Abstract
Wuhan nodavirus (WhNV) is a newly identified member of the Nodaviridae family with a bipartite genome of positive-sense RNAs. A nonstructural protein encoded by subgenomic RNA3 of nodaviruses, B2, serves as a potent RNA silencing suppressor (RSS) by sequestering RNA duplexes. We have previously demonstrated that WhNV B2 blocks RNA silencing in cultured Drosophila cells. However, the molecular mechanism by which WhNV B2 functions remains unknown. Here, we successfully established an RNA silencing system in cells derived from Pieris rapae, a natural host of WhNV, by introducing into these cells double-stranded RNA (dsRNA)-expressing plasmids or chemically synthesized small interfering RNAs (siRNAs). Using this system, we revealed that the WhNV B2 protein inhibited Dicer-mediated dsRNA cleavage and the incorporation of siRNA into the RNA-induced silencing complex (RISC) by sequestering dsRNA and siRNA. Based on the modeled B2 3-dimensional structure, serial single alanine replacement mutations and N-terminal deletion analyses showed that the RNA-binding domain of B2 is formed by its helices α2 and α3, while helix α1 mediates B2 dimerization. Furthermore, positive feedback between RNA binding and B2 dimerization was uncovered by gel shift assay and far-Western blotting, revealing that B2 dimerization is required for its binding to RNA, whereas RNA binding to B2 in turn promotes its dimerization. All together, our findings uncovered a novel RNA-binding mode of WhNV B2 and provided evidence that the promotion effect of RNA binding on dimerization exists in a viral RSS protein.
INTRODUCTION
Originally regarded as a mechanism of eukaryotic posttranscriptional gene regulation, RNA interference (RNAi) is emerging as an important antiviral defense in a wide range of organisms, from plants to animals (1, 3, 11). In eukaryotic cells, the accumulation of virus-derived long double-stranded RNA (dsRNA) induces RNAi, resulting in the cleavage of dsRNA into approximately 21- to 23-bp small interfering RNAs (siRNAs) and the subsequent degradation of homologous mRNAs (6, 55). The notion of RNAi as a natural immune response first took shape in plants and was later shown in animals (4, 52). In invertebrates like insects, RNAi-mediated immunity is the primary response against viral infection (39, 51), whereas in vertebrates, a network of defenses is orchestrated to combat viruses, in which RNAi-mediated immunity is interrelated with other innate immunity pathways, such as the interferon (IFN), dsRNA-inducible protein kinase R (PKR), and oligoadenylate synthetase/RNase L pathways (16, 27, 41, 45).
To escape from the host RNAi-mediated antiviral defense, viruses encode RNA silencing suppressor (RSS) proteins that employ a variety of tactics to undermine the RNAi pathway. For instance, p19 of tombusviruses, NS3 of rice hoja blanca virus, and p14 of aureusvirus sequester siRNAs (19, 26, 42) or long dsRNAs (35). Others, like turnip crinkle virus coat protein and cucumovirus 2b protein, target divergent components of the RNAi machinery (38, 57). Moreover, in addition to suppressing RNAi, some RSS proteins from mammalian viruses, such as influenza A virus NS1, vaccinia virus E3L, and Ebola virus VP35, also have IFN or PKR antagonistic properties to fight against the network of interrelated innate immune responses (5, 7, 10, 30, 50).
In insect viruses, the B2 protein from flock house virus (FHV), the best-studied member of the Nodaviridae family, is among the first identified broad-spectrum RSS proteins. Originally isolated from grass grubs, FHV encodes a B2 protein that antagonizes RNAi in a host-independent manner, thus ensuring viral survival in cells derived from plants, worms, and other insects (28, 34, 44). Although FHV B2 lacks the canonical dsRNA-binding domain (dsRBD), it forms an all-helix homodimer that binds to both dsRNA and siRNA by a four-helix bundle fold, thereby inhibiting siRNA production and the incorporation of siRNA into the RNA-induced silencing complex (RISC) (2, 12, 31). Notably, the Nodamura virus (NoV) B2 protein, which is highly similar to FHV B2 in structure, employs a similar four-helix bundle fold to recognize RNA (24). However, it is unknown whether this RNA-binding mode is applicable to other nodaviral B2 proteins, especially those that are structurally distinct from FHV B2.
Wuhan nodavirus (WhNV), an unclassified member of the Nodaviridae family, was isolated by our group from Pieris rapae larvae in a suburban cabbage field of Wuhan, Hubei, China (33). In previous studies, we determined the genomic sequence of WhNV and characterized its physicochemical properties (32, 33). WhNV has a bipartite genome that contains RNA1 (3,149 nucleotides [nt]) and RNA2 (1,562 nt), both of which are single-stranded, positive-sense RNAs and are copackaged into a single virion. RNA1 encodes protein A, which is an RNA-dependent RNA polymerase (RdRp), whereas RNA2 encodes the coat protein precursor Proα. In addition to protein A, the genomic RNA1 of WhNV also carries two putative open reading frames (ORFs) for B1 and B2 proteins, like those encoded by other nodaviruses. However, our previous work showed that the putative ORF for B1 was not detected during viral infection (9). On the other hand, a subgenomic RNA derived from RNA1 with the 5′ end at nt 2780 by the mechanism of internal initiation, named sgRNA3, encodes the 85-amino-acid (aa) B2 protein that suppresses RNA silencing in cultured Drosophila cells (9, 37). Although the WhNV B2 protein plays key roles in viral survival against RNAi, the molecular mechanism by which WhNV B2 functions is still poorly understood. Furthermore, WhNV B2 shares low sequence homology and structural similarity with FHV B2, which limits the usefulness of FHV B2 structural data for WhNV B2 studies. Thus, it is imperative to understand how WhNV B2 functions as an RSS protein.
In this study, we successfully developed an RNA silencing system by inducing dsRNA-expressing plasmids or chemically synthesized siRNAs into Pieris rapae embryo-derived cells (Pr-E). These efforts enabled us to elucidate the detailed RNAi suppression mechanism of WhNV B2 in its natural host. By utilizing this experimental system, we demonstrated that WhNV B2 efficiently inhibits Dicer-mediated dsRNA cleavage and the incorporation of siRNA into the RISC by dsRNA and siRNA sequestration. Furthermore, a novel six-helical fold was found to be employed by the WhNV B2 dimer to recognize RNA duplexes. More importantly, beyond the scope of WhNV or the Nodaviridae family, the current study revealed not only that B2 dimerization is required for binding to RNA but also that RNA binding could in turn promote B2 dimerization, which may be a general mechanism in viral RSS proteins. In addition, we proposed a model for interpreting this promotion effect of RNA binding on B2 dimerization.
MATERIALS AND METHODS
Plasmids.
The dsRNA-expressing plasmids pRISE-ds-EGFP, a hairpin-shaped dsRNA expression plasmid that targets the enhanced green fluorescent protein (EGFP) ORF region of nucleotides 1 to 500 (23), and pWAGAL4, a yeast transcription factor GAL4 expression plasmid required for dsRNA expression (20), were kindly gifted by Yuji Kageyama (Nara Institute of Science and Technology, Takayama, Ikoma, Japan) and Yasushi Hiromi (National Institute of Genetics, Mishama, Japan), respectively. The negative-control plasmid, pRISE-ds-Fluc, which targets the firefly luciferase (Fluc) ORF region of nt 498 to 871, was constructed as described previously (23). In addition, the siRNAs targeting EGFP and Fluc (siRNA-EGFP and siRNA-Fluc, respectively) were prepared by chemical synthesis (RiboBio, Guangzhou, China), deprotected, and stored according to the manufacturer's protocol. The oligonucleotides are shown in Table 1.
Table 1.
