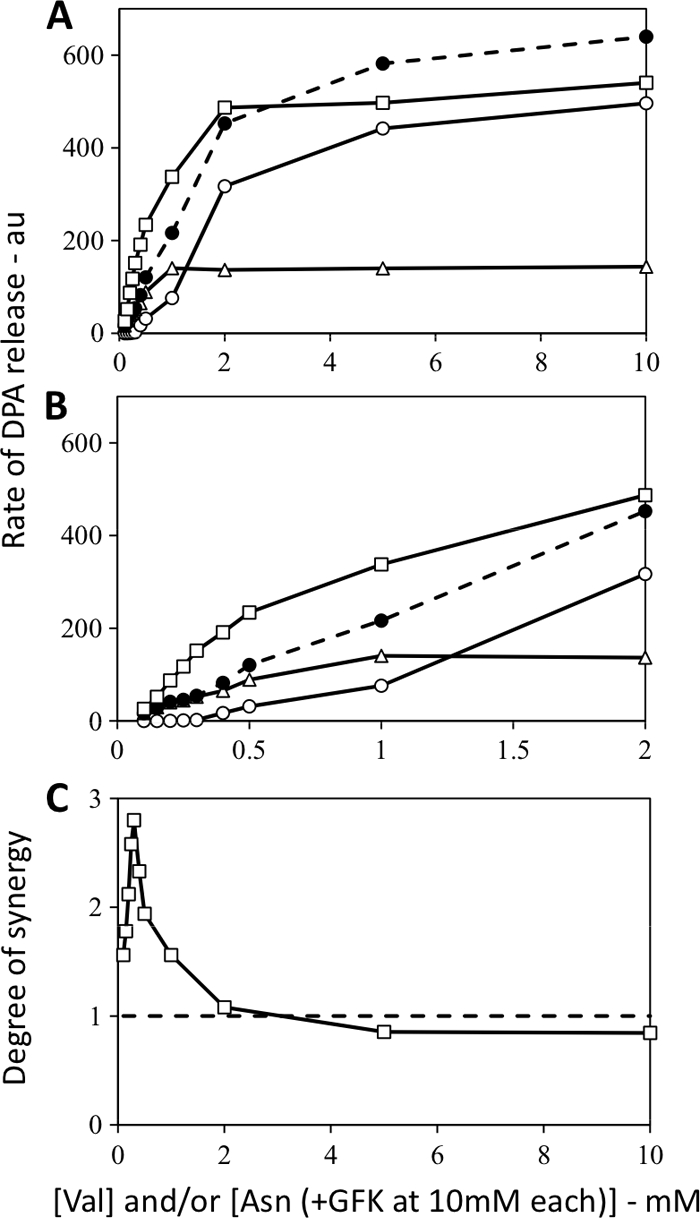
(A to C) Rates of DPA release from wild-type B. subtilis spores germinating with l-valine and/or AGFK and degree of synergy when both germinants were used. Spores of B. subtilis (wild-type) strain PS533 were germinated with various concentrations of l-valine and/or l-asparagine, with the GFK components each at 10 mM in germinations with AGFK (note the different scales on the x axes in panels A and B), and rates of DPA release were determined and are given in arbitrary units (au) as described in Materials and Methods. Symbols in panels A and B: ○, l-valine germination; ▵, AGFK germination; •, sum of the rates with l-valine and AGFK germination alone; □, experimental curve with AGFK plus l-valine germination. (C) Synergy is present when the degree of synergy (defined in Materials and Methods) is greater than 1.
Similar experiments were carried out with spores of strain FB10 that contains a point mutation in the gerB gene termed gerB* that allows the GerB* GR to trigger germination in response to l-asparagine alone with no need for the GerK GR (16). In this case, rates of spore germination with mixtures of low concentrations of l-asparagine and l-valine were much higher than the sum of the rates with each germinant concentration individually (Fig. 2A to C), again indicative of strong synergism between the GerA and GerB* GRs with this germinant mixture. Rates of germination with high concentrations of the l-asparagine-l-valine mixture were also lower than the sum of the germination rates with the high germinant concentrations individually (Fig. 2A to C).
Fig. 2.
