Abstract
Noncoding Y RNAs are essential for the initiation of chromosomal DNA replication in mammalian cell extracts, but their role in this process during early vertebrate development is unknown. Here, we use antisense morpholino nucleotides (MOs) to investigate Y RNA function in Xenopus laevis and zebrafish embryos. We show that embryos in which Y RNA function is inhibited by MOs develop normally until the midblastula transition (MBT) but then fail to replicate their DNA and die before gastrulation. Consistent with this observation, Y RNA function is not required for DNA replication in Xenopus egg extracts but is required for replication in a post-MBT cell line. Y RNAs do not bind chromatin in karyomeres before MBT, but they associate with interphase nuclei after MBT in an origin recognition complex (ORC)-dependent manner. Y RNA-specific MOs inhibit the association of Y RNAs with ORC, Cdt1, and HMGA1a proteins, suggesting that these molecular associations are essential for Y RNA function in DNA replication. The MBT is thus a transition point between Y RNA-independent and Y RNA-dependent control of vertebrate DNA replication. Our data suggest that in vertebrates Y RNAs function as a developmentally regulated layer of control over the evolutionarily conserved eukaryotic DNA replication machinery.
INTRODUCTION
Noncoding RNAs regulate gene expression, heterochromatin formation, and development (2, 8, 20, 50), and they are now emerging as regulators of the initiation of DNA replication in several eukaryotic species (10, 27, 38, 47). Initiation is regulated by the association of evolutionarily conserved proteins with replication origins during the G1 phase of the cell cycle (1, 4, 35, 58). Initially, the origin recognition complex (ORC) associates with Cdc6, Cdt1, and MCM2-7 proteins, forming the prereplicative complex (pre-RC). Initiation then requires the activity of the cyclin-dependent kinases (CDK) and Cdc7-Dbf4 kinase and involves the stepwise recruitment of CDC45, GINS, and further proteins forming preinitiation and initiation complexes, which ultimately lead to the establishment of active DNA replication forks in S phase.
In addition to these proteins, initiation of human DNA replication requires the function of a class of noncoding RNAs, termed Y RNAs (10, 11, 28). Y RNAs associate with unreplicated chromatin in human cell nuclei and associate, and colocalize, with several proteins that are essential for the initiation of DNA replication, including ORC and Cdt1 (61). After initiation, they are displaced from sites of replicated chromatin, suggesting that they act as DNA replication licensing factors (61).
Y RNAs are structured stem-loop RNAs of 70 to 120 nucleotides, and they have been conserved in vertebrate evolution (40, 49, 51). Humans and Xenopus laevis have four Y RNAs (hY1, hY3, hY4, and hY5 and xY3, xY4, xY5, and xYα RNA, respectively), whereas zebrafish have only one (zY1 RNA). Vertebrate Y RNAs can replace each other in a human somatic cell DNA replication system due to an evolutionarily conserved structural motif in the stem of all vertebrate Y RNAs, which is necessary and sufficient for Y RNA function (17). Although these results suggest that Y RNA function in DNA replication is conserved in evolution, a functional role for Y RNAs is still to be established in nonhuman vertebrates.
The regulation of DNA replication changes considerably during early vertebrate development, and it is not known if this involves Y RNA function. During early amphibian development, several cellular parameters change abruptly at the midblastula stage (53). After 12 rapid and synchronous cell divisions without gap phases, cell division cycles in Xenopus become asynchronous and much slower, with discernible G1 and G2 phases, cell motility becomes detectable, and the bulk transcription of zygotic genes begins (7, 43, 44). This coordinated change in cell behavior is called the midblastula transition (MBT) (43). Cell cycle control of DNA replication also changes at MBT in Xenopus. Before MBT, individual chromosomes are surrounded by nuclear membranes during mitotic anaphase, forming separate units termed karyomeres (31, 39). DNA replication initiates within these, allowing the S phase to overlap with mitosis (31). After MBT, karyomeres no longer appear, and chromosomal DNA replication initiates site specifically within late-G1-phase nuclei (21, 31). Regulatory factors that discriminate between early embryonic (pre-MBT) and later (post-MBT) modes of DNA replication have not been identified.
Here we investigated the requirement for noncoding Y RNAs in DNA replication during early development in Xenopus laevis and zebrafish. We use Y RNA-specific antisense morpholino oligonucleotides (MOs) to show that Y RNA function becomes essential for embryo viability and chromosomal DNA replication at MBT but is not required before this point. We demonstrate here that Y RNAs interact with chromatin only after MBT in an ORC-dependent manner, and Y RNA-specific MOs disrupt complex formation between Y RNAs and ORC/Cdt1. Thus, the MBT is a transition point between the Y RNA-independent control of vertebrate DNA replication in eggs and early embryos and the Y RNA-dependent control in later embryos and adult cells.
MATERIALS AND METHODS
Embryo culture and microinjection.
Xenopus laevis embryos were obtained by artificial fertilization. They were maintained in 10% normal amphibian medium (NAM) (54) and staged as described previously (45). Xenopus embryos were injected at the one-cell stage with 100 ng antisense MOs dissolved in water. Zebrafish embryos were obtained through natural spawning and maintained and staged as described previously (59). They were injected in the yolk at the one-cell stage with 10 ng of MOs.
MOs were obtained from GeneTools, LLC; their sequences are listed in Table 1. coMO was the standard control and was obtained from GeneTools.
Table 1.
Sequences of antisense MOs
| MO | Sequence |
|---|---|
| Xenopus xY3 | 5′-GAGGGGAGAAGAACAAAGAAGTCTG-3′ |
| Xenopus xY4 | 5′-AGGTCAGCAGTGGGGTGAAATAGAA-3′ |
| Xenopus xY5 | 5′-TAGTCAATGGCAACGGTGGG-3′ |
| Xenopus xYα | 5′-TCTGTAAGTGGGAACTATTGATTCG-3′ |
| Zebrafish zY1 | 5′-GGAGAGAAGAAACAAGGAGTTCTGT-3′ |
| ORC2-MO1 | 5′-GAGCTTCATGGCTCATGTTTGACGT-3′ |
| ORC2-MO2 | 5′-TACCTCCCGCAAAACTTTGAACCGC-3′ |
Cell culture and RNA interference.
Xenopus laevis XL177 cells were cultivated at 23°C as asynchronously proliferating monolayers in 70% modified Leibovitz L15 medium with Glutamax (Sigma) supplemented with 10% fetal bovine serum, 10 U/ml penicillin, and 0.1 mg/ml streptomycin (14). Small interfering RNAs (siRNAs) were chemically synthesized as detailed previously (10), using the DNA oligonucleotides detailed in Table 2.
Table 2.
DNA oligonucleotides used for synthesis of siRNAs
| Oligonucleotide | Sequence |
|---|---|
| xY3a fw | 5′-TTGCCACCATTAATTGATTACCCTGTCTC-3′ |
| xY3a rev | 5′-GTAATCAATTAATGGTGGCAACCTGTCTC-3′ |
| xY3b fw | 5′-ACTTCTTTGTTCTTCTCCCCTCCTGTCTC-3′ |
| xY3b rev | 5′-AGGGGAGAAGAACAAAGAAGTCCTGTCTC-3′ |
| xY4 fw | 5′-TTATCCAAATCATTCAGTTAGCCTGTCTC-3′ |
| xY4 rev | 5′-CTAACTGAATGATTTGGATAACCTGTCTC-3′ |
| Luciferase fw | 5′-AACTTACGCTGAGTACTTCGACCTGTCTC-3′ |
| Luciferase rev | 5′-AATCGAAGTACTCAGCGTAAGCCTGTCTC-3′ |
For RNA interference, proliferating subconfluent XL177 cells were transfected with the indicated siRNAs as described elsewhere for mammalian cells (10).
Isolation and quantification of RNA.
Total RNA was isolated from embryos by using the TriPure reagent (Roche), followed by LiCl precipitation. Y RNA expression was validated by quantitative reverse transcription-PCR (qRT-PCR) using the LightCycler 480 (Roche). mRNA encoding full-length Venus protein (42) was transcribed with SP6 RNA polymerase from a pCS2 vector that was linearized with NotI. A 100-pg aliquot of Venus mRNA was added to 2.5 μg of total embryo RNA. Reverse transcription was carried out using the Transcriptor First-Strand cDNA kit (Roche) followed by quantitative real-time PCR using the LightCycler 480 SYBR green I master kit (Roche). Primer sequences are listed in Table 3.
Table 3.