Sequences and purpose of the primers used in this work
| Primera | Sequence (5′ to 3′)b | Purpose |
|---|---|---|
| B2-For | GAATTCATGAACGACAACCAGAAAC (EcoRI) | Construction of B2-encoded plasmids for prokaryotic expression and eukaryotic transient expression |
| B2-Rev | TCTAGAGAGTTTCGATGGGTCTGCC (XbaI) | |
| B2ΔN20- For | GAATTCACCATGGCGACCCGAACGCTGGT (EcoRI) | |
| B2ΔN20-Rev 1 | TCTAGAGAGTTTCGATGGGTCTGCC (XbaI) | |
| B2ΔN20-Rev 2 | CTCGAGTTAGAGTTTCGATGGGTCTGCC (XhoI) | |
| B2R17A- For | GAATTCACCATGGCGAACGACAACCAGAAACAAGCATTGACAGCGTACCAAGCACTGGTCgccCGCCAAGT (EcoRI) | |
| B2R17A-Rev | TCTAGAGAGTTTCGATGGGTCTGCC (XbaI) | |
| B2R18A- For | GAATTCACCATGGCGAACGACAACCAGAAACAAGCATTGACAGCGTACCAAGCACTGGTCCGCgccCAAGTAAT (EcoRI) | |
| B2R18A-Rev | TCTAGAGAGTTTCGATGGGTCTGCC (XbaI) | |
| B2R24A- For | GAATTCACCATGGCGAACGACAACCAGAAACAAGCATTGACAGCGTACCAAGCACTGGTCCGCCGCCAAGTAATGGCGACCgccACG (EcoRI) | |
| B2R24A-Rev | TCTAGAGAGTTTCGATGGGTCTGCC (XbaI) | |
| B2R40A- For* | TCAAAAGGCAAGCGGAgccCTAAAGGCC | |
| B2R40A-Rev* | TAGggcCCCGCTTGCCTTTTGAAGGGCC | |
| B2R58A- For* | CGATCCCGCCACACTTCGTCTCAgccAT | |
| B2R58A-Rev* | CATggcTGAGACGAAGTGTGGCGGGATC | |
| B2K6A- For | GAATTCACCATGGCGAACGACAACCAGgccCAAGC (EcoRI) | |
| B2K6A-Rev | TCTAGAGAGTTTCGATGGGTCTGCC (XbaI) | |
| B2K36A- For* | CAAgccGCAAGCGGAAGGCTAAAGGC | |
| B2K36A-Rev* | CTTTAGCCTTCCGCTTGCggcTT | |
| B2K42A- For* | TAgccGCCGTAGGCCTCGAACCGC | |
| B2K42A-Rev* | GCGGTTCGAGGCCTACGGCggcTAGCCTT | |
| B2K75A- For | GAATTCACCATGGCGAACGACAACCAGAAAC (EcoRI) | |
| B2K75A-Rev | TCTAGAGAGTTTCGATGGGTCTGCCTCCTCGCTTGGggcCG (XbaI) | |
| B2K84A- For | GAATTCACCATGGCGAACGACAACCAGAAAC (EcoRI) | |
| B2K84A-Rev | TCTAGAGAGggcCGATGGGTCTGCC (XbaI) | |
| siRNA-EGFP | Chemical synthesis of siRNAs that induce RNA interference in Pr-E cells | |
| Homologous sequence | AAGCTGACCCTGAAGTTCATC | |
| Sense strand | p-GCUGACCCUGAAGUUCAUCUU | |
| Antisense strand | UUCGACUGGGACUUCAAGUAG-p | |
| siRNA-Fluc | ||
| Homologous sequence | GATTATGTCCGGTTATGTA | |
| Sense strand | p-GAUUAUGUCCGGUUAUGUAUU | |
| Antisense strand | UUCUAAUACAGGCCAAUACAU-p | |
| Anti EGFP-For | GATCCGCCACAACATCGAGGACGGC | Construction of in vitro- transcription templates for preparing probes and dsRNA |
| Anti EGFP-Rev | TAATACGACTCACTATAGGTTACTTGTACAGCTCGTCCATGCC | |
| Anti siRNA-For | GAGGGCGATGCCACCTACGGCAAGCTGACCCTGAAGTTCATC | |
| Anti siRNA-Rev | TAATACGACTCACTATAGGCAGCTTGCCGGTGGTGCAGATGAACTTCAGGGTCAGCTT | |
| Sense siRNA-For | TAATACGACTCACTATAGGGAGGGCGATGCCACCTACGGCAAGCTGACCCTGAAGTTCATC | |
| Sense siRNA-Rev | CAGCTTGCCGGTGGTGCAGATGAACTTCAGGGTCAGCTT | |
| T7-dsRNA-For | TAATACGACTCACTATAGGATGGTGAGCTAGGGCGAGGA | |
| T7-dsRNA-Rev | TAATACGACTCACTATAGGTTGAAGTTCACCTTGATGCC |
The GenBank accession numbers of the B2 proteins used in the current study are listed in parentheses as follows: FHV (X77156), NoV (AAF97861), BBV (AAA42746), BoV (AAK15752), GGNNV (AAK21878), SJNNV (BAA84211), and RGNNV (AAX77408). Sequence-specific primers are designed according to GenBank accession numbers AY962576 (WhNV RNA1) and U55762 (EGFP), and primers with asterisks are designed for overlapping PCR.
Underlined characters indicate restriction endonuclease sites, and the types are shown in parentheses. Substituted nucleotides for mutagenesis are shown with lowercase characters. The T7 polymerase promoter is shown in italics. The EGFP ORF region is amplified from pEGFP-N1 (Clontech). The Kozak translation initiation sequence designed according to the pAc 5.1 (Invitrogen) handbook is indicated with boldfaced characters.
For RNAi suppression assays, pAc 5.1/V5-His A (Invitrogen, Carlsbad, CA) was used to construct plasmids that express the EGFP reporter gene, WhNV B2 protein wild type (WT), and its mutants. The EGFP and B2 WT expression plasmids, pAcEGFP and pAcWhB2-85, were constructed in our previous work (9). Mutations were introduced into the WhNV B2 coding region by PCR-mediated mutagenesis, with appropriate primers containing the desired nucleotide changes (Table 1) and subsequently cloned into pAc 5.1/V5-His A, according to our standard protocol (9).
In our previous work, we constructed pETB2-85, a plasmid that expresses the B2 protein with a His(6) tag in bacteria (9). To generate the plasmid expressing the B2ΔN20 protein with a His(6) tag, pET-B2ΔN20, we amplified a DNA fragment of the B2 ORF and cloned it into pET-28a (Novagen, Germany) using corresponding primers (Table 1). To generate plasmids that express B2 and its mutated proteins with N-terminal maltose-binding protein (MBP) fusion in bacteria, the encoding regions of these proteins (which had been cloned into pAc 5.1/V5-His A) were cleaved from the EcoRI and XbaI sites and subsequently inserted into pMAL-c2X (New England BioLabs, Ipswich, MA).
Cell culture and transfection.
The Pr-E cell line, which was derived from Pieris rapae embryos (29), was generously provided by Zehua Yu (Huazhong Normal University, Wuhan, Hubei, China) and cultured in semisuspension at 27°C in Grace's medium (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gibco). Before use, Pr-E cells were plated in six-well plates and grown overnight to reach 80% confluence, which corresponded to about 1 × 106 cells. After overnight incubation, transfection was conducted using FuGene HD transfection reagent (Roche, Basel, Switzerland), according to the manufacturer's protocol. For transfection of plasmids, 1 μg of each plasmid was used, and for siRNA-transfection, 1 to 40 nM siRNA was used to determine the optimal concentration. Cells were harvested at 72 h posttransfection.
Western blot analysis.
Cells were harvested in cell lysis buffer [65 mM Tris-HCl (pH 6.8), 3% SDS, 5% β-mercaptoethanol, 10% glycerol, and a protease inhibitor cocktail (Roche)], and the whole-cell extracts were then subjected to 12% SDS-PAGE and Western blotting, according to our standard procedures (9). A rabbit anti-B2 polyclonal antiserum was previously described and used at a dilution of 1:500 (9).
Immunoprecipitation.
Cells were lysed with NETN buffer [20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, and 0.5% NP-40] for 20 min at 4°C. Lysates were clarified at 12,000 rpm for 10 min at 4°C, incubated with 20 μg of antibodies (anti-His monoclonal antibody or preimmune mouse antisera) and 30 μl of pretreated protein-G agarose beads (Roche), and then rotated slowly at 4°C for 1 h. The antibody-bound complexes were captured via a 10-s pulse in the microcentrifuge and washed three times in 1 ml of NETN buffer. RNA was extracted from the captured complexes using a Poly-Gel RNA extraction kit (Omega Biotek, Norcross, GA) and prepared for Northern blot analysis.
Northern blot analysis.
Total RNA was extracted from cells using TRIzol reagent (Invitrogen), according to the manufacturer's protocol. For EGFP mRNA detection, total RNA was subjected to electrophoresis in 1.5% denaturing agarose gels containing 2.2 M formaldehyde and then transferred to a Hybond-A nylon membrane (Amersham Pharmacia, Piscataway, NJ) as previously described (9). For siRNA detection, the RNA was separated on 8 M urea-15% polyacrylamide gels and subjected to RNA blot analysis of low-molecular-weight RNAs. The probe for detection of EGFP mRNA was complementary to the EGFP ORF region of nucleotides 501 to 720, which does not overlap with the RNAi target sequence. For dsRNA and siRNA detection, either sense strands or antisense strands were detected using two complementary probes that correspond to the target sites of siRNA produced from EGFP-specific dsRNA as previously described (46). All probes were labeled with digoxigenin (DIG)-UTP (Roche) by in vitro transcription and purified according to our standard protocol (37). The oligonucleotides are listed in Table 1.
