Abstract
We investigated the antiviral activity and gene induction properties of interferon gamma (IFN-γ) compared to type I IFN (IFNa1) in Atlantic salmon. IFN-γ protected salmon cells against infectious pancreatic necrosis virus (IPNV)-induced cytopathic effect (CPE), reduced virus titers, and inhibited the synthesis of the viral structural protein VP3. Moreover, IFN-γ showed potent antiviral activity against salmonid alphavirus 3 (SAV3) measured as a reduction in virus nsP1 transcripts. IFN-γ (a type II IFN) had less specific antiviral activity against IPNV than IFNa1, showing a half-maximal effective concentration of 1.6 ng/ml versus 31 pg/ml determined in the CPE reduction assay. Compared to IFNa1, IFN-γ was a more effective inducer of the antiviral protein GBP, several interferon regulatory transcription factors (IRFs), and the chemokine IP-10. The antiviral activity of IFN-γ may also in part be ascribed to upregulation of Mx, ISG15, and viperin. These are typical type I IFN-induced genes in mammals and were also more strongly induced by IFNa1 than by IFN-γ in salmon cells. Fish and mammalian IFN-γ thus show strikingly similar gene induction properties. Interestingly, the antiviral activity of IFN-γ against IPNV and SAV3 and its ability to induce Mx and ISG15 markedly decreased in the presence of neutralizing antiserum against IFNa1. In contrast, antiIFNa1 had no effect on the induction of IRF-1 and IP-10 by IFN-γ. This suggests that the antiviral activity of IFN-γ is partially dependent on IFNa induction. However, because antiIFNa1 could not abolish the IFN-γ-mediated induction of Mx and ISG15 completely, IFN-γ may possibly also induce such genes directly.
INTRODUCTION
Interferons (IFNs) were originally identified as proteins that induce an antiviral state in cells, but they also have important regulatory functions in the immune system (51). Type I IFN (predominantly IFN-α and IFN-β) and type II IFN (IFN-γ) play critical roles in innate and adaptive immune response against viral infection in mammals (30, 32). IFN-α/β are produced by most cells upon virus infection. In contrast, IFN-γ is produced primarily by natural killer (NK) cells during innate responses, and by CD4+ T helper 1 (Th1) cells and CD8+ cytotoxic T cells during adaptive immune responses (44). IFN-γ is regarded as the typical Th1 cytokine because it directs differentiation of naive CD4+ cells toward a Th1 phenotype and is a major product of Th1 cells (45).
IFN-α/β and IFN-γ bind to distinct receptors, which mediate signaling through distinct, but overlapping JAK-STAT pathways resulting in transcriptional activation of IFN-stimulated genes (ISGs) (51). The major transcription factor formed after IFN-α/β stimulation is ISGF3, which is a hetero-trimer composed of phosphorylated STAT1 and STAT2, and interferon regulatory factor 9 (IRF-9) (36). ISGF3 binds to the IFN-stimulated response element (ISRE), a promoter element found in IFN-stimulated genes such as Mx and ISG15 (18). In contrast, the transcription factor formed after IFN-γ stimulation is a STAT1 homodimer, which activates ISGs containing gamma activation site promoter elements found in guanylate-binding protein (GBP) and IRF-1 (9).
The IFN systems in fish and mammals are similar but do also display important differences. Most striking is the difference in type I IFN, which during evolution appeared first in fish as intron-containing genes but was apparently reintroduced into the genomes of amniotes by a retrotransposition event and developed into a new multigene family lacking introns (26). The Atlantic salmon genome contains a cluster of at least 11 type I IFN genes encoding three subtypes of IFNs named IFNa, IFNb, and IFNc (54). IFNa is the predominant IFN produced by most cells and induces Mx and ISG15 and antiviral activity against infectious pancreatic necrosis virus (IPNV) (3, 24, 39, 42).
IFN-γ has been identified from several fish species, including rainbow trout and Atlantic salmon (19, 28, 38, 53, 61, 62). In contrast to the type I IFNs, fish and mammalian IFN-γ are similar in exon/intron structure and display gene synteny. However, some fish species also possess a second IFN-γ subtype named IFN gamma rel, which is quite different from the classical IFN-γ (14). Rainbow trout and carp IFN-γ have several functional properties in common with mammalian IFN-γ including the ability to enhance respiratory burst activity, nitric oxide production, and phagocytosis of bacteria in macrophages (2, 14, 61). Moreover, like mammalian IFN-γ, trout IFN-γ induces the expression of IFN-γ inducible protein 10 (IP-10), major histocompatibility complex class II β-chain, and STAT1 and signals through STAT1 (50, 61).
Far less is known about the antiviral properties of fish IFN-γ, although similar to mammals, IFN-γ was shown to induce the antiviral gene GBP in trout (41). In the present study we have studied the antiviral activity of IFN-γ against IPNV and salmonid alphaviruses (SAV), both of which cause high losses in Norwegian aquaculture of Atlantic salmon. IPNV is a naked double-stranded RNA virus, which belongs to the Birnaviridae family and is closely related to infectious bursal disease virus, which is a major problem in chicken farming (34). IPNV kills salmon fry in freshwater and smolts shortly after release into seawater (46). SAV are enveloped positive-sense single-stranded RNA viruses, which are pathogens of salmonids. At present, SAV encompass six subtypes, of which SAV3 is the cause of pancreas disease of Atlantic salmon in Norwegian seawater farms (22). SAV belong to the genus Alphavirus within the family Togaviridae and are phylogenetically related to arthropod-borne alphavirus groups such as the Semliki Forest virus group and the Sindbis virus group (35).
We show here that IFN-γ induces antiviral activity against both IPNV and SAV3 in salmon. This is in contrast to a recent report, which stated that salmon IFN-γ had no ability to inhibit replication of SAV3 (57). Gene induction studies demonstrated that IFN-γ is a less potent inducer of the typical antiviral genes Mx, ISG15, viperin, and PKR compared to IFNa1 but is a more effective inducer of GBP and several IRFs than IFNa1. Interestingly, the antiviral activity of IFN-γ against IPNV and its ability to induce Mx and ISG15 was found to be partially mediated by the induction of IFNa.
MATERIALS AND METHODS
Cells.
Atlantic salmon TO cells were kindly provided by Heidrun Wergeland, University of Bergen (56). TO and Chinook salmon embryo cells (CHSE-214) were maintained at 20°C and 5% CO2 in Eagle minimal essential medium (EMEM; Invitrogen Life Technologies) supplemented with 100 μg of streptomycin/ml, 100 U of penicillin, 4 mM l-glutamine, and 5 or 8% fetal bovine serum, respectively. Atlantic salmon ASK-2 cells (11) were kindly provided by Birgit Dannevig, Norwegian Veterinary Institute, and were maintained in L-15 medium (Invitrogen) supplemented with 100 μg of streptomycin/ml, 100 U of penicillin, and MEM nonessential amino acids with 5% FBS at 16°C.
IFNs.
