Abstract
Transforming growth factor β (TGF-β) plays a critical role in tissue fibrosis. The duration and intensity of TGF-β signaling are tightly regulated. Here we report that TSC-22 (TGF-β-stimulated clone 22) facilitates TGF-β signaling by antagonizing Smad7 activity to increase receptor stability. TSC-22 enhances TGF-β-induced Smad2/3 phosphorylation and transcriptional responsiveness. The stimulatory effect of TSC-22 is dependent on Smad7, as silencing Smad7 expression abolishes it. TSC-22 interacts with TGF-β type I receptor TβRI and Smad7 in mutually exclusive ways and disrupts the association of Smad7/Smurfs with TβRI, thereby preventing ubiquitination and degradation of the receptor. We also found that TSC-22 can promote the differentiation of cardiac myofibroblasts by increasing expression of the fibrotic genes for α-smooth muscle actin (α-SMA), PAI-1, fibronectin, and collagen I, which is consistent with upregulation of TSC-22, phospho-Smad2/3, and the fibrotic genes in isoproterenol-induced rat myocardial fibrotic hearts. Taken together with the notion that TGF-β induces TSC-22 expression, our findings suggest that TSC-22 regulates TGF-β signaling via a positive-feedback mechanism and may contribute to myocardial fibrosis.
INTRODUCTION
It has been well documented that transforming growth factor β (TGF-β) signaling plays a central role in tissue fibrosis, including cardiac fibrosis (3, 20, 26). The function of the mammalian heart is regulated by coordinated and dynamic interactions between cardiomyocytes and cardiac fibroblasts. Cardiac fibroblasts are involved in heart remodeling that occurs in response to pathological irritants, including hypertension and fibrosis, under conditions in which these cells are induced to proliferate and differentiate into cardiac myofibroblasts (20, 27). TGF-β functions as one of the most important cytokines in cardiac fibroblast activation and myocardial fibrosis by inducing cardiac fibroblast proliferation and migration, cardiac myofibroblast differentiation, and cell matrix deposition (3, 20).
TGF-β signals through two types of membrane-bound serine/threonine kinase receptors and intracellular Smad proteins (10, 22). Ligand binding results in the formation of the receptor heteromeric complex, phosphorylation of R-Smad (Smad2/3) by type I receptor TβRI/ALK5 (activin receptor-like kinase 5), accumulation of the Smad2/3-Smad4 complex in the nucleus, and (ultimately) modulation of gene expression. In distinction from R-Smad and Smad4, which mediate TGF-β signaling, Smad7 and Smad6, the inhibitory Smads (I-Smads), impede TGF-β signaling via a feedback mechanism (21, 37, 39). Among the multiple mechanisms underlying the inhibitory effect of Smad7 on TGF-β signaling, a major means is that of recruiting the E3 ubiquitin ligases Smurf1 and Smurf2 to control receptor stability (8, 17).
TSC-22 (TGF-β-stimulated clone 22) was first identified in MC3T3E1 mouse osteoblastic cells as a TGF-β target gene (30). Subsequently, it was found to be induced by TGF-β and other cytokines in other cell lines (reviewed by Kawamata et al. in reference 18). Accumulated evidence suggests that TSC-22 has an antiproliferative activity and is downregulated in several types of tumor cells, including prostate cancer cells, salivary gland cancer cells, hepatoma cells, astrocytic tumor cells, and large granular lymphocyte leukemia cells (12, 23, 28, 31, 36, 40). Consistently, gene targeting of TSC-22 enhanced the proliferation of hematopoietic precursor cells (HPCs) (40). In addition, TSC-22 was also reported to promote cell differentiation and apoptosis (7, 18). Moreover, the Drosophila homolog of TSC-22 was suggested to be in the same genetic pathway as Dpp (a bone morphogenetic protein [BMP] homolog) during fly eye development (33), and it was also shown to induce erythroid cell differentiation by facilitating TGF-β signaling (4). TSC-22 is an evolutionarily conserved cytoplasmic protein and belongs to the TSC-22 protein family, which is characterized by the conserved TSC box and a leucine zipper (LZ) motif in the middle, while the N termini and the C termini in family members are divergent (18, 19).
In this study, we identified TSC-22 in a yeast two-hybrid screening for proteins interacting with TβRI and found that TSC-22 promotes TGF-β signaling via a positive-feedback mechanism. Mechanistically, TSC-22 sequesters Smad7/Smurfs from binding to activated TβRI and thereby inhibits Smad7/Smurf-induced ubiquitination and degradation of the receptor. Our data also revealed that TSC-22 promotes cardiac myofibroblast differentiation and that its upregulation is correlated with myocardial fibrosis. These results provide a novel insight into the regulation of TGF-β signaling and indicate the possible involvement of TSC-22 in cardiac fibrosis.
MATERIALS AND METHODS
Plasmids and antibodies.
Human TSC-22 cDNA was subcloned into pCMVmyc, pCMV5, pcDNA3.1(+), lentivirus vector, and pGEX4T1 with or without various tags. Truncation mutants of TSC-22 were generated by PCR and subsequent subcloning into pCMVmyc vector. A short hairpin RNA (shRNA) construct to knock down human TSC-22 expression was generated by subcloning into pSuper vector of the double nucleotide sequences annealed from the following oligonucleotides: forward primer, 5′-GATCCCCGTGTGGTAGCTATTGACAATTCAAGAGATTGTCAATAGCTACCACACTTTTTA-3′; reverse primer, 5′-AGCTTAAAAAGTGTGGTAGCTATTGACAATCTCTTGAATTGTCAATAGCTACCACACGGG-3′. The inserted sequence in this shRNA construct was mutated to 5′-GATCCCCGTGTGGTAGCTATCGACAATTCAAGAGATTGTCGATAGCTACCACACTTTTTA-3′ by PCR to target rat TSC-22. Smurf1/2 constructs and the shRNA construct targeting human Smurf2 were gifts from Xin-Hua Feng. Other constructs were described previously (25, 38, 41). TSC-22 and Smad7 antibodies were generated by immunizing rabbit with full-length TSC-22 protein and the N-terminal domain of Smad7 (amino acids [aa] 1 to 259), respectively. Anti-Flag antibody (M2) was purchased from Sigma, and the other antibodies were from Santa Cruz Biotechnology.
Cell culture and transfection.
HEK293 cells, HEK293T cells, HaCaT cells, mouse embryonic fibroblast (MEF) cells, and primary rat cardiac fibroblasts were maintained in Dulbecco's minimum essential medium (DMEM), while L17 cells were maintained in minimum essential medium, supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified, 5% CO2 incubator. Cell transfection was conducted with VigoFect (Vigorous Biotechnology, Beijing, China) or Lipofectamine 2000 (Invitrogen).
Lentivirus infection.
For the production of defective lentivirus, HEK293T cells were transfected using Lipofectamine 2000 with three plasmids, lentivirus–TSC-22 (or lentivirus-green fluorescent protein [lentivirus-GFP] control vector), pCMVΔ8.9, and pM.G (VSVG), at a ratio of 2:1.5:1. The culture supernatants were collected at 48 h posttransfection, and viral particles were concentrated by centrifugation. Cells were infected with lentivirus particles in the presence of Polybrene (final concentration, 5 μg/ml). After 48 h, infection efficiency was examined on the basis of GFP expression and immunoblotting.