Sequences of primers used for quantitative RT-PCR
| Primer | Sequence |
|---|---|
| xY3fw | 5′-GGCTGGTCCGAAGGCAGTGG-3′ |
| xY3rev | 5′-GGGAAGCAGTGGGAGGGGAG-3′ |
| xY4fw | 5′-GGTTGGTCCGAAAGTTGTGG-3′ |
| xY4rev | 5′-GGTCAGCAGTGGGGTGAAAT-3′ |
| xY5fw | 5′-AGTTGGTCCGATAATGGTGG-3′ |
| xY5rev | 5′-GGCAACGGTGGGGGGAATTT-3′ |
| xYαfw | 5′-AGTTGGTCCGTGTACGGTGG-3′ |
| xYαrev | 5′-CAAATCTGTAAGTGGGAACT-3′ |
| zY1fw | 5′-GGCTGGTCCGAAGGCGGT-3′ |
| zY1rev | 5′-TGGGAGAGGGGAGAGAAG-3′ |
| 5Sfw | 5′-CCCTGAAAGTGCCTGATCTC-3′ |
| 5Srev | 5′-CACCTTTTGGCTCCATGACT-3′ |
| Venusfw | 5′-ACGTAAACGGCCACAAGTTC-3′ |
| Venusrev | 5′-AAGTCGTGCTGCTTCATGTG-3′ |
| GS17fw | 5′-TCCTCATTATGCCAGTCCAACTT-3′ |
| GS17rev | 5′-CCGTAAACTTGTGTAGCACCGC-3′ |
| HistoneH1fw | AACTTTGTTACCGGGCTCCT |
| HistoneH1rev | AGCGACTCTTCTCTGCTTGC |
| MCM3fw | TTGCAGACAGAGGAGTGGTG |
| MCM3rev | GTACGCTACATCGGGCATTT |
| ORC2fw | TTATCAGCAATGTCGCGAAG |
| ORC2rev | CACTCCATCCATGCCTTTTT |
Estimated concentrations of target Y RNA (in molecules per embryo) were calculated based on the crossing points of target Y RNA and “spiked” Venus mRNA and the efficiency of the PCR.
Preparation and quantification of bulk embryonic DNA and rRNA.
For quantification, total DNA and rRNA were isolated from microinjected Xenopus laevis embryos and analyzed by agarose gel electrophoresis (52). Three embryos were pooled per data point. The total nucleic acid content of an equivalent of 0.125 embryos was loaded per lane of a 0.6% agarose gel and subsequently visualized by ethidium bromide staining. Fluorescent signals were quantified with ImageJ software (v1.38; http://rsb.info.nih.gov/ij), using nonsaturated charge-coupled-device image files in TIFF format.
Synthesis and modification of wild-type and mutant Y RNAs.
Sequences for vertebrate Y RNA genes have been published (15, 40, 49). Expression constructs containing full-length cDNA sequences encoding wild-type and mutant Y RNAs under the control of a 5′ SP6 promoter site were generated by PCR amplification using synthetic DNA templates (Sigma-Genosys) as described previously (17). Y RNAs were synthesized by in vitro transcription using SP6 RNA polymerase and purified by ion-exchange chromatography as described previously (10, 61).
Purified Y RNA was oxidized at its 3′ end and covalently coupled to Alexa Fluor 488 hydrazide (Invitrogen) or to adipic acid dihydrazide-agarose beads (Sigma) as described previously (61).
The association of proteins with Y RNA agarose beads was analyzed by pull-down experiments and Western blotting as described previously (61). MOs were added to the bead suspensions at 1 ng/μl prior to incubation with cell extract.
DNA replication in vitro.
(i) Vertebrate somatic cell system. Cell culture, cell synchronization, preparation of template nuclei, extract fractionation, DNA replication in vitro, and analysis of DNA replication reactions were performed as described previously (10, 17, 26, 56, 57). Bromodeoxyuridine (BrdU) incorporation into Xenopus laevis XL177 cells was determined as described for mammalian cells (10, 11, 17).
(ii) Xenopus egg extract system. Demembranated sperm nuclei from Xenopus laevis were prepared as described previously (41). Low-speed extracts from activated Xenopus laevis eggs (LSS) were prepared according to (6), with minor modifications (33). DNA replication reactions were set up according to the methods described in reference 6, with the following modifications. Sperm nuclei were incubated in 20 μl of egg extract at 2.3 ng DNA/μl (i.e., 730 nuclei/μl at 3.15 pg DNA per haploid nucleus), supplemented with 1.2 μl of an energy-regenerating solution (0.8 M creatine phosphate, 2.5 mg/ml creatine kinase [both from Roche]). For detection of DNA replication by immunofluorescence microscopy, 0.4 μl of 1 mM digoxigenin-11-dUTP (Roche) was added per 20 μl of LSS extract; for quantification of DNA replication, 0.02 MBq of [α-33P]dCTP was added. Standard incubation conditions were 70 min at ambient temperature (20 to 22°C), unless indicated otherwise.
Xenopus Y RNAs were specifically degraded by endogenous RNase H activity by pretreating LSS extract with 3 μM antisense DNA oligonucleotides (Sigma-Genosys) for 30 min at ambient temperature (20 to 22°C), according to the methods described in reference 37. The sequences of the DNA antisense oligonucleotides are reported in Table 4; the standard T7 DNA sequencing primer served as a negative control.
Table 4.
Sequences of DNA antisense oligonucleotides
| DNA oligonucleotide | Sequence |
|---|---|
| Anti-xY3 | 5′-GAGGGGAGAAGAACAAAGAAGTCTG-3′ |
| Anti-xY4 | 5′-AGAAGGTTAGTGATACTAACTG-3′ |
| Anti-xY5 | 5′-GGGAATTTCGTAAACAAACGGT-3′ |
| Anti-xYα | 5′-TCTGTAAGTGGGAACTATTGATTCG-3′ |
The concentration of individual DNA oligonucleotides in the mix of anti-xY RNA oligonucleotides reflected the proportion of the relative abundance of the endogenous xY RNA in the early embryo (1.75 μM anti-xY3, 0.75 μM anti-xY4, and 0.5 μM anti-xY5). MOs were added at 1 μg/μl without prior preincubation.
Nuclear decondensation and DNA replication were visualized by confocal immunofluorescence microscopy using fluorescein isothiocyanate (FITC)-conjugated antidigoxigenin antibodies after fixation in 4% paraformaldehyde, as described previously (57). DNA replication was quantified by precipitation in trichloroacetic acid and scintillation counting or by integrated pixel intensity analysis (see below).
Xenopus embryo lysate system.
For preparing embryo lysates, about 200 microinjected embryos were lysed at the indicated stage by centrifugation, and the two liquid interphase fractions containing embryo cytosol with karyomeres and/or interphase nuclei were harvested as described previously (22). For pulse-labeling of nascent DNA, and 50 μl of lysate was supplemented with 3 μl of the energy-regenerating solution and 0.4 μl of 1 mM digoxigenin-11-dUTP (Roche) and incubated for 10 min at ambient temperature. DNA replication was visualized by confocal immunofluorescence microscopy, and total DNA was counterstained with propidium iodide (57). Identical microscopic settings were used for all panels. Karyomeres and interphase nuclei in pregastrula embryo lysates were identified according to size (39).
Knockdown of endogenous and expression of MO-insensitive ORC2 protein.
To knock down endogenous ORC2 protein levels, Xenopus embryos were injected at the one cell stage with ORC2-MO1 or MO2. They were harvested at stage 9 and solubilized in 1% NP-40, 150 mM NaCl, 20 mM Tris (pH 7.5), 2 mM EDTA, 50 mM NaF, 1 mM sodium pyrophosphate, supplemented with protease inhibitors (Roche Molecular Biochemicals). Proteins were extracted from yolk and cell debris with freon, separated by SDS-PAGE, and visualized by Western blotting.
An ORC2 open reading frame lacking the binding site for MO1 was generated from a full-length Xenopus laevis ORC2 cDNA clone (Thermo Fisher Scientific) by PCR with the following two primers: 5′-TTTATCGATATGAATGGCACTAAGCTTTTCAAGG-3′ and 5′-AAACTCGAGTCAAGTGTCTTTGTCTTCCATCTC-3′.
The PCR product was cloned in the ClaI and XhoI restriction sites of pCS2. This construct was linearized with NotI, RNA was transcribed with SP6 RNA polymerase, and 500 pg of this mRNA was coinjected with or without MO1 or Alexa Fluor 488-conjugated xY3 RNA. The expressed ORC2 protein is truncated by 9 amino acid residues at its N terminus.
Chromatin association of Y RNAs and immunofluorescence microscopy.
Between 50 and 100 Xenopus embryos were injected at the one-cell stage with 3 to 10 ng of Alexa Fluor 488-conjugated xY3 RNA, MOs, or a mixture of both and prefixed for 10 min at the indicated developmental stages in 10% NAM containing 1% formaldehyde. Embryos were subsequently lysed by centrifugation, and the interphase fraction containing karyomeres and/or nuclei was harvested as described previously (22). Nuclei were fixed in 4% paraformaldehyde in PBS for 5 min at room temperature and spun through a solution of 30% sucrose in PBS onto poly-l-lysine-coated coverslips. Samples were analyzed by confocal immunofluorescence microscopy as described previously (56), using the following antibodies against human proteins that would cross-react with Xenopus laevis proteins: ORC2 (from A. Schepers, Department of Gene Vectors, Munich, Germany), pan-MCM (BD Pharmingen), and CDC45 (from H.-P. Nasheuer, National University of Ireland, Galway, Ireland).
Computing.
Predicted secondary structures for all RNAs were calculated from the full-length nucleotide sequence using the Mfold v3.2 RNA folding algorithm (http://frontend.bioinfo.rpi.edu/applications/mfold/cgi-bin/rna-form1.cgi) under default conditions (34, 62). The thermodynamically most stable structures are presented. t tests (two-tailed, two-sample test of unequal variance) and analysis of variance (ANOVA; single-factor, between-groups test; α= 0.05) were performed with Microsoft Excel mac v.X software. Quantification of chromatin-associated Alexa Fluor-conjugated Y RNAs and per nucleus incorporation of digoxigenin-11-dUTP were determined by measuring the integrated pixel densities of individual nuclei on original grey-scale TIFF files of single-channel confocal images with ImageJ v1.43r software (http://rsb.info.hih.gov.ij), using plug-ins obtained from MacBiophotonics (http://www.macbiophotonics.ca). Background subtraction was applied with 2 standard deviations from the mean prior to analysis.