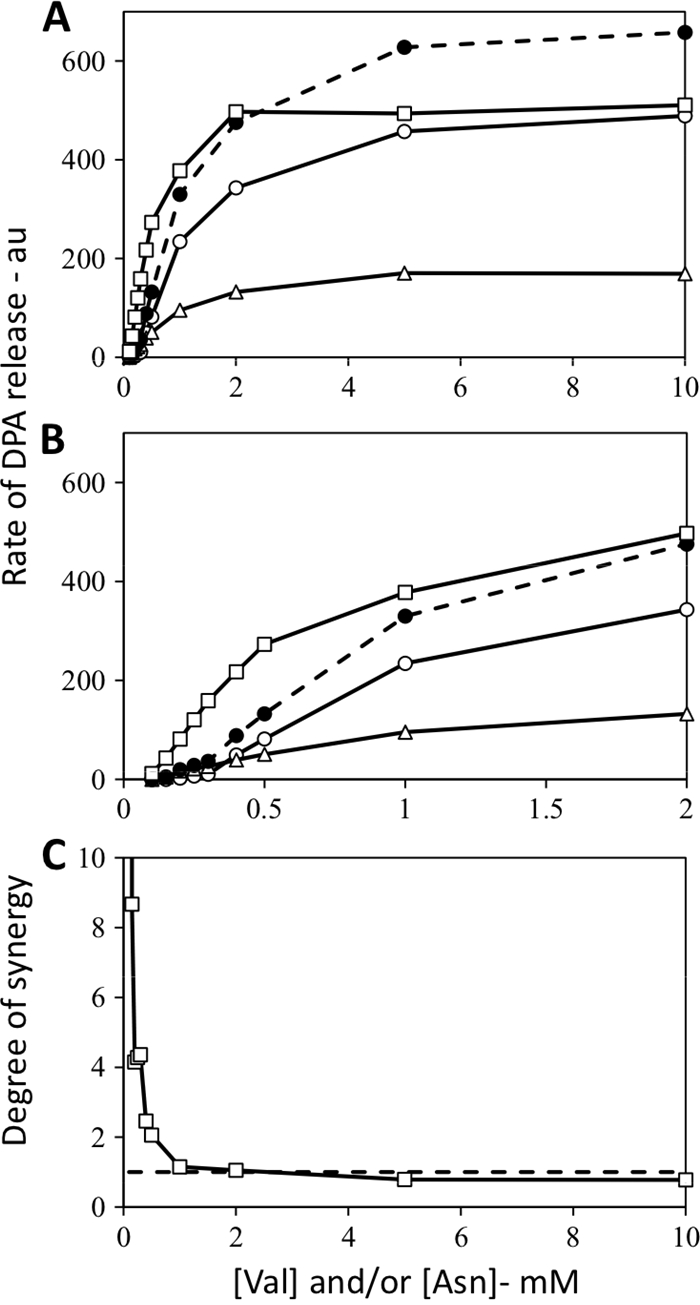
(A to C) Rates of DPA release from gerB* B. subtilis spores germinating with l-valine and/or l-asparagine and degree of synergy when both germinants were used. Spores of B. subtilis FB10 (has the GerB* GR) were germinated with various concentrations of l-valine and/or l-asparagine (note the different scales on the x axes in panels A and B), and rates of DPA release were determined and are given in arbitrary units (au) as described in Materials and Methods. Symbols in panels A and B: ○, l-valine germination; ▵, l-asparagine germination; •, sum of the rates with l-valine and l-asparagine germination alone; □, experimental curve with l-valine plus l-asparagine germination. Note that the FB10 spores used in this experiment were prepared separately from those used in the experiments shown in Fig. 4, 5, and 6.
Lack of synergism in spore germination with multiple germinants if only one GR is present.
The synergism noted above between low concentrations of multiple germinants acting on multiple GRs was striking. The obvious question then is what causes this synergism. One possibility is that the multiple germinants not only activate their cognate GRs but also have direct effects on other GRs; for example, AGFK or one or more of its components, in particular, l-asparagine, might have direct effects on the GerA GR. Indeed, in spores of some species, including B. subtilis, multiple germinants have been suggested to bind to individual GRs (1, 2, 7, 9). To examine this possibility, we used spores containing only a single GR and repeated the germination with multiple nutrient germinants, either alone or together. This analysis showed clearly that neither the GerA GR alone (activated by l-valine) nor the GerB* GR alone (activated by l-asparagine) exhibited any significant synergism with the multiple germinants that gave such synergism when acting on spores with a full GR complement (Fig. 3A to C). Consequently, the synergism seen between mixtures of nutrient germinants is not due to the activation of GRs through noncognate germinants.
Fig. 3.
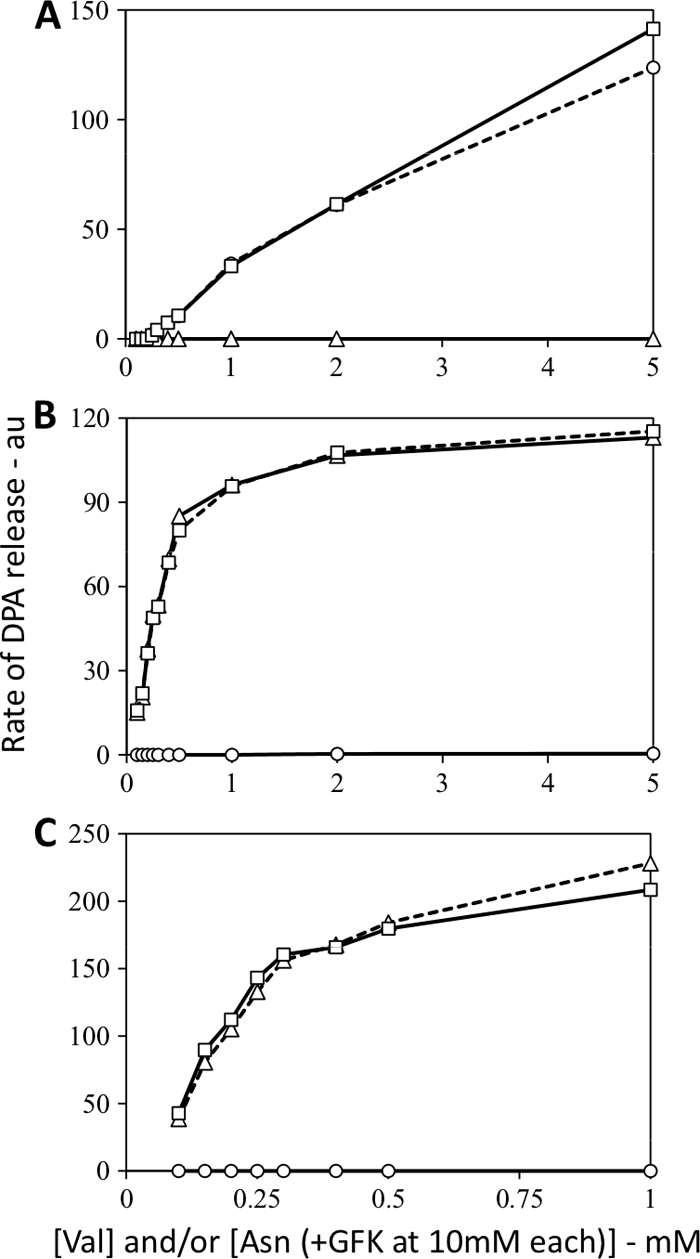
(A to C) Rates of DPA release from B. subtilis spores containing only one GR but germinating with multiple germinants. Spores of B. subtilis strains (A, FB87 [lacks the GerB and GerK GRs]; B, FB20 [lacks the GerA GR]; C, PS3665 [has the GerB* GR but lacks the GerA and GerK GRs]) were germinated with various concentrations of l-valine and/or l-asparagine with the GFK components each at 10 mM in germinations with l-asparagine (A and B) or l-valine and/or l-asparagine (C). Rates of DPA release were determined and are given in arbitrary units (au) as described in Materials and Methods. Symbols: ○, l-valine germination; ▵, germination with AGFK or l-asparagine alone; □, experimental curve for germination with l-valine plus AGFK or l-asparagine.
