Abstract
Elite controllers spontaneously maintain undetectable levels of HIV-1 replication for reasons that remain unclear. Here, we show that in elite controllers, direct ex vivo infection of purified CD4 T cells without prior in vitro activation results in disproportionately low levels of integrated HIV-1 DNA relative to the quantity of reverse transcripts, while the levels of two-long terminal repeat (2-LTR) circles were excessively elevated relative to those of integrated HIV-1 DNA. This indicates that chromosomal HIV-1 integration is inhibited in ex vivo-infected CD4 T cells from elite controllers. This defect in HIV-1 integration was unrelated to p21, a host protein that can restrict early HIV-1 replication steps, and was not visible following infection of in vitro-activated CD4 T cells from elite controllers. These data contribute to increasing evidence that intrinsic inhibition of specific HIV-1 replication steps plays an important role in the ability of elite controllers to maintain undetectable viral loads.
TEXT
Despite encouraging findings in the recent RV144 HIV-1 vaccine study (13), correlates of immune protection against HIV-1 remain poorly understood and continue to represent a high-priority area of research (7). Since individuals who spontaneously clear HIV-1 infection do not exist, efforts to identify effective mechanisms of immune defense have focused on elite controllers, a group of persons who maintain undetectable levels of viral replication in the absence of antiretroviral therapy, although residual low-level viremia remains detectable in most of these patients by ultrasensitive detection techniques (12). Current views suggest that effective suppression of HIV-1 replication in these patients is likely to involve a synergistic interplay between multiple innate and adaptive immune defense mechanisms and may be facilitated by specific polymorphisms in the human HLA class I gene locus (8, 11). Strong, highly functional HIV-1-specific CD8 T cell responses have been described for the majority of elite controllers (2), and these cells are able to effectively restrict HIV-1 replication, at least in in vitro experiments (15). In addition to HIV-1-specific T cell responses, recent studies from two separate laboratories indicated that cell-intrinsic inhibition of HIV-1 replication steps can also importantly contribute to HIV-1 immune defense in elite controllers (6, 14). In these investigations, it was shown that in vitro infection of CD4 T cells from elite controllers consistently resulted in lower levels of HIV-1 replication than in vitro infection of CD4 T cells from progressors and healthy volunteers. This reduced susceptibility to HIV-1 involved inhibition of early viral replication steps and was associated with a selective upregulation of p21, a host protein from the cyclin-dependent kinase inhibitor family that can modulate HIV-1 replication in macrophages (1), hematopoietic stem cells (16), and CD4 T cells (6). Intrinsic inhibition of HIV-1 replication steps in elite controllers was also suggested by a recent study in which HIV-1 DNA was quantified in direct ex vivo assessments of purified CD4 T cells (9). These investigations demonstrated that in comparison to HIV-1 patients with highly-active antiretroviral therapy (HAART)-mediated suppression of viral replication, elite controllers had significantly lower levels of chromosomally integrated HIV-1 DNA but elevated levels of HIV-1 2-long terminal repeat (2-LTR) circles, an episomal HIV-1 DNA form that results from aborted integration of HIV-1 DNA into host chromosomes. This pattern closely resembles alterations in HIV-1 replication dynamics observed after exposure to pharmaceutical HIV-1 integrase inhibitors and suggests that at least under specific circumstances, HIV-1 integration is restricted in elite controllers. However, following ex vivo infection of CD4 T cells from elite controllers, using spinoculation protocols, no evidence for cell-intrinsic inhibition of HIV-1 integration in CD4 T cells from elite controllers was found (9). Yet, intrinsic restriction of HIV-1 replication may not be visible after infection of CD4 T cells by spinoculation (14).