RESULTS
Expression of xY RNAs during early development of Xenopus laevis.
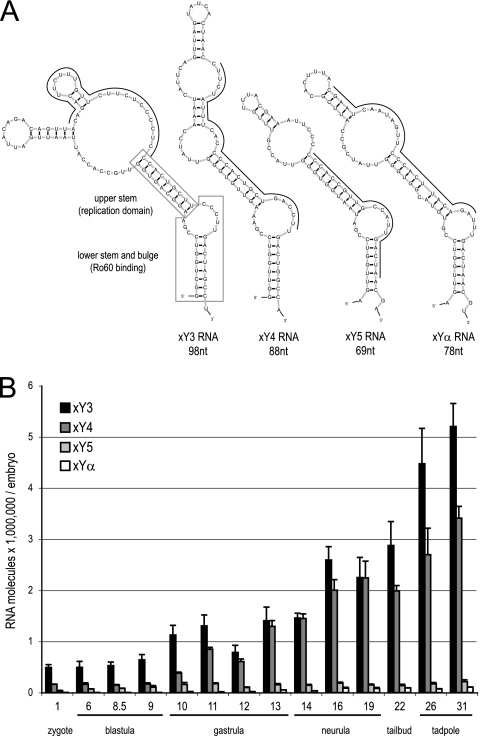
Four noncoding xY RNAs are present in Xenopus laevis (Fig. 1A). We first determined the temporal expression patterns of xY RNAs during early development by qRT-PCR (Fig. 1B). All four xY RNAs were present at all stages from zygote to tadpole. Levels of maternal transcripts did not change until the MBT at stage 8.5. The most abundant Y RNA was xY3, with about 500,000 molecules per embryo before MBT (Fig. 1B). Expression levels of xY4, xY5, and xYα RNA were 3-fold, 8-fold, and 182-fold lower, respectively. After MBT, the expression levels of all four xY RNAs increased (Fig. 1B). Thus, Y RNAs are present in substantial numbers throughout early development, and their zygotic expression is activated at MBT.
Fig. 1.
Y RNA expression during early Xenopus laevis development. (A) Nucleotide sequences and predicted secondary structures of Xenopus laevis Y RNAs (xY3, xY4, xY5, and xYα RNA). Positions of nucleotide sequences complementary to antisense MOs are indicated by thin black lines, and boxes show positions of evolutionarily conserved domains for xY3 RNA. (B) Temporal expression patterns of the indicated xY RNAs during development. Total RNA was prepared from embryos of each of the indicated developmental stages, and the abundance of each xY RNA per embryo was determined by quantitative RT-PCR.
Inhibition of Y RNA function is embryonic lethal in Xenopus.
To analyze the function of Y RNAs during Xenopus laevis development, we designed antisense MOs against the four xY RNAs (Fig. 1A). MOs hybridize with complementary RNA sequences, leading to an inhibition of the biological functions of the targeted RNA by disruption of secondary structures or by blocking interactions with proteins or other nucleic acids (13). MOs are stable because of their resistance to nucleolytic degradation. Conventionally, MOs are used to deplete cellular protein pools by inhibiting translation of their coding mRNAs (13). In this study, we adapted this technology to inhibit noncoding Y RNA function directly.
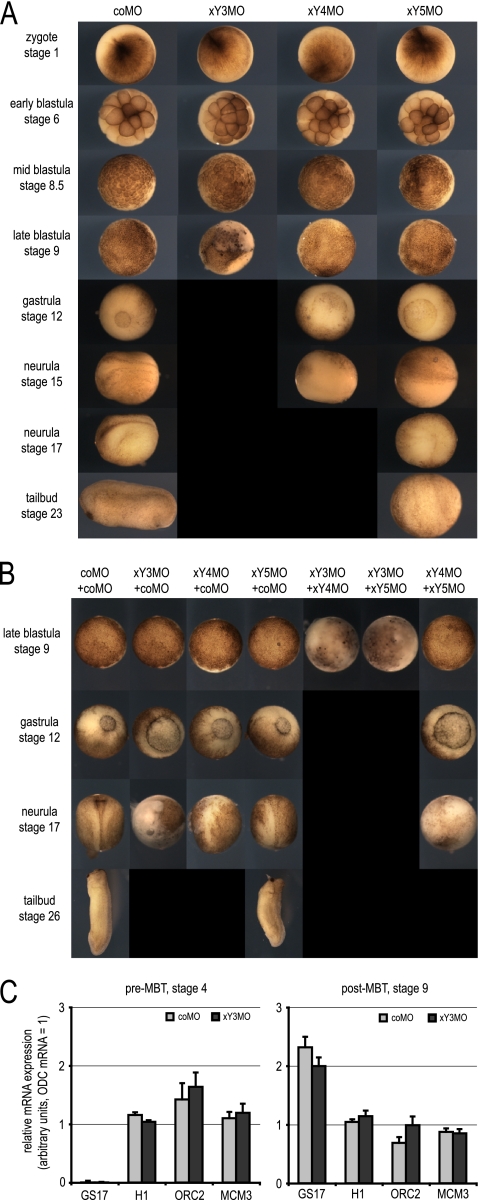
Injection of individual MOs against xY3, xY4, or xY5 RNA each caused a lethal phenotype (Fig. 2A). Injected embryos developed normally until MBT and then showed a delay in development and subsequently died. Embryos injected with xY3MO died soon after MBT at stage 9. Embryos injected with xY4MO and xY5MO showed delayed gastrulation and failed to develop a neural fold. They did not survive beyond the time at which control embryos had reached early neurula and tail bud stages, respectively (Fig. 2A). Injection with an MO against xYα RNA, which is expressed at much lower levels, did not detectably affect early development (data not shown). We note that the more abundant the targeted xY RNA, the earlier the onset of embryo lethality after xYMO injection.
Fig. 2.
Inhibition of Y RNA function is embryonic lethal after the midblastula transition in Xenopus laevis. (A) Phenotypes of developing Xenopus laevis embryos after microinjection of xY RNA-specific MOs. The indicated MOs (100 ng/embryo) were injected at stage 1, and representative embryos were photographed at the indicated stages. Black panels indicate that corresponding embryos had died and disintegrated by that stage. (B) Dose-dependent and additive lethal defects after comicroinjection of different xY RNA-specific antisense MOs. The indicated MOs (50 ng plus 50 μg/embryo) were microinjected at stage 1, and representative embryos were analyzed as described above. All embryos developed normally until stage 8.5. (C) Inhibition of xY RNA function does not affect zygotic transcription at the midblastula transition. Embryos were injected at stage 1 and harvested before MBT, when control embryos had reached stage 4, and after MBT, when control embryos had reached stage 9. Expression levels of GS17, histone H1, ORC2, and MCM3 mRNAs were determined by qRT-PCR relative to ODC mRNA.
We also investigated whether the phenotypes obtained upon depletion of a single xY RNA are dose dependent or additive. Injection of half the amount of each of any one xYMO, complemented with the same amount of the control MO (coMO), reduced the severity of the developmental phenotype and delayed its onset (Fig. 2B). Coinjection of xY3MO with either xY4MO or xY5MO, or coinjection of xY4MO with xY5MO, caused more severe phenotypes with advanced embryonic lethality than coinjection of any of these xYMOs with coMO (Fig. 2B). Therefore, xYMOs have dose-dependent and additive inhibitory effects on development, and the earliest embryo lethality after xY RNA inactivation is at stage 9, just after MBT.
xY RNA function is not required for the onset of transcription at MBT, nor for maintaining mRNA expression levels.
To test whether embryo lethality resulting from xYMO injection is due to an inhibition of transcription at MBT, or to a failure to maintain mRNA expression levels, we measured mRNA levels following injection of either coMO or xY3MO before and after MBT (Fig. 2C). GS17 mRNA, a marker for the onset of transcription (24), was not expressed before MBT at stage 4 but became expressed to similar levels at stage 9 following injection of either coMO or xY3MO (Fig. 2C). Levels of mRNAs coding for histone H1, which is incorporated into chromatin only after MBT, were similar before and after MBT following injection of either coMO or xY3MO. Finally, expression levels of mRNAs coding for the pre-RC subunits ORC2 and MCM3 were also similar after injection of either coMO or xY3MO, before and after MBT. Therefore, xY RNA function is not required for either the onset of transcription at MBT or for the maintenance of several mRNAs before or after MBT.
xYMOs target and inhibit xY RNA function specifically.
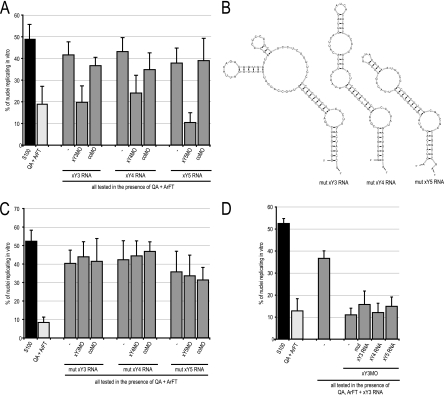
MOs can have unspecific off-target effects, and it is important to control for their specificity by using mismatch MOs and rescue experiments (13). Xenopus Y RNAs can functionally substitute for human hY RNAs to initiate chromosomal DNA replication in a human cell-free system (17). We used this established biochemical system to control for the specificity of xYMOs (Fig. 3).
