Abstract
Virus-specific cytotoxic T lymphocytes (CTL) with high levels of functional avidity have been associated with viral clearance in hepatitis C virus infection and with enhanced antiviral protective immunity in animal models. However, the role of functional avidity as a determinant of HIV-specific CTL efficacy remains to be assessed. Here we measured the functional avidities of HIV-specific CTL responses targeting 20 different, optimally defined CTL epitopes restricted by 13 different HLA class I alleles in a cohort comprising 44 HIV controllers and 68 HIV noncontrollers. Responses restricted by HLA-B alleles and responses targeting epitopes located in HIV Gag exhibited significantly higher functional avidities than responses restricted by HLA-A or HLA-C molecules (P = 0.0003) or responses targeting epitopes outside Gag (P < 0.0001). The functional avidities of Gag-specific and HLA-B-restricted responses were higher in HIV controllers than in noncontrollers (P = 0.014 and P = 0.018) and were not restored in HIV noncontrollers initiating antiretroviral therapy. T-cell receptor (TCR) analyses revealed narrower TCR repertoires in higher-avidity CTL populations, which were dominated by public TCR sequences in HIV controllers. Together, these data link the presence of high-avidity Gag-specific and HLA-B-restricted CTL responses with viral suppression in vivo and provide new insights into the immune parameters that mediate spontaneous control of HIV infection.
INTRODUCTION
A small number of human immunodeficiency virus (HIV)-infected individuals, comprising approximately 0.5% of all cases, are able to control the infection in the absence of antiretroviral treatment. Such individuals exhibit low to undetectable virus loads in the peripheral blood and show either no or minimal signs of HIV disease progression; they are therefore referred to as “controllers” or “long-term nonprogressors” (LTNP) (26). Elucidating the mechanisms that are involved in the relative control of viral replication in these individuals is essential for our understanding of HIV pathogenesis and could crucially inform vaccine development (18).
CD8+ cytotoxic T lymphocytes (CTL) are considered an important component of immune defense against HIV and viral pathogens in general. The most compelling evidence for their protective role in HIV infection comes from the following: (i) studies that show a temporal association between the appearance of CTL responses and the initial early decline of plasma viremia (45), (ii) CD8 T-cell depletion experiments in the nonhuman primate simian immunodeficiency virus (SIV) model (70), and (iii) the finding that certain HLA class I alleles are enriched in subjects with delayed disease progression (reviewed in reference 59). Interestingly, a recent genome-wide association analysis comparing HIV controllers and noncontrollers identified specific amino acids in the peptide binding grooves of HLA-B molecules that could enable these alleles to restrict particularly potent CTL responses (64). However, despite the large number of studies characterizing HIV-specific T-cell responses, the hallmarks of a truly protective immune response remain unclear.
Neither the total breadth nor the magnitude of the overall virus-specific CTL response associates with the outcome of HIV disease (1, 32). However, CTL responses restricted by specific HLA-B alleles and responses that target the Gag protein appear to be particularly effective in vivo (43, 87). Nonetheless, some individuals with progressive disease also carry protective HLA alleles and mount broad Gag-specific CTL responses, indicating the presence of other factors that determine in vivo immune efficacy. One parameter that has repeatedly been associated with the control of viral replication in animal models is the functional avidity of virus-specific CTL (2, 9, 27). High-functional-avidity T cells feature a superior ability to recognize virally infected cells even at low surface antigen densities and deploy effector functions at lower cognate antigen concentration thresholds than do low-avidity T-cell populations (10, 14). In humans, such high-avidity CTL have been associated with clearance of hepatitis C virus (HCV) infection and broad recognition of HCV epitope variants (86). Although not necessarily directly linked, these data suggest that high-avidity CTL responses may be especially effective at controlling infections with highly variable pathogens, since variant recognition may be crucial for the limitation of immune escape (25). Furthermore, in HIV infection, the Nef protein downregulates HLA class I molecules on the surfaces of infected cells; in this setting, high-avidity CTL responses could provide a crucial advantage by recognizing HIV-infected cells despite the reduction in surface antigen density (8, 9, 24). The presence of high-avidity CD4+ T cells in HIV controllers and the emergence of escape mutants as a direct consequence of immune selection pressure mediated by high-avidity CTL provide support for the important role of functionally avid responses in the control of HIV/SIV replication (49, 61, 82).
In the current study, we directly tested the role of functional avidity as a determinant of CTL efficacy in the control of HIV replication. Furthermore, we examined the relationship between this parameter and antigen specificity, in terms of both the targeted HIV-derived epitopes and their associated HLA class I restriction elements.
MATERIALS AND METHODS
Study subjects.
All study participants were recruited from previously described cohorts in the Boston, MA, area (33, 64). HIV loads were determined using Roche Amplicor tests with a lower detection limit of 50 copies/ml. HLA typing was performed as described previously using sequence-specific primer PCR (SSP-PCR) (30). The Partners Human Research Committee at Massachusetts General Hospital approved all human subject research protocols, and all subjects provided written informed consent prior to enrollment. Responses were compared between 44 subjects with spontaneous control of HIV replication to less than 2,000 RNA copies/ml (“controllers”; median viral load < 50 copies/ml; range, <50 to 2,000 copies/ml) and 68 untreated subjects with viral loads greater than 3,000 RNA copies/ml (“noncontrollers”; median viral load, 38,450 copies/ml, range, 3,900 to 604,000 copies/ml). For experiments investigating the effects of highly active antiretroviral therapy (HAART)-induced viral suppression on CTL function, the functional avidities and magnitudes of CTL responses were determined for 14 treated individuals before and after the initiation of HAART; the median time on treatment at the second time point was 56.4 weeks (range, 1 to 150 weeks).
Peptide epitopes.
A total of 20 well-defined and commonly recognized HIV CTL epitopes listed at the Los Alamos HIV Immunology Database were included in the present study (15, 33). These epitopes are restricted by 13 different HLA-A (n = 4), HLA-B (n = 7) and HLA-C (n = 2) molecules and are summarized in Table 1. The antigenic peptides were selected to allow testing of epitopes with the following characteristics: (i) they are restricted by alleles commonly found in both HIV-controllers and noncontrollers, (ii) they are targeted by a substantial proportion of individuals expressing the restricting allele(s), and (iii) they can be presented in the context of different alleles (15, 33). For additional experiments on the role of HLA alleles as determinants of functional avidity, epitopes for which promiscuous presentation on multiple class I alleles has been demonstrated (33) and which are also presented by HLA alleles associated with controlled and noncontrolled HIV infection (60) were used as indicated in the respective section.
Table 1.
Epitopes tested for functional avidity
| Sequence | HLA | Protein |
|---|---|---|
| YFPDWQNYT | A01 | Nef |
| FLGKIWPSYK | A02 | Pol (p15) |
| ILKEPVHGV | A02 | Pol (RT) |
| SLYNTVATL | A02 | Gag (p17) |
| AIFQSSMTK | A03 | Pol (RT) |
| QVPLRPMTYK | A03 | Nef |
| AIFQSSMTK | A11 | Pol (RT) |
| QVPLRPMTYK | A11 | Nef |
| TPGPGVRYPL | B07 | Nef |
| TPQDLNTML | B07 | Gag (p24) |
| TPQVPLRPM | B07 | Nef |
| EIYKRWII | B08 | Gag (p24) |
| ELRSLYNTV | B08 | Gag (p17) |
| FLKEKGGL | B08 | Nef |
| TPQDLNTML | B42 | Gag (p24) |
| AEQASQDVKNW | B44 | Gag (p24) |
| QASQEVKNW | B53 | Gag (p24) |
| KAFSPEVIPMF | B57 | Gag (p24) |
| SFNCGGEFF | Cw04 | Env (gp120) |
| KRQEILDLWVY | Cw07 | Nef |
IFN-γ ELISpot and functional avidity assays.