We also observed a subadditive response at high germinant concentrations. In principle, this may indicate either that the response is partially saturated with respect to one or both germinants or that there is an inhibitory effect when inputs are combined. For the same reason that the observed synergism is not due to the activation of GRs through noncognate germinants, we argue that the subadditive responses at high germinant concentrations were due not to the inhibition of GRs through noncognate germinants but rather to saturation of the rate of DPA release.
Synergism in germination between the GerA and GerB* GRs when GerD is absent or GerA is overexpressed.
In order to further examine the phenomenon of synergism between GRs, we examined the effects of either a gerD mutation or overexpression of the GerA GR on synergism in germination via the GerA and GerB* GRs. The GerD protein is essential for normal GR-mediated spore germination, and its absence results in large decreases in rates of spores' nutrient germination, most likely by increasing the lag time between germinant addition and initiation of DPA release (20, 25, 28). As expected, a gerD mutation significantly decreased rates of germination of gerB* spores with either l-asparagine or l-valine (compare Fig. 4A and B). However, the absence of GerD did not eliminate the synergism in germination via the GerA and GerB* GRs, although in gerB* gerD spores the highest levels of synergism between these GRs were at higher germinant concentrations than with gerB* spores that had GerD (Fig. 4C).
Fig. 4.
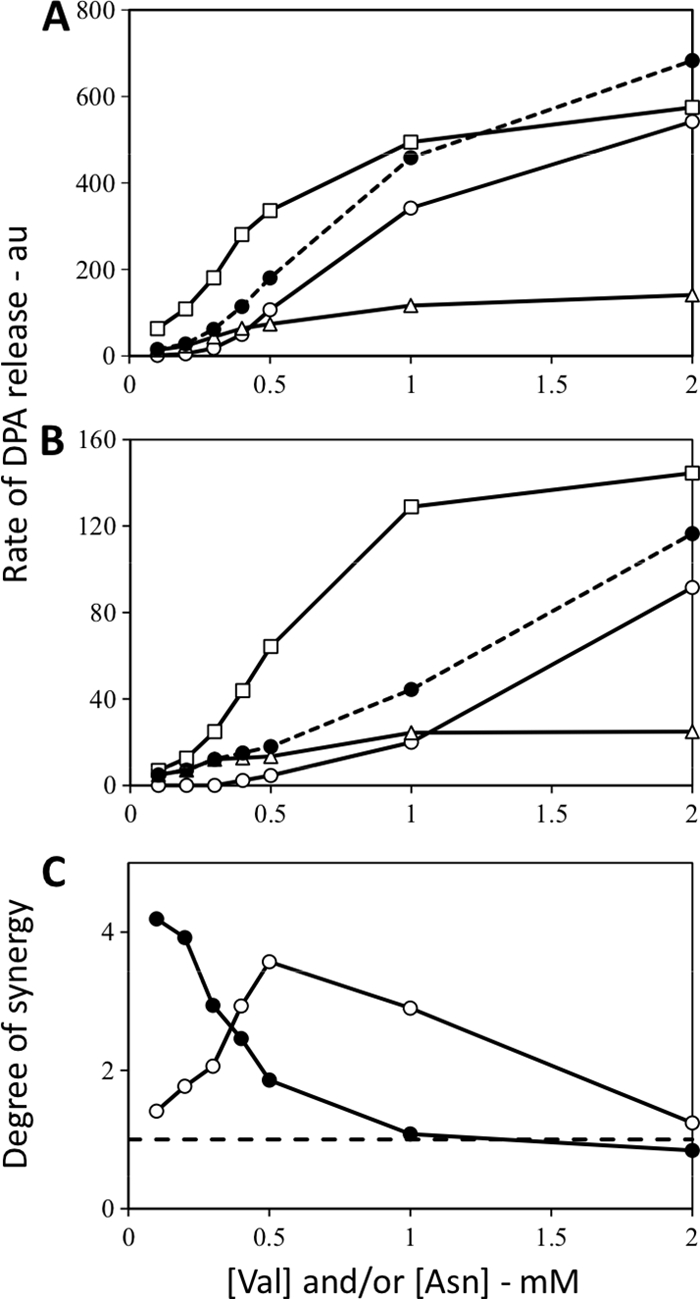
(A to C) Rates of DPA release from B. subtilis spores carrying the GerB* GR with or without GerD and germinating with multiple germinants and degrees of synergy when mixtures of germinants were used. Spores of B. subtilis strains (A, FB10 [has the GerB* GR]; B, PS3929 [has the GerB* GR and lacks GerD]) were germinated with various concentrations of l-valine and/or l-asparagine, and rates of DPA release were determined and are given in arbitrary units (au) as described in Materials and Methods. Symbols in panels A and B: ○, l-valine germination; ▵, germination with l-asparagine alone; □, experimental curve for germination with l-valine plus l-asparagine; •, sum of the rates with l-valine and l-asparagine germination alone. Symbols in panel C: •, FB10 spores; ○, PS3929 spores. Note that the FB10 spores used in this experiment were prepared separately from those used in the experiments shown in Fig. 2, 5, and 6.