Fig. 3.
xYMOs inhibit xY RNA function specifically. (A) xY RNA-specific MOs inhibit xY RNA-dependent initiation of chromosomal DNA replication in a cell-free system. Template nuclei from late-G1-phase human cells were incubated with protein fractions QA and ArFT in the presence of the indicated xY RNAs and MOs. Unfractionated human cell extract containing endogenous hY RNAs (S100) served as a positive control. Proportions of replicating nuclei were determined by immunofluorescence microscopy; mean values and standard deviations are shown. (B) Nucleotide sequences and predicted secondary structures of mutant xY RNAs (mut xY3, -4, and -5 RNA). The predicted structures of mutant xY RNAs are identical to their corresponding wild-type xY RNAs, and mutations are limited to sequences complementary to the respective MOs in the corresponding wild-type xY RNAs (c.f. Fig. 1A). The essential domain for DNA replication is preserved in these mutants. (C) Mutant xY RNAs initiate chromosomal replication in the human cell-free system, but their activity is not affected by xYMOs. (D) xY RNA/xYMO complexes are dominant-negative inhibitors of chromosomal DNA replication, which cannot be overcome by addition of mut xY3, wild-type xY4, or xY5 RNAs.
Late-G1-phase template nuclei from mammalian cells initiate semiconservative chromosomal DNA replication in this system upon incubation in cytosolic extract from proliferating human cells (25, 26). Fractionation of the extract by anion-exchange chromatography yields two essential protein fractions, termed QA and ArFT, that support initiation of DNA replication in the presence of purified vertebrate Y RNAs (10, 17). These fractions alone supported replication in only 10 to 20% of template nuclei, while addition of pure xY RNAs increased proportions of replicating nuclei significantly to 40 to 50% (Fig. 3A) (t tests, P < 0.02), as described previously (17). Addition of xYMOs returned percentages of replicating nuclei to background levels (Fig. 3A) (t tests, P < 0.02), while addition of coMO had no significant effect (Fig. 3A) (t tests, P > 0.2). These data show that xYMOs target their complementary xY RNAs, resulting in the inactivation of their function in the initiation of chromosomal DNA replication.
We next assessed the specificity of these xYMOs, asking whether they would also inhibit the activity of modified xY RNAs in which the complementary target nucleotide sequences were mutated. We designed mutant versions of xY3, xY4, and xY5 RNAs, in which the overall structures and essential nucleotide sequences in the upper stem required for DNA replication were maintained while the target sequences of the xYMOs were different. The nucleotide sequences and predicted structures of these mutant xY RNAs (mut xY3, mut xY4, and mut xY5 RNA) are shown in Fig. 3B. As expected, these three mutant xY RNAs supported the initiation of chromosomal DNA replication in the human cell-free system (Fig. 3C). Neither xYMOs nor coMO, however, inhibited the initiation of DNA replication mediated by these mutant xY RNAs, showing that the xYMOs used in this study specifically inactivate the function of their complementary wild-type xY RNAs.
We were unable to rescue the inhibitory effects of these MOs by addition of wild-type or mutant xY RNAs (Fig. 3D), and coinjection of xYMOs with nontargeted mutant xY RNAs did not rescue the lethal phenotypes in early embryos (data not shown). This lack of rescue is not an uncommon observation in experiments using antisense MOs (13). It is possible that xY RNAs and their complementary xYMOs form stable dominant-negative inhibitor complexes, which cannot be overcome by simple addition of excess Y RNA.
Y RNA is essential for early development in zebrafish after MBT.
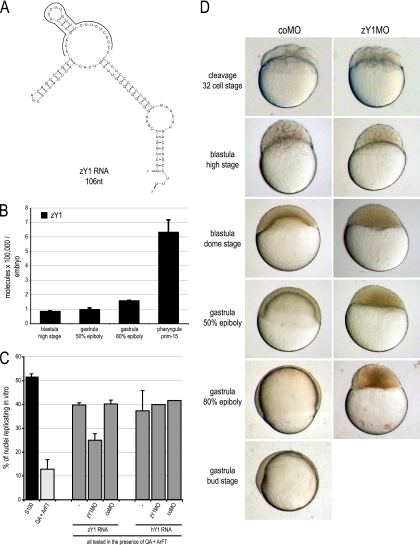
To address the evolutionary conservation of Y RNA function in vertebrate early development, we investigated whether the requirement of Y RNAs is unique to Xenopus laevis or whether they are also required in another vertebrate model system, the zebrafish Danio rerio (Fig. 4).
Fig. 4.
Inhibition of Y RNA function in zebrafish is embryonic lethal at the midblastula transition. (A) Nucleotide sequence and predicted secondary structure of zebrafish Y1 RNA (zY1 RNA). The positions of nucleotides complementary to zY1MO are indicated by a gray bar. (B) Quantification of zY1 RNA expression during early zebrafish development based on qRT-PCR. Total RNA was prepared from 10 embryos of each indicated developmental stage, and the abundance of zY1 RNA per embryo was determined by quantitative RT-PCR. (C) zY1MO targets and inhibits zY1 RNA function specifically in a human cell-free DNA replication system. Template nuclei from late-G1-phase human cells were incubated with protein fractions QA and ArFT and with zY1 or hY1 RNAs in the presence of MOs as indicated. Data were analyzed as for Fig. 3. Addition of zY1MO inhibited only the effect of zY1 RNA significantly (t test, P = 0.005), while the coMO had no significant effect (t tests, P > 0.7). (D) Inhibition of zY1 RNA function is embryonic lethal in zebrafish. The indicated MOs were injected at the one-cell stage. Representative embryos were photographed at the time postinjection when control-injected embryos had reached the indicated developmental stage. No differences between coMO- and zY1MO-injected embryos were observed before the 32-cell stage shown. A white panel indicates that no viable embryos were present at that particular stage of development.
Zebrafish contain only one Y RNA gene, which is homologous to Y1 RNA (40, 49). The nucleotide sequence and predicted secondary structure of zY1 RNA is shown in Fig. 4A. Maternally expressed zY1 RNA is present in early embryos before MBT, and its levels increase after the onset of zygotic gene expression at MBT (Fig. 4B). A morpholino oligonucleotide directed against zY1 RNA (zY1MO) inhibited zY1 RNA function in the human cell-free DNA replication system, demonstrating target specificity (Fig. 4C). Injection of zY1MO into fertilized zebrafish eggs arrested development of early zebrafish embryos at the midblastula stage between the high and dome stages (Fig. 4D). As in Xenopus, these arrested embryos did not survive beyond the time when control embryos had reached late gastrula stages, establishing an essential function of zY1 RNA for early zebrafish development after MBT.
We conclude that Y RNAs perform an essential function in early zebrafish and Xenopus development and that the onset after MBT is evolutionarily conserved.
xY RNA function becomes essential for DNA replication after MBT.
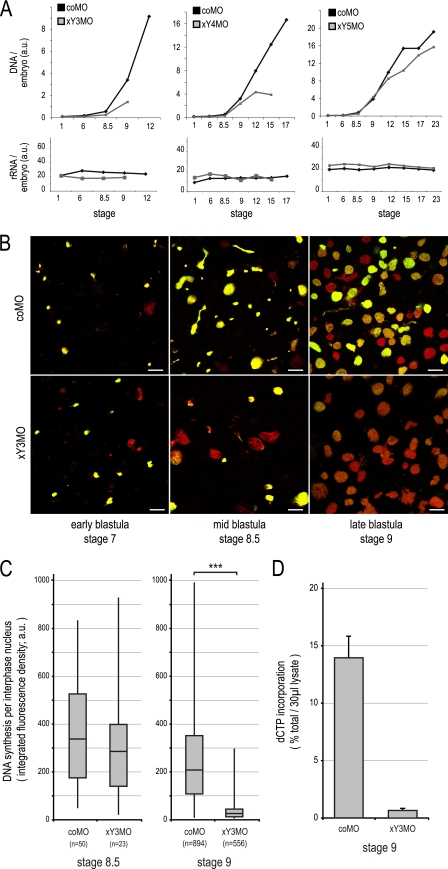
We next investigated the mechanism by which xYMOs cause embryonic death after MBT and asked whether inactivation of xY RNA function inhibits DNA replication during early development in Xenopus laevis (Fig. 5). The chromosomal DNA content of developing embryos showed an increase after injection of the coMO due to increasing cell numbers, as expected (Fig. 5A). Embryos injected with xY3MO, xY4MO, and xY5MO showed normal DNA contents up to MBT but contained much-reduced levels thereafter. As a control, the expression levels of rRNA did not detectably change during early development, as described previously (7), and they were not affected by injection of either coMO or xYMOs (Fig. 5A). Therefore, inhibition of xY RNA function results in reduced accumulation of chromosomal DNA content after MBT, preceding the death of the embryo.