Recombinant Atlantic salmon IFNa1 and rainbow trout IFN-γ were produced by modification of a protocol described previously (61). Briefly, the coding sequences of the mature salmon IFNa1 (39) and rainbow trout IFN-γ (61) were synthesized in the bacterial genetic code by GenScript USA, Inc. The sequences were amplified by PCR using primers with BamHI and HindIII cutting sites (Table 1). The PCR conditions were 94°C for 2 min and then 30 cycles of 94°C for 30 s, 69°C for 30 s, and 72°C for 45 s, followed by 72°C for 7 min. The PCR products were separated on a 1.2% agarose gel, purified using a Qiagen gel extraction kit (Qiagen), and digested with restriction enzymes BamHI and HindIII. The digested fragments were ligated into the pQE30 expression vector (Qiagen) and transformed into Escherichia coli M15 cells (Qiagen). To get the maximum soluble proteins, 0.2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was used in the induction, and the temperature for the induction stage was lowered to 28°C. The bacteria were harvested and sonicated on ice in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10% glycerol [pH 8.0]), and the recombinant proteins were purified using the Ni-NTA resin column (Qiagen) with Triton X-114 in the first phase of washing. The purity of the recombinant IFNs was checked on a 4 to 12% precast SDS-PAGE gel (Invitrogen Life Technologies) stained with SimpleBlue (Invitrogen). Protein concentrations were measured with a QuickStart Bradford protein assay kit (Bio-Rad) with bovine serum albumin as a standard.
Table 1.
Primers for protein expression and real-time PCR
| Primer | Sequence (5′–3′) | Application |
|---|---|---|
| nsP1F | CCGGCCCTGAACCAGTT | qPCR |
| nsP1R | GTAGCCAAGTGGGAGAAAGCT | |
| viperinF | TCCTTGATGTTGGCGTGGAA | qPCR |
| viperinR | GCATGTCAGCTTTGCTCCACA | |
| IP10F | TGGTCAAGTTGGAGACGGTCA | qPCR |
| IP10R | TGGAACGCATGGACACATTG | |
| GBPF | TGAACATGCACCGACTGGACT | qPCR |
| GBPR | TCTTCAGACGGCTTTCCTGGTA | |
| ISG15F | ACAGTTCAGCCACACACCACTG | qPCR |
| ISG15R | GCTCCCATTGACACCTAACAGC | |
| IRF9F | ACCCATCCAGCAAACTCATCAT | qPCR |
| IRF9R | ATGTCGCGGAGGTAGGTCATT | |
| IRF8F | AAGGTTCCTCCAAGCTCTCCAG | qPCR |
| IRF8R | TTTGCTCTTGGCTGTGCTGAG | |
| IRF1F | AGGCTAATTTCCGCTGTGCA | qPCR |
| IRF1R | TTTTGTAGACGCGCACTGCT | |
| IRF3F | TGGACCAATCAGGAGCGAAC | qPCR |
| IRF3R | AGCCCACGCCTTGAAAATAA | |
| IRF7F | GTCGTCAAGGTGGTTCCCCT | qPCR |
| IRF7R | TGGGAGATCTGCAGGCTGAT | |
| GADPHF | GAAGGGAATCAAAGTCGTTGC | qPCR |
| GADPHR | CCATGCTCACCTCACCCTTGT | |
| MxF | TGCAACCACAGAGGCTTTGAA | qPCR |
| MxR | GGCTTGGTCAGGATGCCTAAT | |
| IFNaF | TGCAGTATGCAGAGCGTGTG | qPCR |
| IFNaR | TCTCCTCCCATCTGGTCCAG | |
| omIFNgF | GGGATCCGCTCAGTTCACATCAATTAAC | Protein expression |
| omIFNgR | CAAGCTTCTACATGATGTGTGATTTGAG | |
| ssIFNaF | GCGGATCCTGTGACTGGATCCGACACCACT | Protein expression |
| ssIFNaR | GCAAGCTTTCATCAGTACATCTGTGCTGCAAG |
Viruses.
IPNV serotype Sp N1 (7) was propagated in TO and CHSE-214 cells. Virus was harvested from the medium and titrated in CHSE-214 cells using the 50% tissue culture infective dose (TCID50) method (37). SAV3 was obtained from Birgit Dannevig at Norwegian Veterinary Institute, Oslo, Norway. SAV3 was propagated by inoculating 80% confluent CHSE-214 cells maintained with growth medium supplemented with 2% FBS. The virus stock contained 43,200 copies of virus nsP1 per μl, as determined by absolute quantitative real-time reverse transcription-PCR (RT-PCR) (see below). Infections were carried out by absorbing the virus suspension to the cells in serum-free medium for 2 to 3 h, after which the cells were rinsed once with phosphate-buffered saline (PBS) and incubated for the indicated periods in medium containing supplements and 2% FBS.
Virus titration.
IPNV titers were determined by endpoint titration on CHSE-214 cells and calculated by the TCID50 method (37).
Antibodies.
A monoclonal antibody against the VP3 protein of IPNV (antiVP3) was kindly provided by K. E. Christie, Intervet Norbio, Bergen, Norway. Polyclonal antibodies against salmon IFNa1 (antiIFNa1) and salmon ISG15 were obtained as described previously (3, 42). Polyclonal rabbit antibody was prepared against Atlantic salmon Mx1 (40) expressed in E. coli by Hilde Hansen, Norwegian College of Fishery Science, University of Tromsø. The polyclonal antibody against actin was from Sigma.
RNA isolation, cDNA synthesis, and real-time PCR.
Total RNA was isolated with TRIzol (Invitrogen) from TO cells stimulated for 24 h with the indicated concentrations of IFNa1 and IFN-γ with or without 1% antiIFNa1 antiserum. Then, 1 μg of RNA was reverse transcribed in a 40-μl reaction volume using a cDNA synthesis kit according to the manufacturer's instructions (Fermentas Life Sciences). Primers for real-time PCR were designed by Applied Biosystems protocols and are listed in Table 1. The primers for salmon IFNb and IFNc were as described previously (54).
Quantitative PCR was performed by using a ABI Prism 7000 sequence detection system (Applied Biosystems). For each gene, 2 μl of cDNA was used as a template in a mixture of specific primers (final concentration, 500 nM) and 10 μl of SYBR green PCR Master Mix (Applied Biosystems) in a final volume of 20 μl. The mixtures were first incubated at 95°C for 10 min, followed by 40 amplification cycles of 10 s at 95°C and 60 s at 60°C. The specificity of the PCR products from each primer pair was confirmed by the melting-curve analysis and subsequent agarose gel electrophoresis. Relative quantifications of gene transcripts were performed by the 2−ΔΔCT method (33). All quantifications were normalized to the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
Western blot analysis.
TO cells in 24-well plates were stimulated with 100 ng of recombinant trout IFN-γ/ml for the indicated time periods. The cells were rinsed with PBS before the lysates were harvested in SDS sample buffer (160 mM Tris-HCl [pH 6.8], 10% β-mercaptoethanol, 2% SDS, 20% glycerol, 0.1% bromophenol blue). Inhibition of IPNV replication was studied by immunoblotting of VP3 as follows. TO cells were stimulated with 100 ng of IFN-γ/ml with or without 1% antiIFNa1 antiserum for 24 h preinfection with IPNV at a multiplicity of infection (MOI) of 0.1. For samples containing antiIFNa1 antiserum, 1% antiIFNa1 antiserum was also added to the medium after adsorption of the virus and left until harvesting. Supernatants and lysates were harvested at the indicated time points postinfection. Lysates were boiled for 10 min and subjected to Western blotting by applying the NuPAGE system (Invitrogen). Blotting and incubation with antiVP3 (1:1,000), Mx1 (1:3,000), and actin antibody (1:1,000) was performed according to the manufacturer's instructions. Blots were stripped in 0.2 M NaOH for 10 min and incubated with primary ISG15 antibody (1:20,000). Secondary antibody was goat anti-rabbit- or anti-mouse horseradish peroxidase-antibody (Santa Cruz Biotechnology) diluted 1:25,000. Detection was performed by using the SuperSignal West Pico chemiluminescent substrate and a molecular imaging system from Carestream Health, Inc., Rochester, NY. Quantification of blots was done utilizing the Carestream MI software according to the manufacturer's instructions.