Phylogenetic analysis.
Phylogenetic and molecular evolutionary analysis was performed using Clustal W 1.8 and PHYLOWIN95 programs as described in our previous work (32). The GenBank accession numbers of the nodaviral B2 proteins used in the current study are listed in Table 1.
Expression and purification of recombinant proteins.
The recombinant protein MBP-B2 and its mutants as well as the negative-control protein MBP were expressed in Escherichia coli TB1 at 37°C, and the cells were harvested after being incubated for 4 h in the presence of 0.25 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG). Proteins were purified using amylose resin (New England BioLabs), according to the manufacturer's protocol. Purified proteins were concentrated using Amicon Ultra-15 filters (Millipore, Schwalbach, Germany) and stored in 100 mM NaCl and 50 mM Tris-HCl (pH 7.4) at −20°C. Expression was generated and purification of His-B2ΔN20 was conducted according to our standard protocol (9). All proteins were quantified by an UV-visible spectrophotometer (Shimadzu, Kyoto, Japan).
Modeling and secondary structure analysis of the WhNV B2 protein.
The 3-dimensional structure of WhNV B2 was modeled by submitting its amino acid sequence to the HMMSTR/Rosetta server (from Robetta, University of Washington [http://robetta.bakerlab.org/]) (8). Five models were obtained, and the best one was chosen as a template based on its score, assessed by submitting it to the SWISS-MODEL Server (from Swiss Institute of Bioinformatics and the Biozentrum, University of Basel, Switzerland [http://swissmodel.expasy.org]). The figures of the WhNV B2 and FHV B2 3-D structures were made by using PyMOL program 1.1 (DeLano Scientific LLC, South San Francisco, CA) from coordinate files.
To perform circular dichroism (CD) spectroscopic studies on the WhNV B2 protein, MBP-B2 was cleaved with factor Xa (wt/wt ratio of 1%) in 50 mM phosphate-buffered saline (pH 7.4) at 27°C for 24 h. B2 protein, essentially free from its MBP fusion partner, was eluted with 300 mM NaCl during DEAE-Sepharose ion-exchange chromatography, according to the manufacturer's protocol (New England BioLabs), and then concentrated at 0.2 mg/ml in 50 mM phosphate-buffered saline (pH 7.4) using Amicon Ultra-15 filters (Millipore). The CD spectra from 185 nm to 250 nm were recorded immediately on a J-810 CD spectropolarimeter (JASCO, Essex, United Kingdom) at room temperature, with an average of three scans at a speed of 100 nm/min for each sample. The standard sample, myoglobin from equine skeletal muscle, was purchased from Sigma-Aldrich (Saint Louis, MO).
Protein-RNA interaction assays.
A 500-bp dsRNA, which was designed to mimic the transcripts of pRISE-ds-EGFP, was generated by annealing two complementary T7 transcripts in vitro. Primers incorporating 5′-end T7 RNA polymerase promoters into the PCR products were used to amplify the EGFP ORF region of nucleotides 1 to 500 (Table 1). The PCR products were gel purified with a QIAquick kit (Qiagen, Valencia, CA) and then used as a template for RNA synthesis with T7 RNA polymerase (Promega, Madison, WI). The DNA template was digested by RQ1 RNase-free DNase (Promega), and RNAs were subsequently extracted with TRIzol reagent (Invitrogen). Purified dsRNA was quantitated by an UV-visible spectrophotometer (Shimadzu).
Gel shift assays were performed using purified MBP, MBP-B2, or its mutants at a concentration of 30 μM (100 μM for siRNA) and 0.1 μM RNAs in buffer containing 50 mM Tris-HCl (pH 7.4) and 100 mM NaCl. After incubation for 30 min at 25°C, reaction mixtures were separated on 1.2% Tris-borate-EDTA (TBE)–agarose gels, and the RNA was visualized by staining with ethidium bromide at 1 μg/ml.
RNase III cleavage inhibition assays were conducted using bacterium-expressed glutathione S-transferase fusion RNase III (generously gifted by Yi Zhang, Wuhan University, Wuhan, China). Reactions were performed in 20-μl volumes containing 0.1 μM EGFP-derived 500-bp dsRNA, 1× RNase III buffer [20 mM Tris-HCl, 0.5 mM EDTA, 5 mM MgCl2, 1 mM dithiothreitol (DTT), 140 mM NaCl, 2.7 mM KCl (pH 7.9)], and 30 μM MBP, MBP-B2, or its mutants. Following 30 min of preincubation at 25°C, allowing B2 to bind to dsRNA, 1 U of RNase III was added, and the reaction mixtures were incubated at 37°C for 30 min. Reaction products were resolved by TBE-agarose gel electrophoresis on 1.2% gels, and the RNA was stained with ethidium bromide at 1 μg/ml.
Chemical cross-linking assays.
For chemical cross-linking assays, 2 μg MBP-B2, MBP-B2ΔN20, or MBP was incubated with 20 μl cross-linking buffer containing 20 mM Tris-HCl (pH 7.5), 50 mM MgCl2, 10 mM DTT, 1% glycerol, and 3% glutaraldehyde at 4°C for 30 min. The reaction mixture was separated on a 12% denaturing polyacrylamide gel and then electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes using B2 polyclonal antibody for Western blotting. The gel was stained with Coomassie brilliant blue R250.
Far-Western blot assays.
Far-Western blot assays were conducted according to our standard protocol (53). Bacterial expressions of His-B2 and His-B2ΔN20 were separated by SDS-PAGE, electrophoretically transferred to PVDF membranes, and gradually renatured at 4°C in HEPES buffer [20 mM HEPES-KOH, 50 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, and 10% glycerol (pH 7.4)] containing 5% nonfat milk. The membranes were washed and incubated with 100 μg MBP-B2 or its mutants alone in 4 ml incubation buffer [10 mM Tris-HCl, 100 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, and 1% nonfat milk powder (pH 7.4)] for 3 h at room temperature with or without 50 μg 500-bp dsRNA. The membranes were subsequently washed three times (10 min/time) with incubation buffer and incubated with anti-MBP antibody (New England BioLabs) at a 1:10,000 dilution for 1 h at room temperature. The membranes were again washed three times (10 min/time) with incubation buffer and subsequently incubated with alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (1:2,000 dilution) for 1 h at room temperature. After the blots were washed three more times, color development was carried out with BCIP/NBT (Promega) as a substrate.
RESULTS
WhNV B2 suppresses RNAi induced by plasmid-carrying dsRNA or synthetic siRNA in Pr-E cells.
Our previous work revealed that WhNV B2 is a potent RNAi suppressor in cultured Drosophila cells (9). To study the molecular mechanism by which WhNV B2 suppresses RNAi, a silencing experimental system in the natural host environment of WhNV is desired. For this purpose, we cotransfected insect expression vector for EGFP with either EGFP-specific dsRNA-expressing plasmid (anti-EGFP dsRNA) or chemically synthesized siRNA in Pr-E cells, which are derived from the embryos of the natural host of WhNV, Pieris rapae (29, 33).
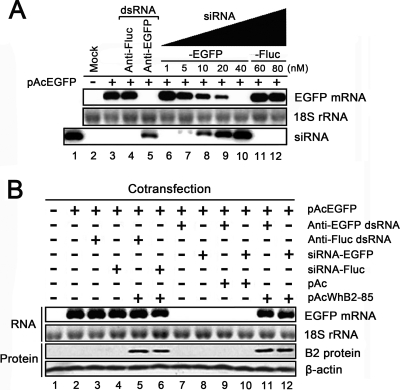
After cotransfection, the reduction of EGFP mRNA levels and the formation of siRNA in Pr-E cells were determined by Northern blot analysis. The EGFP-specific dsRNA mostly eliminated the EGFP transcripts (Fig. 1 A, top lane 5), whereas the irrelevant dsRNA targeting Fluc did not affect the levels of EGFP mRNA (Fig. 1A, top lane 4). On the other hand, EGFP-specific dsRNA caused siRNA production in Pr-E cells (Fig. 1A, bottom lane 5), indicating that the elimination of EGFP mRNA was due to RNAi. The chemically synthesized siRNA inhibited EGFP mRNA in a dose-dependent manner (Fig. 1A, top lanes 6 to 10), whereas higher concentrations of Fluc-specific siRNA had no effect on EGFP mRNA levels (Fig. 1A, top lanes 11 and 12). All together, these results demonstrate that plasmid-carrying dsRNA or chemically synthesized siRNA can induce gene-specific RNAi in Pr-E cells.
Fig. 1.