Fig. 5.
xY RNA function becomes essential for DNA replication in Xenopus embryos after MBT. (A) Injection of xY RNA-specific MOs results in reduced embryonic DNA content after the midblastula transition. Control- and xY RNA-specific MOs were injected at stage 1, and total nucleic acid was isolated when control embryos had reached the indicated stages. DNA and rRNA quantification of a representative experiment is plotted. a.u., arbitrary units. (B) Visualization of chromosomal DNA replication around MBT by confocal immunofluorescence microscopy. Embryos were injected at stage 1, and lysates were prepared when control embryos had reached the indicated stages. Nascent DNA was pulse-labeled in embryo lysates with digoxigenin-dUTP, and replicated DNA was stained with FITC-conjugated antidigoxigenin antibodies (green signal). Total DNA was stained with propidium iodide (red signal); the overlap of red and green gives a yellow signal. Bars, 20 μm. (C) Quantification of digoxigenin-dUTP incorporation by chromosomal DNA replication in interphase nuclei at and after MBT. Box-and-whisker plots of nascent DNA signal intensities are shown for the indicated nuclei at stages 8.5 and 9. Combined distributions from two independent experiments are plotted. A statistically significant difference between matched populations is indicated (***) (ANOVA, P = 4.7 × 10−3). (D) Quantification of radioactively labeled dCTP into replicating nuclei after MBT. Nascent DNA was pulse-labeled in embryo lysates with [33P]dCTP and quantified by trichloroacetic acid precipitation and scintillation counting.
To investigate whether DNA replication is directly affected by xY RNA inactivation in blastula embryos, we prepared embryo lysates (22) and measured run-on replication in the endogenous karyomeres and interphase nuclei (Fig. 5B to D). In control embryos before MBT (stage 7), more than 90% of all replicating structures were karyomeres with diameters of 2 to 6 μm (Fig. 5B, top row). At MBT (stage 8.5), a mixture of replicating karyomeres and interphase nuclei with diameters of 10 to 20 μm was observed. After MBT (stage 9), karyomeres disappeared and DNA replication was apparent in 70 to 80% of the interphase nuclei. These data are consistent with earlier analyses of pregastrula embryo ultrastructure and DNA replication dynamics (31, 39). After injection of xY3MO, DNA replication was not detectably affected in karyomeres in stage 7 pre-MBT embryos (Fig. 5B, bottom row). At MBT, percentages of replicating karyomeres remained unchanged. The proportion of replicating interphase nuclei (i.e., S-phase nuclei) decreased about 2-fold, but the amount of nucleotide incorporation per S-phase nucleus did not change significantly at this stage (Fig. 5C, left panel) (ANOVA, P = 0.27). After MBT, but before the embryos died, a severe inhibition of DNA replication was apparent in all interphase nuclei (Fig. 5B). The proportion of S-phase nuclei decreased about 2-fold, and residual DNA replication in these S-phase nuclei was restricted to a few intranuclear sites. Significantly, the incorporation of nucleotides per S-phase nucleus had decreased about 10-fold at this stage (Fig. 5C, right panel) (ANOVA, P = 4.7 × 10−113). This 10-fold reduction of DNA synthesis after MBT was confirmed by quantifying the incorporation of radiolabeled dCTP into acid-insoluble DNA (Fig. 5D).
We also considered the possibility that this inhibition of DNA replication and occurrence of embryo death after MBT might be due to accumulated DNA damage suffered before MBT. We performed terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining in stage-8.5 embryo lysates to detect DNA strand breaks, but we were unable to observe any significant increase of signal upon injection of either coMO or xY3MO (data not shown).
From these data, we conclude that xY RNAs are required for chromosomal DNA replication only after MBT, concurrent with the disappearance of karyomeres and with the establishment of interphase nuclei as templates for initiation.
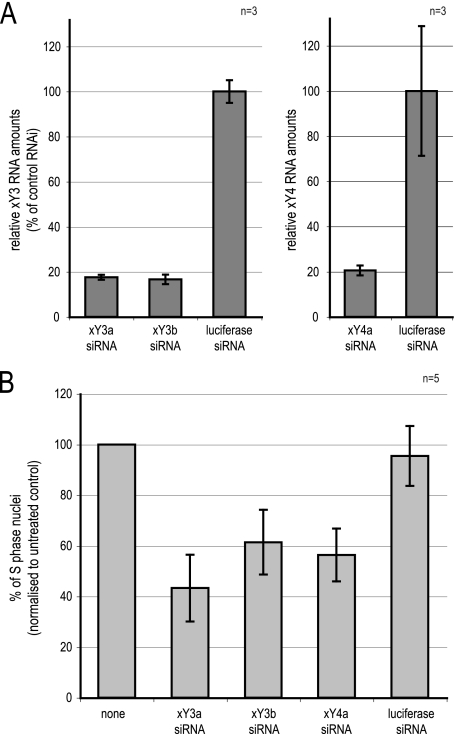
To corroborate this conclusion independently, we analyzed by RNA interference (RNAi) the requirement of xY RNA function in XL177 cells (Fig. 6), which are derived from epithelial tissue of stage 40 post-MBT Xenopus laevis tadpoles (14). Transfection of siRNAs against xY3 and xY4 RNAs in asynchronously proliferating XL177 cells reduced the intracellular levels of the targeted xY RNAs to about 20% of the control (Fig. 6A), resulting in the reduction of replicating S-phase cells in the population to about 50% (Fig. 6B). These data support an essential Y RNA function for DNA replication in post-MBT cells, to a similar extent as demonstrated in mammalian cells (10, 11, 17).
Fig. 6.
Y RNA depletion inhibits DNA replication in Xenopus XL177 cells. (A) Quantification of relative xY RNA levels after RNAi. Asynchronously proliferating XL177 cells were transfected with siRNAs against xY3 (siRNAs xY3a and xY3b) and xY4 RNA (siRNA xY4a). A nontarget siRNA against firefly luciferase was used as a control. At 24 h after RNAi, the indicated xY RNAs were quantified relative to 5S rRNA by qRT-PCR. Data were normalized to the firefly luciferase values. Mean values and standard deviations of 3 qPCRs of a representative experiment are shown. (B) Quantification of replicating S-phase cells after RNAi in vivo. At 23 h after transfection, replicating cell nuclei were pulse-labeled for 1 h with BrdU. At 24 post-RNAi, percentages of BrdU-incorporating cell nuclei were determined by immunofluorescence microscopy. Percentages of replicating S-phase nuclei were normalized to an untreated control (none) for each independent experiment. Mean values and standard deviations of 5 independent experiments are shown. Absolute values were 24 ± 13.2% S-phase cells in the untreated controls.
DNA replication in Xenopus egg extract does not require Y RNA function.
Xenopus egg extracts mimic the regulation of DNA replication before MBT (3, 6). Our results therefore suggest that Y RNA function would not be required for DNA replication in egg extracts. We tested this prediction by asking whether inactivation or degradation of xY RNAs affects DNA replication in Xenopus egg extracts (Fig. 7).
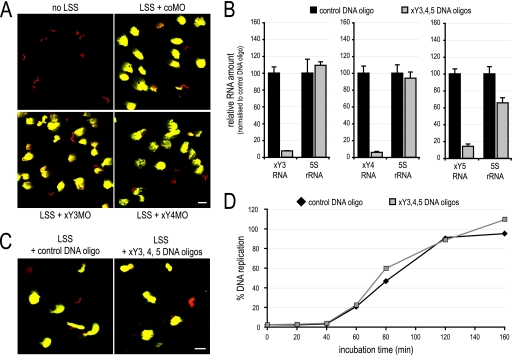
Fig. 7.
DNA replication in Xenopus egg extracts does not require Y RNA function. (A) Nuclear decondensation and DNA replication in the presence of MOs. Xenopus sperm chromatin was incubated in buffer or Xenopus egg extract (low-speed supernatant [LSS]) supplemented with the indicated MOs. DNA replication was analyzed as detailed for Fig. 5B. Bars, 20 μm. (B) Degradation of endogenous xY RNAs. Xenopus egg extract was preincubated in the presence of the indicated DNA antisense oligonucleotides to target RNase H activity to the complementary RNA. Total RNA was prepared from the treated egg extracts, and abundances of the indicated RNAs were determined by qRT-PCR. Data are expressed as percentages of the control-treated extracts. (C) Visualization of nuclear decondensation and DNA replication after Y RNA degradation. Sperm chromatin was incubated in egg extract (LSS) that was pretreated with the indicated DNA antisense oligonucleotides. Data were analyzed as for panel A. (D) Time course of DNA replication in control- and xY RNA-depleted egg extracts. Sperm chromatin was incubated for the indicated time in pretreated egg extracts, and DNA replication was quantified. Incorporation is expressed as the percentage of input template DNA; mean values of two parallel data acquisitions of one representative experiment are shown.
Demembranated sperm nuclei decondensed and replicated their DNA upon incubation in low-speed extract from activated Xenopus laevis eggs (Fig. 7A). Addition of either xY3MO or xY4MO had no effect on nuclear decondensation or DNA replication, even at a 10-fold-higher concentration than that used in microinjection experiments (Fig. 7A). Identical negative results were obtained upon addition of xY5MO, xYαMO, or a combination of all xYMOs (data not shown). To corroborate these findings, we also depleted xY RNA levels in egg extracts by specific degradation of xY RNAs. Addition of an antisense DNA oligonucleotide to Xenopus egg extract directs RNase H activity to the DNA/RNA hybrid, resulting in the degradation of the targeted RNA within minutes (37). We thus degraded xY3, xY4, and xY5 RNA in Xenopus egg extracts by addition of antisense DNA oligonucleotides (Fig. 7B) and found that sperm chromatin decondensation and DNA replication were unaffected (Fig. 7C). Furthermore, DNA replication showed identical kinetics of nucleotide incorporation after xY3 to -5 RNA degradation as in the control (Fig. 7D).