To overcome this possible limitation, we performed a detailed investigation of HIV-1 reverse transcription and integration in directly ex vivo-isolated CD4 T cells that were infected without spinoculation or prior to in vitro activation. For this purpose, CD4 T cells from elite controllers (HIV-1 viral load, <50/74 copies/ml; CD4 T cell count, 618/μl [363 to 1,001/μl]) recruited from the International HIV Controllers Study (www.hivcontrollers.org) and reference cohorts of HIV-1-negative volunteers and untreated HIV-1 progressors (viral load, 98,000 copies/ml [7,560 to 449,000 copies/ml]; CD4 T cell count, 488/μl [199 to 1,000/μl]) were ex vivo purified by negative immunomagnetic selection (purity, >90%). Afterwards, cells were infected with a yellow fluorescence protein (YFP)-encoding vesicular stomatitis virus G protein (VSV-G) pseudotyped HIV-1 virus (3) (50% tissue culture infective dose [TCID50] of 5,000) that infects cells independently of coreceptor-mediated entry processes and causes only a single round of infection, thus allowing for detailed assessments of individual early HIV-1 replication steps (4). After two washes, cells were plated at a concentration of 5 × 105 cells/ml in 24-well round-bottom plates in RPMI medium supplemented with 10% fetal calf serum (FCS) but without the addition of exogenous interleukin-2 (IL-2). Forty-eight hours after infection, cell lysates were collected and subjected to quantification of HIV-1 late reverse transcripts (LRT) and 2-LTR circles; chromosomally integrated HIV-1 was detected in cell lysates collected 96 h after infection using PCR protocols described in our previous work (5, 6).
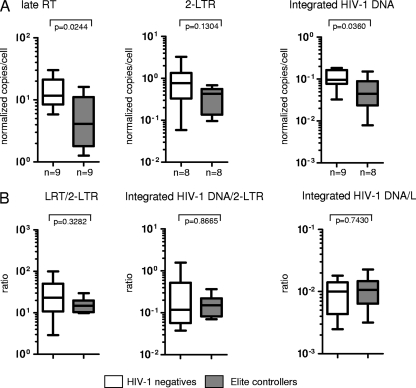
Following infection of ex vivo-isolated CD4 T cells with YFP-encoding VSV pseudotyped HIV-1, the proportions of YFP-positive CD4 T cells were 0.58% (0.17 to 1.4%) in elite controllers, 1.4% (0.77 to 2.14%) in progressors, and 1.79% (1.07 to 2.58%) in HIV-1-negative persons. As summarized in Fig. 1, infection of CD4 T cells resulted in significantly lower levels of LRT and integrated HIV-1 DNA in CD4 T cells from elite controllers than in cells from HIV-1-negative persons or progressors, as described in our earlier findings (6). In contrast, no significant differences were found between 2-LTR quantities from elite controllers and those from the two reference cohorts. These data resulted in significantly reduced ratios of LRT to 2-LTR and integrated DNA to 2-LTR in elite controllers in comparison to those for HIV-1-negative persons or progressors, indicating that relative to LRT and integrated HIV-1 DNA quantities, 2-LTR circles were disproportionately elevated in elite controllers. Moreover, ratios of integrated DNA to LRT were also significantly lower in elite controllers than in HIV-1-negative persons or progressors, consistent with a disproportionate decrease of integrated HIV-1 DNA relative to LRT levels in elite controllers. Overall, this altered pattern of early HIV-1 replication products strongly suggests a defect at the level of HIV-1 integration in ex vivo-infected CD4 T cells from elite controllers.
Fig. 1.
Inhibition of HIV-1 integration in purified, ex vivo-infected CD4 T cells from elite controllers. CD4 T cells from elite controllers, HIV-1-negative persons, or HIV-1 progressors were ex vivo infected with HIV-1 without prior in vitro activation. (A) Summary of the quantity of LRTs, 2-LTR circles, and integrated HIV-1 DNA in the three study cohorts. RT, reverse transcripts. (B) Ratios of the indicated HIV-1 DNA forms from the three study cohorts. Autologous HIV-1 DNA levels in CD4 T cells without exogenous HIV-1 infection were subtracted from corresponding HIV-1 DNA quantities after ex vivo infection. Significance was tested using Mann-Whitney U tests. Data are presented as box-and-whisker plots, indicating the median, interquartile ranges, and minimum and maximum values.