WhNV B2 inhibits gene-specific RNAi induced by plasmid-carrying dsRNA or chemically synthesized siRNA in Pr-E cells. (A) Assay for RNA silencing of EGFP in Pr-E cells. The EGFP expression plasmid pAcEGFP was cotransfected with EGFP-specific dsRNA-expressing plasmid (anti-EGFP dsRNA) or synthesized siRNA (siRNA-EGFP) at multiple concentrations (1 to 40 nM) that were indicated above each lane. At 72 h postcotransfection, RNA was harvested from Pr-E cells to determine the reduction in EGFP transcripts and the production of siRNA by Northern blot analysis. We loaded 5 nM synthetic siRNA-EGFP as a positive control (lane 1), indicating the location of the bands corresponding to cellular siRNAs produced from EGFP-specific dsRNA. Irrelevant dsRNA-expressing plasmid (anti-Fluc dsRNA) or negative siRNA (siRNA-Fluc) was transfected as a negative control, respectively (lanes 4, 11, and 12). (B) Northern blotting shows the levels of EGFP mRNA when cotransfected with anti-EGFP dsRNA or siRNA-EGFP (40 nM) in the absence or presence of WhNV B2. Cells assayed as described for panel A were cotransfected with B2-expressing plasmid pAcWhB2-85 (lanes 5, 6, 11, and 12) or empty vector pAc (lanes 9 and 10). The mRNA levels of EGFP and the expression of B2 protein were determined by Northern blotting and Western blotting, with 18S rRNA and β-actin as loading controls, respectively.
Having established the sensitive RNAi system in cells derived from the natural host of WhNV, we next examined the RNAi suppression property of WhNV B2. The presence of WhNV B2 mostly abolished the remarkable reduction of EGFP mRNA levels caused by EGFP-specific dsRNA or siRNA (Fig. 1B, top lanes 11 and 12). In contrast, the presence of WhNV B2 did not increase EGFP mRNA levels in irrelevant dsRNA- or negative siRNA-transfected cells (Fig. 1B, lanes 5 and 6), demonstrating that WhNV B2 suppresses RNAi rather than promotes EGFP transcription. Taken together, we conclude that WhNV B2 efficiently blocks RNAi induced by plasmid-carrying dsRNA or chemically synthesized siRNA in Pr-E cells.
WhNV B2 inhibits Dicer-mediated siRNA generation and the incorporation of siRNA into the RISC.
siRNA-based RNAi is a two-step pathway involving Dicer-mediated dsRNA cleavage into siRNA and subsequent siRNA-induced RISC assembly (55). Given the observation that the RNAi pathway induced by dsRNA or siRNA was undermined by WhNV B2, we speculated that B2 would block both of the aforementioned steps in the RNAi pathway. To test this hypothesis, RNA harvested from a portion of the cells assayed, shown in Fig. 1B, was subjected to Northern blot analysis to determine the ratio of pre-Dicer dsRNA to post-Dicer siRNAs in the presence or absence of B2 (Fig. 2 A). As anticipated, EGFP-specific dsRNA was completely digested into siRNAs when B2 was absent (Fig. 2A, lane 2). On the contrary, a dramatic increase in the ratio of 500-bp dsRNA to ∼20-bp Dicer-processed siRNA was observed in the presence of B2 (Fig. 2A, lane 3). Notably, this dsRNA cleavage effect was also observed when pAcEGFP was not transfected (Fig. 2A, lanes 4 and 5), indicating that the presence of target mRNA is not required for Dicer-mediated siRNA generation, which could be disrupted by WhNV B2.
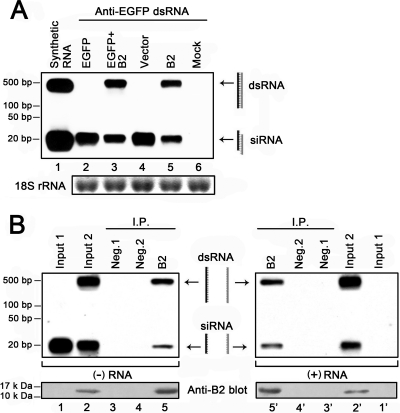
Fig. 2.
WhNV B2 prevents the dsRNA precursor from undergoing Dicer-mediated cleavage and stabilizes siRNA duplexes in Pr-E cells. (A) In the presence (B2) or absence (vector) of WhNV B2, the relative ratio of dsRNA/siRNA in cells was determined by Northern blotting. RNA harvested from a portion of the cells analyzed as shown in Fig. 1B was subjected to Northern blotting with a DIG-labeled probe that recognized both EGFP-specific dsRNA and Dicer-processed siRNAs (indicated by cartons and arrows). We used 18S rRNA as a loading control. Lane 1, in vitro-transcribed 500-bp dsRNA and chemically synthesized 21- to 23-bp siRNA, indicating the location of their cellular transcripts, EGFP-specific dsRNA, and its digestion products siRNA in the gel. Lanes 2 to 5, cells were cotransfected with EGFP-specific dsRNA expression plasmid (anti-EGFP dsRNA) and other corresponding plasmids as indicated. Lane 6, cells were mock transfected. (B) Northern blot analysis of RNA precipitated with B2. Cells assayed as shown in panel A, lanes 4 and 5, in which anti-EGFP dsRNA was cotransfected with empty vector or the B2 expression plasmid, were lysed and immunoprecipitated with anti-His monoclonal antibody recognizing the His(6) tag of the empty vector (Neg. 2) and His-tagged B2 or with control antiserum (Neg. 1). RNA extracted from the immunoprecipitates (IP) was subjected to Northern blotting with two complementary probes that recognized either sense-strand (+) RNA (lanes 3′ to 5′) or antisense-strand (−) RNA (lanes 3 to 5), indicated by cartons and arrows. Total RNAs directly harvested from lysates of cells assayed as described for panel A (vector and B2) were loaded as inputs (Input 1 and Input 2). The presence of B2 in the immunoprecipitated complex was confirmed by Western blotting using anti-B2 polyclonal antibodies.
When siRNA duplexes are incorporated into the RISC, the passenger strand is preferentially excluded from the RISC and rapidly degraded, thereby librating the guide strand (22, 43). By sequestering both the guide strands and passenger strands, many RSS proteins derived from plant viruses, such as P19 and P1/HC Pro, stabilize siRNA duplexes, thus blocking the incorporation of siRNA into the RISC (25, 54). To investigate whether WhNV B2 shares this strategy, we examined if B2 could hijack and stabilize post-Dicer siRNA duplexes in cells. Cells in which the EGFP-specific dsRNA-expressing plasmid was cotransfected with empty vector or the B2 expression plasmid (Fig. 2A, lanes 4 and 5) were lysed and immunoprecipitated with anti-His monoclonal antibody recognizing the His(6) tag of the empty vector (Neg. 2) and His-tagged B2 or with control antiserum (Fig. 2b, Neg. 1). RNA harvested from the immunoprecipitates (IP) was subjected to Northern blot analysis with two probes recognizing either antisense strands (Fig. 2B, left, lanes 1 to 5) or sense strands (Fig. 2B, right, lanes 1′ to 5′) of duplex RNA, respectively.
In the absence of WhNV B2, passenger-strand siRNA was below the detection limit (Fig. 2B, lane 1′). However, the presence of B2 caused an aberrant accumulation of passenger-strand siRNA (Fig. 2B, lane 2′), demonstrating that WhNV B2 can stabilize siRNA duplexes. If WhNV B2 can associate with ∼20-bp siRNA duplexes, the siRNA/dsRNA ratios of IP detected with both probes should be identical, since both probes recognize the dsRNA precursor with an approximately equal affinity. The siRNA/dsRNA ratios of the antisense strand and sense strand precipitated by B2 were approximately equal (Fig. 2B, compare lane 5 to lane 5′), indicating that WhNV B2 associates with both pre-Dicer dsRNA and post-Dicer siRNA duplexes in cells. In contrast, neither of the two negative controls precipitated detectable levels of RNAs (Fig. 2B, lanes 3, 3′, 4, and 4′). Interestingly, the amount of B2-precipited siRNA duplexes was less than that of B2-precipited dsRNA, since the siRNA/dsRNA ratio of IP was dramatically lower than that of the input control (Fig. 2B, compare lane 3 to lane 5), implying that WhNV B2 has a relatively lower affinity for siRNA duplexes than dsRNA. All together, these results revealed that WhNV B2 associates with dsRNA and siRNA duplexes, thus protecting dsRNA from Dicer-mediated cleavage and inhibiting the incorporation of siRNA into the RISC.
WhNV B2 is far from other nodaviral B2 proteins in the phylogenetic tree and shares low structural homology with FHV B2.