RNA isolation and quantitative RT-PCR.
Total RNA was prepared with TRIzol reagent (Invitrogen), and cDNA was synthesized from 1 μg of RNA with Revertra Ace (Toyobo). Quantitative reverse transcription-PCR (RT-PCR) was performed using the SYBR green detection method and an Mx3000p quantitative PCR system (Stratagene). The primers used were as follows: for human GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′; for human TSC-22, 5′-ATGGTGTAGACCAGTGGCGAT-3′ and 5′-CACAGAAGCGTTTTCAGTCCC-3′; for human c-myc, 5′-TCTCCTTGCAGCTGCTTAG-3′ and 5′-GTCGTAGTCGAGGTCATAG-3′; for human p21, 5′-TGGAGACTCTCAGGGTCGAAAA-3′ and 5′-GCGTTTGGAGTGGTAGAAATCTG-3′; for human p15, 5′-CACCGTTGGCCGTAAACTTAAC-3′ and 5′-TAATGAAGCTGAGCCCAGGTCT-3′; for human PAI-1, 5′-GAGACAGGCAGCTCGGATTC-3′ and 5′-GGCCTCCCAAAGTGCATTAC-3′; for human or rat Smad7, 5′-ACCCGATGGATTTTCTCAAAC-3′ and 5′-GCCAGATAATTCGTTCCCCC-3′; for rat GAPDH, 5′-GGTCGGTGTGAACGGATTTG-3′ and 5′-GCCTTCTCCATGGTGGTGAA-3′; for rat TSC-22, 5′-ATGGTGTAGACCAGTGGCGAT-3′ and 5′-CACGGAAGCGTTTTCAGTTCC-3′; for rat α-SMA, 5′-CGATAGAACACGGCATCATC-3′ and 5′-CATCAGGCAGTTCGTAGCTC-3′; for rat collagen I, 5′-CGATGGCTGCACGAGTCACACCAG-3′ and 5′-GTTGGGATGGAGGGAGTTTAC-3′; for rat PAI-1, 5′-CCTCCTCATCCTGCCTAAGTTC-3′ and 5′-GCCGCTCTCGTTCACCTC-3′; and for rat fibronectin, 5′-GTGAAGAACGAGGAGGATGTG-3′ and 5′-GTGATGGCGGATGATGTAGC-3′). Gene expression was normalized against GAPDH mRNA. Each sample was measured in triplicate experiments. Data were analyzed using Microsoft Excel software.
Reporter assays, immunoblotting, immunoprecipitation, and immunofluorescence.
Reporter assays, immunoblotting, and immunoprecipitation were performed as described previously (38). Reporter assays were performed in triplicate, and the data are presented as means ± standard deviations (SD) after normalization to Renilla activity. Immunofluorescence was carried out per a previously reported method (41), and the results were examined using a FluoView FV10i confocal laser scanning microscope (Olympus). Protein colocalization was analyzed using Image-Pro Plus (IPP) software (version 6.0; Media Cybernetics).
In vivo ubiquitination assay.
HEK293T cells were transfected with myc-His-ubiquitin along with other expression vectors as indicated. At 40 h posttransfection, cells were treated with 50 μM MG132 for 4 h and lysed with lysis solution (6 M guanidine, 50 mM Tris-HCl, 250 mM NaCl, 5 mM imidazole [pH 8.0]), protease inhibitors, and 50 μM MG132. The resulting lysate was sonicated before addition of 20 μl of nickel-nitrilotriacetic acid beads (Qiagen), and the mixture was rotated overnight at 4°C. Subsequently, the beads were washed twice with buffer A (3 M guanidine, 50 mM Tris-HCl, 250 mM NaCl, 5 mM imidazole [pH 8.0]) and twice with buffer B (50 mM Tris-HCl, 250 mM NaCl, 5 mM imidazole, 0.2% Triton X-100 [pH 8.0]) and then subjected to immunoblotting analysis.
GST pulldown assay.
To test the direct binding of glutathione S-transferase–TSC-22 (GST-TSC-22) to TβRI (intracellular domain [ICD]), 1 μg of each purified protein was mixed in TNE lysis buffer (50 mM Tris [pH 7.5], 140 mM NaCl, 1 mM EDTA) with 30 μl of glutathione-Sepharose beads. The mixture was then incubated at 4°C for 2 h with gentle agitation. After the beads were washed 4 times with TNE lysis buffer, 40 μl of loading buffer was added and the samples were analyzed by immunoblotting.
Isolation of primary rat cardiac fibroblasts.
Primary cardiac fibroblasts were isolated from neonatal Sprague-Dawley rats. Hearts were quickly removed under sterile conditions, rinsed in cold phosphate-buffered saline (PBS), minced, and digested with trypsin (0.25% trypsin and 0.02% EDTA in PBS [pH 8.0]) at 37°C for 1 h. The digestion product was then suspended in equal volumes of DMEM–10% FBS followed by filtration with a cell sieve and centrifugation at 1,000 rpm for 10 min. Subsequently, the cells were resuspended in DMEM–10% FBS and plated in 6-well plates. The unattached or weakly adherent cells, including myocytes and endothelial cells, were removed by changing the medium. The animal study was approved by the Animal Care Committee, Tsinghua University.
Myocardial fibrosis model and tissue preparation.
Thirty adult male Sprague Dawley rats (200 to 300 g) were divided into 2 groups. Rats of the first group (n = 20) were subcutaneously injected with isoproterenol (ISO) (5 mg/kg of body weight) daily for 2 weeks, while rats from the control group (n = 10) were injected with an equal volume of saline solution. The rats were sacrificed after 1 month. ISO-injected rats showed a survival rate of 50%. For tissue preparation, rats were anesthetized with 10% chloral hydrate (300 mg/kg). Then, sternotomy was performed and the hearts were rapidly removed. Tissue from the left ventricle was rapidly frozen in liquid nitrogen and stored at −80°C for protein extraction and RNA isolation or fixed in 4% paraformaldehyde and embedded in paraffin for staining or immunohistochemistry.
Protein extraction and RNA isolation from rat hearts.
To extract proteins, cardiac tissues from the left ventricles of rat hearts were homogenized in lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 0.5% NP-40, 5 mM EDTA, 40 mM NaF, 1 mM sodium vanadate, 10 mM sodium pyrophosphate, 20 mM β-glycerol phosphate, and protease inhibitors). The homogenate was sonicated 5 times for 5 s each time, followed by 10 min of incubation on ice and 15 min of centrifugation at 13,000 rpm at 4°C. The supernatant was used for protein level detection by immunoblotting. For RNA isolation, cardiac tissues from the left ventricles were homogenized in 1 ml of TRIzol reagent per 50 to 100 mg of tissue. The samples were then centrifuged at 13,000 rpm for 15 min at 4°C. After incubation for 5 min at room temperature, 0.2 ml of chloroform was added. The samples were shaken vigorously for 15 s and incubated for 5 min at room temperature, followed by centrifugation at 12,000 × g for 15 min at 4°C. The aqueous phase was then collected, and RNA was precipitated by mixing with an equal volume of isopropanol and centrifugation.