Anti-IPNV assay by the CPE reduction method.
IFN activity in cell supernatants was determined by their ability to induce protection against IPNV-induced cytopathic effect (CPE) (3). Recombinant IFNs were 2-fold serially diluted in EMEM with 5% FBS (salmon IFNa1 from 2 ng/ml to 7.8 pg/ml and trout IFN-γ from 50 to 0.1 ng/ml), and 100 μl of each dilution was added to quadruplicate wells of subconfluent TO cells or ASK cells in 96-well plates. To determine whether IFNa was involved in the antiviral activity of IFN-γ, a neutralizing anti-IFNa antibody was used as previously described (3). Briefly, antiIFNa1 antiserum or preserum control at a dilution of 1:100 was added and present in the medium during IFN-γ stimulation and during the whole virus infection procedure.
After 24 h, the culture medium was removed and the cells were infected with IPNV (MOI of 0.1) in 100 μl of EMEM without FBS. Virus was absorbed for 2 h before 100 μl of medium with 4% serum was added. When complete destruction of unstimulated infected cells occurred after approximately 4 days, all of the cells were washed with PBS and fixed and stained by incubation with 1% (wt/vol) crystal violet in 20% ethanol for 10 min. The cells were then washed three times with distilled water and air dried before the stain was dissolved by the addition of 100 μl of 50% ethanol containing 0.05 M sodium citrate and 0.05 M citric acid. The absorbance was then determined at 550 nm.
Anti-SAV3 assay by measuring nsP1 transcripts.
TO cells were seeded in 24-well plates (64,000 cells/well) and grown at 16°C. Cells in triplicate wells were kept unstimulated or stimulated with IFN-γ from 25 to 0.03 ng/ml with or without 1% anti-IFNa antiserum or stimulated with 1 ng of IFNa1/ml. After 24 h, the culture medium was removed, and the cells were infected with SAV3 (216,000 nsP1 transcripts) in 500 μl of L-15 medium without FBS. Two hours later, another 500 μl of L-15 medium with 4% FBS was added, and the cells were incubated at 16°C. SAV3 replication was analyzed 1, 8, and 14 days after infection by absolute quantification of virus nsP1 transcripts using real-time RT-PCR as described in the manual supplied by Applied Biosystems. The primers are listed in Table 1. Briefly, the nsP1 fragment (107 bp) was cloned into the pCRT4 TOPO TA vector (Invitrogen), and the concentration of purified plasmid was measured by using a NanoDrop 1000 spectrophotometer (Thermo Scientific). The plasmid copy numbers was calculated according to 1 μg of 1,000-bp DNA containing 9.1 × 1011 molecules, and serial dilutions of plasmid (102 to 108 copies) were used as standards in each real-time PCR run. For comparison of nsP1 copy numbers between different time points postinfection, the number of nsP1 transcripts in non-IFN-treated, virus-infected cells at day 1 was taken as 1.
Statistical analysis.
Statistical analysis was performed on log transformed TCID50 values and quantified Western blot signal values. The GraphPad Prism4 software (GraphPad Software, Inc., San Diego, CA) was used to run one-way analysis of variance for the different treatment groups within each time point, followed by a Bonferroni post test. A P value of <0.05 was considered significant.
RESULTS
Antiviral activity against IPNV of IFN-γ compared to IFNa1.
In the initial experiments, the salmon IFN-γ gene (GenBank accession number AAW21707) was cloned and expressed in E. coli using the pQE-30 vector according to the protocol described for rainbow trout IFN-γ (61). However, because salmon IFN-γ hardly expressed as a soluble protein, rainbow trout IFN-γ was used in the following experiments due to its high sequence homology to salmon IFN-γ. For comparison, Atlantic salmon IFNa1, a type I IFN, was also expressed in E. coli using the same protocol as that used for trout IFN-γ. Figure 1 shows SDS-PAGE results for the purified recombinant rainbow trout IFN-γ and salmon IFNa1.
Fig. 1.
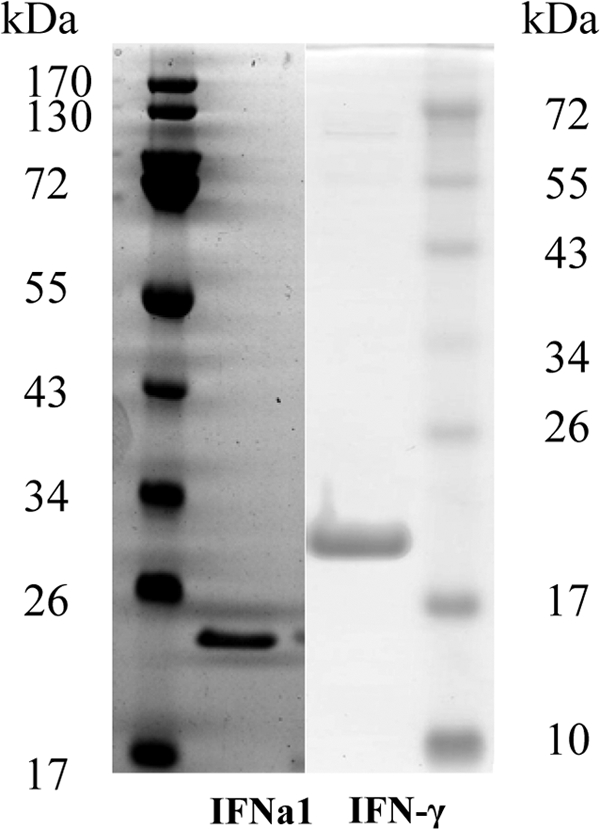
SDS-PAGE of recombinant Atlantic salmon IFNa1 and rainbow trout IFN-γ. Recombinant proteins were produced in E. coli and purified by Ni-NTA affinity chromatography. IFNa1 (100 ng) and IFN-γ (100 ng) were subjected to SDS-PAGE, along with protein standards. Gels were stained with SimpleBlue.