To better characterize the nature of WhNV B2, we compared its amino acid sequence with the sequences of other B2 proteins from alphanodaviruses (such as NoV, FHV, black beetle virus [BBV], and Boolarra virus [BoV]) and betanodaviruses (such as striped jack nervous necrosis virus [SJNNV], greasy grouper nervous necrosis virus [GGNNV], and red-spotted grouper nervous necrosis virus [RGNNV]) using Clustal W and PHYLOWIN95 programs. According to phylogenetic analysis (Fig. 3), WhNV is the most distantly related to any other nodaviruses and forms an independent evolutionary branch of the phylogenetic tree of the Nodaviridae family. This result is consistent with our previous findings, which indicated that WhNV is the prototype member of a new genus of the Nodaviridae family (32).
Fig. 3.
Phylogenetic analysis of the B2 protein from the Nodaviridae family shows that WhNV B2 forms an independent evolution branch. The neighbor-joining algorithm was used to compare the amino acid sequences of the nodaviral B2 protein. The bars show evolutionary distances as computed (described in Materials and Methods).
To further compare WhNV B2 with FHV B2, whose crystal structure has been solved and extensively studied (12, 31), we modeled the 3-dimensional structure of WhNV B2 by submitting its amino acid sequence to the HMMSTR/Rosetta server. Compared to the three-alpha-helix structure of FHV B2, the WhNV B2 monomer is composed of five alpha helices (α1, residues 4 to 16; α2, residues 21 to 44; α3, residues 49 to 61; α4, residues 65 to 71; and α5, residues 76 to 80) (Fig. 4). The predicted helical structure of WhNV B2 was validated by circular dichroism (CD) spectroscopic analysis, since its CD spectrum exhibits a positive absorption peak at 192 nm and two negative peaks at 208 nm/222 nm, which are characteristics of the classical alpha-helical protein, myoglobin (N. Qi et al., http://www.bio.whu.edu.cn/UploadFiles/supplementary%20figures.pdf). The low structural homology of WhNV B2 with FHV B2 limits the usefulness of the structural data of FHV B2 for WhNV B2 investigation, implying that the RNA-binding mode of FHV B2 maybe not applicable to WhNV B2.
Fig. 4.
Comparison of 3-dimensional structures between WhNV B2 and FHV B2. The WhNV B2 3-dimensional structure was modeled by the HMMSTR/Rosetta server, as described in Materials and Methods. The figures of WhNV B2 and FHV B2 3-dimensional structures were generated using the PyMOL program from coordinate files, with the alpha-helices indicated. (A) The 3-dimensional structure of FHV B2; (B) modeled 3-dimensional structure of WhNV B2.
Positively charged-to-alanine scanning mutagenesis and N-terminal deletion of WhNV B2.
Given that the structural information of FHV B2 has limited utility in the structural analysis of WhNV B2, uncovering the functional domains of WhNV B2 is required to define its RNA-binding mode. Previous studies reported that RNA-binding proteins employ positively charged residues arginine and lysine to mediate electrostatic interaction with the phosphate backbone of dsRNA (12, 17, 31, 40). Interestingly, sequence analysis of the putative ORF of WhNV B2 showed that there are 10 positively charged residues, among which 6 are located at helices α1 to α3 (Fig. 5 A). In addition, we previously reported that the N-terminal 1- to 20-amino acid (aa) region of WhNV B2, which comprises helix α1, mediates its RNAi suppression property (9); however, its mechanism is still unknown. For this purpose, we performed positively charged-to-alanine scanning mutagenesis and deleted the N-terminal 1- to 20-aa region to map the functional domains of WhNV B2 (Fig. 5A). Vectors pMAL-c2x and pET-28a were used to express N-terminal MBP- and C-terminal His(6)-tagged fusion proteins, respectively, and the bacterium-expressed proteins were purified (Fig. 5B). The recombinant WhNV B2 protein and its mutants would be valuable tools for studying B2 functions and defining its functional domains in future research.
Fig. 5.
Positively charged-to-alanine scanning mutagenesis and N-terminal deletion of the WhNV B2 protein. (A) Sequence analysis of the B2 ORF and the mutagenesis strategy, with the five alpha-helical regions indicated by “α,” the sites of replacement with alanine indicated by a star, and the N-terminal deletion regions indicated by “▴.” The 11 B2 mutants are shown as R17A, R18A, R24A, R40A, R58A, K6A, K36A, K42A, K75A, K84A, and B2ΔN20. (B) Bacterium-expressed and purified proteins were subjected to 12% SDS-PAGE, followed by Coomassie brilliant blue R250 staining.
WhNV B2 directly binds to RNA duplexes and prevents dsRNA from RNase III-mediated cleavage in vitro.
Having demonstrated that WhNV B2 precipitates duplex RNA in Pr-E cells (Fig. 2B), it is intriguing to examine whether this protein directly binds to RNA duplexes in vitro. To this end, gel shift assays were conducted by incubating 0.1 μM in vitro-transcribed 500-bp dsRNA or 0.1 μM synthetic siRNA-EGFP, which mimics cellular pre-Dicer dsRNA or post-Dicer siRNA, with 30 nM recombinant MBP-B2 or MBP, respectively. As expected, almost all dsRNA underwent a distinct mobility shift when incubated with 30 nM MBP-B2 (Fig. 6 A, top lane 2), whereas MBP alone did not induce any shift under the same conditions (Fig. 6A, top lane 3). Interestingly, 30 nM MBP-B2 induced no detectable mobility shift in siRNA (data not shown). At a higher concentration of MBP-B2 (100 nM), nonbound siRNA and two shifted siRNA bands underwent mobility shifts (Fig. 6A, bottom lane 2). This result was consistent with those of immunoprecipitation assays (Fig. 2B), indicating that WhNV B2 has a higher affinity for dsRNA than for siRNA.
Fig. 6.
In vitro RNA-binding properties of the WhNV B2 protein and its mutants. (A) Gel shift assay for WT and mutated B2 proteins. In vitro-transcribed EGFP-derived 500-bp dsRNA (top lanes) and synthesized siRNA-EGFP (bottom lanes) at a concentration of 0.1 μM were incubated with 30 μM (100 μM for siRNA) MBP-B2, MBP, or its mutants as indicated above each lane. After 30 min of incubation at 25°C, products were separated on 1.2% TBE-agarose gels, and the RNA was visualized by staining with ethidium bromide. (B) RNase III cleavage protection assay for the B2 WT and its mutants. After incubation with 30 μM MBP-B2, MBP, or its mutants as indicated above, 0.1 μM 500-bp dsRNA was incubated with 1 U of RNase III at 37°C for 30 min. The synthesized siRNA-EGFP was loaded as a positive control (lane 1), indicating the location of the bands corresponding to the RNase III digestion products. (C) RNase III cleavage protection assay for the B2 protein at multiple concentrations. The experimental conditions used were identical to those indicated for panel B, except that the concentration of MBP-B2 was increased from 0 μM to 30 μM, as indicated above each lane, which was positively correlated with the increasing ratios of protected dsRNA to RNase III-processed siRNA.
Next, we examined the capability of WhNV B2 to protect dsRNA from RNase III-mediated cleavage. RNase III is a Dicer homolog and was used as the Dicer substitute for in vitro experiments as previously reported (17, 21, 56). Before being digested by RNase III, an in vitro-transcribed 500-bp dsRNA precursor was preincubated with MBP-B2 or MBP. In the presence of MBP-B2, dsRNA formed a high-molecular-weight complex and was then protected from RNase III digestion (Fig. 6B, lane 4), whereas MBP alone did not prevent dsRNA from being cleaved into approximately 21- to 23-bp siRNA (Fig. 6B, lane 5), the size of which was almost identical to the loaded synthetic siRNA-EGFP (Fig. 6B, lane 1). Additionally, at increasing concentrations of MBP-B2, the relative ratio of protected dsRNA to cleavage-generated siRNA was increased in a dose-dependent manner (Fig. 6C, lanes 3 to 6). These results clearly demonstrate that WhNV B2 blocks the in vitro RNase III cleavage activity by sequestering the dsRNA precursor directly.
Positively charged residues Arg 24, Arg 58, and the N-terminal of WhNV B2 are critical for the RNA-binding activity.
Crystal structure determination has shown that the FHV B2 dimer binds to dsRNA by a four-helix bundle fold, which is formed by an antiparallel association of two monomers (12, 31). The RNA-binding domain of the FHV B2 dimer is located at helices α2 and α2′, in which positively charged residues Lys 47 and Arg 54 mediate electrostatic interaction with the phosphate backbone of dsRNA (12, 31). Since the primary RNA-binding interface is often located at alpha helices, which are highly positively charged, identifying the positively charged residues required for the RNA-binding activity of WhNV B2 is critical for defining its RNA-binding domain. With this in mind, we introduced single replacement of each of the 10 positively charged residues of WhNV B2 with alanine to identify the RNA-binding essential residues.