Egg extracts initiate DNA replication in permeabilized mammalian cell nuclei (18, 32). To investigate whether Y RNA function is required for this, we incubated Dounce-ruptured late-G1-phase human cell nuclei in Xenopus egg extracts in the presence of coMO or xYMOs. Identical proportions of 88% ± 1% of these nuclei replicated the DNA under both conditions.
We conclude that DNA replication in Xenopus egg extracts does not require Y RNA function, which is consistent with the fact that these extracts are derived from pre-MBT embryos.
xY3 RNA associates with chromatin after MBT, but not before.
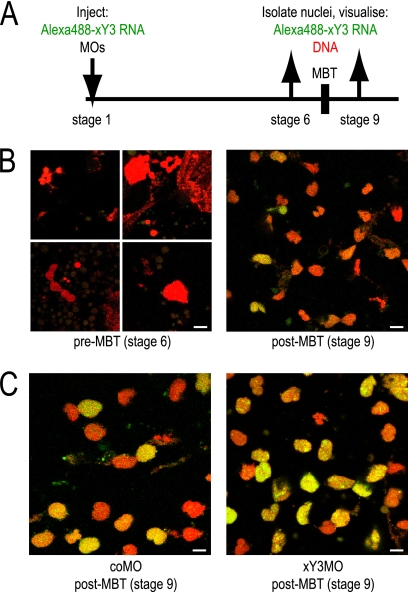
Y RNAs associate with unreplicated chromatin in proliferating human cell nuclei but are displaced from these sites once DNA replication is initiated in a Y RNA-dependent manner (61). We therefore investigated by microinjection of fluorescently labeled xY3 RNA whether chromatin association of xY RNA is regulated during Xenopus laevis development (Fig. 8A). xY3 RNA did not associate with karyomere structures before MBT at stage 6 (Fig. 8B, left panels). In contrast, xY3 RNA did associate with more than 50% of interphase nuclei after MBT at stage 9 (Fig. 8B, right panel). We conclude that the association of Y RNA with chromatin is regulated during Xenopus development and occurs only after MBT, when DNA replication becomes dependent on Y RNAs.
Fig. 8.
xY3 RNA associates with chromatin after MBT, but not before. (A) Experimental design. (B) xY3 RNA associates with chromatin only after MBT. Alexa Fluor 488-conjugated xY3 RNA was directly visualized by confocal fluorescence microscopy (green signal), and DNA was counterstained with propidium iodide (red signal). (C) xY3MO does not prevent chromatin association of xY3 RNA after the MBT. Bars, 10 μm.
It is important to note, however, that the association of xY3 RNA with post-MBT nuclei was not disrupted by coinjection of xY3MO (Fig. 8C), suggesting that MOs do not simply inhibit Y RNA function by preventing their association with chromatin. In the next experiments, we therefore investigated the mechanism by which Y RNA-specific MOs inhibit DNA replication after MBT.
Y RNA-specific MOs disrupt association between Y RNAs and pre-RC proteins.
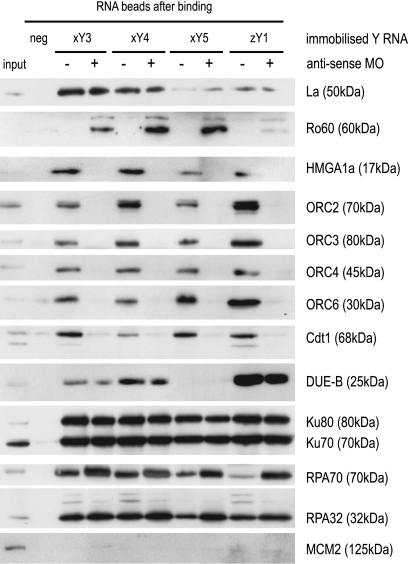
Human Y RNAs associate with ORC, Cdt1, and other proteins involved in the initiation of chromosomal DNA replication (61). Therefore, we investigated whether Y RNA-specific MOs inhibit this association (Fig. 9). Purified xY3, xY4, xY5, and zY1 RNAs were coupled to agarose beads and incubated with cell extracts in the presence of coMO or the Y RNA-specific MOs, and associated proteins were analyzed by Western blotting.
Fig. 9.
Y RNA-specific MOs disrupt association between Y RNAs and pre-RC proteins. The four indicated Y RNAs (xY3, xY4, xY5, and zY1) were covalently coupled to adipic acid dihydrazide agarose beads and incubated with HeLa cell extracts in the presence of the indicated antisense MOs (+) or coMO (−). 6B Sepharose beads were used as negative controls (neg). Beads were isolated and washed, and associated proteins were identified by Western blotting. As a reference, 3% of the input cell extract was loaded on the gel. The apparent molecular mass is given for each protein.
La protein, which binds to the 3′ terminus of Y RNAs (60), associated with all Y RNAs in the presence of either MO (Fig. 9). Ro60 protein binds to the bulged stem structure of Y RNAs and has been implicated in RNA surveillance and quality control via binding to misfolded RNAs (9, 16, 48, 55). In contrast to La, Ro60 associated very weakly with Y RNAs in the presence of coMO, but very strongly in the presence of Y RNA-specific MOs (Fig. 9). This specificity of Ro60 for functionally inactive Y RNA/MO complexes supports the model that MOs inactivate target Y RNA function by disrupting their native secondary structure.
Importantly, the ORC, the pre-RC protein Cdt1, and the ORC-binding chromatin protein HMGA1a all associated strongly with all tested Y RNAs in the presence of coMO, but not in the presence of Y RNA-specific MOs (Fig. 9). In contrast, other initiation proteins known to interact with human Y RNAs (61), namely, DUE-B, Ku70/80, and RPA, all associated with Y RNAs in the presence of either coMO or Y RNA-specific MOs. As a negative control, MCM2 protein did not associate with any Y RNA under any of these conditions, as seen previously for human Y RNAs (61). We conclude that the association of Y RNAs with ORC, Cdt1, and HMGA1a is specifically disrupted by MOs, strongly suggesting that these interactions are functionally relevant for the role of Y RNAs for chromosomal DNA replication after MBT.
Association of Y RNAs with post-MBT chromatin is ORC dependent.
Y RNA-specific MOs do not inhibit association of Y RNAs with post-MBT chromatin (Fig. 8C), but they do disrupt the interaction between Y RNAs and ORC/Cdt1 (Fig. 9). We therefore tested the hypothesis that chromatin-associated Y RNAs are required for pre-RC or pre-IC loading after MBT. Injection of xY3MO did not prevent association of ORC, MCM2-7, or Cdc45 with chromatin in post-MBT embryos (data not shown), demonstrating that Y RNA function is not required for pre-RC or pre-IC loading.
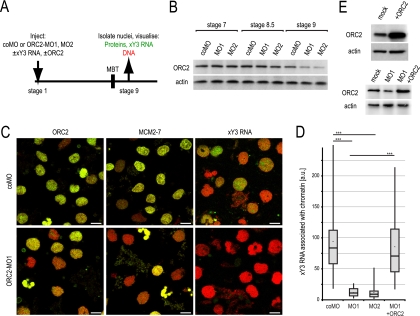
Therefore, we went on to investigate whether the inverse was the case and tested if ORC is required for the association of Y RNAs with chromatin after MBT (Fig. 10). Expression of ORC2 was knocked down by injection of two antisense morpholino oligonucleotides, MO1 and MO2 (Fig. 10A), resulting in a clear reduction of endogenous ORC2 protein at stage 9 (Fig. 10B) and in embryo lethality during gastrulation (data not shown). As expected, this ORC2 depletion led to reduced pools of chromatin-associated ORC2 and MCM2-7 at stage 9 (Fig. 10C) and to a strong and significant inhibition of the association of xY3 RNA with post-MBT chromatin (Fig. 10C and D). By coinjecting an ORC2 mRNA that lacks the ORC2-MO1 binding site, we could rescue expression of ORC2 protein (Fig. 10E) and association of xY3 RNA with chromatin (Fig. 10D). We thus conclude that the association of xY3 RNA with chromatin after the MBT is ORC dependent.
Fig. 10.
ORC is required for Y RNA loading onto post-MBT chromatin. (A) Experimental design. (B) Knockdown of ORC2 expression. Xenopus laevis embryos were injected with coMO or two MOs against ORC2 protein (MO1 and MO2), lysates were prepared at the indicated stages, and ORC2 protein was investigated by Western blotting. Actin was used as a loading control. (C) Y RNA association after MBT depends on ORC. After injection of coMO or MO1, the association of ORC2, MCM2-7, and Alexa Fluor 488-conjugated xY3 RNA with chromatin was visualized by fluorescence microscopy (green signals); DNA was counterstained with propidium iodide (red signals). Bars, 10 μm. (D) Quantification of chromatin-associated xY3 RNA. For each individual nucleus, the amount of chromatin-associated Alexa Fluor 488-conjugated xY3 RNA was measured as the integrated fluorescence intensity, as detailed in Materials and Methods. Box-and-whisker plots for each distribution are shown; the median is indicated by a horizontal line, and the mean is shown by the asterisk within the boxed 25th to 75th percentile. Statistically significant differences between matched distributions are bracketed and are indicated by triple asterisks (t tests, P < 1.5 × 10−18). (E) Exogenous expression of ORC2 protein in stage 9 embryos. Stage 1 embryos were injected with a recombinant ORC2 mRNA lacking the binding site for MO1 in the absence or presence of MO1 (top and bottom panel, respectively). ORC2 protein was detected by Western blotting.