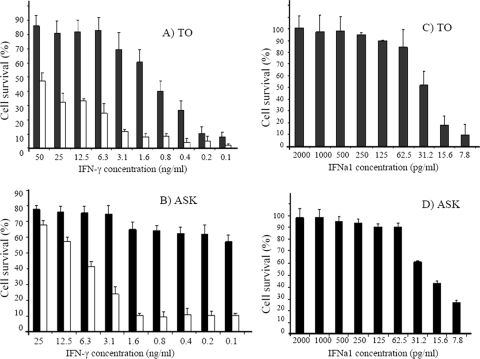
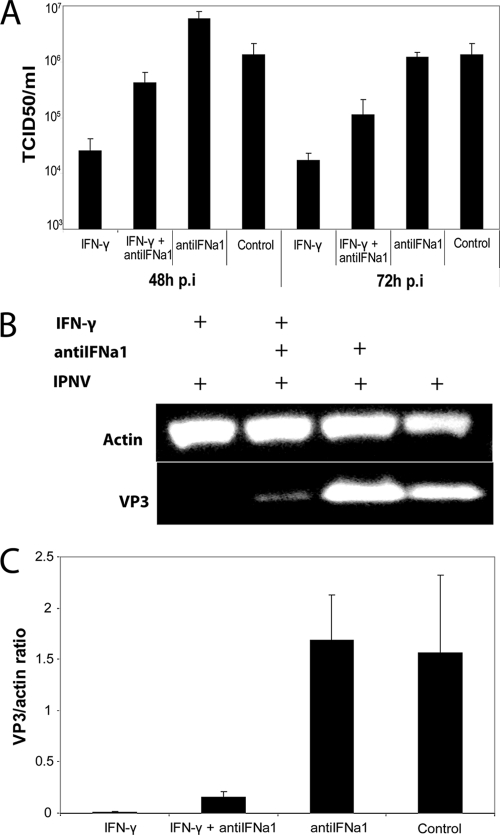
Antiviral activity of IFN-γ and IFNa1 against IPNV were tested in TO and ASK cells using the CPE reduction method (Fig. 2). IFN-γ showed potent antiviral activity in TO cells where the effective concentration that induced 50% protection of the cells (EC50) was determined to be ∼1.6 ng/ml (Fig. 2A). An even better protective effect of IFN-γ was observed in ASK cells with an EC50 of <0.1 ng/ml (Fig. 2B). As expected for a type I IFN, IFNa1 showed stronger antiviral activity than IFN-γ, with an EC50 of ∼31 pg of IFNa1/ml (Fig. 2C and D). The virus titers and synthesis of the IPNV structural protein VP3 were also compared in IFN-γ treated and nontreated TO cells after infection with IPNV. Treatment of TO cells with 100 ng of IFN-γ/ml resulted in an approximately 50- to 100-fold reduction in IPNV titers at 48 and 72 h postinfection (p.i.) compared to the control, with differences that were significant (P < 0.05) (Fig. 3A). Moreover, virus protein expression was also inhibited by IFN-γ treatment (Fig. 3B), since a significant (P < 0.05) reduction in VP3 levels was detected in IFN-γ-stimulated cells at 48 h p.i. compared to nontreated, infected cells. The VP3/actin ratio was ∼150-fold higher in the control compared to IFN-γ-treated cells (Fig. 3C). The antiviral activity of the IFN-γ preparation is not due to impurities in the lipopolysaccharide or nucleic acids, because heat-inactivated IFN-γ lost its ability to induce antiviral activity against IPNV (data not shown).
Fig. 2.
Antiviral activity of trout IFN-γ and salmon IFNa1 against IPNV measured as protection against the development of virus-induced CPE. Quadruplicate wells of TO cells (A and C) and ASK cells (B and D) were stimulated with different concentrations of recombinant IFN-γ (A and B) or IFNa1 (C and D) for 24 h at 17°C (■). Cells were also stimulated with IFN-γ in the presence of 1% antiIFNa1 antiserum (□). The cells were then infected with IPNV at an MOI of 0.1. When full CPE was observed in infected nontreated cells, cell survival was estimated by crystal violet staining (optical density at 550 nm). Nontreated noninfected cells served as the positive control (100% survival).
Fig. 3.
Antiviral activity of IFN-γ measured as the reduction in virus titers and the expression of viral VP3 protein. (A) TO cells were pretreated for 24 h with 100 ng of IFN-γ/ml with or without antiIFNa1, before infection with IPNV (MOI of 0.1). Supernatants were harvested 48 and 72 h p.i. and titrated on CHSE cells. The results show the mean TCID50/ml values of three replicate wells from the same setup. Control was untreated, infected cells. (B) TO cells were pretreated for 24 h with 100 ng of IFN-γ/ml with or without antiIFNa1, before infection with IPNV (MOI of 0.1). Lysates were harvested 48 h p.i. and subjected to Western blot analysis. The figure shows results from one representative parallel from the same setup as in panel A. (C) The actin and VP3 bands from the Western blot analysis in panel B were quantified, and the results show the mean VP3/actin ratio of three replicate wells from the same setup. The control was untreated, infected cells.
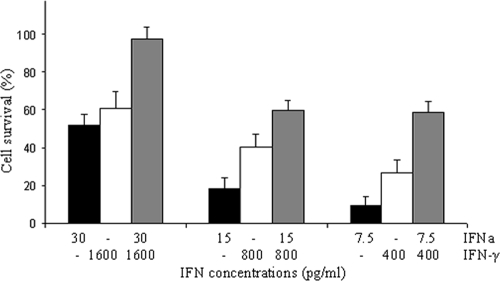
Finally, IFN-γ and IFNa1 were found to act synergistically in antiviral activity against IPNV (Fig. 4). Combination of IFN-γ (0.4 to 1.6 ng/ml) and IFNa1 (7.5 to 30 pg/ml) induced a greater protection of cells against IPNV-induced CPE than did each IFN alone.
Fig. 4.
Synergistic activity of IFN-γ and IFNa1 against IPNV in TO cells. TO cells were stimulated with recombinant IFNa (7.5 to 30 pg/ml), IFN-γ (400 to 1,600 pg/ml), or a combination of IFNa and IFN-γ (■, IFNa; □, IFN-γ;  , IFN-γ and IFNa). Antiviral analysis was performed as previously described. After 24 h of stimulation, the culture medium was removed, and the cells were infected with IPNV at an MOI of 0.1. At 4 days after IPNV infection, cell survival was estimated by crystal violet staining (optical density at 550 nm).
, IFN-γ and IFNa). Antiviral analysis was performed as previously described. After 24 h of stimulation, the culture medium was removed, and the cells were infected with IPNV at an MOI of 0.1. At 4 days after IPNV infection, cell survival was estimated by crystal violet staining (optical density at 550 nm).
The anti-IPNV activity of IFN-γ is partially dependent on IFNa.
A priori, the antiviral activity of IFN-γ could be due to induction of type I IFN (8). To test this hypothesis in the salmon model, TO cells were stimulated with IFN-γ in the presence or absence of neutralizing antiserum generated against IFNa1 (antiIFNa1) (3). AntiIFNa1 reduced the antiviral activity of IFN-γ substantially (Fig. 2A and B). In the presence of antiIFNa1, the minimum doses of IFN-γ resulting in 50% survivals of IPNV-infected TO cells and ASK cells were 50 and 12.5 ng/ml, respectively (Fig. 2A and B, white bars). This is 30 to 125 times greater than the concentration of IFN-γ giving 50% protection of cells in the absence of antiIFNa1. AntiIFNa1 also had a significant (P < 0.05) negative impact on the ability of IFN-γ to reduce IPNV titers (Fig. 3A). Furthermore, the reduction in antiviral activity of IFN-γ by anti-IFNa was also evident from the effect on viral protein expression (Fig. 3B and C). Thus, while VP3 expression was almost abolished in IFN-γ-treated cells at 48 h p.i., cells treated with IFN-γ in the presence of antiIFNa1 showed a VP3 expression ∼15-fold higher than the IFN-γ-treated cells (Fig. 3C).