Masson staining and immunohistochemistry.
After fixation for 24 h in 4% paraformaldehyde, the heart blocks were dehydrated, embedded in paraffin, and cut into 4-μm-thick slices. For Masson staining, slices were heated overnight at 37°C, dewaxed, and stained with Masson dye. For immunohistochemistry, slices were deparaffinized with xylene. Endogenous peroxidase was blocked with 0.3% H2O2 for 15 min. After treatment with blocking goat serum for 15 min, sections were incubated overnight with α-SMA antibody (1:200) and phospho-Smad2 antibody (1:200) and then with biotinylated-link secondary antibody and peroxidase-labeled streptavidin followed by a diaminobenzidine (substrate of peroxidase) revelation and counterstaining with Mayer's hematoxylin. Slices were analyzed under a microscope.
RESULTS
TSC-22 promotes TGF-β signaling.
In a yeast two-hybrid screening for proteins interacting with TGF-β type I receptor TβRI/ALK5, we identified TSC-22 (data not shown). To investigate the role of TSC-22 in TGF-β signaling, we first examined the effect of TSC-22 on TGF-β-induced reporter expression in HaCaT cells. As shown in Fig. 1 A to C, TGF-β1 stimulated the expression of three TGF-β-responsive reporters, CAGA-luciferase, ARE-luciferase, and 3TP-luciferse, and ectopic expression of TSC-22 further enhanced this response. In addition, TSC-22 promoted the transcriptional responsiveness of TGF-β in HEK293 cells (Fig. 1D) and also promoted reporter expression induced by constitutively active TGF-β type I receptor (ca-TβRI) (data not shown). These data indicate that TSC-22 can promote TGF-β signaling.
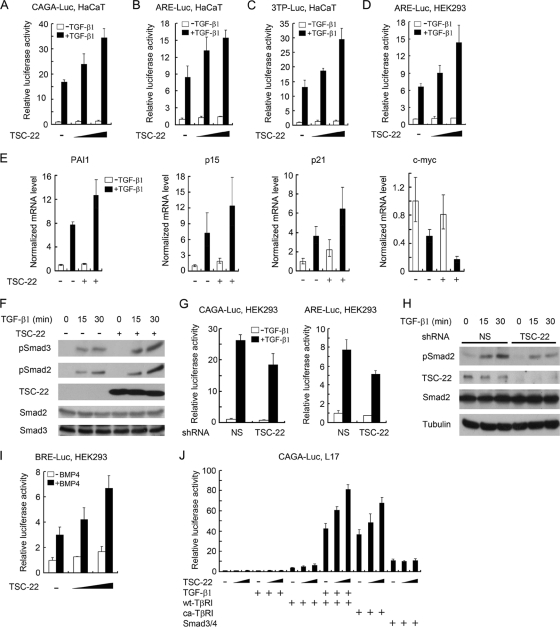
Fig. 1.
TSC-22 promotes TGF-β signaling. (A to C) TSC-22 increases TGF-β-induced expression of reporter genes in HaCaT cells. HaCaT cells transfected with various reporters (CAGA-luciferase [CAGA-Luc] [200 ng] [A], ARE-luciferase [300 ng]/FAST2 [150 ng] [B], and 3TP-luciferase [200 ng] [C]) together with Renilla luciferase (20 ng) and TSC-22 (100 or 200 ng) were treated with 100 pM TGF-β1 for 20 h and harvested for luciferase activity measurements. (D) TSC-22 increases TGF-β-induced expression of ARE-luciferase reporter in HEK293 cells. (E) TSC-22 enhances expression of TGF-β target genes in HaCaT cells. After infection with TSC-22 lentivirus, HaCaT cells were treated with TGF-β1 for 20 h and then harvested for quantitative RT-PCR. (F) TSC-22 enhances TGF-β-induced phospho-Smad2/3 levels. After infection with TSC-22 lentivirus, HaCaT cells were treated with TGF-β1 for the indicated times. Phospho-Smad2/3 and protein expression levels were analyzed by immunoblotting. (G) TSC-22 knockdown reduces TGF-β-induced expression of reporters. HEK293 cells were transfected with various reporters as described for panels A and B and with nonspecific (NS) shRNA or TSC-22-specific shRNA (50 ng). After treatment with 100 pM TGF-β1 for 20 h, the cells were harvested for luciferase activity measurements. (H) TSC-22 knockdown reduces TGF-β-mediated phospho-Smad2 level in HEK293 cells. (I) TSC-22 increases BMP4-induced expression of BRE-luciferase in HEK293 cells. HEK293 cells transfected with plasmids encoding BRE-luciferase (200 ng), Renilla luciferase (20 ng), and TSC-22 (100 ng or 200 ng) were treated with 10 ng of BMP4/ml for 20 h and harvested for luciferase determinations. (J) TSC-22 enhances reporter expression induced by TβRI but not by Smads. L17 cells transfected with the reporter and wt-TβRI, ca-TβRI (50 ng), Smad3/4 (25 ng each), or TSC-22 (100 or 200 ng) were treated with 100 pM TGF-β1 for 20 h before being harvested for luciferase determinations. In all of the reporter assay experiments, empty vectors were used to equalize the total amounts of plasmids in each sample. Each experiment was performed in triplicate, and the data are presented as means ± SD after normalization to Renilla activity.
To confirm the promoting function of TSC-22 for TGF-β signaling, we examined expression of several TGF-β target genes in HaCaT cells and observed that lentivirus-mediated overexpression of TSC-22 further increased TGF-β-mediated upregulation of PAI-1, p15, and p21 and downregulation of c-myc (Fig. 1E). Consistently, TGF-β-induced phosphorylation of Smad2 and Smad3, two intracellular transducers of TGF-β signaling, was enhanced by TSC-22 overexpression (Fig. 1F). Conversely, TSC-22 knockdown impaired TGF-β-induced expression of CAGA-luciferase and ARE-luciferase (Fig. 1G) and decreased Smad2 phosphorylation in HEK293 cells (Fig. 1H).
We also found that TSC-22 enhanced expression of the bone morphogenetic protein (BMP)-responsive reporter BRE-luciferase induced by BMP4 (Fig. 1I) or by a constitutively active BMP type I receptor (ca-ALK6/ca-BMPRIB) (data not shown), suggesting that TSC-22 may be a general positive regulator of TGF-β superfamily signaling.
The data presented above suggested that TSC-22 can promote TGF-β/BMP signaling at the type I receptor level. We then asked whether TSC-22 can facilitate Smad-induced reporter expression in TβRI-deficient L17 cells (2). As shown previously, introduction of wild-type (wt) TβRI could restore the transcriptional activity of TGF-β (Fig. 1G). Coexpression of TSC-22 enhanced this activity further. Moreover, TSC-22 also facilitated ca-TβRI-induced reporter expression. Although overexpression of Smad3 and Smad4 alone activated the reporter to certain extent, TSC-22 was unable to further increase the reporter expression. These results indicate that TSC-22 regulates TGF-β signaling at the receptor level and upstream of Smads.