Gel shift assays showed that different B2 mutants exhibited tremendous diversity in their effects on the RNA-binding activities. Obviously, mutation of Arg 24 or Arg 58 completely abolished their RNA-binding activities, as no mobility shift was observed (Fig. 6A, lanes 6 and 8). In contrast, mutation of the other three arginine residues did not result in a loss of the B2-RNA complex (Fig. 6A, lanes 4, 5, and 7). All five lysine-to-alanine mutants retained their RNA-binding activities, suggesting that the lysine residues of WhNV B2 are not essential for binding to RNA (Fig. 6A, lanes 10 to 14). Consistently, in RNase III cleavage protection assays, only mutants R24A and R58A failed to prevent dsRNA from being digested into siRNAs (Fig. 6B, lanes 8 and 10). Interestingly, we found that the RNA-binding essential residues, Arg 24 and Arg 58, were located at helices α2 and α3, which are highly positively charged (three arginines and two lysines included), indicating that the RNA-binding domain of WhNV B2 is formed primarily by helices α2 and α3.
In addition, deletion of N-terminal residues 1 to 20 of B2 also resulted in a complete abolishment of its ability to sequester and to protect dsRNA from RNase III-mediated cleavage (Fig. 6A, lane 9, and B, lane 11). Notably, the RNA-binding essential residues (Arg 24 and Arg 58) are not included in the N-terminal 1- to 20-aa region, suggesting that this N-terminal region of B2 does not directly contribute to form its RNA-binding interface but is functionally essential for its RNA-binding activity.
The RNA-binding activity of WhNV B2 mediates its RNAi suppression property.
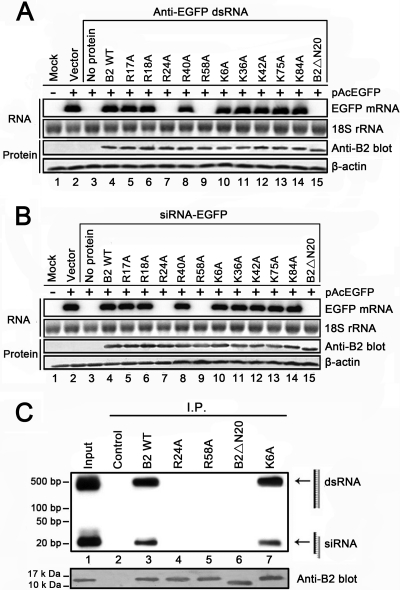
To confirm that binding to RNA is required for the silencing suppression activity of WhNV B2, we next examined the impact of RNA-binding-deficient mutations on its silencing suppression property. As expected, the RNA-binding-deficient mutants R24A, R58A, and B2ΔN20 exhibited a complete loss of their capability to suppress RNAi induced by plasmid-carrying dsRNA (Fig. 7 A, lanes 7, 9, and 15) or synthesized siRNA (Fig. 7B, lanes 7, 9, and 15), whereas WT B2 and other mutants, which retain their RNA-binding activities, markedly suppressed RNAi (Fig. 7A and B, lanes 4, 5, 6, 8, and 10 to 14). Consistently, in immunoprecipitation assays, compared to the B2 WT and the unaffected mutant K6A, none of the three RNAi suppression-deficient mutants precipitated dsRNA or siRNA duplexes in Pr-E cells (Fig. 7C, lanes 4 to 6). Taken together, these results clearly support the idea that WhNV B2 suppresses RNAi by dsRNA and siRNA sequestration.
Fig. 7.
Suppression of plasmid-carrying dsRNA or synthesized siRNA-induced RNAi in Pr-E cells by WT and mutated B2 proteins. The experimental conditions used were previously described for Fig. 1B. The mRNA levels of reporter gene EGFP and the expression of B2 proteins were detected by Northern and Western blot analyses, respectively. 18S rRNA and β-actin were used as loading controls. (A) Suppression of RNAi induced by plasmid-carrying dsRNA. EGFP expression plasmid, pAcEGFP, and EGFP-specific dsRNA expression plasmid, and anti-EGFP dsRNA were cotransfected into Pr-E cells, together with WT or mutated B2 expression plasmid as indicated. (B) Suppression of RNAi induced by synthetic siRNA. pAcEGFP and 40 nM synthetic siRNA-EGFP were cotransfected into Pr-E cells, together with WT or mutated B2 expression plasmid as indicated. (C) Northern blot analysis of RNA precipitated with mutated B2 proteins. The experimental conditions used were previously described for Fig. 2B. RNA extracted from the immunoprecipitates (IP) of a portion of cells assayed as shown in panel A, as indicated by the B2 WT (lane 3) and R24A, R58A, B2ΔN20, and K6A (lanes 4 to 7), was subjected to Northern blotting with a probe recognizing both EGFP-specific dsRNA and Dicer-processed siRNAs, indicated by cartons and arrows. Total RNA directly harvested from cell lysates was loaded as input (lane 1), and RNA extracted from the IP that immunoprecipitated with control serum was loaded as a negative control (lane 2). The presence of B2 and its mutants in the captured complex was confirmed by Western blotting using anti-B2 polyclonal antibodies.
Positive feedback between RNA binding and dimerization of WhNV B2.
Previous studies have reported that FHV B2 and NoV B2 bind to RNA duplexes as homodimers (12, 18, 24). With this in mind, we asked whether WhNV B2 also exists as a homodimer. In addition, since we determined that the RNA-binding essential region, N-terminal aa 1 to 20, is not responsible for forming the primary RNA-binding interface, we sought to examine whether this N-terminal region mediates WhNV B2 dimerization. To this end, following a 30-min treatment with chemical cross-linking reagent glutaraldehyde, 2 μg MBP, MBP-B2, or MBP-B2ΔN20 (deletion of aa 1 to 20) mutant was separated on a 12% denaturing polyacrylamide gel to test their capabilities to form homodimers in the absence of dsRNA. MBP-B2 was cross-linked and existed as a homodimer (Fig. 8 A, lane 4), since a high-molecular-mass complex around 110 kDa was observed. In contrast, neither MBP nor MBP-B2ΔN20 was cross-linked (Fig. 8A, lanes 1 and 2). Western blot analysis using anti-B2 polyclonal antibodies (Fig. 8A, lanes 5 and 6) further confirmed the previous observation. However, oligomers were not observed. These findings demonstrate that WhNV B2 exists as a homodimer in vitro in the absence of duplex RNA. Additionally, the N-terminal 1- to 20-aa region, containing helix α1, was found to be indispensable for B2 dimerization, indicating that helix α1 is involved in B2 dimerization. Given that the deletion of the N-terminal dimerization essential region of B2 resulted in a complete abolishment of its RNA-binding activity, we thus conclude that B2 dimerization is required for its binding to RNA.
Fig. 8.
Dimerization analysis of the WhNV B2 protein and its mutants. (A) Chemical cross-linking assays. (Left) Following a 30-min treatment with the cross-linking agent glutaraldehyde, 2 μg MBP-B2, MBP-B2ΔN20, or MBP was separated by 12% SDS-PAGE. (Right) Western blotting using anti-B2 polyclonal antibodies was conducted for further confirmation. The locations of the B2 monomer and dimer are indicated. (B) Far-Western blotting showing B2 self-interaction between two different recombinant B2 proteins. After being separated by 12% SDS-PAGE and transferred to PVDF membranes, 2 μg renatured His-B2 or His-B2ΔN20 was overlaid with 100 μg MBP-B2 or MBP as indicated above the panel and probed with anti-MBP antibody. Lane 1, MBP, a positive control to test the valence of MBP antibody used for blotting; lane 2, His-B2; lane 3, His-B2ΔN20; and lane 4, His-B2 overlaid with MBP as a negative control to eliminate the possibility of an interaction between B2 and MBP. (C) Far-Western blotting showing B2 self-interaction between His-B2 and MBP-B2 or its mutants in the absence (dsRNA −) or presence (dsRNA +) of 50 μg 500-bp dsRNA. As overlaid proteins, MBP-B2 or its mutants are indicated above the panel.
Next, we examined the dimerization activity of B2 in the context of dsRNA using far-Western blotting. His-B2 or His-B2ΔN20 was incubated with MBP-B2 or its mutants in the absence or presence of 500-bp dsRNA. His-B2 was blotted with anti-MBP antibody after incubation with MBP-B2 (Fig. 8B, lane 2), showing that these two different recombinant B2 proteins were clearly associated in the absence of dsRNA. In contrast, this effect was not observed with His-B2ΔN20, since deletion of the N-terminal residues 1 to 20 leads to an abolishment of B2 dimerization (Fig. 8B, lane 3). Interestingly, in the presence of dsRNA, B2 self-interaction was more intensive (Fig. 8C, compare top lane 1 to bottom lane 1). All together, these results indicate that although B2 could form homodimers in the absence of RNA, RNA binding to B2 dramatically promotes its dimerization.