Antiviral activity of IFN-γ against SAV3.
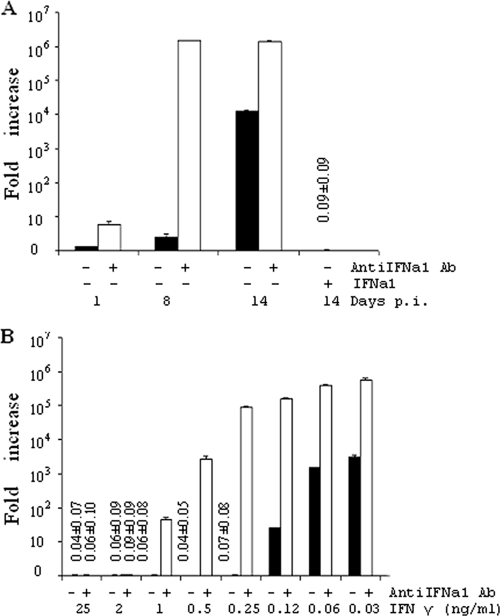
During the 14 days of SAV3 infection of TO cells, no clear CPE was observed. SAV3 replication was thus tracked by measuring nsP1 transcripts by quantitative PCR (qPCR). In the nonstimulated virus-infected cells, the nsP1 transcripts increased 4-fold between days 1 and 8 and 104-fold by day 14 (Fig. 5). SAV3 replication was effectively inhibited by both IFN-γ (1.6 ng/ml) and IFNa1 (1 ng/ml), resulting in a complete elimination of nsP1 transcripts or a reduction in transcripts to <10% of the day 1 levels in infected control cells. Interestingly, the presence of antiIFNa1 during infection of cells caused a marked increase in virus replication. In the presence of antiIFNa1, nsP1 transcripts were 7.6, 105, and 102 times higher than in infected control cells at days 1, 8, and 14 p.i., respectively. This suggests that infection with SAV3 induces IFNa in the cells, which then slows down replication of the virus in support of previous work (58). IFN-γ abolished SAV3 transcription at concentrations from 0.25 to 25 ng/ml (Fig. 5). AntiIFNa1 reduced the antiviral activity against SAV3 of IFN-γ at 1 ng/ml and lower concentrations. In the case of SAV3, the dependency on IFNa for antiviral activity of IFN-γ is, however, less certain since the virus itself induces IFNa (58).
Fig. 5.
Inhibition of SAV3 replication by IFN-γ and IFNa1. (A) TO cells were infected with SAV3 in the absence (■) or presence (□) of antiIFNa1 antibody or 24 h after stimulation with IFNa1 (1 ng/ml). (B) TO cells were infected with SAV3 after stimulating with IFN-γ (24 h) with or without antiIFNa1 and sampled 14 days p.i. Bars show the fold increase in virus nsP1 transcripts measured by qPCR related to non-IFN-treated cells at day 1 p.i. Treatments and time points for sampling are indicated underneath bars (n = 3).
IFN-γ and IFNa1 show different profiles in the induction of antiviral genes, IFN regulatory factors, and the chemokine IP-10.
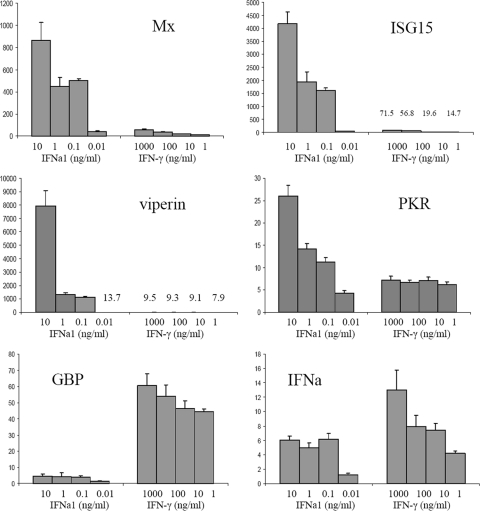
To compare gene induction by IFNa1 and IFN-γ, we chose antiviral genes that in mammals are typically induced by type I IFN (Mx, ISG15, viperin, and PKR) and type II IFN (GBP). We also studied the expression of other IFN regulatory factors—IRF-1, IRF-3, IRF-7, IRF-8, and IRF-9—since they are involved in the regulation of type I IFN and IFN-induced antiviral genes. The chemokine IP-10 (also named CXCL10) was included because it is strongly induced by IFN-γ in mammals (25). Moreover, since antiIFNa1 reduced the antiviral activity of IFN-γ against IPNV, we wanted to find out whether IFN-γ induced the expression of IFNa1 and the other two type I IFNs, IFNb and IFNc. Gene expression was measured by qPCR. Although both IFN-γ and IFNa1 upregulated the expression of all of the tested genes, most of the genes showed different expression characteristics in response to the two IFNs (Fig. 6 and 7). IFN-γ showed a dose-dependent effect on the transcription of most genes from 1 to 1,000 ng/ml, while IFNa1 showed a dose-dependent effect at much lower concentrations (0.01 to 10 ng/ml). Mx protein was upregulated 850-fold by 10 ng of IFNa1/ml but only 20-fold by 10 ng of IFN-γ/ml. Even at a concentration of 0.1 ng/ml, Mx was upregulated 500-fold by IFNa1 but was still only 50-fold upregulated by IFN-γ at 1 μg/ml. The difference in transcriptional response was even more pronounced for viperin, which increased 7,000 times by treatment with IFNa1 (10 ng/ml). In contrast, viperin transcripts only increased 8-fold after stimulation with 1 μg of IFN-γ/ml. ISG15 was upregulated 1,500- to 4,000-fold with 0.1 to 10 ng of IFNa1/ml, whereas ISG15 was upregulated only 15- to 71-fold with 1 to 1,000 ng of IFN-γ/ml (Fig. 6). PKR was constitutively expressed in TO cells (CT of 29) but showed a dose-dependent response to IFNa1 being upregulated 26 times after treatment with 10 ng of IFNa1/ml. PKR transcripts increased 7-fold after treatment with 1 to 1,000 ng of IFN-γ/ml. Similar to mammals, GBP was more strongly upregulated by IFN-γ than by type I IFN. Thus, GBP transcripts increased 45- to 60-fold by stimulation of TO cells with 1 to 1,000 ng of IFN-γ/ml. In contrast, GBP increased only 4-fold with 10 ng of IFNa1/ml. Interestingly, both IFN-γ and IFNa1 were able to upregulate expression of IFNa 6- to 7-fold at 10 ng/ml. The fact that IFN-γ is able to induce IFNa1 thus supports the hypothesis that the antiviral effects of IFN-γ are in part due to the induction of IFNa1. In contrast, IFN-γ did not induce IFNb and IFNc (data not shown).
Fig. 6.
Effect of IFN-γ and IFNa1 on the expression of antiviral genes in TO cells 24 h after stimulation. Values are the fold increase in transcripts compared to nonstimulated cells (n = 3).