TSC-22 associates with the TGF-β receptor complex.
As TSC-22 was identified as interacting with TβRI in a yeast two-hybrid screening, we wanted first to confirm the interaction between TSC-22 and TβRI at the endogenous protein level. HEK293 cells treated with or without TGF-β1 for 2 h were subjected to anti-TβRI immunoprecipitation followed by anti-TSC-22 immunoblotting. As shown in Fig. 2A, TSC-22 showed a weak interaction with TβRI in the basal state, but this interaction was dramatically enhanced by TGF-β1 stimulation. The notion that TSC-22 interacts with TβRI in yeast suggests that the two proteins have a direct interaction. Indeed, in vitro GST pulldown assays performed with recombinant proteins verified the direct interaction between TSC-22 and the intracellular domain (ICD) of TβRI (Fig. 2B).
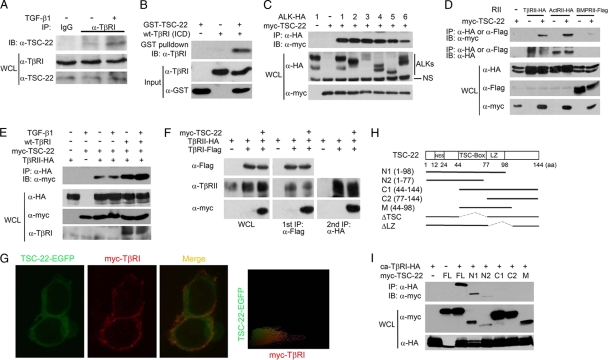
Fig. 2.
TSC-22 directly interacts with TβRI. (A) TGF-β enhances the TβRI-TSC-22 interaction at the endogenous protein level. HEK293 cells were treated with or without 100 pM TGF-β1 for 2 h and harvested for immunoprecipitation (IP) with control rabbit IgG or anti-TβRI antibody followed by anti-TSC-22 immunoblotting (IB). WCL, whole-cell lysate. (B) Direct interaction between TSC-22 and the intracellular domain (ICD) of TβRI. Purified recombinant GST-TSC-22 and TβRI-ICD (1 μg each) were subjected to GST pulldown assays. (C) TSC-22 associates with the type I receptors of the TGF-β family. After transfection with the indicated constructs (2 μg each) for 48 h, HEK293T cells were harvested for anti-hemagglutinin (anti-HA) immunoprecipitation (IP) followed by anti-Myc immunoblotting (IB). Protein expression was examined with whole-cell lysates (WCL) by immunoblotting. (D) TSC-22 associates with the type II receptors of the TGF-β family. (E) TGF-β and TβRI enhance the association between TSC-22 and TβRII. After transfection with indicated constructs for 48 h, HEK293T cells were treated with 100 pM TGF-β1 for 2 h before being harvested for coimmunoprecipitation. (F) TSC-22 forms a ternary complex with TβRI and TβRII. HEK293T cells transfected with indicated constructs were harvested for the first immunoprecipitation with anti-Flag antibody. The immunocomplex was then eluted by the use of Flag peptide and subjected to anti-HA immunoprecipitation followed by immunoblotting (right panel). Aliquots of whole-cell lysates and the first immunoprecipitants were analyzed by immunoblotting (left and middle panels). (G) TSC-22 substantially colocalizes with TβRI along the plasma membrane. HEK293T cells transfected with TSC-22-enhanced GFP (TSC-22-EGFP) and myc-TβRI were treated with 100 pM TGF-β1 for 2 h before being harvested for immunofluorescence analysis. TSC-22 localization was detected by direct EGFP immunofluorescence, and TβRI localization was determined indirectly using anti-myc antibody followed by a tetramethyl rhodamine isocyanate (TRITC)-conjugated second antibody and visualized under a fluorescence microscope. Colocalization was measured by IPP software; yellow coloring indicates the colocalization. (H) Schematic diagram of TSC-22 and its mutants. (I) The N terminus of TSC-22 mediates its interaction with ca-TβRI. The experiment was performed as described for panel A.
Next, we examined the specificity of the interaction between TSC-22 and the receptors of the TGF-β family. Coimmunoprecipitation revealed that TSC-22 was coimmunoprecipitated with all the examined type I receptors (ALK1, ALK2, ALK3/BMPRIA, ALK4/ActRIB, ALK5/TβRI, and ALK6/BMPRIB) (Fig. 2C), as well as with the type II receptors TβRII, ActRII, and BMPRII (Fig. 2D). Interestingly, the interaction of TSC-22 with TβRII was enhanced by coexpression of TβRI (Fig. 2E); similarly, TβRII increased the interaction between TSC-22 and TβRI (data not shown). Since ectopic expression of TβRI and TβRII can form a complex in vivo (9), these results indicate that TSC-22 prefers to associate with the TβRI-TβRII heterocomplex. To consolidate this assumption, we carried out a two-step sequential immunoprecipitation experiment. As shown in Fig. 2F, both TβRII and TSC-22 were coprecipitated with TβRI, and TSC-22 was found to associate with TβRII in the second round of immunoprecipitation, indicating that the three proteins form a ternary complex in vivo. In addition, immunofluorescence analysis revealed that TSC-22 was diffusively distributed in the cytoplasm and that some of it showed substantial colocalization with TβRI along the plasma membrane (Fig. 2G).
TSC-22 belongs to the TSC-22 protein family, with the conserved TSC box and a leucine zipper (LZ) motif in the middle and flexible N-terminal and C-terminal regions (18, 19). Domain mapping analysis revealed that the N terminus of TSC-22 mediates its association with TβRI (Fig. 2H and I).
TSC-22 relieves the inhibitory effects of Smad7 and Smurfs on TGF-β signaling.
We then examined the interaction of TSC-22 with Smad proteins. As shown in Fig. 3 A and B, TSC-22 was coimmunoprecipitated only with Smad6 and Smad7, and their interactions were enhanced by ligand treatment. Furthermore, the interaction between endogenous Smad7 and TSC-22 was detected only in the presence of TGF-β (Fig. 3C). These data indicate that the interaction between TSC-22 and I-Smads is regulated by TGF-β or BMP signaling.
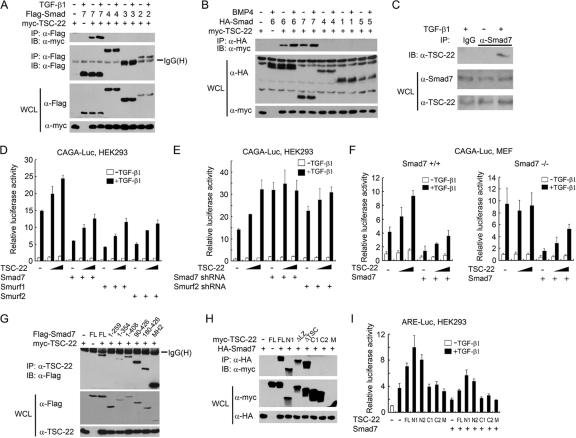
Fig. 3.