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood using density gradient centrifugation and tested ex vivo in gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assays using single epitopes at a concentration of 14 μg/ml (11). Functional avidity was determined as described previously (86). In brief, unexpanded PBMC were thawed and rested for 4 h at 37°C and then stimulated in IFN-γ ELISpot assays with peptides added in serial 10-fold dilutions ranging from 100 μg/ml to 10 pg/ml (33). A positive (phytohemagglutinin) control well and at least four negative (no peptide) control wells were included on each plate. Stimulations were set up in duplicates or triplicates if cell availability allowed. Results are expressed as spot-forming cells (SFC) per million input cells. The threshold for a positive response was defined as at least five spots per well at a peptide concentration of 10 μg/ml and ≥3× mean background. Functional avidity was calculated by sigmoidal dose (SD)-response curves fitted to the ELISpot data using the GraphPad Prism software program (version 4.0).
T-cell receptor (TCR) repertoire analysis.
Antigenic stimulation was performed on thawed PBMC. Individual HIV peptides were used at 10 μg/ml to stimulate HIV-specific CD8+ T cells for 14 h at 37°C in the presence of costimulation with anti-CD28 and anti-CD49d monoclonal antibodies (1-μg/ml [each] final concentration; BD Biosciences). In every experiment, a negative control (costimulation alone with anti-CD28/anti-CD49d) was included to account for spontaneous stimulation, and a positive control (Staphylococcus enterotoxin B [SEB], 1 μg/ml, final; Sigma-Aldrich) was included to ensure that the cells were responsive. After stimulation, the cells were surface stained with the following monoclonal antibodies: anti-CD3-fluorescein isothiocyanate (FITC), anti-CD8-Pacific Blue, anti-CD14-Cy5PE, anti-CD19-Cy5PE, anti-CD25-phycoerythrin (PE), and anti-CD69-allophycocyanin (APC) (all from BD Biosciences). Viable antigen-specific CD8+ T cells were identified on the basis of CD3, CD8, CD25, and CD69 coexpression as described previously for reactive T-cell populations (66). Staining for CD14 and CD19 was used to exclude macrophages and B cells from the analysis. Responding cell populations were sorted at 70 lb/in2 directly into 1.5-ml microtubes (Sarstedt) containing 100 μl RNAlater (Applied Biosystems) using a modified FACSAria flow cytometer (BD Biosciences); sort purity was >98% in all cases, and at least 1,000 cells were collected for each experimental condition. Unbiased clonotypic analysis of all expressed TRB gene products was then conducted using a nonnested template-switch-anchored reverse transcriptase PCR (RT-PCR) as described previously (28, 67). The entire procedure was performed blinded to the clinical status of the subjects in all cases.
Statistical analysis.
The GraphPad Prism (version 4.0) software for Macintosh was used for the 50% SD (SD50) determinations. The Mann-Whitney test was used to compare the functional avidities of CTL responses between the two study groups; the Kruskal-Wallis test and Dunn's posttest were used to compare the functional avidities of CTL responses across different restricting HLA alleles and different viral proteins. A Spearman rank test was used to assess the relationship between the functional avidities or magnitude of the CTL responses and the time after initiation of HAART.
RESULTS
HLA-B-restricted and Gag-derived epitopes are targeted by CTL responses with high levels of functional avidity.
A number of studies in humans and animal models have aimed to understand the impact that different major histocompatibility complex (MHC) class I molecules exert on the quality and quantity of the evolving CTL response (15, 42, 54, 64). This issue is of particular importance in HIV infection, for which specific HLA class I alleles have been associated with improved in vivo control of viral replication (reviewed in references 59 and 60). However, direct links between the attributes of CTL-mediated immunity and the clinical control of HIV infection have been addressed only in the context of a few selected HLA alleles and individual epitopes or on the basis of responses elicited by peptide pools spanning whole viral proteins (4, 5, 13, 14, 40, 44, 49, 51, 55). To overcome these limitations, we assessed the functional avidities of CTL responses to 20 optimally defined HIV-derived epitopes in a cohort of 112 HIV-infected individuals with variable disease progression rates and heterogeneous HLA backgrounds. The epitopes included in this study spanned CTL targets in the HIV Gag, Pol, Nef, and Env proteins, thereby also enabling comparison of functional avidities across specificities from distinct viral gene products (Table 1). Of the 112 HIV-infected individuals tested, functional avidities were defined for a total of 199 distinct CTL responses, including 121 responses detected in 68 noncontrollers and 78 responses detected in 44 controllers.
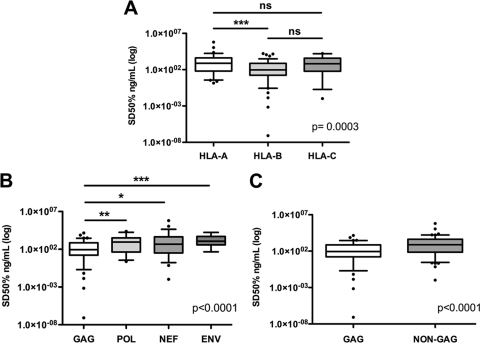
To test whether functional avidity was affected by the restricting HLA molecule or the viral protein from which it was derived, SD50 values were compared between responses restricted by HLA-A, -B, or -C alleles and between epitopes located in different viral proteins. CTL responses restricted by HLA-B exhibited significantly higher functional avidities (median, 93.9 ng/ml; range, 8.37 × 10−8 to 16,312 ng/ml) than those restricted by HLA-A (median, 761.6 ng/ml; range, 1.45 to 625,806 ng/ml; P < 0.001). Although the median SD50 was also higher than HLA-C allele-restricted responses, this comparison did not reach statistical significance (median, 651.5 ng/ml; range, 0.01 to 17,255 ng/ml) (Fig. 1A). When responses to different HIV proteins were compared, Gag-specific responses displayed higher functional avidities than responses directed against other individual proteins or all non-Gag-derived epitopes combined (P < 0.0001) (Fig. 1B and C). Together, these data support an important role for HLA-B-restricted responses in HIV control (42) and suggest that the significantly higher functional avidities of Gag-specific responses might contribute to their previously described efficacy (43, 87).
Fig. 1.