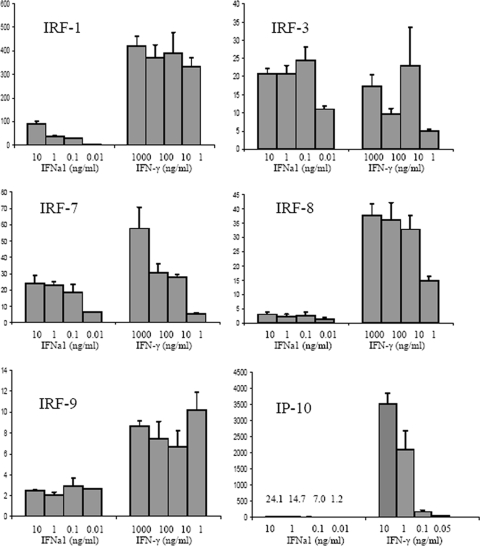
Fig. 7.
Effect of IFN-γ and IFNa1 on expression of IRFs and IP-10 in TO cells 24 h after stimulation. Values are the fold increase in transcripts compared to nonstimulated cells (n = 3).
Interestingly, several of the IRFs showed a higher increase in transcripts upon stimulation with IFN-γ than with IFNa1. IRF-1 transcription was most highly induced by IFN-γ, increasing more than 300 times in response to 1 and 10 ng of IFN-γ/ml. In contrast, IFNa1 induced IRF-1 90-fold at 10 ng/ml and 36-fold at 1 ng/ml. Also, IRF-7, IRF-8, and IRF-9 were more strongly induced by IFN-γ than by IFNa1. On the other hand, there was no clear difference between IFN-γ and IFNa1 in the induction of IRF-3 at 10 ng/ml, but IFNa1 was the more effective inducer at lower concentrations. Finally, the chemokine IP-10 was rather specifically induced by IFN-γ compared to IFNa1, at least at the 24-h time point. At 10 ng/ml, IFN-γ induced IP-10 expression 3,500-fold, while IFNa1 only induced expression 24-fold (Fig. 7).
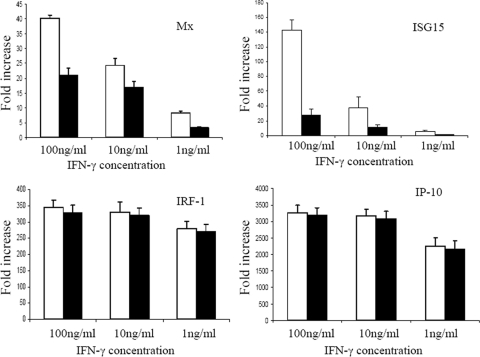
AntiIFNa1 reduced IFN-γ induction of Mx and ISG15 but not the induction of IRF-1 or IP-10.
To test the influence of antiIFNa1 on the expression of IFN-γ-induced genes, we studied the expression of IRF-1, IP-10, Mx, and ISG15 transcripts in IFN-γ-treated TO cells in the presence or absence of antiIFNa1 (Fig. 8). Adding antiIFNa1 caused ca. 50 and 78% reductions in the transcript levels of Mx and ISG15, respectively, upon stimulation with 100 ng of IFN-γ/ml (Fig. 8). In contrast, the expression of IRF-1 and IP-10 in response to IFN-γ was not affected by the presence of antiIFNa1. These results show that while the induction of Mx and ISG15 apparently is partially dependent on the induction of IFNa, IFN-γ induction of IRF-1 and IP-10 is independent of IFNa.
Fig. 8.
Effects of antiIFNa1 antibody on IFN-γ induction of Mx, ISG15, IP-10, and IRF-1 transcripts. TO cells were stimulated with 100, 10, and 1 ng of IFN-γ/ml, respectively, with 1% antiIFNa1 serum (■) or control serum (□) and sampled at 10 h after stimulation. Values are the fold increase in transcripts compared to nonstimulated cells (n = 3).
Effect of antiIFNa1 on the IFN-γ-induced expression of Mx and ISG15 proteins.
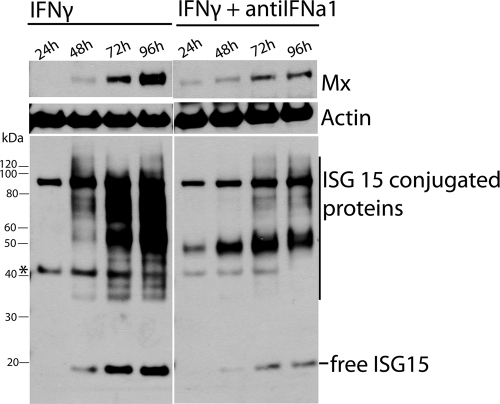
We also studied the effect of IFN-γ on the expression of Mx and ISG15 at the protein level and whether antiIFNa1 had an influence on protein expression. As shown in Fig. 9, IFN-γ increased the expression of Mx protein and free ISG15 and ISG15 conjugates between 24 and 96 h. Mx and ISG15 protein levels were markedly diminished at 72 and 96 h in cells stimulated in the presence of antiIFNa1 compared to cells stimulated with IFN-γ alone (Fig. 9), which further supports that the induction of these genes by IFN-γ is partially dependent on IFNa.
Fig. 9.
Time course study of Mx and ISG15 protein expression was conducted in TO cells stimulated with 100 ng of IFN-γ/ml with or without 1% antiIFNa1 anti-serum. Lysates were harvested at the indicated time points ranging from 24 to 96 h and subjected to Western blot analysis. The blot was incubated with antibodies against Mx (1:3,000) and actin (1:1,000) and developed before stripping and reincubation with antibody against ISG15 (1:20,000). The asterisk indicates the remnants of actin antibody, which was not completely removed during the stripping procedure.
DISCUSSION
Little is previously known about the antiviral properties of fish IFN-γ. The present study demonstrates that IFN-γ has strong antiviral activity against the naked double-stranded RNA virus IPNV and the enveloped single-stranded RNA virus SAV3 in Atlantic salmon cells. Both viruses cause high losses of farmed salmon. IFN-γ protected salmon cells against IPNV-induced CPE, reduced virus titers, and inhibited synthesis of the viral structural protein VP3. Moreover, IFN-γ showed potent antiviral activity against SAV3 measured as reduction in virus nsP1 transcripts. IFN-γ had 50-fold less specific antiviral activity against IPNV compared to IFNa1 in TO cells, showing an EC50 of 1.6 ng/ml versus 31 pg/ml in the CPE reduction assay. Differences in antiviral activity of IFN-γ and type I IFNs is likely due to differential induction of genes since these IFN utilize different receptors and thus activate different JAK/STAT pathways. IFN-γ and IFNa1 were indeed shown to induce distinct expression profiles of selected genes in Atlantic salmon. IFNa1 induced the highest transcript levels of the antiviral proteins Mx, ISG15, and viperin, which are much more strongly induced by type I IFNs than by IFN-γ also in mammals (6, 15, 17). Mx is a GTPase with broad antiviral activity in mammals and does also display antiviral activity against IPNV in salmonid cells (16, 24). ISG15 is an ubiquitin homolog, which conjugates to various host proteins both in mammals and fish, although its exact antiviral mechanism is still unknown (17, 42). Viperin was first identified in rainbow trout as a virus-induced gene (vig1) and was later shown to inhibit virus release or replication by binding to lipid droplets in mammals (5, 12). Viperin and ISG15 were identified as inhibitors of the alphavirus Sindbis virus and may thus play a role in the IFN inhibition of SAV3 (58).