Overexpression of the GerA receptor in gerB* spores by placing the gerA operon under the control of the moderately strong, forespore-specific sspD promoter slightly but significantly increased the rate of these spores' germination with l-valine (compare Fig. 5A and B), as expected (6), and greatly decreased spore germination with l-asparagine via the GerB* GR (Fig. 5). However, despite the low rates of l-asparagine germination of gerB* spores with elevated GerA levels, the rates of germination of these spores with mixtures of l-valine and l-asparagine were almost identical to the rates of germination of gerB* spores with normal GerA levels (compare Fig. 5A and B). Consequently, the degree of synergism seen in germination via the GerB* GR and the overexpressed GerA GR was actually higher than that seen in spores that had a normal GerA level (Fig. 5C).
Fig. 5.
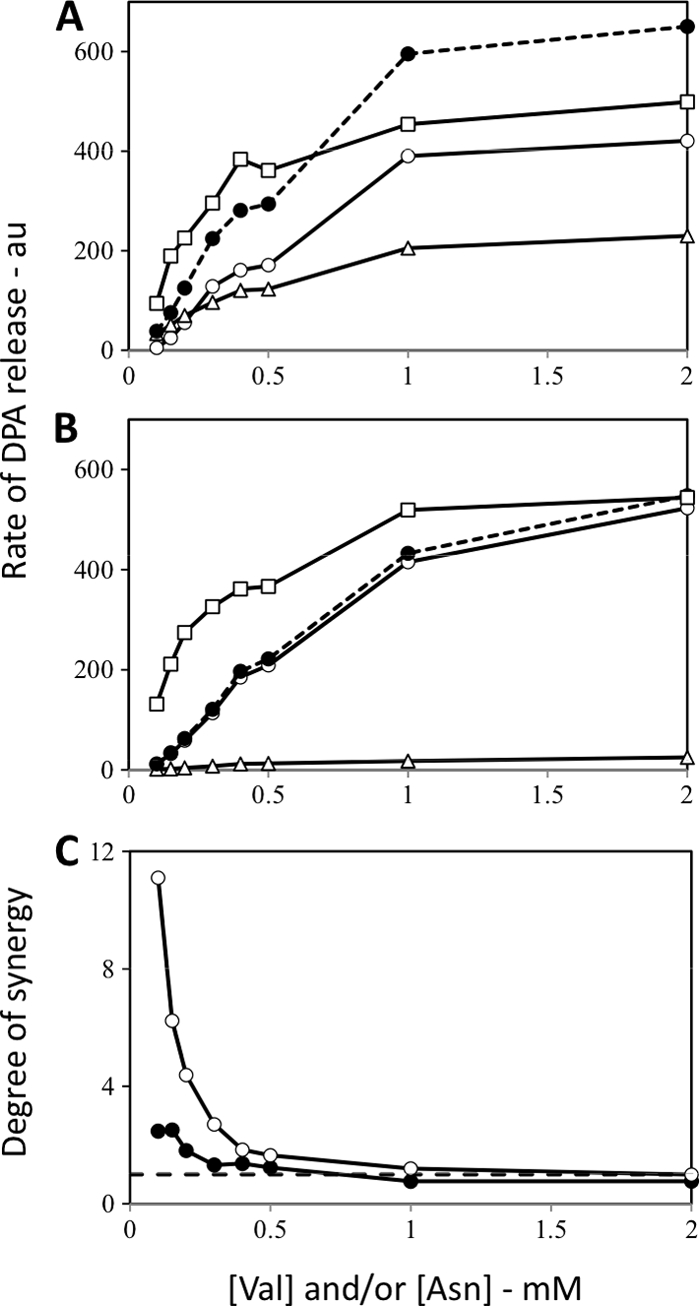
(A to C) Rates of DPA release from B. subtilis spores carrying the GerB* GR with or without the overexpressed GerA GR and germinating with multiple germinants and degrees of synergy when mixtures of germinants were used. Spores of B. subtilis strains (A, FB10 [has the GerB* GR]; B, PS3501 [has the GerB* GR, and the GerA GR is overexpressed]) were germinated with various concentrations of l-valine and/or l-asparagine, and rates of DPA release were determined and are given in arbitrary units (au) as described in Materials and Methods. Symbols in panels A and B: ○, l-valine germination; ▵, germination with l-asparagine alone; □, experimental curve for germination with l-valine plus l-asparagine; •, sum of the rates with l-valine and l-asparagine germination alone. Symbols in panel C: •, FB10 spores; ○, PS3501 spores. Note that the FB10 spores used in this experiment were prepared separately from those used in the experiments shown in Fig. 2, 4, and 6.
Synergism in commitment to germinate between the GerA and GerB* GRs.