CTL responses targeting HLA-B-restricted and Gag-derived epitopes exhibit high levels of functional avidity. Epitope-specific responses were analyzed for 112 HIV-infected individuals (68 noncontrollers and 44 controllers). (A) The median functional avidity of 99 HLA-B-restricted responses was compared with the median functional avidities of 76 HLA-A-restricted and 24 HLA-C-restricted responses. (B and C) Stratification for epitope localization by HIV protein. The median functional avidity of responses targeting Gag-derived epitopes was compared with the median functional avidities of responses directed against epitopes derived from Pol, Nef, and Env. The box plots represent the functional avidities of all epitopes tested in each group; median values, 25th and 75th percentiles, and ranges are displayed. The Kruskal-Wallis test followed by Dunn's posttest for multiple comparisons was used to assess significance. ns, not significant; *, P = 0.01 to 0.05; **, P = 0.001 to 0.01; ***, P < 0.001.
HIV controllers mount CTL responses with higher functional avidities than noncontrollers.
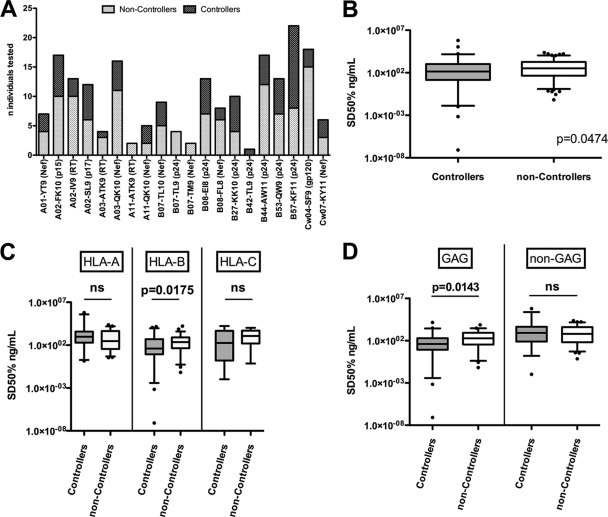
To further assess the relevance of functional avidity in HIV control, we determined whether epitope-specific CTL responses in controllers exhibited intrinsically higher avidities than those in noncontrollers. Where possible, comparable numbers of CTL responses were tested for each epitope in either group (Fig. 2A). When the SD50 values of all responses for HIV controllers (n = 78 responses) and noncontrollers (n = 121 responses) were compared, significantly higher levels of functional avidity were detected for controllers (median, 140.3 ng/ml; range, 8.37 × 10−8 to 625,806 ng/ml) than for noncontrollers (median, 327.5 ng/ml; range, 0.066 to 23,168 ng/ml; P = 0.047) (Fig. 2B). Stratification by HLA allele demonstrated that the functional avidities of responses restricted by HLA-B (P = 0.0175) but not HLA-A (P = 0.38) or HLA-C (P = 0.43) were significantly higher for HIV controllers than for noncontrollers (Fig. 2C). In both groups, we found no significant correlation between the time since initial HIV infection and functional avidity (data not shown). Furthermore, when responses were grouped according to epitope source, only Gag-specific responses exhibited significantly different functional avidities between HIV controllers and noncontrollers (P = 0.0143) (Fig. 2D).
Fig. 2.
HIV controllers mount CTL responses with higher functional avidities than noncontrollers. (A) Stacked bars indicate the numbers of individuals (controllers or noncontrollers) tested for each epitope. (B) Comparing all responses, controllers exhibited significantly higher functional avidities than noncontrollers. (C) Functional avidities are shown for controllers and noncontrollers after stratification of responses according to the restricting HLA molecule. Epitope-specific responses restricted by HLA-B but not HLA-A or HLA-C showed higher functional avidities with controllers (gray-shaded boxes) than with noncontrollers (white boxes). (D) Gag-derived epitopes but not non-Gag-derived epitopes elicited responses with higher functional avidities with controllers (gray-shaded boxes) than with noncontrollers (white boxes). ns, not significant.
Functional avidities and CTL response magnitudes decline with suppression of viral replication by antiretroviral treatment.
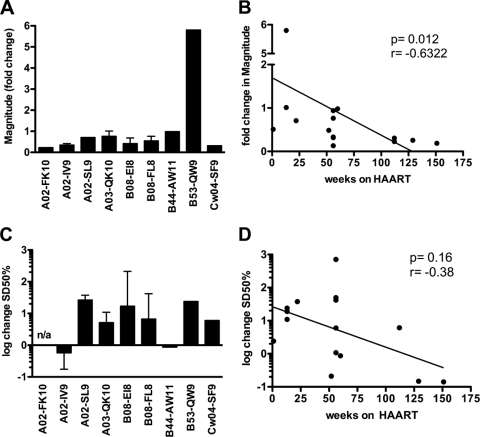
Previous studies have suggested that viral load may drive some of the observed differences in HIV-specific T-cell reactivities (32, 73). To test whether low functional avidities could be driven by high viral loads and consequently would be restored to higher levels of functional avidity by antigen removal, the functional avidities of CTL responses to 8 different epitopes were assessed for 12 untreated individuals with chronic HIV infection over a period of up to 3 years (median, 56.4 weeks; range, 1 to 151 weeks; total number of epitope-specific responses tested, 14) both before and after the initiation of highly active antiretroviral treatment (HAART). As expected from previous reports (23, 62, 63), the magnitude of 13/14 responses declined over time during the treatment period (P = 0.003) (Fig. 3A). Indeed, the duration of HAART was inversely associated with the reduction in CTL response magnitudes, supporting previously described gradual decreases over the period of suppressed antigenemia (P = 0.012) (Fig. 3B). Rather unexpectedly, however, the functional avidities of these responses were higher at the pretreatment time points (median SD50, 146.3 ng/ml) than for samples obtained during HAART (median SD50, 324.9 ng/ml; P = 0.051) (Fig. 3C). Functional avidity seemed to decline in relation to the duration of HAART treatment; this correlation, however, was not significant (Fig. 3D) (P = 0.16). Although treatment-induced viral suppression may not have the same effects on viral avidity as viral suppression by the immune system, these data suggest that antigen suppression during the chronic stages of uncontrolled HIV infection does not restore higher levels of functional avidity and that CTL response magnitude and functional avidity could be differentially regulated.
Fig. 3.
HAART-mediated suppression of viral replication does not select for high-avidity CTL responses. Functional avidities and magnitudes for a total of 14 different epitope-specific CTL responses were quantified for 12 individuals with chronic HIV infection before and after the initiation of HAART. For epitopes tested for more than one individual, mean values and standard deviations are indicated. (A) CTL response magnitudes were measured in IFN-γ ELISpot assays before and after the initiation of HAART; fold change between the untreated and treated time points is displayed in each case. (B) Changes in CTL response magnitudes versus time after the initiation of HAART (Spearman rank test). (C) Functional avidities of CTL responses were measured before and after the initiation of HAART; log change between the untreated and treated time points is displayed in each case. (D) Changes in the functional avidities of CTL responses versus time after the initiation of HAART (Spearman rank test).
CTL responses to protective epitopes restricted by HLA-B27 and HLA-B57 exhibit the highest functional avidities.