Prior studies have shown that p21, a host protein from the cyclin-dependent kinase inhibitor family that is highly upregulated in CD4 T cells from elite controllers, can inhibit HIV-1 integration in hematopoietic stem cells (16) and may also be involved in restriction of early HIV-1 replication steps in macrophages (1) and CD4 T cells (6). To analyze whether p21 is involved in the observed inhibition of HIV-1 integration in ex vivo-infected CD4 T cells from elite controllers, we performed HIV-1 infection experiments with ex vivo-isolated CD4 T cells in the presence of a small molecule inhibitor of p21 (#15; concentration of 2 μM) that selectively eliminates p21 through proteasomal degradation (10); control cells were treated with the carrier dimethyl sulfoxide (DMSO) only. In line with prior work (6), the addition of the p21 inhibitor had no effect on LTR, 2-LTR circles, and integrated HIV-1 DNA in CD4 T cells from HIV-1-negative persons, likely as a result of low-baseline p21 expression in these individuals (Fig. 2). However, following inhibition of p21 in CD4 T cells from elite controllers, LRT and 2-LTR circles significantly increased and reached levels similar to those in HIV-1-negative persons; this suggests that p21 inhibition can overcome intrinsic restriction at the level of HIV-1 reverse transcription in elite controllers. In contrast, integrated HIV-1 DNA only weakly increased after p21 inhibition in CD4 T cells from elite controllers and remained significantly lower than in control cells from HIV-1-negative persons. Moreover, inhibition of p21 resulted in lower ratios of integrated DNA to 2-LTR and integrated DNA to LRT than in control cells, indicating that the disproportionate decrease of integrated HIV-1 DNA relative to LRT and 2-LTR circles described above cannot be corrected by the silencing of p21 (Fig. 2). Overall, this suggests that the intrinsic inhibition of HIV-1 integration in ex vivo-infected CD4 T cells from elite controllers is unrelated to p21.
Fig. 2.
Inhibition of HIV-1 integration in ex vivo-infected CD4 T cells from elite controllers is unrelated to p21. CD4 T cells from elite controllers or HIV-1-negative persons were ex vivo-infected with HIV-1 without prior in vitro activation. Experiments were performed in the presence of a small molecule inhibitor of p21 (striped bars) or the carrier DMSO as a control (solid bars). (A) Quantitation of levels of LRT, 2-LTR, and integrated HIV-1 DNA in the two study cohorts. (B) Corresponding ratios of indicated HIV-1 DNA forms. Significance was tested by Mann-Whitney U tests or paired Wilcoxon tests, as appropriate. Data are presented as box-and-whisker plots, indicating the median, interquartile ranges, and minimum and maximum values.
We subsequently investigated whether an inhibition of HIV-1 integration is also detectable in CD4 T cells from elite controllers that were infected after in vitro activation. Our previous work has shown that following in vitro activation, CD4 T cells from elite controllers were significantly less susceptible to HIV-1 infection (6); this appeared to be related to blockages at the level of HIV-1 reverse transcription and mRNA transcription, while a possible inhibition of viral integration in these cells remained unclear. To investigate this further, we activated CD4 T cells from elite controllers and HIV-1-negative persons using CD3/CD8-bispecific antibodies (0.5 μg/ml) and IL-2 (50 IU/ml) as described before. After 5 days, CD4 T cell populations without contaminating CD8 T cells (<0.1%) were infected with the VSV-G pseudotyped HIV-1 virus (TCID50 of 1,000) and plated at a concentration of 5 × 105 cells/ml in RPMI medium supplemented with 10% FCS and IL-2. Cell lysates collected after 18 h were used for quantification of LRTs and 2-LTR circles, while samples obtained after 48 h were used for assessments of integrated HIV-1 DNA. Overall, we observed that LRT and integrated DNA levels were significantly lower in elite controllers than in HIV-1-negative persons, as demonstrated previously (6). The levels of 2-LTR circles showed a similar pattern and were also reduced in elite controllers compared to those in HIV-1-negative persons (Fig. 3). Ratios of LRT to 2-LTR, integrated HIV-1 DNA to 2-LTR, and LRT to integrated HIV-1 DNA were not significantly different between the two study cohorts, suggesting that reduced levels of integrated HIV-1 DNA in ex vivo-activated CD4 T cells from elite controllers represent a consequence of reduced HIV-1 reverse transcripts and not an independent restriction at the level of HIV-1 integration.
Fig. 3.
Uncompromised HIV-1 integration in CD4 T cells from elite controllers infected after in vitro activation. CD4 T cells from elite controllers or HIV-1-negative persons were activated with CD3/CD8-bispecific antibodies and IL-2 for 5 days before being infected with HIV-1. (A) Summary of the quantities of late reverse transcripts, 2-LTR circles, and integrated HIV-1 DNA in the two study cohorts. (B) Ratios of the indicated HIV-1 forms in the two study cohorts. Significance was tested using Mann-Whitney U tests. Data are presented as box-and-whisker plots, indicating the median, interquartile ranges, and minimum and maximum values.