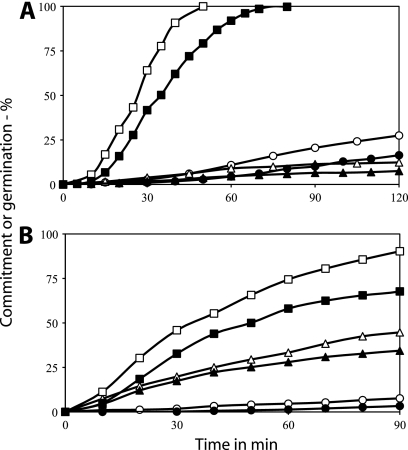
While the results described above indicate that stimulation of different GRs can result in synergism in the triggering of spore germination, the mechanism of this synergism is not clear. One event that occurs prior to DPA release in spore germination via GRs is commitment, whereby spores are irreversibly committed to continue through germination even if germinant binding to spores is abolished. While the mechanism of commitment of spores to germinate is not clear, it seems likely that variability in the timing of this event between individual spores is the major variable causing heterogeneity in the timing of DPA release between individuals in germinating spore populations (27, 28). To test whether rates of commitment also exhibit synergism with mixtures of germinants that stimulate different GRs, PS533 (wild-type) spores were germinated with low concentrations of l-valine alone, AGFK alone, or a mixture of l-valine and AGFK and the rates of the spores' commitment and germination (i.e., DPA release) were measured (Fig. 6A). It was clear that rates of commitment also exhibited synergism with low levels of the mixture of these germinants. Similar results were obtained when FB10 spores (gerB*) were germinated with low concentrations of l-asparagine or l-valine or a mixture of these two germinants (Fig. 6B).
Fig. 6.
(A and B) Commitment and germination of B. subtilis spores germinating with multiple germinants. Spores of B. subtilis strains (A, PS533 [wild type]; B, FB10 [gerB*]) were germinated as described in the text with 0.4 mM l-valine (○ and •), 0.4 mM l-asparagine and each GFK component at 10 mM (▵ and ▴), or 0.4 mM l-valine plus l-asparagine at 0.4 mM and each GFK component at 10 mM (□ and ▪) (A) or with 0.4 mM l-valine (○ and •), 0.1 mM l-asparagine (▵ and ▴), or 0.4 mM l-valine plus 0.1 mM l-asparagine (□ and ▪) (B). During the course of germination, the percentages of spores that had committed (○ ▵, and □) and released DPA (•, ▴, and ▪) were measured as described in Materials and Methods.
Lack of synergism in germination between l-valine and dodecylamine.
The results given above indicated that there consistently was synergism in germination between separate GRs. An obvious question is whether this synergism is absolutely dependent on the GRs or due to a particular germination event, such as DPA release. Since some nonnutrient germinants trigger spore germination independently of GRs (23), the presence or absence of synergism in germination triggered by such a germinant plus a germinant that acts via a GR could indicate whether synergism acts only via GRs. The nonnutrient we examined was the surfactant dodecylamine, which triggers B. subtilis spore germination even if all GRs are absent (21). However, analysis of the rates of B. subtilis spore germination with low concentrations of l-valine plus dodecylamine did not show any synergism between these two germinants (Fig. 7A). Indeed, the rates of germination with mixtures of l-valine and dodecylamine were generally lower than the sum of the rates of spore germination with both germinants individually, and this was especially obvious at high l-valine concentrations (Fig. 7B). The latter finding suggests that dodecylamine inhibits l-valine germination, and this was certainly the case (Fig. 7B), although l-valine may have inhibited dodecylamine germination somewhat as well (Fig. 7A). It is perhaps notable that dodecylamine has been shown to inhibit B. subtilis spore germination by high pressures, including pressures that trigger spore germination via the GRs (4).
Fig. 7.
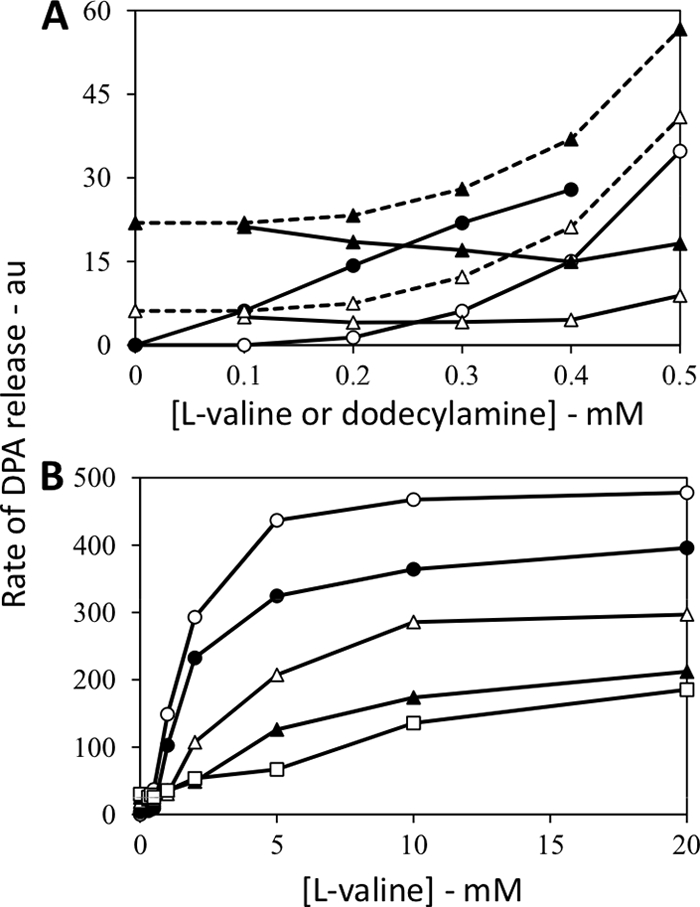
(A and B) Rates of germination of B. subtilis spores with l-valine and dodecylamine, individually or together. Spores of B. subtilis (wild-type) strain PS533 were germinated at 42°C with various concentrations of l-valine and/or dodecylamine (note the different scales on the x axes in panels A and B), and rates of DPA release were determined and are given in arbitrary units (au) as described in Materials and Methods. Symbols in panel A: ○, l-valine germination; •, dodecylamine germination; ▵ and solid line, experimental curve for germination with l-valine plus 0.1 mM dodecylamine; ▵ and dashed line, theoretical curve for the sum of the rates of germination with l-valine and 0.1 mM dodecylamine individually; ▴ and solid line, experimental curve for germination with l-valine plus 0.3 mM dodecylamine; ▴ and dashed line, theoretical curve for the sum of the rates of germination with l-valine and 0.3 mM dodecylamine individually. Symbols for l-valine germination in panel B: ○, no dodecylamine; ▵, 0.1 mM dodecylamine; □, 0.2 mM dodecylamine; ▴, 0.3 mM dodecylamine; •, 0.4 mM dodecylamine. Results similar to those shown here were obtained in two independent experiments.