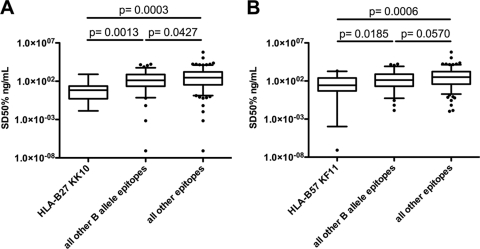
Among the many peptides presented by HLA class I alleles associated with superior HIV control, only a few optimally defined epitopes have been specifically implicated in the suppression of viral replication in vivo (21, 31, 35, 36). The most consistent of these are the HLA-B27-restricted KRWIILGLNK epitope (B27-KK10) and the HLA-B57-restricted KAFSPEVIPMF epitope (B57-KF11), both of which are located in HIV Gag p24. Since responses to these epitopes may contribute disproportionately to the observed association between Gag-specific responses and relative HIV control, we assessed the functional avidities of these responses in detail. Consistent with their apparent in vivo efficacy, both the B27-KK10 and B57-KF11 epitopes elicited in vitro responses with significantly higher functional avidities than those for the other epitopes, independent of clinical status (P = 0.0003 and P = 0.0006 for B27-KK10 and B57-KF11, respectively) (Fig. 4A and B). Of note, the median functional avidities of responses to these two specific epitopes were not significantly different for controllers from those for noncontrollers (13.46 ng/ml versus 201.4 ng/ml for B27-KK10 [P = 0.268]; 152.0 ng/ml versus 443.9 ng/ml for B57-KF11 [P = 0.200]; data not shown). This could be attributed partly to smaller samples sizes for this subanalysis but may also indicate that other mechanisms contribute to the lack of viral control in the rare individuals who express protective HLA alleles but fail to control the infection (20, 57).
Fig. 4.
Responses restricted by HLA alleles associated with superior control of HIV infection exhibit high levels of functional avidity. The functional avidities of CTL responses specific for two Gag-derived epitopes restricted by the protective alleles HLA-B27 and HLA-B57 were compared either with those measured for all other tested epitope-specific responses, regardless of allelic restriction, or with those for all other HLA-B-restricted responses. CTL responses to B27-KK10 (A) or B57-KF11 (B) showed higher median functional avidities than either other HLA-B-restricted responses or all other responses studied. The box plots represent the functional avidities of all epitopes tested in each group; median values, 25th and 75th percentiles, and ranges are displayed.
To assess whether CTL responses to these two epitopes were also driving the overall associations between high levels of functional avidity, HLA-B restriction, and the targeting of epitopes in Gag, we repeated the analyses after excluding B27-KK10 and B57-KF11 from the data set. Despite the reduced number of CTL responses analyzed, HLA-B-restricted responses still exhibited higher functional avidities than responses restricted by HLA-A alleles (P = 0.02). Similarly, the statistically significant difference in the functional avidities of responses to Gag-derived versus non-Gag-derived epitopes persisted (P = 0.0069). Thus, while the B27-KK10 and B57-KF11 responses showed the most pronounced effects, in line with their particular protective efficacy in vivo, the remaining HLA-B-restricted and Gag-derived epitope-specific responses still showed significantly higher functional avidities than responses to epitopes restricted by HLA-A alleles and responses to non-Gag-derived epitopes, respectively.
High functional avidities to specific epitopes relate to the presenting HLA allele.
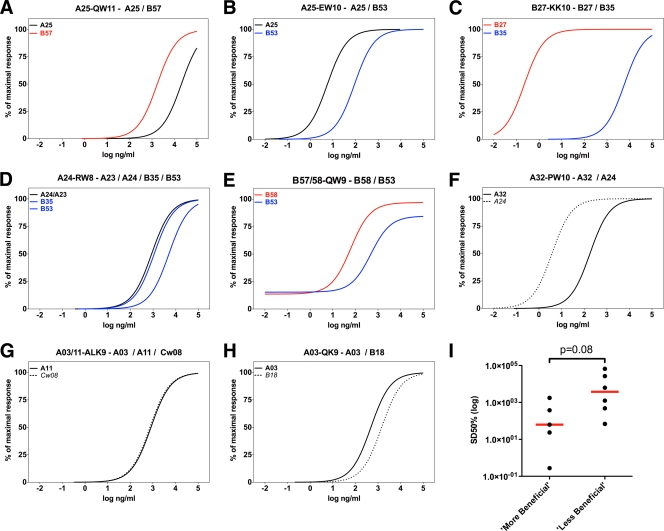
Several previous studies have demonstrated that promiscuous presentation of specific CTL epitopes by different HLA class I alleles is not uncommon (22, 30, 33, 51, 53, 77). Indeed, the vast majority of known optimal CTL epitopes derived from HIV and Epstein-Barr virus (EBV) are effectively presented by several different and unrelated HLA class I alleles (33). Employing such promiscuous epitopes allows the impact of the presenting HLA class I molecule on the quality of the epitope-specific CTL response to be studied more directly, avoiding potential biases related to protein abundance and epitope processing. Based on an earlier report (33), epitopes presented by HLA alleles that are differentially associated with HIV disease control were selected for this purpose (52, 60). The functional avidities of responses to five such epitopes were compared in the context of their originally described and alternative restricting alleles (Fig. 5A to H). As controls, an additional three epitopes were included that are promiscuously presented by HLA class I alleles for which no strong association with HIV control, either good or bad, has been reported. In all cases tested, the alleles associated with better control of HIV replication restricted responses with higher functional avidities than the alleles associated with a less beneficial HIV disease outcome, showing a strong trend toward statistical significance (P = 0.08) (Fig. 5I). The functional avidities of CTL responses restricted by the original or alternative alleles were not significantly different (P = 0.2) (not shown), demonstrating that responses restricted by “alternative” HLA class I alleles can represent functionally relevant responses. Collectively, these data indicate that the nature of the presenting HLA allele can profoundly influence the functional avidities of the CTL responses that it restricts. This observation is in line with recent data describing an important role for the presenting HLA molecule in HIV control (64) and a previous analysis of HIV clade C-derived epitopes presented in the context of different alleles in the HLA-B7 supertype, in which the more beneficial allele restricted responses with higher functional avidities (49).
Fig. 5.
Responses to promiscuously presented epitopes exhibit higher functional avidities in the context of HLA class I alleles associated with better disease outcome. The functional avidities of CTL responses to epitopes presented in the context of different HLA class I alleles were determined for untreated individuals with chronic HIV infection. (A to E) CTL responses in individuals expressing HLA class I alleles associated with better control (“good alleles,” red lines) or accelerated disease progression (“bad alleles,” blue lines) were compared with responses to the corresponding epitopes presented in the context of alleles with neutral disease outcome (black lines). (F to H) Epitopes promiscuously presented on alleles associated with average rate of HIV disease progression were included as controls. (I) To allow exploratory statistical comparisons, avidities of the response restricted by the “more beneficial” allele were compared to the avidities of those for the “less beneficial” allele for each epitope tested in different HLA contexts. Responses to epitopes restricted by HLA class I alleles associated with a more beneficial disease outcome showed a strong trend toward higher functional avidities than those of the corresponding responses restricted by less beneficial alleles. Horizontal red bars in panel I represent median values.
High-avidity responses in controllers exhibit narrow and highly skewed TCR repertoires.