IFN-γ also upregulated Mx, ISG15, and viperin transcripts in salmon, but at much lower levels compared to IFNa1 24 h after stimulation. On the other hand, IFN-γ treatment of salmon cells resulted in a marked increase in Mx and ISG15 proteins 48 to 96 h after stimulation, which indicates a later response time compared to IFNa1. Induction of ISG15 by IFN-γ resulted in conjugation of ISG15 to multiple cellular proteins similar to that shown for salmon IFNa1 previously (42).
The double-stranded RNA activated kinase PKR was constitutively expressed in salmon cells and was upregulated by stimulation with both IFN-γ and IFNa1, which is similar to expression properties of PKR in mammals (10). PKR inhibits viral replication by phosphorylating the initiation factor eIF2α chain, which leads to the inhibition of protein synthesis (43). Antiviral activity of PKR was recently demonstrated in fish (60).
GBP was confirmed here to be a typical IFN-γ-induced gene also in fish, with transcript levels in TO cells increased more than 45-fold by stimulation with IFN-γ compared to 4-fold by stimulation with IFNa1. GBP is a GTPase that is recognized as an IFN-γ-induced antiviral protein in mammals and is induced by IFN-γ in rainbow trout (1, 41).
Taken together, the antiviral activity induced by IFN-γ in salmon is likely due to upregulation of PKR, GBP, and several genes that are normally induced by type I IFN such as Mx, ISG15, and viperin. The lower antiviral activity of IFN-γ compared to IFNa1 may be attributed to lower and later induction of the latter group of antiviral genes. Even in mammals, there is little information about typical IFN-γ-induced antiviral genes other than GBP (45).
The fact that IFN-γ induced an increase in IFNa1 transcripts raised the question of whether the antiviral activity of IFN-γ is due to the induction of IFNa. To explore this possibility, the antiviral activity of IFN-γ was measured in the presence or absence of an antiserum which effectively neutralizes the antiviral activity of IFNa1 (3). Indeed, in the presence of antiIFNa1, IFN-γ showed not only reduced antiviral activity against IPNV but also induced lower amounts of Mx and ISG15 at the transcript and protein level. IPNV itself does not induce IFN or Mx in TO or CHSE cells (20, 21, 42). In fact, proteins encoded by IPNV inhibit both activation of the IFNa1 promoter (unpublished results) and inhibit IFNa1-mediated signaling (49). Anti-IFNa1 also seemed to reduce the antiviral activity of IFN-γ against SAV3, but here the mechanism is less clear since the virus itself is known to induce IFN. Altogether, the antiviral activity of IFN-γ in salmonid fish is at least partially dependent on IFNa1, potentially due to the induction of IFNa, which subsequently induces Mx and other antiviral genes. On the other hand, IFN-γ may induce some Mx transcription independent of IFNa1 since it was impossible to abolish Mx transcription completely by adding antiIFNa1 to IFN-γ. Since IFN-γ neither induces IFNb nor IFNc in TO cells, it is possible that IFN-γ activates ISGF3 to some extent in salmon, as has been shown for mice (27). Salmon cells deficient in IFNa receptor would be required to settle these questions, but this receptor has thus far not been identified in salmonid fish.
In human cells, the antiviral activity of IFN-γ appears to be independent of autocrine or paracrine type I IFN induction of antiviral genes (8, 29). The induction of antiviral genes by human IFN-γ was instead found to be dependent on the formation of ISGF3II, which contains nonphosphorylated STAT2. Whether this mechanism is important in fish has to be examined in future studies. The antiviral activity of IFN-γ in mice is partially dependent on the constitutive expression of type I IFN but not on the induction of type I IFN (55). An efficient IFN-γ response has been suggested to be dependent on the association of the IFN-γ receptor with the type I IFN receptor bound to type I IFN (55) or to be dependent on upregulation of STAT1 by low-level constitutively expressed type I IFN (13). Upregulation of STAT1 by IFNa1 also occurs in salmon (23, 50). We reasoned that if the lowering of antiviral activity of IFN-γ by antiIFNa1 in salmon is due to suboptimal assembly of the IFN-γ receptor complex or to lowering of STAT1 levels, then the antiIFNa1 antibody should also lower induction of typical IFN-γ induced genes such as IRF-1 and IP-10. However, although IFN-γ in the presence of antiIFNa1 induced lower levels of Mx and ISG15 transcripts compared to IFN-γ alone, there was no change in the induction of IRF-1 and IP-10 (Fig. 8). This suggests that in salmon, constitutively expressed IFNa1 is neither important for the function of the IFN-γ receptor nor for the maintenance of sufficient STAT1 levels. Taken together, the dependency of type I IFN for the antiviral activity of IFN-γ seems different in Atlantic salmon, humans, and mice.
We confirmed that in salmon the transcription factor IRF-1 and the chemokine IP-10 (CXCL10) were much more strongly induced by IFN-γ than by IFNa1 and thus represent signature genes for the IFN-γ response also in fish. Compared to IFNa1, IFN-γ also induced higher levels of IRF-7, IRF-8, and IRF-9, while IFN-γ and IFNa1 induced similar levels of IRF-3. IRF-1, IRF-3, and IRF-7 are involved in the transcriptional activation of IFN-β in mammals (31). In salmon, IRF-1, IRF-3, and IRF-7b activate the IFNa1 promoter (4). In mammals, IRF-1 has recently been shown to directly activate the transcription of viperin and to mediate the induction of viperin after IFN-γ stimulation (52). The interaction between IRF-8 and TRAF6 modulates TLR signaling in mammals and may contribute to the cross talk between IFN-γ and TLR signal pathways (59). IRF-9 is an important component of the ISGF3 complex which mediates the transcription of type I IFN-induced genes. Previous work has demonstrated the upregulation of STAT1 by IFN-γ (50). Taken together, IFN-γ induction of IRFs and STAT1 may prime cells for a more swift response to viral infections. Previous research showed that IFN-γ also upregulated the transcription of TLR8 and TLR9 homologs in salmon, which should further contribute to the priming of cells for an enhanced antiviral response (47, 48).
A recent report claimed that salmon IFN-γ has no antiviral activity against SAV3, which is in disagreement with the present results (57). However, the reason for the difference in results is probably that recombinant salmon IFN-γ produced in E. coli from inclusion bodies is inactive. Salmon IFN-γ prepared this way induced only 30- and 12-fold increases in IP-10 at 1.65 μg/ml and 330 ng/ml, respectively (57). We obtained similar results with salmon IFN-γ produced from inclusion bodies in E. coli (data not shown). In contrast, trout IFN-γ produced as a soluble protein induced a 2,000-fold increase in IP-10 transcripts at a concentration of 1 ng/ml. Unfortunately, the yield of soluble salmon IFN-γ produced by the same protocol as for trout IFN-γ was very small and not suitable for purification (data not shown). It is highly unlikely that Atlantic salmon possesses an inactive IFN-γ. Our interpretation is thus that the sequence difference between trout and salmon IFN-γ may explain the difficulty in producing a recombinant bioactive preparation of the latter.