TSC-22 interferes with the inhibitory effect of Smad7/Smurfs on TGF-β signaling. (A and B) TGF-β/BMP enhances the interaction of TSC-22 with Smad6/7. HEK293T cells transfected with the indicated constructs (2 μg each) were treated with 100 pM TGF-β1 or 400 pM BMP4 for 2 h and harvested for coimmunoprecipitation. (C) TGF-β induces endogenous TSC-22-Smad7 interaction. HEK293 cells were treated with or without 100 pM TGF-β1 for 2 h before being harvested for coimmunoprecipitation. (D) TSC-22 attenuates the inhibitory effect of Smad7/Smurfs on TGF-β-induced expression of CAGA-luciferase. HEK293 cells were transfected with plasmids encoding CAGA-luciferase (200 ng), Renilla luciferase (20 ng), Smad7 (50 ng), Smurf1 (20 ng), Smurf2 (20 ng), and TSC-22 (100 or 200 ng) and then treated with 100 pM TGF-β1 for 20 h before being harvested for luciferase assays. (E) Smad7 is required by TSC-22 to promote TGF-β signaling. HEK293 cells were transfected with reporters, shRNA (50 ng), and TSC-22 (100 or 200 ng) and treated with or without 100 pM TGF-β1 for 20 h and harvested for luciferase assays. NS, nonspecific. (F) The function of TSC-22 in regulating TGF-β signaling depends on Smad7. Reporter assays were carried out with Smad7+/+ or Smad7−/− MEFs. (G and H) HEK293T cells were transfected with the indicated constructs for 48 h before being harvested for coimmunoprecipitation assays. (I) Reporter assays were performed as described for panel D.
One of the major mechanisms underlying the inhibitory effect of Smad7 on TGF-β signaling operates through accelerating TβRI turnover by recruiting ubiquitin E3 ligases such as Smurf1 and Smurf2 (21). The interaction of TSC-22 with Smad7 suggests a possible functional connection between TSC-22 and Smad7/Smurfs. To investigate this possibility, TGF-β-induced CAGA-luciferase activity was evaluated when Smad7, Smurf1, or Smurf2 was overexpressed. As shown in Fig. 3D, Smad7, Smurf1, and Smurf2 exhibited a robust inhibitory effect on TGF-β signaling. However, this inhibitory effect was reduced by the presence of TSC-22 in a dose-dependent manner. Intriguingly, knockdown of Smad7 by shRNA curbed the promoting effect of TSC-22 on TGF-β-induced reporter expression, and silencing of Smurf2 also reduced the promoting effect of TSC-22 on TGF-β signaling (Fig. 3E), indicating that the function of TSC-22 in promoting TGF-β signaling is dependent on Smad7. To consolidate this assumption, reporter assays were performed using wild-type and Smad7−/− mouse embryonic fibroblasts (MEFs). As expected, Smad7−/− cells exhibited enhanced TGF-β signaling (Fig. 3F). As observed in HaCaT and HEK293 cell experiments, TSC-22 enhanced TGF-β signaling in wild-type MEF cells. In contrast, this enhancement was not observed in Smad7−/− cells. Smad7 expression in these knockout cells attenuated TGF-β signaling, and TSC-22 again alleviated the antagonizing effect of Smad7. Taken together, these results suggest that TSC-22 facilitates TGF-β signaling by relieving the inhibitory effect of Smad7.
Domain mapping revealed that the first 44 amino acid residues at the N terminus of TSC-22 were important for its interaction with the MH2 domain (amino acids 260 to 426) of Smad7 (Fig. 3G and H). Consistent with that finding, the N terminus of TSC-22 is required to facilitate TGF-β signaling (Fig. 3I).
TSC-22 sequesters Smad7/Smurfs from degrading TβRI.
The data reported above indicate that TSC-22 facilitates TGF-β signaling through interactions with the receptor complex and Smad7 and relieves the inhibitory functions of Smad7 and Smurfs. Since Smad7 recruits Smurf1/2 to ubiquitinate activated TβRI, we asked whether TSC-22 affects Smurf-mediated TβRI ubiquitination. In accordance with previous reports, the basal ubiquitination level of ca-TβRI was dramatically increased in the presence of Smurf1 or Smurf2 (Fig. 4 A). However, the ubiquitination level was reduced by TSC-22. Consistently, TSC-22 attenuated Smad7/Smurf-induced degradation of ca-TβRI (Fig. 4B).
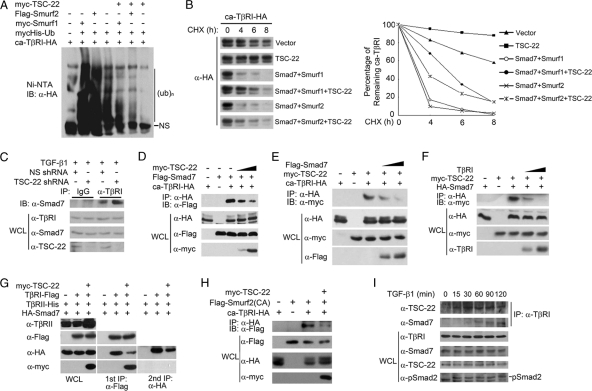
Fig. 4.
TSC-22 attenuates Smad7/Smurf-induced TβRI ubiquitination and degradation. (A) TSC-22 inhibits Smurf1/2-induced ubiquitination of TβRI. HEK293T cells transfected with the indicated constructs (2 μg each) were treated with 50 μM MG132 for 4 h before being harvested for precipitation with nickel beads followed by anti-HA immunoblotting. (B) TSC-22 attenuates Smad7/Smurf-induced turnover of TβRI. HEK293T cells were transfected with the indicated constructs, treated with 50 μg/ml cycloheximide (CHX) for the indicated time periods, and then harvested for anti-HA immunoblotting. The results were quantified by the use of Bandscan software. (C) TSC-22 knockdown enhances the association of Smad7 with TβRI. HEK293T cells were transfected with nonspecific or TSC-22 shRNA, treated with 100 pM TGF-β1 for 2 h, and harvested for coimmunoprecipitation. (D) TSC-22 impairs the association of Smad7 with TβRI. At 48 h posttransfection with the indicated constructs, HEK293T cells were harvested for coimmunoprecipitation assays. (E) Smad7 inhibits the association between TβRI and TSC-22. (F) TβRI decreases the binding of TSC-22 to Smad7. (G) TSC-22 associates with TβRI and Smad7 in different complexes. The experiment was performed as described for Fig. 2F. (H) TSC-22 impairs the association of Smurf2 (C716A) with TβRI in HEK293T cells. (F) Association of TSC-22 and Smad7 with TβRI. HaCaT cells treated with 100 pM TGF-β1 for the indicated time were harvested for coimmunoprecipitation assays.