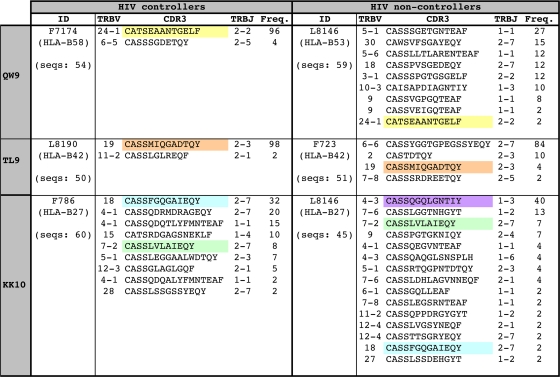
Next, we examined the functional avidities of CTL responses in relation to their composite T-cell receptor (TCR) repertoires, which act as counterparts to the targeted peptide/MHC complex and determine the consequences of antigen engagement. The definition of functional avidity directly ex vivo is based on the antigen concentration-dependent reactivity of the entire epitope-specific CTL population, which potentially comprises multiple cognate TCR clonotypes. Some of these clonotypes may be shared between individuals, even in CTL populations that target the same epitope presented by different HLA molecules (51, 77, 79). Such “public” clonotypes display variable degrees of interindividual sharing and have been associated with protective effects in response to a conserved immunodominant Gag-derived epitope during acute SIV infection (65, 78, 81). To determine whether the high-avidity responses in controllers were linked to particular features of the corresponding antigen-specific TCR repertoires, quantitative molecular analyses of clonotype composition were conducted for three paired epitope-specific responses that were each detectable in one HIV controller and an HIV noncontroller: (i) the HLA-B53/B58-restricted QW9 epitope (Fig. 6, top panels), (ii) the HLA-B42-restricted TL9 epitope (Fig. 6, middle panels), and (iii) the HLA-B27-restricted KK10 epitope (Fig. 6, bottom panels). For all three epitope-specific responses, the HIV controllers exhibited narrower TCR repertoires than the noncontrollers. Furthermore, these narrower repertoires were markedly skewed in two of the controllers (Fig. 6, F7174 and L8190). The public TCR sequences that were shared between the three pairs of individuals in all cases dominated the TCR in the HIV controllers.
Fig. 6.
High-avidity CTL responses in controllers are associated with narrow TCR repertoires. CDR3β amino acid sequence, TRBV and TRBJ usage, and relative frequency are shown for all clonotypes within each epitope-specific response. For each epitope, one controller and one noncontroller pair was studied. Public clonotypes are color coded in the CDR3 sequence column and were identified as TCRβ amino acid sequences observed for more than one individual with reference to an extensive database including unpublished data and data from previous studies (4, 12, 34, 49, 50, 58, 80, 85). The IMGT nomenclature is used (48).
The functional avidities of the KK10-specific responses restricted by HLA-B27 and the QW9-specific responses restricted by HLA-B53/58 were higher in the controllers than in the noncontrollers (data not shown), despite similar peptide affinities for the different restriction elements in the latter case (46 nM versus 9.7 nM) (15). In contrast, the functional avidity of the TL9-specific response restricted by HLA-B42 for the noncontroller was slightly higher than that of the corresponding response for the controller (27.7 ng/ml versus 79.5 ng/ml; data not shown). However, in this case, the noncontroller displayed a narrow and highly skewed TCR repertoire (Fig. 6, F723). Thus, although limited to three pairs of epitope-specific responses, these data indicate that high-avidity CTL responses tend to be mediated by narrower TCR repertoires, which are not invariably associated with, but may contribute to, superior in vivo HIV control.
DISCUSSION
A number of recent studies have suggested an important role for the functional avidity of CTL responses in the control of viral infections (7, 86). However, many reports have been based on responses to single epitope/HLA allele combinations or have used T-cell cultures after in vitro expansion, which could bias the resulting avidity measurements (4, 38, 86). Furthermore, a comprehensive assessment of HIV-specific CTL responses with respect to the role of functional avidity in the control of viral replication is lacking. To address these issues, we studied unexpanded CTL directly ex vivo in a cohort of more than 100 HIV-infected individuals with varying disease status and broad HLA class I allele distribution. Our data confirm previous reports based on selected epitopes that link the presence of high-avidity CTL populations to viral control and further support the important role of HLA-B-restricted responses targeting Gag-derived epitopes in antiviral immunity (4, 6, 16, 38, 42, 44, 46).
Although directly supporting human experimental data are scarce, it is likely that several different mechanisms are responsible for such a superior immune function of HLA-B-restricted CTL responses and the induction and maintenance of responses of elevated functional avidity. These possibly include efficient epitope processing, the characteristics of the presented peptide on B alleles, and a particularly effective TCR recognition of presented epitopes in the context of HLA-B alleles. The HLA-B-restricted HIV epitope repertoire is thought to be enriched for epitopes with increased sequence conservation, which might limit viral escape from CTL-mediated immune pressure and select for a narrower TCR repertoire of high avidity (29). Such selective presentation of more conserved epitopes on HLA-B alleles could result from the need for specific anchor residues for B alleles that require amino acids that pose stringent functional and structural constraints on the viral protein and thus complicate viral escape. Whether this would be specifically true for the protective HLA-B alleles remains to be tested. Other than the need for specific anchor residues, it is also plausible that structural differences of the MHC molecules may influence the interaction between the TCR and the peptide-MHC complex (pMHC) (76), which could explain CTL responses of higher avidity to epitopes presented by HLA B alleles (78, 83).
Aside from HLA restriction, the fine specificity of HIV-specific CTL has also been associated with relative control of viral replication. In accordance with several previous studies (43, 53, 87), the data presented here also link Gag-specific responses with relative viral control and, likely of relevance in mechanistic terms, with high levels of functional avidity. Since Gag degradation products from the infecting viral particle are presented very early after infection, high-avidity responses to such cross-presented antigens could mediate the effective elimination of infected cells at a relatively low surface density of the represented epitopes and before the production of progenitor virus (69). Furthermore, Gag is one of the most conserved proteins in HIV and may contain a disproportionally high number of epitopes that bind to alleles associated with superior HIV control, particularly HLA-B27 and HLA-B57. Since these epitopes are among the most immunodominant ones in individuals expressing these alleles (15, 33), they may be focusing the most beneficial response onto this protein (17). Taken together, our data thus might suggest that high-avidity CTL to Gag-derived, HLA-B-restricted epitopes contribute to relative HIV control by targeting epitopes that (i) are expressed early in infection, (ii) could elicit cytolytic effector functions at low epitope abundance on the surface (74), (iii) are located in conserved regions with restricted viral escape pathways, and (iv) might result in a more efficacious TCR activation in the context of HLA-B alleles.
The differences in the median functional avidities between controllers and noncontrollers, although statistically significant, were surprisingly modest. This may be due in part to the evident variability in SD50 values between different epitope-specific responses. In addition, IFN-γ production is relatively preserved in exhausted T cells and may be subject to comparatively low activation thresholds (13, 73). Thus, determining SD50 values for additional effector functions may reveal more marked differences between study groups, epitopes, and restricting HLA alleles. Furthermore, functional avidity as tested in our present study reflects the summation of CTL response and epitope presentation, without taking into consideration several additional factors that are likely important in determining the in vivo efficiency of these responses. For example, epitope abundance on the surface of an infected cell will also contribute to the in vivo effectiveness of the CTL response independently of the experimentally measured functional avidity using synthetic peptides. This amount of presented epitopes on the cell surface is in turn impacted by several factors, such as antigenic protein expression levels, epitope processing preferences, intracellular epitope stability, HLA binding, HLA expression kinetics, and likely others (75). Data on these factors are incomplete to date and are difficult to take into account experimentally all at the same time, thus limiting the conclusions that can be drawn from analyzing individual factors in isolation. In vitro testing of functional avidity in the context of HIV-infected cells might be one approach to bypass some of the limitations of synthetic peptide-based assays and to reflect the in vivo situation more directly. Practical limitations, however, would limit the feasibility of such studies for larger, genetically heterogeneous host populations. Furthermore, epitopes with the highest functional avidity in the present study are not necessarily the epitopes with the highest intracellular stability (47), which suggests that epitope stability is a possibly independent factor, aside from avidity and others, that will define the in vivo effectiveness of a CTL response.