In this study, we analyzed early HIV-1 replication steps in purified CD4 T cells that were infected directly ex vivo without prior activation or spinoculation. We showed that exogenous HIV-1 infection of directly ex vivo-isolated CD4 T cells from elite controllers leads to disproportionate reductions in the levels of integrated HIV-1 DNA relative to those of LRT and 2-LTR circles; moreover, the levels of 2-LTR circles were disproportionately increased relative to those of LRT and integrated HIV-1 DNA. This specific pattern is consistent with a block at the level of chromosomal HIV-1 integration and corresponds well to the recent description of increases in 2-LTR quantities relative to that of chromosomally integrated HIV-1 DNA in ex vivo-isolated CD4 T cells from elite controllers (9). In combination, these studies strongly suggest that at least under specific circumstances, the efficacy of HIV-1 integration can be markedly reduced in CD4 T cells from elite controllers and warrant further studies to identify molecular mechanisms that contribute to such a block. Notably, chromosomal integration of HIV-1 DNA depends on a number of different host proteins, and alterations in the expression or function of such proteins may lead to conditions that only insufficiently support HIV-1 integration in elite controllers. Moreover, it is possible that specific molecular inhibitors that block host proteins required for effective HIV-1 integration and in this way exert an indirect effect on chromosomal HIV-1 integration are available in CD4 T cells from elite controllers. Importantly, activation levels of CD4 T cells from our elite controller cohort were slightly elevated in comparison to those of HIV-1-negative persons, as determined by surface expression levels of HLA-DR in direct ex vivo assessments (data not shown); this indicates that defective HIV-1 integration in CD4 T cells from these patients cannot be attributed simply to reduced activation of CD4 T cells in elite controllers. Overall, the studies presented here contribute to increasing evidence that intrinsic restriction of HIV-1 replication plays an important role in the ability of elite controllers to maintain undetectable viral loads and may stimulate future mechanistic studies to identify cell-intrinsic inhibitors of chromosomal HIV-1 integration in CD4 T cells from elite controllers.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (AI093203 to M.L. and AI078799 and AI089339 to X.G.Y.). M.L. and X.G.Y. are both recipients of the Doris Duke Clinical Scientist Development Award. M.J.B. is supported by a Fellowship Award from the European Molecular Biology Laboratory (EMBL). R.W. is supported by the NIH (grants CA135401 and DK082690) and by the Medical Service of the U.S. Department of Veterans Affairs.
Footnotes
Published ahead of print on 6 July 2011.
REFERENCES
- 1. Bergamaschi A., et al. 2009. The CDK inhibitor p21Cip1/WAF1 is induced by FcγR activation and restricts the replication of human immunodeficiency virus type 1 and related primate lentiviruses in human macrophages. J. Virol. 83:12253–12265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Betts M. R., et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brass A. L., et al. 2008. Identification of host proteins required for HIV infection through a functional genomic screen. Science 319:921–926 [DOI] [PubMed] [Google Scholar]
- 4. Butler S. L., Hansen M. S., Bushman F. D. 2001. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7:631–634 [DOI] [PubMed] [Google Scholar]
- 5. Buzón M. J., et al. 2010. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat. Med. 16:460–465 [DOI] [PubMed] [Google Scholar]
- 6. Chen H., et al. 2011. CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J. Clin. Invest. 121:1549–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Council of the Global HIV Vaccine Enterprise 2010. The 2010 scientific strategic plan of the Global HIV Vaccine Enterprise. Nat. Med. 16:981–989 [DOI] [PubMed] [Google Scholar]
- 8. Fellay J., et al. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317:944–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graf E. H., et al. 2011. Elite suppressors harbor low levels of integrated HIV DNA and high levels of 2-LTR circular HIV DNA compared to HIV+ patients on and off HAART. PLoS Pathog. 7:e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park S. H., Wang X., Liu R., Lam K. S., Weiss R. H. 2008. High throughput screening of a small molecule one-bead-one-compound combinatorial library to identify attenuators of p21 as chemotherapy sensitizers. Cancer Biol. Ther. 7:2015–2022 [DOI] [PubMed] [Google Scholar]
- 11. Pereyra F., et al. 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pereyra F., et al. 2009. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J. Infect. Dis. 200:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rerks-Ngarm S., et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 14. Sáez-Cirión A., et al. 3 June 2011, posting date Restriction of HIV-1 replication in macrophages and CD4+ T cells from HIV controllers. Blood [Epub ahead of print.] doi: 10.1182/blood-2010-12-327106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sáez-Cirión A., et al. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. U. S. A. 104:6776–6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J., Scadden D. T., Crumpacker C. S. 2007. Primitive hematopoietic cells resist HIV-1 infection via p21. J. Clin. Invest. 117:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]