We further found that TSC-22 knockdown enhanced the association of Smad7 with TβRI at the endogenous level in response to TGF-β (Fig. 4C), while TSC-22 overexpression impaired TβRI-Smad7 interactions in a dose-dependent manner (Fig. 4D). Therefore, it is possible that TSC-22 associates with Smad7 and TβRI in a competitive way. Indeed, Smad7 decreased the interaction between TSC-22 and TβRI (Fig. 4E), and TβRI inhibited the Smad7–TSC-22 association (Fig. 4F). This finding was further supported by the sequential immunoprecipitation result that the three proteins could not form a ternary complex (Fig. 4G), indicating that TβRI and Smad7 are in different complexes of TSC-22. In addition, ectopic expression of TSC-22 also reduced the interaction between ca-TβRI and a Smurf2 mutant (C716A) (Fig. 4H) that is deficient in E3 ubiquitin ligase activity but retains Smad7 binding ability (8). Together, these results indicate that TSC-22 interacts with Smad7 and TβRI in a mutually exclusive manner and blocks the recruitment of Smad7 and Smurfs to TβRI.
Since both TSC-22 and Smad7 associate with TβRI and are functionally connected in regulating the stability of TβRI, we then examined the dynamics of these associations. As shown in Fig. 4I, a weak interaction between TSC-22 and TβRI could be detected in HaCaT cells, and this interaction was enhanced by TGF-β1 stimulation at 15 min. However, the association between Smad7 and TβRI appeared at 30 min and was more obvious at 1 h after TGF-β1 treatment, suggesting that association of TSC-22 with TβRI occurs more rapidly than association with Smad7.
TSC-22 promotes TGF-β-induced cardiac myofibroblast differentiation and is involved in myocardial fibrosis.
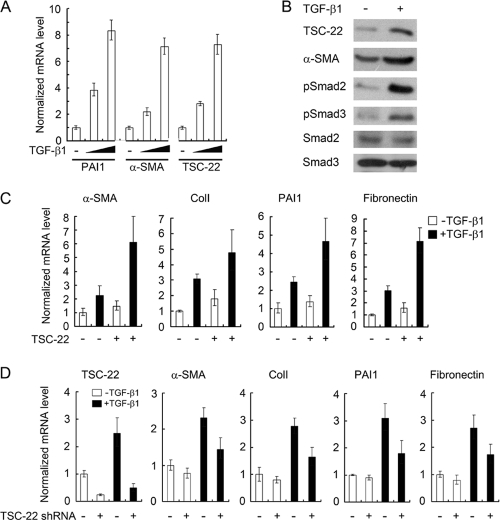
Aberrantly elevated TGF-β activity has been associated with cardiac fibrosis. TGF-β can induce the differentiation of cardiac fibroblasts into myofibroblasts and stimulate extracellular matrix deposition (3, 20). We investigated whether TSC-22 is involved in this process by the use of primary cardiac fibroblasts isolated from the neonatal rat heart. After treatment with TGF-β1 for 20 h, the cells were harvested for gene expression analysis by quantitative RT-PCR or immunoblotting. As shown in Fig. 5 A and B, TGF-β1 induced the expression of both PAI-1, a known TGF-β target involved in fibrosis, and α-smooth muscle actin (α-SMA), a marker of myofibroblasts. Interestingly, TSC-22 was also upregulated by TGF-β at the mRNA and protein levels, indicating that TSC-22 is a TGF-β target gene in cardiac fibroblasts.
Fig. 5.
TSC-22 promotes TGF-β-induced cardiac myofibroblast differentiation. (A) TGF-β upregulates the expression of TSC-22, PAI-1, and α-SMA in primary cardiac fibroblasts from neonatal rats. After treatment with TGF-β1 (100 or 300 pM) for 20 h, the cells were harvested for quantitative RT-PCR. (B) TGF-β enhances the protein levels of TSC-22, PAI-1, and α-SMA in primary rat cardiac fibroblasts. Cells were treated with 100 pM TGF-β1 for 20 h and harvested for immunoblotting. (C) Overexpression of TSC-22 promotes TGF-β-induced cardiac myofibroblast differentiation. Primary rat cardiac fibroblasts were infected with TSC-22 lentivirus. After 24 h, the cells were treated with 100 pM TGF-β1 for 20 h and harvested for quantitative RT-PCR. (D) Knockdown of TSC-22 inhibits TGF-β-induced cardiac myofibroblast differentiation. Primary rat cardiac fibroblasts were transfected with nonspecific or TSC-22 shRNA. At 24 h posttransfection, the cells were treated with TGF-β1 and harvested for quantitative RT-PCR. ColI, collagen I.
We next investigated whether TSC-22 can promote TGF-β-induced cardiac myofibroblast differentiation. As shown in Fig. 5C, lentivirus-mediated overexpression of TSC-22 indeed enhanced the TGF-β-induced expression of α-SMA, PAI-1, collagen I, and fibronectin. Consistently, TSC-22 knockdown decreased their expression (Fig. 5D). These results indicate that TSC-22 promotes cardiac myofibroblast differentiation by facilitation of TGF-β signaling.
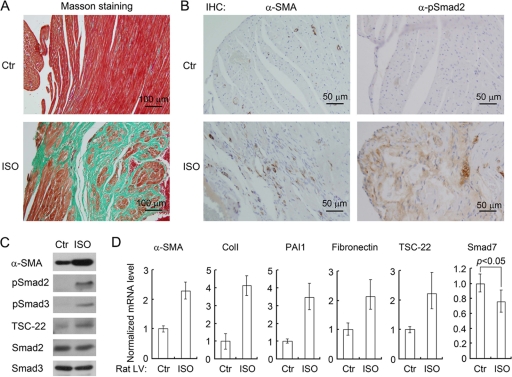
To further study the function of TSC-22 in cardiac fibrosis, we set up a rat myocardial fibrosis model by injection of isoproterenol (ISO) (1). Hearts from ISO-injected rats exhibited cardiac hypertrophy and myocardial fibrosis, as indicated by high levels of collagen (Masson staining) and of α-SMA (immunohistochemistry) (Fig. 6 A and B). In agreement with previous reports (20, 29), TGF-β signaling was activated in the left ventricles of the ISO hearts, as shown by phospho-Smad2 staining (Fig. 6B). Examination of protein expression also revealed that the ISO hearts exhibited higher levels of TSC-22, phospho-Smad2/3, and α-SMA (Fig. 6C). Consistently, the mRNA levels of TSC-22, α-SMA, PAI-1, collagen I, and fibronectin were increased in the ISO hearts, whereas Smad7 expression was decreased (Fig. 6D). Together, these results demonstrated that elevated TSC-22 levels correlate with decreased Smad7 levels, high TGF-β signaling levels, and enhanced expression of fibrotic genes in ISO-induced myocardial fibrosis.
Fig. 6.
TSC-22 is upregulated in ISO-induced myocardial fibrosis. (A) Masson staining analysis of the left ventricles of rat hearts. Blue staining indicates collagen fibers. (B) Upregulation of α-SMA and phospho-Smad2 in the left ventricles of ISO hearts as shown by immunohistochemistry. IHC, immunohistochemistry. (C) Upregulation of α-SMA, TSC-22, phospho-Smad2, and phospho-Smad3 in ISO hearts as shown by immunoblotting. (D) mRNA expression in the left ventricles of rat hearts. Total RNA isolated from the left ventricles (LV) was subjected to quantitative RT-PCR. Ctr, control; ColI, collagen I.