Further complicating our understanding of the relative importances of specific CTL populations and HLA restriction for HIV control are factors such as epitope competition in individuals with different HLA backgrounds, variable epitope dominance patterns, the possible effects of other coinfections, and often extensive sequence variation in the targeted HIV epitope itself (19). In the present study, we attempted to eliminate some of these potential biases by assessing functional avidities for CTL responses targeting the same epitopes presented by several different HLA class I alleles, particularly those associated with different HIV disease progression rates. For all tested epitopes with promiscuous HLA restriction, the alleles associated with superior HIV disease control restricted responses with higher functional avidities than the less beneficial alleles, even if the responses in the beneficial alleles were tested in samples from a noncontroller individual. These findings suggest that the restricting allele may be a more potent determinant of functional avidity within the CTL population than the bound peptide, which would be in line with recent data that link specific residues within beneficial HLA allele sequences with relative viral control (64).
Functional avidity is an indirect measure of the strength of the interaction between the T cell and its target cell (e.g., an HIV-infected cell) and depends among other factors on the monomeric affinity of the clonotypic TCR for cognate pMHC antigen (72). In paired analyses of epitope-specific CTL responses, high levels of functional avidity were associated with narrower TCR repertoires in our study. This observation is consistent with interclonal competition for antigen, which has been shown to drive oligoclonal and highly skewed epitope-specific TCR repertoires in persistent DNA virus infections (67). Previous work has associated public TCR usage in response to the protective Mamu-A*01-restricted Gag CM9 epitope during acute infection with relative control of SIV replication (65). The observation may be due to an intrinsically higher level of cross-reactivity among public clonotypes, which are also present in the naive T-cell pool at greater precursor frequencies and can therefore be mobilized more readily (68). It remains to be determined, however, whether the greater representation of public clonotypes in controllers is associated with functional avidity per se and how the allele's intrinsic ability to restrict cross-reactive responses could impact these results. In addition, differential TCRα chain pairing (56) could potentially complicate the overall interpretation. Indeed, such pairing promiscuity could explain why the same public TCRβ sequences were subdominant in the three noncontrollers studied here. Alternatively, these clonotypes may be inefficiently selected in noncontrollers due to greater immune dysregulation or could perhaps be waning due to higher rates of senescence and clonal turnover under conditions of rampant viral replication (4). Such ongoing viral replication also increases viral diversity, and this might lead to the emergence of escape variants that can induce new T-cell populations at later time points in infection (3, 37). These variant-specific CTL populations may possess functional avidity different from that of the wild-type-specific CTL population and may broaden the TCR repertoire of the epitope-specific response. This possibility cannot be ruled out in the present study, where responses were not studied longitudinally and under inclusion of all autologous viral epitope variants. As a consequence, the elevated functional avidity in HIV controllers may reflect a certain bias caused by measuring epitope-specific responses in HIV controllers that target nonmutated epitopes and score with a higher avidity than responses in HIV noncontrollers that may harbor more-avid responses reactive with autologous epitope variants but which may not be detected in our assay system using consensus-based peptides.
Most studies aimed at understanding the characteristics of protective immune responses to HIV are confronted with difficulties in disentangling cause versus consequence of the tested markers. In the SIV model, a progressive decline of functional avidity has been observed with increasing time of infection (61), which has been suggested to reflect early escape of epitopes targeted by high-avidity responses. This would be in line with recent data on a selected HLA-B*27 epitope showing the gradual escape from high-avidity CTL responses (39). To indirectly address this point in our analyses and to test whether or not removal of antigen would result in the (re)emergence of high-avidity responses, chronically HIV infected individuals were studied before and during HAART therapy. Somewhat surprisingly, a decline rather than a recuperation of functional avidity was observed, suggesting that reduction of high antigen doses is not sufficient to select for high-avidity responses. Obviously, HAART-induced suppression and spontaneous viral control are very different situations, and the data may simply be a reflection of the previously active viral replication and its effects on the CTL function during the pre-HAART period. In particular, ongoing viral replication could have led to immune alterations in chronic progressive infection, which might no longer be reversible upon HAART initiation, including post-TCR signaling pathway dysfunction, increased cell turnover, and likely other mechanisms (41, 71). Alternatively, high-avidity effector CTL may be more affected by antigen removal than their low-avidity counterparts and/or the persisting memory pool may be skewed toward certain clonotypes upon the initiation of HAART. However, the clonotypic composition of epitope-specific T-cell populations is thought to remain relatively stable during HAART (84), which would tend to invoke interclonal qualitative differences. In this regard, one potential explanation for the observed reduction could be the preferential activation of memory T cells that are cross-reactive with other persistent pathogens and thus might not exhibit the highest functional avidities for the cognate HIV antigen (19). Clearly, longitudinal studies of untreated individuals starting in acute infection simultaneously controlling for changes in TCR repertoire, viral sequence evolution, phenotypic T-cell characteristics, immune dysfunction, and functional avidity will be required to resolve these questions.
Despite some limitations, the present study provides strong evidence, on a cross-sectional basis and in the context of a broad HLA background, for a direct association between the presence of high-functional-avidity CTL responses and relative control of HIV replication in vivo. These findings have substantial implications for HIV vaccine design, which may need to focus on the induction of CTL responses with high levels of functional avidity through the mobilization of optimal epitope-specific TCR repertoires and targeting highly conserved regions to reduce immune escape.
ACKNOWLEDGMENTS
This work was funded by a National Institutes of Health grant to C.B. and D.E.K. (R01 067 077) and in part by the Spanish FIPSE grant 36-0737-09. C.T.B. is supported by a research grant from the University of Basel, Basel, Switzerland, and the Janggen-Pöhn Foundation, Switzerland. D.A.P. is a Medical Research Council (United Kingdom) senior clinical fellow. B.M. holds a research fellowship grant from the FIS (Rio Hortega; CM08/00020), Madrid, Spain. C.B. is a senior ICREA research professor.
C.T.B. thanks C. Hess for ongoing support.
Footnotes
Published ahead of print on 13 July 2011.