DISCUSSION
Here we show that Y RNAs are essential for DNA replication in Xenopus and zebrafish, establishing that their function is evolutionarily conserved in vertebrates. Injection of Y RNA-specific antisense MOs into fertilized eggs of both species results in the death of the developing embryo just after MBT. This mortality is associated with an inhibition of DNA replication. Surprisingly, DNA replication is not sensitive to an inhibition of Y RNA function before MBT, as demonstrated both in whole embryos and in egg and embryo extracts. Y RNAs associate with post-MBT chromatin in an ORC-dependent manner, and Y RNA-specific MOs disrupt the association between Y RNAs and the initiation proteins ORC/Cdt1. Our observations therefore demonstrate that DNA replication is regulated differently before and after MBT. As discussed below, the MBT is a transition point between Y RNA-independent control of vertebrate DNA replication in eggs and early embryos and Y RNA-dependent control in later embryos and adult cells.
DNA replication before and after MBT.
Why does the requirement for Y RNAs differ before and after MBT? One clue may come from the observation that Xenopus egg extracts contain an activity that overrides aspects of the regulation of DNA replication in somatic cells. For example, permeabilized mammalian cell nuclei incubated in Xenopus egg extracts no longer initiate DNA replication with a preference for particular origin sequences (18, 32). We have shown here that such nuclei initiate DNA replication in Xenopus egg extracts with and without prior inactivation of xY RNA function. In contrast, when incubated in somatic human cell extracts, these nuclei initiate DNA replication only in the presence of Y RNAs (10, 28), and they do so in an origin-specific manner (23). Xenopus egg extracts, therefore, overcome the requirement of Y RNAs for DNA replication in a dominant manner. The identity of the factor responsible for this effect is unknown. Its inactivation at MBT may be caused by the increase in the nucleus-to-cytoplasm ratio occurring during early development (43), or its expression or biochemical activity may be lost at MBT.
The Xenopus egg extract is a powerful and well-established experimental system for studying the regulation of DNA replication before MBT (3, 6). Nevertheless, our data now raise the question as to whether it is also a suitable model system for the control of the initiation step of DNA replication in somatic vertebrate cells. We would certainly argue that it is unsuitable to study the mechanistic role of Y RNAs in DNA replication.
At MBT, the nuclear structure supporting chromosomal DNA replication changes dramatically. During early cleavages, DNA replication begins in individual karyomeres at the mitotic anaphase (31, 39). Replication does not require any particular DNA sequence, and it occurs at a regular spacing of about 10 kb (6, 19, 21, 22, 36). After MBT, from cycle 13 onwards, karyomeres no longer exist and DNA replication initiates in conventional G1-phase nuclei (31). The length of an individual S phase increases significantly, the spacing between initiation events increases and, within the rRNA gene cluster, DNA replication initiates in nontranscribed spacers rather than in transcribed genes (21). Here we showed that, in contrast to replication before MBT, initiation of DNA replication after MBT requires Y RNA function. Thus, the requirement for noncoding Y RNA function after MBT defines the onset of a differently regulated pathway for vertebrate DNA replication initiation control.
Mode of action of Y RNAs.
The ORC has been reported to employ noncoding RNA function to regulate the initiation of DNA replication in diverse biological systems (12, 27, 38, 46, 47). Here, we show that ORC is required to recruit noncoding Y RNAs to post-MBT chromatin in vertebrates, and we have also demonstrated that the interaction between ORC and Y RNAs is functionally relevant for DNA replication after MBT. It is important to note, however, that other factors are also likely to mediate the association of Y RNAs with chromatin, because xYMOs do not prevent the association of Y RNAs with chromatin, despite preventing their interaction with the ORC. It is therefore possible that misfolded or inactivated Y RNAs bind chromatin nonspecifically or through alternative pathways. This may involve increased binding to Ro60 protein as reported here, as part of a Ro60-dependent surveillance pathway for noncoding RNAs (9, 16, 48, 55). In support of such a dispatch pathway for dysfunctional Y RNAs, we recently showed that RNPs containing Ro60 and Y RNAs are not involved in DNA replication control in the human cell-free system (29).
Noncoding RNAs other than Y RNAs, such as structured G-rich RNAs and the rRNA-derived 26T RNA, have been shown to play specific regulatory roles in recruiting ORC to eukaryotic DNA replication origins (27, 38, 46, 47). We have shown here that Y RNAs do not recruit ORC to chromatin, but conversely, that ORC is required for recruiting Y RNAs to chromatin. This shows that Y RNAs have a different function in the ORC-dependent regulation of the initiation of DNA replication than do noncoding G-rich and 26T RNAs.
In human somatic cells, Y RNAs mark unreplicated chromatin and are displaced from replicated chromatin locally after the Y RNA-dependent initiation step (61). This suggests that Y RNAs fulfill the “activator” function in the original licensing factor model for once-per-cell-cycle control of eukaryotic DNA replication (5, 30), like the pre-RC proteins Cdt1 and MCM2-7. Unlike the established pre-RC proteins, Y RNAs are not essential for DNA replication before the MBT in Xenopus and zebrafish. It is thus tempting to speculate that Y RNAs have evolved in vertebrates as an additional, and developmentally regulated, layer of DNA replication control over and above the evolutionarily conserved eukaryotic DNA replication machinery. This may provide a further safeguard against uncontrolled initiation and genome instability in cell division cycles during post-MBT development.
ACKNOWLEDGMENTS
We thank John Gurdon and Kazutaka Murata for providing demembranated Xenopus laevis sperm chromatin and Tony Mills for initial advice on Xenopus egg extract preparation and on the Xenopus DNA replication system. We also thank Alexander Langley, John Gurdon, Olivier Hyrien, and Tony Mills for critical reading and discussions.
This work was supported by Cancer Research UK (project grant C1471/A8448), the UK Medical Research Council (program number U117597140) and the Wellcome Trust. The University of Cambridge Department of Zoology confocal suite was financed by the Wellcome Trust and the Newton Trust.
Footnotes
Published ahead of print on 26 July 2011.