DISCUSSION
The results of the experiments described above indicated that at low concentrations of mixtures of nutrient germinants that act via GRs, there is strong synergism between the effects of the individual components of nutrient germinant mixtures, such that rates of germination are much higher than the sums of the rates obtained with the individual germinants. Similar results were seen in the absolute levels of germination as measured by the level of DPA release, which is proportional to the number of spores germinated (see the supplemental material). This certainly seems to be a reasonable adaptive response, as the presence of mixtures of nutrient germinants would presumably signal an environment more conducive to vegetative growth than one with fewer germinants present. In the following, we discuss the mechanistic implications of the observed synergy.
Both the percentage and rate of spore germination increase as the germinant concentration is increased. As far as is known, the only effect that different germinant concentrations have is activating different numbers of GRs on a spore, thus suggesting that spores are “counting” the “active” GRs and germinating when there are enough. (Note that different GRs may have different efficacies in triggering germination, so that the counting is likely to be weighted.) The time it takes to germinate should also decrease as the count increases. This “counting model” is supported by the fact that overexpressing the GRs increases the rate and percentage of germination with the corresponding germinants (6). Recent experiments have also shown that GR numbers vary from spore to spore and vary between different GR types in the same spore (13), suggesting that the observed heterogeneity of germination kinetics could arise from heterogeneity of GR expression.
If we assume that the counting model is correct, the synergy observed in our experiments can be explained with the help of two Venn diagrams of the spore population (Fig. 8). In the presence of a single nutrient germinant, only those spores with sufficient numbers of the corresponding GRs activated can be germinated (boxes labeled I in Fig. 8). With a different single nutrient, a different fraction (boxes labeled II in Fig. 8) of the same population can be germinated, and there can be an overlap between the two populations, as shown. With a mixture of the two nutrient germinants, those spores in regions I and II will still germinate and will do so more quickly because each spore will have a higher number of active GRs. In addition, spores in region III, which did not have enough receptors to germinate with a single nutrient germinant, will now be able to germinate because the total number of receptors activated by both germinants is sufficient to trigger germination. If there is also a cooperative interaction between the GRs involved, the spores contained in region IV will also germinate.
Fig. 8.
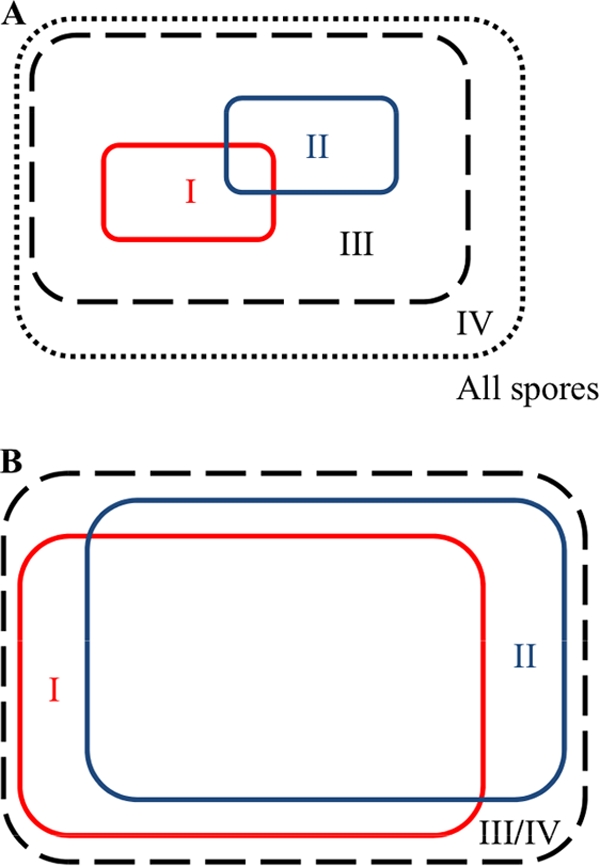
Venn diagrams of spore germination at low (A) and high (B) nutrient germinant concentrations. The outermost rectangular box represents all of the spores in a population. Regions I (red box) and II (blue box) represent the spores that could be germinated at given concentrations by two different single nutrient germinants, respectively, that activate different types of GRs, and there could be overlap between the two subpopulations, as indicated. Spores in each of these populations will germinate faster when a mixture of both germinants is present at the same corresponding concentrations because the total number of active GRs on each spore will be higher. In addition, spores in region III (dashed box) will now also have enough active GRs to undergo germination. If there is also cooperative interaction between the different GRs involved in triggering germination, the additional spores contained in region IV (dotted box) will germinate. Regions I to IV encompass the whole population as germinant concentrations approach saturation, and synergy disappears.