REFERENCES
- 1. Addo M. M., et al. 2003. Comprehensive epitope analysis of HIV-1-specific T cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander-Miller M. A., Leggatt G. R., Berzofsky J. A. 1996. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 93:4102–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen T. M., et al. 2005. De novo generation of escape variant-specific CD8+ T-cell responses following cytotoxic T-lymphocyte escape in chronic human immunodeficiency virus type 1 infection. J. Virol. 79:12952–12960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Almeida J. R., et al. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204:2473–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Almeida J. R., et al. 2009. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood 113:6351–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Altfeld M., et al. 2006. HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8(+) T cell response against HIV-1. PLoS Med. 3:e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bangham C. R. 2009. CTL quality and the control of human retroviral infections. Eur. J. Immunol. 39:1700–1712 [DOI] [PubMed] [Google Scholar]
- 8. Belyakov I. M., Isakov D., Zhu Q., Dzutsev A., Berzofsky J. A. 2007. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against simian/human immunodeficiency viral depletion of mucosal CD4+ T cells. J. Immunol. 178:7211–7221 [DOI] [PubMed] [Google Scholar]
- 9. Belyakov I. M., et al. 2006. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood 107:3258–3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bennett M. S., Ng H. L., Dagarag M., Ali A., Yang O. O. 2007. Epitope-dependent avidity thresholds for cytotoxic T-lymphocyte clearance of virus-infected cells. J. Virol. 81:4973–4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berger C. T., et al. 2010. Viral adaptation to immune selection pressure by HLA class I-restricted CTL responses targeting epitopes in HIV frameshift sequences. J. Exp. Med. 207:61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Betts M. R., et al. 2005. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced T cell responses and their role in protection after HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 102:4512–4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Betts M. R., et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T-cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Betts M. R., et al. 2004. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J. Immunol. 172:6407–6417 [DOI] [PubMed] [Google Scholar]
- 15. Bihl F., et al. 2006. Impact of HLA-B alleles, epitope binding affinity, functional avidity, and viral coinfection on the immunodominance of virus-specific CTL responses. J. Immunol. 176:4094–4101 [DOI] [PubMed] [Google Scholar]
- 16. Boon A. C., et al. 2004. Preferential HLA usage in the influenza virus-specific CTL response. J. Immunol. 172:4435–4443 [DOI] [PubMed] [Google Scholar]
- 17. Borghans J. A., Molgaard A., de Boer R. J., Kesmir C. 2007. HLA alleles associated with slow progression to AIDS truly prefer to present HIV-1 p24. PLoS One 2:e920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brander C., Frahm N., Walker B. D. 2006. The challenges of host and viral diversity in HIV vaccine design. Curr. Opin. Immunol. 18:430–437 [DOI] [PubMed] [Google Scholar]
- 19. Brehm M. A., et al. 2002. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat. Immunol. 3:627–634 [DOI] [PubMed] [Google Scholar]
- 20. Brockman M. A., et al. 2010. Early selection in Gag by protective HLA alleles contributes to reduced HIV-1 replication capacity that may be largely compensated for in chronic infection. J. Virol. 84:11937–11949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brumme Z. L., et al. 2008. Human leukocyte antigen-specific polymorphisms in HIV-1 Gag and their association with viral load in chronic untreated infection. AIDS 22:1277–1286 [DOI] [PubMed] [Google Scholar]
- 22. Burrows S. R., et al. 2003. Promiscuous CTL recognition of viral epitopes on multiple human leukocyte antigens: biological validation of the proposed HLA A24 supertype. J. Immunol. 171:1407–1412 [DOI] [PubMed] [Google Scholar]
- 23. Casazza J. P., Betts M. R., Picker L. J., Koup R. A. 2001. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J. Virol. 75:6508–6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen G. B., et al. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661–671 [DOI] [PubMed] [Google Scholar]
- 25. Davenport M. P., Price D. A., McMichael A. J. 2007. The T cell repertoire in infection and vaccination: implications for control of persistent viruses. Curr. Opin. Immunol. 19:294–300 [DOI] [PubMed] [Google Scholar]
- 26. Deeks S. G., Walker B. D. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406–416 [DOI] [PubMed] [Google Scholar]
- 27. Derby M., Alexander-Miller M., Tse R., Berzofsky J. 2001. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J. Immunol. 166:1690–1697 [DOI] [PubMed] [Google Scholar]
- 28. Douek D. C., et al. 2002. A novel approach to the analysis of specificity, clonality, and frequency of HIV-specific T cell responses reveals a potential mechanism for control of viral escape. J. Immunol. 168:3099–3104 [DOI] [PubMed] [Google Scholar]
- 29. Fontaine Costa A. I., Rao X., Lechenadec E., van Baarle D., Kesmir C. 2010. HLA-B molecules target more conserved regions of the HIV-1 proteome. AIDS 24:211–215 [DOI] [PubMed] [Google Scholar]
- 30. Frahm N., et al. 2005. HLA-B63 presents HLA-B57/B58-restricted cytotoxic T-lymphocyte epitopes and is associated with low human immunodeficiency virus load. J. Virol. 79:10218–10225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frahm N., et al. 2006. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat. Immunol. 7:173–178 [DOI] [PubMed] [Google Scholar]
- 32. Frahm N., et al. 2004. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J. Virol. 78:2187–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frahm N., et al. 2007. Extensive HLA class I allele promiscuity among viral CTL epitopes. Eur. J. Immunol. 37:2419–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Geldmacher C., et al. 2009. Minor viral and host genetic polymorphisms can dramatically impact the biologic outcome of an epitope-specific CD8 T-cell response. Blood 114:1553–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goulder P. J., et al. 2001. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature 412:334–338 [DOI] [PubMed] [Google Scholar]
- 36. Goulder P. J., et al. 1996. Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res. Hum. Retroviruses 12:1691–1698 [DOI] [PubMed] [Google Scholar]
- 37. Haas G., et al. 1996. Dynamics of viral variants in HIV-1 Nef and specific cytotoxic T lymphocytes in vivo. J. Immunol. 157:4212–4221 [PubMed] [Google Scholar]
- 38. Harari A., et al. 2007. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc. Natl. Acad. Sci. U. S. A. 104:16233–16238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iglesias M. C., et al. 6 July 2011. Escape from highly effective public CD8+ T-cell clonotypes by HIV. Blood. doi:10.1182/blood-2011-01-328781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jagannathan P., et al. 2009. Comparisons of CD8+ T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. J. Virol. 83:2728–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaufmann D. E., Walker B. D. 2008. Programmed death-1 as a factor in immune exhaustion and activation in HIV infection. Curr. Opin. HIV AIDS 3:362–367 [DOI] [PubMed] [Google Scholar]
- 42. Kiepiela P., et al. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432:769–775 [DOI] [PubMed] [Google Scholar]
- 43. Kiepiela P., et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53 [DOI] [PubMed] [Google Scholar]
- 44. Kosmrlj A., et al. 2010. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature 465:350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koup R. A., et al. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lacey S. F., et al. 2003. Relative dominance of HLA-B*07 restricted CD8+ T-lymphocyte immune responses to human cytomegalovirus pp65 in persons sharing HLA-A*02 and HLA-B*07 alleles. Hum. Immunol. 64:440–452 [DOI] [PubMed] [Google Scholar]