DISCUSSION
The duration and intensity of TGF-β signaling are tightly controlled, and deregulation of TGF-β signaling is associated with various diseases. Dozens of proteins have been reported to modulate TGF-β signaling, and most of them have been found to play a negative role (13, 15, 21). In this report, we show that TSC-22 regulates TGF-β signaling through a positive-feedback mechanism. TSC-22 associates with TβRI and Smad7 in mutually exclusive ways and decreases the association of Smad7/Smurfs with activated TβRI, thereby stabilizing the receptor and promoting TGF-β signaling.
TSC-22 functions as a positive regulator of TGF-β signaling.
TSC-22 was originally identified as a TGF-β-responsive gene in mouse osteoblastic MC3T3E1 cells (30). Subsequently, it was also found to be induced by TGF-β in several other cell lines, such as HSC-39 human gastric carcinoma cells, TYS salivary gland cancer cells, and FaO hepatoma cells (18). It has been reported that TSC-22 inhibits cell proliferation and induces differentiation and apoptosis (5, 24). TSC-22 is evolutionally conserved, and shortsighted/bunched, the Drosophila homolog of TSC-22, was shown to function in the same genetic pathway as Dpp, a BMP family member (33). All of these results suggest that a functional connection may exist between TGF-β and TSC-22. Indeed, TSC-22 has been shown to enhance TGF-β signaling (4). In this study, we found that TGF-β upregulates TSC-22 expression both in HaCaT cells and in primary rat cardiac fibroblasts. In turn, TSC-22 enhances TGF-β-induced Smad2/3 phosphorylation and promotes the transcriptional activity of TGF-β. Together, these results indicate that TSC-22 promotes TGF-β signaling in a positive-feedback manner that may play an important role in development of TGF-β-related diseases such as tissue fibrosis (see below).
TSC-22 belongs to a group of proteins that contain a leucine zipper motif and a TSC box in the middle with divergent N termini and C termini (18, 19). We found that its N-terminal region (aa 1 to 44) is important for both its interaction with TβRI and Smad7 and promotion of TGF-β signaling. In addition to encoding the isoform (144 aa) studied here, the TSC-22 gene has been reported to encode a long isoform (1,073 aa) due to alternative splicing. The long isoform, which has a long and different N terminus (11, 14, 19), has no effect on the transcriptional activity of TGF-β (data not shown). Thus, the presence of the short isoform of TSC-22 may be unique in this family to facilitate TGF-β signaling via its specific N terminus.
TSC-22 prevents the Smad7-Smurf complex from associating with TGF-β receptors.
It was previously reported that TSC-22 can interact with Smad4 (4), but we found that TSC-22 can interact directly with TβRI and with the inhibitory Smad7 and Smad6. These inhibitory Smads are critical regulators of TGF-β signaling through negative-feedback mechanisms (21, 37). We showed that TSC-22 interferes with the inhibitory effects of Smad7 and Smurf1/2 on TGF-β signaling, indicating that TSC-22 is a novel antagonist of Smad7. In support of the notion that TSC-22 functions at the receptor level, reporter assays performed using TβRI-deficient L17 cells demonstrated that TSC-22 increased TGF-β- and ca-TβRI-mediated expression of CAGA-luciferase, whereas it had no effect on that mediated by Smad3/4. TSC-22 attenuates Smad7/Smurf-mediated ubiquitination and degradation of TβRI. Through the mutually exclusive association with TβRI and Smad7, TSC-22 could effectively prevent TβRI from associating with Smad7/Smurfs and consequent degradation.
Since Smad7 and TSC-22 regulate TGF-β signaling negatively and positively, respectively, the balance between TSC-22 and Smad7 may determine the intensity and duration of TGF-β signaling. We observed that association of TβRI with TSC-22 occurs more rapidly than association with Smad7, indicating that TSC-22 may be important to prevent early downregulation of TGF-β signaling by Smad7. Both TSC-22 and Smad7 are target genes of TGF-β. Therefore, their expression kinetics could influence the outcome of TGF-β signaling. We found that Smad7 expression was more rapidly induced by TGF-β than that of TSC-22 at the mRNA level but that TSC-22 induction continued longer (data not shown). This result was likely due to the fact that Smad7 is a direct target gene of TGF-β (6, 32, 35), whereas TSC-22 was induced by TGF-β at the posttranscriptional level (16, 34). Thus, TSC-22 may be important for the long-term and sustained activation of TGF-β signaling.
The role of TSC-22 in cardiac fibrosis.
TGF-β plays a critical role in cardiac fibrosis (3, 20, 26). TSC-22 promotes the TGF-β-induced expression of α-SMA, PAI-1, collagen I, and fibronectin. α-SMA is a marker for myofibroblast differentiation, and PAI-1, collagen I, and fibronectin are well-known fibrotic factors (20, 26). Moreover, compared with the hearts from the control rats, the hearts from the ISO-induced rat myocardial fibrosis model showed higher expression of the α-SMA, PAI-1, collagen I, and fibronectin fibrotic genes. TSC-22 expression was also increased at both the mRNA and the protein levels in the ISO-treated hearts. These results indicate that TSC-22 is positively involved in myocardial fibrosis and functions by facilitating TGF-β signaling and promoting cardiac myofibroblast differentiation. Our findings suggest that TSC-22 could be a therapeutic target of myocardial fibrosis.
ACKNOWLEDGMENTS
We thank Yan Chen for Smad7−/− MEFs, Xin-Hua Feng for Smad7 and Smurf1/2 constructs, and Zhe Cai for technical assistance.
This work was supported by grants from the National Natural Science Foundation of China (30930050 and 30921004), the 973 Program of Ministry of Science and Technology of China (2010CB833706 and 2011CB943803), and the Tsinghua University Initiative Scientific Research Program (2010THZ0) to Y.-G.C.
Footnotes
Published ahead of print on 26 July 2011.