Figures 8A and B show the relative sizes of these regions at low and high germinant concentration, respectively. At low germinant concentrations (Fig. 8A), regions I and II contain spores that have a relatively high expression of GRs responsive to each type of germinant and encompass a small portion of the total population. Region III may be substantially larger than regions I and II because while most spores do not have enough receptors to germinate at low single-nutrient concentrations, there are a larger number of spores that can achieve the germination threshold when two populations of active receptors are combined. At saturating germinant concentrations (Fig. 8B), most spores are able to germinate and each region extends nearly over the whole population. The response appears subadditive under such saturating conditions because adding an additional amount of a germinant of any type has little effect on the observed levels of germination. Finally, we also observed that in some cases the degree of synergy decreases at low concentrations (Fig. 1C and 4C; see Fig. S1A, S2B, and S3B in the supplemental material). This can be understood by considering the case of very low germinant concentrations, where the levels of active GRs on most spores are also very low even if multiple germinants are present; thus, multiple germinants do not trigger significantly more spores than single germinants.
In addition to suggesting that the number of “active” GRs in a spore is a major factor in triggering germination, the experiments presented in this paper also provide a starting point to address the question of whether there are direct cooperative interactions between different GRs, i.e., whether the binding of a germinant to one type of GR can facilitate the activation of other types of GRs. Many chemosensory proteins are associated in large arrays in the plasma membrane of bacteria, which allows direct cooperative interactions between different proteins, as has been observed in a number of bacterial species (3, 5, 12, 14). Indeed, recent work has shown that the great majority of a spore's GRs are colocalized in a small region of the spore's inner membrane (13), and this seems likely to promote cooperative interactions. Cooperativity between different GRs is also strongly suggested by the requirement for both GerB and GerK GRs for germination with the AGFK mixture (23). Cooperative interaction between different GRs, although not necessary for the emergence of synergy, can enhance synergy, as shown by the different sizes of regions III and IV in Fig. 8A, because it enables nutrient mixtures to activate more GRs than the sum of the GRs that can be activated by individual nutrients alone. Our experimental observations of synergy so far are not sufficient to determine whether the degree of synergy we observed requires the existence of cooperativity, but preliminary mathematical modeling of FB10 germination shows that cooperativity between GerA and GerB* is not necessary to fit data from experiments using mixtures of l-valine and l-asparagine (not shown). Thus, it appears that the interaction between GerA and GerB* is, at most, weak, but this conclusion may not apply to other GR pairs, since amino acid changes giving rise to the GerB* GRs may disrupt interactions between GerA and GerB, just as they eliminate the GerK GR requirement in order for the GerB GR to respond to l-asparagine alone.
Our observations of synergy also have implications for superdormant spores, i.e., spores that germinate extremely slowly, even at high germinant concentrations (11). According to our assumption that the active GR level is a major factor determining germination, low numbers of active GRs should be a major reason why those spores are superdormant, and they are represented in Fig. 8B by the region outside I or II (depending on which germinant is used to generate the superdormant spores). This is consistent with the findings that superdormancy is not genetic and these spores germinate normally with nonnutrient germinants that do not involve GRs. Superdormant spores also respond poorly to nutrient germinants that trigger other GRs but germinate normally with nutrient mixtures that target multiple GRs. This is consistent with our observation of synergy and also suggests that regions I and II in Fig. 8B should almost be identical, with the spores in region III/IV, but not those in regions I and II, representing the superdormant population that germinates with nutrient combinations. There are at least two factors that could be involved. (i) The expression of the genes encoding different GR subunits is regulated by the same transcription factors during sporulation, including both the same RNA polymerase sigma factor, σG, and the DNA binding protein SpoVT (24), which suggests that there should be a moderate correlation between the levels of different GRs on the same spore, so that spores having low levels of one GR are likely to also have low levels of other GRs. (ii) If there are cooperative interactions between different GRs, e.g., if some fraction of the GRs function as heterooligomers, they may only become active when all of the corresponding germinants are present. If the fraction of heterooligomers is large, even a spore with high levels of GRs could be superdormant, since the number of active GRs would be low with single germinants.
We now come back to our basic assumption that the number of active GRs determines both the extent and the speed of germination. One possible mechanism for this is that any single active GR has a certain probability of triggering germination, and the more active GRs there are, the more likely a spore is to germinate and the faster the process is. (As an analogy, any activated grenade has a certain probability of triggering the explosion of an arsenal, and the more activated grenades there are, the more likely and is the explosion the sooner it will occur.) However, this mechanism predicts that spores are unstable since the GRs may maintain a low level of activity due to either trace levels of germinants in the environment or to spontaneous GR activation, and this contradicts the fact that spores can stay dormant for extremely long periods.
A second possible mechanism is that germination is determined by the total number of active GRs on a spore; i.e., signals from all of the active GRs are integrated inside the spore, and germination is triggered when the total signal strength is above a certain threshold. There is currently no direct evidence for such an “integrator,” although it has been suggested that the GerD protein, loss of which greatly decreases germination via GRs, might serve such a function (20). However, since there was synergism between the GerA and GerB* GRs in germination when GerD was absent, GerD is not likely to be the “integrator” postulated above. In addition, GerD appears not to be present in spores of Clostridium species (19), and although synergism between multiple germinants has not been investigated in spores of Clostridium species, one might expect such a phenomenon to be widespread. A second way that signals from different germinants could be integrated is via some major germination event itself, such as DPA release. The release of the great majority of DPA during the germination of individual spores takes only a few minutes, although this is preceded by a long lag period (Tlag), the length of which varies considerably between individual spores (28). While all of the events that take place during Tlag are not known, there is a slow release of DPA during this period (25). This slow DPA release in Tlag would likely alter spore properties such as core water content and the strain on the spore's peptidoglycan cortex, and these changes could then trigger fast DPA release and completion of spore germination (23, 25). In this scenario, perhaps the whole spore core/cortex functions as the integrator and there is no dedicated integrator molecule.