- 47. Lazaro E., et al. 2011. (in press). Variable HIV peptide stability in human cytosol is critical to epitope presentation and immune escape. J. Clin. Invest. 121:2480–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lefranc M. P. 2003. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 31:307–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leslie A., et al. 2006. Differential selection pressure exerted on HIV by CTL targeting identical epitopes but restricted by distinct HLA alleles from the same HLA supertype. J. Immunol. 177:4699–4708 [DOI] [PubMed] [Google Scholar]
- 50. Lichterfeld M., et al. 2007. A viral CTL escape mutation leading to immunoglobulin-like transcript 4-mediated functional inhibition of myelomonocytic cells. J. Exp. Med. 204:2813–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lichterfeld M., et al. 2006. T cell receptor cross-recognition of an HIV-1 CD8+ T cell epitope presented by closely related alleles from the HLA-A3 superfamily. Int. Immunol. 18:1179–1188 [DOI] [PubMed] [Google Scholar]
- 52. Llano A., Frahm N., Brander C. 2009. How to optimally define optimal cytotoxic T lymphocyte epitopes in HIV infection? In Korber C. B. B., Walker B., Koup R., Moore J., Haynes B., Meyers G. (ed.), HIV molecular immunology database. Theoretical Biology and Biophysics, Los Alamos National Laboratory, Los Alamos, NM [Google Scholar]
- 53. Masemola A. M., et al. 2004. Novel and promiscuous CTL epitopes in conserved regions of Gag targeted by individuals with early subtype C HIV type 1 infection from southern Africa. J. Immunol. 173:4607–4617 [DOI] [PubMed] [Google Scholar]
- 54. Messaoudi I., Guevara Patino J. A., Dyall R., LeMaoult J., Nikolich-Zugich J. 2002. Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science 298:1797–1800 [DOI] [PubMed] [Google Scholar]
- 55. Migueles S. A., et al. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miles J. J., et al. 2006. TCR alpha genes direct MHC restriction in the potent human T cell response to a class I-bound viral epitope. J. Immunol. 177:6804–6814 [DOI] [PubMed] [Google Scholar]
- 57. Miura T., et al. 2009. HLA-associated viral mutations are common in human immunodeficiency virus type 1 elite controllers. J. Virol. 83:3407–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moss P. A., et al. 1995. Persistent high frequency of human immunodeficiency virus-specific cytotoxic T cells in peripheral blood of infected donors. Proc. Natl. Acad. Sci. U. S. A. 92:5773–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mothe B., Ibarrondo J., Llano A., Brander C. 2009. Virological, immune and host genetics markers in the control of HIV infection. Dis. Markers 27:105–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. O'Brien S. J., Gao X., Carrington M. 2001. HLA and AIDS: a cautionary tale. Trends Mol. Med. 7:379–381 [DOI] [PubMed] [Google Scholar]
- 61. O'Connor D. H., et al. 2002. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8:493–499 [DOI] [PubMed] [Google Scholar]
- 62. Ogg G., et al. 1999. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J. Virol. 73:797–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Oxenius A., et al. 2001. Direct ex vivo analysis reveals distinct phenotypic patterns of HIV- specific CD8(+) T lymphocyte activation in response to therapeutic manipulation of virus load. Eur. J. Immunol. 31:1115–1121 [DOI] [PubMed] [Google Scholar]
- 64. Pereyra F., et al. 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Price D. A., et al. 2009. Public clonotype usage identifies protective Gag-specific CD8+ T cell responses in SIV infection. J. Exp. Med. 206:923–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Price D. A., et al. 2008. Induction and evolution of cytomegalovirus-specific CD4+ T cell clonotypes in rhesus macaques. J. Immunol. 180:269–280 [DOI] [PubMed] [Google Scholar]
- 67. Price D. A., et al. 2005. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 202:1349–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Quigley M. F., et al. 2010. Convergent recombination shapes the clonotypic landscape of the naive T-cell repertoire. Proc. Natl. Acad. Sci. U. S. A. 107:19414–19419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sacha J. B., et al. 2007. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol. 178:2746–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schmitz J., et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857–860 [DOI] [PubMed] [Google Scholar]
- 71. Sharma S. K., Alexander-Miller M. A. 2011. Increased sensitivity to antigen in high avidity CD8(+) T cells results from augmented membrane proximal T-cell receptor signal transduction. Immunology 133:307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Snyder J. T., Alexander-Miller M. A., Berzofskyl J. A., Belyakov I. M. 2003. Molecular mechanisms and biological significance of CTL avidity. Curr. HIV Res. 1:287–294 [DOI] [PubMed] [Google Scholar]
- 73. Streeck H., et al. 2008. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sykulev Y., Joo M., Vturina I., Tsomides T. J., Eisen H. N. 1996. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4:565–571 [DOI] [PubMed] [Google Scholar]
- 75. Tenzer S., et al. 2009. Antigen processing influences HIV-specific cytotoxic T lymphocyte immunodominance. Nat. Immunol. 10:636–646 [DOI] [PubMed] [Google Scholar]
- 76. Theodossis A., et al. 2010. Constraints within major histocompatibility complex class I restricted peptides: presentation and consequences for T-cell recognition. Proc. Natl. Acad. Sci. U. S. A. 107:5534–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Threlkeld S. C., et al. 1997. Degenerate and promiscuous recognition by cytotoxic T lymphocyte (CTL) of peptides presented by the MHC class I A3-like superfamily: implications for vaccine development. J. Immunol. 159:1648–1657 [PubMed] [Google Scholar]
- 78. Turner S. J., Doherty P. C., McCluskey J., Rossjohn J. 2006. Structural determinants of T-cell receptor bias in immunity. Nat. Rev. Immunol. 6:883–894 [DOI] [PubMed] [Google Scholar]
- 79. Ueno T., Tomiyama H., Takiguchi M. 2002. Single T cell receptor-mediated recognition of an identical HIV-derived peptide presented by multiple HLA class I molecules. J. Immunol. 169:4961–4969 [DOI] [PubMed] [Google Scholar]
- 80. van Bockel D. J., et al. 2011. Persistent survival of prevalent clonotypes within an immunodominant HIV gag-specific CD8+ T cell response. J. Immunol. 186:359–371 [DOI] [PubMed] [Google Scholar]
- 81. Venturi V., Price D. A., Douek D. C., Davenport M. P. 2008. The molecular basis for public T-cell responses? Nat. Rev. Immunol. 8:231–238 [DOI] [PubMed] [Google Scholar]
- 82. Vingert B., et al. 2010. HIV controller CD4+ T cells respond to minimal amounts of Gag antigen due to high TCR avidity. PLoS Pathog. 6:e1000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Webb A. I., et al. 2004. The structure of H-2K(b) and K(bm8) complexed to a herpes simplex virus determinant: evidence for a conformational switch that governs T cell repertoire selection and viral resistance. J. Immunol. 173:402–409 [DOI] [PubMed] [Google Scholar]
- 84. Weekes M. P., Wills M. R., Sissons J. G., Carmichael A. J. 2006. Large HIV-specific CD8 cytotoxic T-lymphocyte (CTL) clones reduce their overall size but maintain high frequencies of memory CTL following highly active antiretroviral therapy. Immunology 118:25–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wilson J. D., et al. 1998. Oligoclonal expansions of CD8(+) T cells in chronic HIV infection are antigen specific. J. Exp. Med. 188:785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yerly D., et al. 2008. Increased cytotoxic T-lymphocyte epitope variant cross-recognition and functional avidity are associated with hepatitis C virus clearance. J. Virol. 82:3147–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zuniga R., et al. 2006. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J. Virol. 80:3122–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]