REFERENCES
- 1. Benjamin I. J., et al. 1989. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ. Res. 65:657–670 [DOI] [PubMed] [Google Scholar]
- 2. Boyd F. T., Massague J. 1989. Transforming growth factor-beta inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J. Biol. Chem. 264:2272–2278 [PubMed] [Google Scholar]
- 3. Bujak M., Frangogiannis N. G. 2007. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 74:184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi S. J., et al. 2005. Tsc-22 enhances TGF-beta signaling by associating with Smad4 and induces erythroid cell differentiation. Mol. Cell. Biochem. 271:23–28 [DOI] [PubMed] [Google Scholar]
- 5. Coyle B., Freathy C., Gant T. W., Roberts R. A., Cain K. 2003. Characterization of the transforming growth factor-beta 1-induced apoptotic transcriptome in FaO hepatoma cells. J. Biol. Chem. 278:5920–5928 [DOI] [PubMed] [Google Scholar]
- 6. Denissova N. G., Pouponnot C., Long J., He D., Liu F. 2000. Transforming growth factor beta-inducible independent binding of SMAD to the Smad7 promoter. Proc. Natl. Acad. Sci. U. S. A. 97:6397–6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doi Y., Kawamata H., Ono Y., Fujimori T., Imai Y. 2008. Expression and cellular localization of TSC-22 in normal salivary glands and salivary gland tumors: implications for tumor cell differentiation. Oncol. Rep. 19:609–616 [PubMed] [Google Scholar]
- 8. Ebisawa T., et al. 2001. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J. Biol. Chem. 276:12477–12480 [DOI] [PubMed] [Google Scholar]
- 9. Feng X. H., Derynck R. 1996. Ligand-independent activation of transforming growth factor (TGF) beta signaling pathways by heteromeric cytoplasmic domains of TGF-beta receptors. J. Biol. Chem. 271:13123–13129 [DOI] [PubMed] [Google Scholar]
- 10. Feng X. H., Derynck R. 2005. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 21:659–693 [DOI] [PubMed] [Google Scholar]
- 11. Huser C. A., et al. 2010. TSC-22D1 isoforms have opposing roles in mammary epithelial cell survival. Cell Death Differ. 17:304–315 [DOI] [PubMed] [Google Scholar]
- 12. Iida M., Anna C. H., Gaskin N. D., Walker N. J., Devereux T. R. 2007. The putative tumor suppressor Tsc-22 is downregulated early in chemically induced hepatocarcinogenesis and may be a suppressor of Gadd45b. Toxicol. Sci. 99:43–50 [DOI] [PubMed] [Google Scholar]
- 13. Itoh S., ten Dijke P. 2007. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr. Opin. Cell Biol. 19:176–184 [DOI] [PubMed] [Google Scholar]
- 14. Jay P., et al. 1996. Cloning of the human homologue of the TGF beta-stimulated clone 22 gene. Biochem. Biophys. Res. Commun. 222:821–826 [DOI] [PubMed] [Google Scholar]
- 15. Kang J. S., Liu C., Derynck R. 2009. New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol. 19:385–394 [DOI] [PubMed] [Google Scholar]
- 16. Kato M., et al. 2010. Posttranscriptional upregulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-{beta}-induced collagen expression in kidney cells. J. Biol. Chem. 285:34004–34015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kavsak P., et al. 2000. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol. Cell 6:1365–1375 [DOI] [PubMed] [Google Scholar]
- 18. Kawamata H., Fujimori T., Imai Y. 2004. TSC-22 (TGF-beta stimulated clone-22): a novel molecular target for differentiation-inducing therapy in salivary gland cancer. Curr. Cancer Drug Targets 4:521–529 [DOI] [PubMed] [Google Scholar]
- 19. Khoury C. M., et al. 2008. A TSC22-like motif defines a novel antiapoptotic protein family. FEMS Yeast Res. 8:540–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leask A. 2007. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc. Res. 74:207–212 [DOI] [PubMed] [Google Scholar]
- 21. Lönn P., Moren A., Raja E., Dahl M., Moustakas A. 2009. Regulating the stability of TGFbeta receptors and Smads. Cell Res. 19:21–35 [DOI] [PubMed] [Google Scholar]
- 22. Massagué J. 2008. TGFbeta in cancer. Cell 134:215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakashiro K., et al. 1998. Down-regulation of TSC-22 (transforming growth factor beta-stimulated clone 22) markedly enhances the growth of a human salivary gland cancer cell line in vitro and in vivo. Cancer Res. 58:549–555 [PubMed] [Google Scholar]
- 24. Ohta S., Yanagihara K., Nagata K. 1997. Mechanism of apoptotic cell death of human gastric carcinoma cells mediated by transforming growth factor beta. Biochem. J. 324(Pt. 3):777–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Onichtchouk D., et al. 1999. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 401:480–485 [DOI] [PubMed] [Google Scholar]
- 26. Pohlers D., et al. 2009. TGF-beta and fibrosis in different organs—molecular pathway imprints. Biochim. Biophys. Acta 1792:746–756 [DOI] [PubMed] [Google Scholar]
- 27. Porter K. E., Turner N. A. 2009. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol. Ther. 123:255–278 [DOI] [PubMed] [Google Scholar]
- 28. Rentsch C. A., et al. 2006. Differential expression of TGFbeta-stimulated clone 22 in normal prostate and prostate cancer. Int. J. Cancer 118:899–906 [DOI] [PubMed] [Google Scholar]
- 29. Ruiz-Ortega M., Rodriguez-Vita J., Sanchez-Lopez E., Carvajal G., Egido J. 2007. TGF-beta signaling in vascular fibrosis. Cardiovasc. Res. 74:196–206 [DOI] [PubMed] [Google Scholar]
- 30. Shibanuma M., Kuroki T., Nose K. 1992. Isolation of a gene encoding a putative leucine zipper structure that is induced by transforming growth factor beta 1 and other growth factors. J. Biol. Chem. 267:10219–10224 [PubMed] [Google Scholar]
- 31. Shostak K. O., et al. 2003. Downregulation of putative tumor suppressor gene TSC-22 in human brain tumors. J. Surg. Oncol. 82:57–64 [DOI] [PubMed] [Google Scholar]
- 32. Stopa M., et al. 2000. Participation of Smad2, Smad3, and Smad4 in transforming growth factor beta (TGF-beta)-induced activation of Smad7. The TGF-beta response element of the promoter requires functional Smad binding element and E-box sequences for transcriptional regulation. J. Biol. Chem. 275:29308–29317 [DOI] [PubMed] [Google Scholar]
- 33. Treisman J. E., Lai Z. C., Rubin G. M. 1995. Shortsighted acts in the decapentaplegic pathway in Drosophila eye development and has homology to a mouse TGF-beta-responsive gene. Development 121:2835–2845 [DOI] [PubMed] [Google Scholar]
- 34. Uchida D., et al. 2003. Posttranscriptional regulation of TSC-22 (TGF-beta-stimulated clone-22) gene by TGF-beta 1. Biochem. Biophys. Res. Commun. 305:846–854 [DOI] [PubMed] [Google Scholar]
- 35. von Gersdorff G., et al. 2000. Smad3 and Smad4 mediate transcriptional activation of the human Smad7 promoter by transforming growth factor beta. J. Biol. Chem. 275:11320–11326 [DOI] [PubMed] [Google Scholar]
- 36. Xu Y., Iyengar S., Roberts R. L., Shappell S. B., Peehl D. M. 2003. Primary culture model of peroxisome proliferator-activated receptor gamma activity in prostate cancer cells. J. Cell. Physiol. 196:131–143 [DOI] [PubMed] [Google Scholar]
- 37. Yan X., Chen Y. G. 2011. Smad7: not only a regulator, but also a cross-talk mediator of TGF-beta signalling. Biochem. J. 434:1–10 [DOI] [PubMed] [Google Scholar]
- 38. Yan X., et al. 2009. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-beta signaling. J. Biol. Chem. 284:30097–30104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yan X., Liu Z., Chen Y. 2009. Regulation of TGF-beta signaling by Smad7. Acta Biochim. Biophys. Sin. (Shanghai) 41:263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu J., et al. 2009. TSC-22 contributes to hematopoietic precursor cell proliferation and repopulation and is epigenetically silenced in large granular lymphocyte leukemia. Blood 113:5558–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang S., et al. 2007. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol. Cell. Biol. 27:4488–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]