In addition, we found that the RNA-binding essential residues Arg 24 and Arg 58 were not required for B2 dimerization, since all 10 MBP-tagged mutants mediated B2 self-interaction with His-B2 in the absence of dsRNA (Fig. 8C, top lanes 2 to 11). Notably, when dsRNA was present, compared to the B2 WT and eight other RNA-binding unaffected mutants, the dimerization of the RNA-binding-deficient mutants (R24A and R58A) was not enhanced (Fig. 8C, compare top lanes 4 and 6 to bottom lanes 4 and 6), further supporting the idea that RNA binding to B2 promotes its dimerization.
We subsequently deleted the last two helices, α4 and α5, and found that this portion of B2 is not responsible for its RNA-binding or dimerization activities (N. Qi et al., http://www.bio.whu.edu.cn/UploadFiles/supplementary%20figures.pdf). In conclusion, we defined the RNA-binding domain of WhNV B2 and the functionally essential region for B2 dimerization. Helix α1 is functionally required for B2 dimerization, while the RNA-binding domain is formed by helices α2 and α3 (N. Qi et al., http://www.bio.whu.edu.cn/UploadFiles/supplementary%20figures.pdf). Furthermore, our findings revealed a positive feedback between RNA binding and B2 dimerization (N. Qi et al., http://www.bio.whu.edu.cn/UploadFiles/supplementary%20figures.pdf), showing that B2 dimerization is required for its binding to RNA, whereas RNA binding to B2 in turn promotes its dimerization.
DISCUSSION
For viral RSS proteins, several RNA-binding modes have been described. Vaccinia virus E3L protein binds to dsRNA by the α-β-β-β-α fold of the canonical dsRBD (40). On the other hand, the RNA-binding modes of other RSS proteins that lack the canonical dsRBD are mediated by homodimerization. For instance, tombusvirus p19 binds to siRNA as a homodimer by an extended β-sheet surface and a small helical motif (48, 54), whereas the dimerization domain of the influenza virus NS1 protein is coincident with its RNA-binding domain, exhibiting a dimeric six-helical fold to recognize dsRNA (13, 14, 49). Additionally, the FHV B2 dimer binds to RNA by a special four-helix bundle fold that is formed by helices α1 and α2 in each monomer (12, 31). In the highly positively charged helix α2, Lys 47 and Arg 54, which are located at the center of the RNA-binding interface, mediate electrostatic contact with the phosphate-sugar backbone of the dsRNA stem (12, 31).
In our study, of the 10 positively charged residues, Arg 24 and Arg 58 were critical for the RNA-binding activity of WhNV B2 (Fig. 6A). These two RNA-binding essential residues, Arg 24 and Arg 58, are located at helices α2 and α3, respectively. Although the crystal structural information about WhNV B2 is not yet available, it is plausible that the RNA-binding interface is formed by helices α2 and α3, since these two highly positively charged helices (three arginines and two lysines included) are conformationally adjacent, exhibiting a helix-turn-helix-like motif (Fig. 4 and 5). In addition, we found that helix α1 is involved in WhNV B2 dimerization. Therefore, we propose that the WhNV B2 dimer employs a novel six-helical fold (α1, α1′, α2, α2′, α3, and α3′) to recognize RNA, which is different from the four-helix bundle fold of dimeric FHV B2. Further crystallographic study of the B2-RNA complex should be conducted to definitely resolve this novel RNA-binding mode.
The correlation between RNA binding and dimerization has been previously investigated. For instance, the dsRBD of PKR, a dsRNA-dependent protein kinase involved in mammalian antiviral response, was found to be able to mediate its dimerization (15, 47). Besides, this effect also exists in rat ADAR2 protein, an adenosine deaminase that acts on RNA (36). For viral RSS proteins, dimerization of influenza virus NS1 has been shown to correlate with its RNA-binding activity, as the RNA-binding-deficient NS1 mutants cannot form homodimers because of the overlapping between its RNA-binding and dimerization domains (49). Although the idea that the dimerization of RSS proteins is required for their RNA-binding activities has been further supported (12, 13, 48), whether RNA binding to RSS proteins could in turn promote their dimerization remains unknown. In this work, we revealed a positive feedback between RNA binding and dimerization of WhNV B2 and provided evidence that the promotion effect of RNA binding on dimerization exists in a viral RSS protein.
To interpret the promotion effect of RNA binding, we proposed a dynamic equilibrium and positive feedback model (N. Qi et al., http://www.bio.whu.edu.cn/UploadFiles/supplementary%20figures.pdf). In the absence of dsRNA, a small portion of B2 protein spontaneously forms homodimers, which might be conformationally loose and unstable, with a dynamic equilibrium established between the dimerization of monomers and the dedimerization of dimers. When dsRNA is present, RNA binding to this portion of dimeric B2 causes rearrangement and conformational change in the B2 dimer, thereby stabilizing the B2-RNA complex and weakening the dedimerization of the B2 dimer. Consequently, the dynamic equilibrium between the dimerization and the dedimerization of B2 is upset, since more and more B2 proteins exist as dimeric B2-RNA complexes rather than as monomers.
Through forming a complex with pre-Dicer precursor dsRNA and post-Dicer siRNA duplexes, WhNV B2 inhibits Dicer-mediated cleavage of dsRNA and siRNA loading onto the RISC (Fig. 2). In addition, our results suggested that WhNV B2 has a higher affinity for dsRNA than siRNA (Fig. 2B and 6A). However, these findings are inconsistent with our observation that WhNV B2 completely blocked highly sensitive siRNA-induced RNAi in Pr-E cells (Fig. 1B), leading us to question whether WhNV B2 suppresses RNAi by mechanisms other than sequestering siRNA duplexes. It is possible that WhNV B2 directly associates with specific components of RNAi machinery, like Dicer or Argonaute protein, to inhibit the RISC assembly or RISC-mediated mRNA degradation. Further studies are ongoing in our group to identify these potential RNAi suppression mechanisms of WhNV B2.
WhNV B2 is an attractive model for studying viral RSS proteins. As a potent RSS in both Diptera and Lepidoptera insect-derived cells (9), WhNV B2 can serve as a valuable model for elucidating innate immunity in insects. Furthermore, although nodaviral B2 proteins employ a similar strategy for suppressing RNAi, RNA binding by the novel six-alpha-helical fold of WhNV B2 dimer, which is different from the four-helix bundle revealed by FHV B2, would extend our knowledge about the RNA-binding modes of nodaviral B2 proteins. More importantly, we showed that RNA binding could promote B2 dimerization and further interpreted this promotion effect using the dynamic equilibrium and positive feedback model. These efforts significantly deepened our understanding of the molecular mechanisms by which viral RSS proteins suppress RNAi, since the positive feedback between RNA binding and dimerization not only may exist in WhNV or the Nodaviridae family but also may be a general mechanism of dimeric RSS proteins that bind to RNA.
ACKNOWLEDGMENTS
We are grateful to Zehua Yu, Yi Zhang, Yuji Kageyama, and Yasushi Hiromi for generously providing experimental materials. We also thank Markeda Wade (Houston, TX) for professionally editing the manuscript.
This work was supported by the National Natural Science Foundation of China (grant 30870112).
Footnotes
Published ahead of print on 6 July 2011.