Finally, it is possible that the observed synergy may not be unique to nutrient germination, as the same type of downstream germination signal may also come from sources other than GRs. Indeed, some nonnutrients trigger germination without involving the GRs, such as dodecylamine, exogenous Ca-DPA, and certain peptidoglycan fragments. We did not observe synergy between l-valine and dodecylamine (Fig. 7), but the interpretation of this result is complicated by the likely inhibition of l-valine germination by dodecylamine, as seen previously with high-pressure-dependent germination (4). However, the synergy observed in this work has certainly provided a basis for further experiments to dissect the signaling pathways in spore germination.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by a Department of Defense Multidisciplinary University Research Initiative award through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract W911NF-09-1-0286.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 1 July 2011.
REFERENCES
- 1. Akoachere M., et al. 2007. Identification of an in vivo inhibitor of Bacillus anthracis spore germination. J. Biol. Chem. 282:12112–12118 [DOI] [PubMed] [Google Scholar]
- 2. Atluri S., Ragkousi K., Cortezzo D. E., Setlow P. 2006. Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this cooperativity by alterations in the GerB receptor. J. Bacteriol. 188:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bai F., et al. 2010. Conformational spread as a mechanism for cooperativity in the bacterial flagellar switch. Science 327:685–689 [DOI] [PubMed] [Google Scholar]
- 4. Black E. P., et al. 2007. Analysis of factors influencing the rate of germination of spores of Bacillus subtilis by very high pressure. J. Appl. Microbiol. 102:65–76 [DOI] [PubMed] [Google Scholar]
- 5. Briegel A., et al. 2009. Universal architecture of bacterial chemotaxis arrays. Proc. Natl. Acad. Sci. U. S. A. 106:17181–17186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cabrera-Martinez R.-M., Tovar-Rojo F., Vepachedu V. R., Setlow P. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 185:2457–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christie G., Lowe C. R. 2007. Role of chromosomal and plasmid-borne receptor homologs in the response of Bacillus megaterium QM B1551 spores to germinants. J. Bacteriol. 189:4375–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cowan A. E., et al. 2004. Lipids in the inner membrane of dormant spores of Bacillus species are immobile. Proc. Natl. Acad. Sci. U. S. A. 101:7733–7738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisher N., Hanna P. 2005. Characterization of Bacillus anthracis germinant receptors in vitro. J. Bacteriol. 187:8055–8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foerster H. F., Foster J. W. 1966. Response of Bacillus species to combinations of germinative compounds. J. Bacteriol. 91:1168–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghosh S., Setlow P. 2009. Isolation and characterization of superdormant spores of Bacillus species. J. Bacteriol. 191:1787–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greenfield D., et al. 2009. Self-organization of the Escherichia coli chemotaxis network imaged with super-resolution light microscopy. PLoS Biol. 7:c1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Griffiths K. K., Zhang J., Cowan A. E., Yu J., Setlow P. 23 June 2011, posting date Germination proteins in the inner membrane of dormant Bacillus subtilis spores colocalize in a discrete cluster. Mol. Microbiol. [Epub ahead of print.] doi:10.1111/j.1365-2958.2011.07753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hazelbauer G. L., Falke J. J., Parkinson J. S. 2008. Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem. Sci. 33:9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicholson W. L., Setlow P. 1990. Spore germination and outgrowth, p. 391–450 In Harwood C. R., Cutting S. M. (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, England [Google Scholar]
- 16. Paidhungat M., Setlow P. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J. Bacteriol. 181:3341–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paidhungat M., Setlow P. 2000. Role of Ger-proteins in nutrient and non-nutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paidhungat M., Setlow B., Driks A., Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paredes-Sabja, Setlow P., Sarker M. R. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol. 19:85–94 [DOI] [PubMed] [Google Scholar]
- 20. Pelczar P. L., Setlow P. 2007. Role of GerD in germination of Bacillus subtilis spores. J. Bacteriol. 189:1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Setlow B., Cowan A. E., Setlow P. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95:637–648 [DOI] [PubMed] [Google Scholar]
- 22. Setlow B., Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550–556 [DOI] [PubMed] [Google Scholar]
- 24. Setlow P., Johnson E. A. 2007. Spores and their significance, p. 35–67 In Doyle M. P., Beuchat L. R. (ed.), Food microbiology: fundamentals and frontiers, 3rd edition ASM Press, Washington, DC [Google Scholar]
- 25. Wang G., Yi X., Li Y. Q., Setlow P. 2011. Germination of individual spores of Bacillus subtilis with alterations in the GerD and SpoVA proteins important in spore germination. J. Bacteriol. 193:2301–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wax R., Freese E. 1968. Initiation of the germination of Bacillus subtilis spores by a combination of compounds in place of l-alanine. J. Bacteriol. 95:433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yi X., Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J. Bacteriol. 192:3424–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang P., et al. 2010. Factors affecting the variability in time between addition of nutrient germinants and rapid dipicolinic acid release during germination of spores of Bacillus species. J. Bacteriol. 192:3608–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.