REFERENCES
- 1. Aladjem M. I. 2007. Replication in context: dynamic regulation of DNA replication patterns in metazoans. Nat. Rev. Genet. 8:588–600 [DOI] [PubMed] [Google Scholar]
- 2. Amaral P. P., Mattick J. S. 2008. Noncoding RNA in development. Mamm. Genome 19:454–492 [DOI] [PubMed] [Google Scholar]
- 3. Arias E. E., Walter J. C. 2004. Initiation of DNA replication in xenopus egg extracts. Front. Biosci. 9:3029–3045 [DOI] [PubMed] [Google Scholar]
- 4. Arias E. E., Walter J. C. 2007. Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev. 21:497–518 [DOI] [PubMed] [Google Scholar]
- 5. Blow J. J., Dilworth S. M., Dingwall C., Mills A. D., Laskey R. A. 1987. Chromosome replication in cell-free systems from Xenopus eggs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 317:483–494 [DOI] [PubMed] [Google Scholar]
- 6. Blow J. J., Laskey R. A. 1986. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell 47:577–587 [DOI] [PubMed] [Google Scholar]
- 7. Brown D. D., Littna E. 1964. RNA synthesis during the development of Xenopus laevis, the South African clawed toad. J. Mol. Biol. 8:669–687 [DOI] [PubMed] [Google Scholar]
- 8. Carthew R. W., Sontheimer E. J. 2009. Origins and mechanisms of miRNAs and siRNAs. Cell 136:642–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen X., Wolin S. L. 2004. The Ro 60 kDa autoantigen: insights into cellular function and role in autoimmunity. J. Mol. Med. 82:232–239 [DOI] [PubMed] [Google Scholar]
- 10. Christov C. P., Gardiner T. J., Szüts D., Krude T. 2006. Functional requirement of noncoding Y RNAs for human chromosomal DNA replication. Mol. Cell. Biol. 26:6993–7004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christov C. P., Trivier E., Krude T. 2008. Noncoding human Y RNAs are overexpressed in tumours and required for cell proliferation. Br. J. Cancer 98:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donti T. R., Datta S., Sandoval P. Y., Kapler G. M. 2009. Differential targeting of Tetrahymena ORC to ribosomal DNA and non-rDNA replication origins. EMBO J. 28:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisen J. S., Smith J. C. 2008. Controlling morpholino experiments: don't stop making antisense. Development 135:1735–1743 [DOI] [PubMed] [Google Scholar]
- 14. Ellison T. R., Mathisen P. M., Miller L. 1985. Developmental changes in keratin patterns during epidermal maturation. Dev. Biol. 112:329–337 [DOI] [PubMed] [Google Scholar]
- 15. Farris A. D., O'Brien C. A., Harley J. B. 1995. Y3 is the most conserved small RNA component of Ro ribonucleoprotein complexes in vertebrate species. Gene 154:193–198 [DOI] [PubMed] [Google Scholar]
- 16. Fuchs G., Stein A. J., Fu C., Reinisch K. M., Wolin S. L. 2006. Structural and biochemical basis for misfolded RNA recognition by the Ro autoantigen. Nat. Struct. Mol. Biol. 13:1002–1009 [DOI] [PubMed] [Google Scholar]
- 17. Gardiner T. J., Christov C. P., Langley A. R., Krude T. 2009. A conserved motif of vertebrate Y RNAs essential for chromosomal DNA replication. RNA 15:1375–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilbert D. M., Miyazawa H., DePamphilis M. L. 1995. Site-specific initiation of DNA replication in Xenopus egg extract requires nuclear structure. Mol. Cell. Biol. 15:2942–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harland R. M., Laskey R. A. 1980. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell 21:761–771 [DOI] [PubMed] [Google Scholar]
- 20. Hogg J. R., Collins K. 2008. Structured non-coding RNAs and the RNP renaissance. Curr. Opin. Chem. Biol. 12:684–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hyrien O., Maric C., Mechali M. 1995. Transition in specification of embryonic metazoan DNA replication origins. Science 270:994–997 [DOI] [PubMed] [Google Scholar]
- 22. Hyrien O., Mechali M. 1993. Chromosomal replication initiates and terminates at random sequences but at regular intervals in the ribosomal DNA of Xenopus early embryos. EMBO J. 12:4511–4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keller C., Hyrien O., Knippers R., Krude T. 2002. Site-specific and temporally controlled initiation of DNA replication in a human cell-free system. Nucleic Acids Res. 30:2114–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krieg P. A., Melton D. A. 1985. Developmental regulation of a gastrula-specific gene injected into fertilized Xenopus eggs. EMBO J. 4:3463–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krude T. 2006. Initiation of chromosomal DNA replication in mammalian cell-free systems. Cell Cycle 5:2115–2122 [DOI] [PubMed] [Google Scholar]
- 26. Krude T. 2000. Initiation of human DNA replication in vitro using nuclei from cells arrested at an initiation-competent state. J. Biol. Chem. 275:13699–13707 [DOI] [PubMed] [Google Scholar]
- 27. Krude T. 2010. Non-coding RNAs: new players in the field of eukaryotic DNA replication. Subcell. Biochem. 50:105–118 [DOI] [PubMed] [Google Scholar]
- 28. Krude T., Christov C. P., Hyrien O., Marheineke K. 2009. Y RNA functions at the initiation step of mammalian chromosomal DNA replication. J. Cell Sci. 122:2836–2845 [DOI] [PubMed] [Google Scholar]
- 29. Langley A. R., Chambers H., Christov C. P., Krude T. 2010. Ribonucleoprotein particles containing non-coding Y RNAs, Ro60, La and nucleolin are not required for Y RNA function in DNA replication. PLoS One 5:e13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laskey R. A., Harland R. M., Earnshaw W. C., Dingwall C. 1981. Chromatin assembly and the co-ordination of DNA replication in the eukaryotic chromosome, p. 162–167 In Schweiger H. G. (ed.), International Cell Biology 1980–1981. Springer Verlag, Berlin, Germany [Google Scholar]
- 31. Lemaitre J. M., Geraud G., Mechali M. 1998. Dynamics of the genome during early Xenopus laevis development: karyomeres as independent units of replication. J. Cell Biol. 142:1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leno G. H., Downes C. S., Laskey R. A. 1992. The nuclear membrane prevents replication of human G2 nuclei but not G1 nuclei in Xenopus egg extract. Cell 69:151–158 [DOI] [PubMed] [Google Scholar]
- 33. Marheineke K., Goldar A., Krude T., Hyrien O. 2009. Use of DNA combing to study DNA replication in xenopus and human cell-free systems. Methods Mol. Biol. 521:575–603 [DOI] [PubMed] [Google Scholar]
- 34. Mathews D. H., Sabina J., Zuker M., Turner D. H. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911–940 [DOI] [PubMed] [Google Scholar]
- 35. Mechali M. 2010. Eukaryotic DNA replication origins: many choices for appropriate answers. Nat. Rev. Mol. Cell Biol. 11:728–738 [DOI] [PubMed] [Google Scholar]
- 36. Mechali M., Kearsey S. 1984. Lack of specific sequence requirement for DNA replication in Xenopus eggs compared with high sequence specificity in yeast. Cell 38:55–64 [DOI] [PubMed] [Google Scholar]
- 37. Minshull J., Blow J. J., Hunt T. 1989. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell 56:947–956 [DOI] [PubMed] [Google Scholar]
- 38. Mohammad M. M., Donti T. R., Sebastian Yakisich J., Smith A. G., Kapler G. M. 2007. Tetrahymena ORC contains a rRNA fragment that participates in rDNA origin recognition. EMBO J. 26:5048–5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Montag M., Spring H., Trendelenburg M. F. 1988. Structural analysis of the mitotic cycle in pre-gastrula Xenopus embryos. Chromosoma 96:187–196 [DOI] [PubMed] [Google Scholar]
- 40. Mosig A., Guofeng M., Stadler B. M. R., Stadler P. F. 2007. Evolution of the vertebrate Y RNA cluster. Theor. Biosci. 129:9–14 [DOI] [PubMed] [Google Scholar]
- 41. Murray A. W. 1991. Cell cycle extracts. Methods Cell Biol. 36:581–605 [PubMed] [Google Scholar]
- 42. Nagai T., et al. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20:87–90 [DOI] [PubMed] [Google Scholar]
- 43. Newport J., Kirschner M. 1982. A major developmental transition in early Xenopus embryos. I. Characterization and timing of cellular changes at the midblastula stage. Cell 30:675–686 [DOI] [PubMed] [Google Scholar]
- 44. Newport J., Kirschner M. 1982. A major developmental transition in early Xenopus embryos. II. Control of the onset of transcription. Cell 30:687–696 [DOI] [PubMed] [Google Scholar]
- 45. Nieuwkoop P. D., Faber J. 1975. Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis, 2nd ed North-Holland Publishing Co., Amsterdam, Netherlands [Google Scholar]
- 46. Norseen J., Johnson F. B., Lieberman P. M. 2009. Role for G-quadruplex RNA binding by Epstein-Barr virus nuclear antigen 1 in DNA replication and metaphase chromosome attachment. J. Virol. 83:10336–10346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Norseen J., et al. 2008. RNA-dependent recruitment of the origin recognition complex. EMBO J. 27:3024–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Brien C. A., Wolin S. L. 1994. A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev. 8:2891–2903 [DOI] [PubMed] [Google Scholar]
- 49. Perreault J., Perreault J. P., Boire G. 2007. The Ro associated Y RNAs in metazoans: evolution and diversification. Mol. Biol. Evol. 24:1678–1689 [DOI] [PubMed] [Google Scholar]
- 50. Ponting C. P., Oliver P. L., Reik W. 2009. Evolution and functions of long noncoding RNAs. Cell 136:629–641 [DOI] [PubMed] [Google Scholar]
- 51. Pruijn G. J., Wingens P. A., Peters S. L., Thijssen J. P., van Venrooij W. J. 1993. Ro RNP associated Y RNAs are highly conserved among mammals. Biochim. Biophys. Acta 1216:395–401 [DOI] [PubMed] [Google Scholar]
- 52. Rollins M. B., Andrews M. T. 1991. Morphogenesis and regulated gene activity are independent of DNA replication in Xenopus embryos. Development 112:559–569 [DOI] [PubMed] [Google Scholar]
- 53. Signoret J., Lefresne J. 1971. Contribution a l'etude de la segmentation de l'oef d'axolotl. I. Definition de la transition blastuleenne. Ann. Embryol. Morphogen. 4:113–123 (In French.) [Google Scholar]
- 54. Slack J. M. 1984. Regional biosynthetic markers in the early amphibian embryo. J. Embryol. Exp. Morphol. 80:289–319 [PubMed] [Google Scholar]
- 55. Stein A. J., Fuchs G., Fu C., Wolin S. L., Reinisch K. M. 2005. Structural insights into RNA quality control: the Ro autoantigen binds misfolded RNAs via its central cavity. Cell 121:529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Szüts D., Christov C., Kitching L., Krude T. 2005. Distinct populations of human PCNA are required for initiation of chromosomal DNA replication and concurrent DNA repair. Exp. Cell Res. 311:240–250 [DOI] [PubMed] [Google Scholar]
- 57. Szüts D., et al. 2003. RPA is an initiation factor for human chromosomal DNA replication. Nucleic Acids Res. 31:1725–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Takeda D. Y., Dutta A. 2005. DNA replication and progression through S phase. Oncogene 24:2827–2843 [DOI] [PubMed] [Google Scholar]
- 59. Westerfield M. 1993. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio). University of Oregon Press, Eugene, OR [Google Scholar]
- 60. Wolin S. L., Cedervall T. 2002. The La protein. Annu. Rev. Biochem. 71:375–403 [DOI] [PubMed] [Google Scholar]
- 61. Zhang A. T., et al. 2011. Dynamic interaction of Y RNAs with chromatin and initiation proteins during human DNA replication. J. Cell Sci. 124:2058–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]