REFERENCES
- 1. Agrawal N., et al. 2003. RNA interference: biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. 67:657–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aliyari R., et al. 2008. Mechanism of induction and suppression of antiviral immunity directed by virus-derived small RNAs in Drosophila. Cell Host Microbe 4:387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baulcombe D. 2002. RNA silencing. Curr. Biol. 12:R82–R84 [DOI] [PubMed] [Google Scholar]
- 4. Baulcombe D. C. 1996. RNA as a target and an initiator of post-transcriptional gene silencing in transgenic plants. Plant Mol. Biol. 32:79–88 [DOI] [PubMed] [Google Scholar]
- 5. Bergmann M., et al. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 74:6203–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernstein E., Caudy A. A., Hammond S. M., Hannon G. J. 2001. Role for a bidentate RNase in the initiation step of RNA interference. Nature 409:363–366 [DOI] [PubMed] [Google Scholar]
- 7. Bucher E., Hemmes H., de Haan P., Goldbach R., Prins M. 2004. The influenza A virus NS1 protein binds small interfering RNAs and suppresses RNA silencing in plants. J. Gen. Virol. 85:983–991 [DOI] [PubMed] [Google Scholar]
- 8. Bystroff C., Shao Y. 2002. Fully automated ab initio protein structure prediction using I-SITES, HMMSTR and ROSETTA. Bioinformatics 18(Suppl. 1):S54–S61 [DOI] [PubMed] [Google Scholar]
- 9. Cai D., et al. 2010. Characterization of Wuhan nodavirus subgenomic RNA3 and the RNAi inhibition property of its encoded protein B2. Virus Res. 151:153–161 [DOI] [PubMed] [Google Scholar]
- 10. Cardenas W. B., et al. 2006. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J. Virol. 80:5168–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carthew R. W. 2001. Gene silencing by double-stranded RNA. Curr. Opin. Cell Biol. 13:244–248 [DOI] [PubMed] [Google Scholar]
- 12. Chao J. A., et al. 2005. Dual modes of RNA-silencing suppression by Flock House virus protein B2. Nat. Struct. Mol. Biol. 12:952–957 [DOI] [PubMed] [Google Scholar]
- 13. Chien C. Y., et al. 1997. A novel RNA-binding motif in influenza A virus non-structural protein 1. Nat. Struct. Biol. 4:891–895 [DOI] [PubMed] [Google Scholar]
- 14. Chien C. Y., et al. 2004. Biophysical characterization of the complex between double-stranded RNA and the N-terminal domain of the NS1 protein from influenza A virus: evidence for a novel RNA-binding mode. Biochemistry 43:1950–1962 [DOI] [PubMed] [Google Scholar]
- 15. Cosentino G. P., et al. 1995. Double-stranded-RNA-dependent protein kinase and TAR RNA-binding protein form homo- and heterodimers in vivo. Proc. Natl. Acad. Sci. U. S. A. 92:9445–9449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cullen B. R. 2006. Is RNA interference involved in intrinsic antiviral immunity in mammals? Nat. Immunol. 7:563–567 [DOI] [PubMed] [Google Scholar]
- 17. Fenner B. J., Goh W., Kwang J. 2007. Dissection of double-stranded RNA binding protein B2 from betanodavirus. J. Virol. 81:5449–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fenner B. J., Goh W., Kwang J. 2006. Sequestration and protection of double-stranded RNA by the betanodavirus b2 protein. J. Virol. 80:6822–6833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hemmes H., Lakatos L., Goldbach R., Burgyan J., Prins M. 2007. The NS3 protein of Rice hoja blanca tenuivirus suppresses RNA silencing in plant and insect hosts by efficiently binding both siRNAs and miRNAs. RNA 13:1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishikawa T., et al. 1999. DCRY is a Drosophila photoreceptor protein implicated in light entrainment of circadian rhythm. Genes Cells 4:57–65 [DOI] [PubMed] [Google Scholar]
- 21. Ji X. 2008. The mechanism of RNase III action: how dicer dices. Curr. Top. Microbiol. Immunol. 320:99–116 [DOI] [PubMed] [Google Scholar]
- 22. Khvorova A., Reynolds A., Jayasena S. D. 2003. Functional siRNAs and miRNAs exhibit strand bias. Cell 115:209–216 [DOI] [PubMed] [Google Scholar]
- 23. Kondo T., Inagaki S., Yasuda K., Kageyama Y. 2006. Rapid construction of Drosophila RNAi transgenes using pRISE, a P-element-mediated transformation vector exploiting an in vitro recombination system. Genes Genet. Syst. 81:129–134 [DOI] [PubMed] [Google Scholar]
- 24. Korber S., Shaik Syed Ali P., Chen J. C. 2009. Structure of the RNA-binding domain of Nodamura virus protein B2, a suppressor of RNA interference. Biochemistry 48:2307–2309 [DOI] [PubMed] [Google Scholar]
- 25. Lakatos L., et al. 2006. Small RNA binding is a common strategy to suppress RNA silencing by several viral suppressors. EMBO J. 25:2768–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lakatos L., Szittya G., Silhavy D., Burgyan J. 2004. Molecular mechanism of RNA silencing suppression mediated by p19 protein of tombusviruses. EMBO J. 23:876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langland J. O., Cameron J. M., Heck M. C., Jancovich J. K., Jacobs B. L. 2006. Inhibition of PKR by RNA and DNA viruses. Virus Res. 119:100–110 [DOI] [PubMed] [Google Scholar]
- 28. Li H., Li W. X., Ding S. W. 2002. Induction and suppression of RNA silencing by an animal virus. Science 296:1319–1321 [DOI] [PubMed] [Google Scholar]
- 29. Li L. L., Chen Q. H. 1995. Continuous culture of embryos cells from Pieris rapae. J. Central China Normal Univ. 29:364–366 [Google Scholar]
- 30. Li W. X., et al. 2004. Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. Proc. Natl. Acad. Sci. U. S. A. 101:1350–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lingel A., Simon B., Izaurralde E., Sattler M. 2005. The structure of the flock house virus B2 protein, a viral suppressor of RNA interference, shows a novel mode of double-stranded RNA recognition. EMBO Rep. 6:1149–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu C., et al. 2006. Sequence analysis of coat protein gene of Wuhan nodavirus isolated from insect. Virus Res. 121:17–22 [DOI] [PubMed] [Google Scholar]
- 33. Liu C., et al. 2006. Isolation and RNA1 nucleotide sequence determination of a new insect nodavirus from Pieris rapae larvae in Wuhan city, China. Virus Res. 120:28–35 [DOI] [PubMed] [Google Scholar]
- 34. Lu R., et al. 2005. Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature 436:1040–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Merai Z., et al. 2005. Aureusvirus P14 is an efficient RNA silencing suppressor that binds double-stranded RNAs without size specificity. J. Virol. 79:7217–7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poulsen H., et al. 2006. Dimerization of ADAR2 is mediated by the double-stranded RNA binding domain. RNA 12:1350–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qiu Y., et al. 2011. Internal initiation is responsible for the synthesis of Wuhan nodavirus subgenomic RNA. J. Virol. 85:4440–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qu F., Ren T., Morris T. J. 2003. The coat protein of turnip crinkle virus suppresses posttranscriptional gene silencing at an early initiation step. J. Virol. 77:511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robalino J., et al. 2005. Double-stranded RNA induces sequence-specific antiviral silencing in addition to nonspecific immunity in a marine shrimp: convergence of RNA interference and innate immunity in the invertebrate antiviral response? J. Virol. 79:13561–13571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Romano P. R., et al. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 18:7304–7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Samuel C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778–809,table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schnettler E., et al. 2009. The NS3 protein of rice hoja blanca virus complements the RNAi suppressor function of HIV-1 Tat. EMBO Rep. 10:258–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schwarz D. S., et al. 2003. Asymmetry in the assembly of the RNAi enzyme complex. Cell 115:199–208 [DOI] [PubMed] [Google Scholar]
- 44. Selling B. H., Allison R. F., Kaesberg P. 1990. Genomic RNA of an insect virus directs synthesis of infectious virions in plants. Proc. Natl. Acad. Sci. U. S. A. 87:434–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stark G. R., Kerr I. M., Williams B. R., Silverman R. H., Schreiber R. D. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227–264 [DOI] [PubMed] [Google Scholar]
- 46. Sullivan C. S., Ganem D. 2005. A virus-encoded inhibitor that blocks RNA interference in mammalian cells. J. Virol. 79:7371–7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ung T. L., Cao C., Lu J., Ozato K., Dever T. E. 2001. Heterologous dimerization domains functionally substitute for the double-stranded RNA binding domains of the kinase PKR. EMBO J. 20:3728–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vargason J. M., Szittya G., Burgyan J., Hall T. M. 2003. Size selective recognition of siRNA by an RNA silencing suppressor. Cell 115:799–811 [DOI] [PubMed] [Google Scholar]
- 49. Wang W., et al. 1999. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA 5:195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang X., et al. 2000. Influenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferon. J. Virol. 74:11566–11573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang X. H., et al. 2006. RNA interference directs innate immunity against viruses in adult Drosophila. Science 312:452–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Waterhouse P. M., Wang M. B., Lough T. 2001. Gene silencing as an adaptive defence against viruses. Nature 411:834–842 [DOI] [PubMed] [Google Scholar]
- 53. Yang B., et al. 2006. Biochemical characterization of Periplaneta fuliginosa densovirus non-structural protein NS1. Biochem. Biophys. Res. Commun. 342:1188–1196 [DOI] [PubMed] [Google Scholar]
- 54. Ye K., Malinina L., Patel D. J. 2003. Recognition of small interfering RNA by a viral suppressor of RNA silencing. Nature 426:874–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zamore P. D., Tuschl T., Sharp P. A., Bartel D. P. 2000. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101:25–33 [DOI] [PubMed] [Google Scholar]
- 56. Zhang H., Kolb F. A., Jaskiewicz L., Westhof E., Filipowicz W. 2004. Single processing center models for human Dicer and bacterial RNase III. Cell 118:57–68 [DOI] [PubMed] [Google Scholar]
- 57. Zhang X., et al. 2006. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 20:3255–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]