It has been shown that SAV3 induces IFNa in salmon cells, which may be limiting for replication of this virus (57). This is indeed confirmed by the present study, which showed that the addition of antiIFNa1 to SAV3 culture strongly promoted replication of the virus.
In summary, we demonstrate here that IFN-γ has antiviral activity against IPNV and SAV3 in Atlantic salmon. The antiviral activity of IFN-γ in salmon appears to be partially dependent on genes, which in mammals are induced by type I IFNs through the activation of ISGF3. In mammals, IFN-γ can also to some extent induce such genes by the activation of ISGF3 or ISGF3II. Although this may occur in salmon as well, we found that the antiviral activity of IFN-γ and its ability to induce Mx and ISG15 is at least in part dependent on IFNa, possibly through induction.
ACKNOWLEDGMENTS
We thank Hilde Hansen, Norwegian College of Fishery Science, University of Tromsø, for making the polyclonal antibody against Atlantic salmon Mx1.
This study was supported by the Aquaculture Program of the Research Council of Norway (grant 1726619).
Footnotes
Published ahead of print on 22 June 2011.
REFERENCES
- 1. Anderson S. L., Carton J. M., Lou J., Xing L., Rubin B. Y. 1999. Interferon-induced guanylate binding protein-1 (GBP-1) mediates an antiviral effect against vesicular stomatitis virus and encephalomyocarditis virus. Virology 256:8–14 [DOI] [PubMed] [Google Scholar]
- 2. Arts J. A., Tijhaar E. J., Chadzinska M., Savelkoul H. F., Verburg-van Kemenade B. M. 2010. Functional analysis of carp interferon-gamma: evolutionary conservation of classical phagocyte activation. Fish Shellfish Immunol. 29:793–802 [DOI] [PubMed] [Google Scholar]
- 3. Berg K., Svingerud T., Sun B., Robertsen B. 2009. An antiserum against Atlantic salmon IFNa1 detects IFN and neutralizes antiviral activity produced by poly I:C stimulated cells. Dev. Comp. Immunol. 33:638–645 [DOI] [PubMed] [Google Scholar]
- 4. Bergan V., Kileng O., Sun B., Robertsen B. 2010. Regulation and function of interferon regulatory factors of Atlantic salmon. Mol. Immunol. 47:2005–2014 [DOI] [PubMed] [Google Scholar]
- 5. Boudinot P., Massin P., Blanco M., Riffault S., Benmansour A. 1999. Vig-1, a new fish gene induced by the rhabdovirus glycoprotein, has a virus-induced homologue in humans and shares conserved motifs with the MoaA family. J. Virol. 73:1846–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chin K. C., Cresswell P. 2001. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 98:15125–15130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christie K. E., Havarstein L. S., Djupvik H. O., Ness S., Endresen C. 1988. Characterization of a new serotype of infectious pancreatic necrosis virus isolated from Atlantic salmon. Arch. Virol. 103:167–177 [DOI] [PubMed] [Google Scholar]
- 8. Costa-Pereira A. P., et al. 2002. The antiviral response to gamma interferon. J. Virol. 76:9060–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Decker T., Kovarik P., Meinke A. 1997. GAS elements: a few nucleotides with a major impact on cytokine-induced gene expression. J. Interferon Cytokine Res. 17:121–134 [DOI] [PubMed] [Google Scholar]
- 10. Der S. D., Lau A. S. 1995. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc. Natl. Acad. Sci. U. S. A. 92:8841–8845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devold M., Krossoy B., Aspehaug V., Nylund A. 2000. Use of RT-PCR for diagnosis of infectious salmon anaemia virus (ISAV) in carrier sea trout Salmo trutta after experimental infection. Dis. Aquat. Org. 40:9–18 [DOI] [PubMed] [Google Scholar]
- 12. Fitzgerald K. A. 2011. The interferon-inducible gene: viperin. J. Interferon Cytokine Res. 31:131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gough D. J., et al. 2010. Functional crosstalk between type I and II interferon through the regulated expression of STAT1. PLoS Biol. 8:e1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grayfer L., Garcia E. G., Belosevic M. 2010. Comparison of macrophage antimicrobial responses induced by type II interferons of the goldfish (Carassius auratus L.). J. Biol. Chem. 285:23537–23547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haller O., Frese M., Kochs G. 1998. Mx proteins: mediators of innate resistance to RNA viruses. Rev. Sci. Technol. 17:220–230 [DOI] [PubMed] [Google Scholar]
- 16. Haller O., Kochs G. 2002. Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity. Traffic 3:710–717 [DOI] [PubMed] [Google Scholar]
- 17. Harty R. N., Pitha P. M., Okumura A. 2009. Antiviral activity of innate immune protein ISG15. J. Innate Immun. 1:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hug H., Costas M., Staeheli P., Aebi M., Weissmann C. 1988. Organization of the murine Mx gene and characterization of its interferon- and virus-inducible promoter. Mol. Cell. Biol. 8:3065–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Igawa D., Sakai M., Savan R. 2006. An unexpected discovery of two interferon gamma-like genes along with interleukin (IL)-22 and -26 from teleost: IL-22 and -26 genes have been described for the first time outside mammals. Mol. Immunol. 43:999–1009 [DOI] [PubMed] [Google Scholar]
- 20. Jensen I., Robertsen B. 2002. Effect of double-stranded RNA and interferon on the antiviral activity of Atlantic salmon cells against infectious salmon anemia virus and infectious pancreatic necrosis virus. Fish Shellfish Immunol. 13:221–241 [DOI] [PubMed] [Google Scholar]
- 21. Jorgensen J. B., et al. 2007. A recombinant CHSE-214 cell line expressing an Mx1 promoter-reporter system responds to both interferon type I and type II from salmonids and represents a versatile tool to study the IFN-system in teleost fish. Fish Shellfish Immunol. 23:1294–1303 [DOI] [PubMed] [Google Scholar]
- 22. Karlsen M., Villoing S., Rimstad E., Nylund A. 2009. Characterization of untranslated regions of the salmonid alphavirus 3 (SAV3) genome and construction of a SAV3 based replicon. Virol. J. 6:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kileng O., Bergan V., Workenhe S. T., Robertsen B. 2009. Structural and functional studies of an IRF-7-like gene from Atlantic salmon. Dev. Comp. Immunol. 33:18–27 [DOI] [PubMed] [Google Scholar]
- 24. Larsen R., Røkenes T. P., Robertsen B. 2004. Inhibition of infectious pancreatic necrosis virus replication by Atlantic salmon Mx1 protein. J. Virol. 78:7938–7944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luster A. D., Ravetch J. V. 1987. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J. Exp. Med. 166:1084–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lutfalla G., et al. 2003. Comparative genomic analysis reveals independent expansion of a lineage-specific gene family in vertebrates: the class II cytokine receptors and their ligands in mammals and fish. BMC Genomics 4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsumoto M., et al. 1999. Activation of the transcription factor ISGF3 by interferon-gamma. Biol. Chem. 380:699–703 [DOI] [PubMed] [Google Scholar]
- 28. Milev-Milovanovic I., et al. 2006. Identification and expression analysis of interferon gamma genes in channel catfish. Immunogenetics 58:70–80 [DOI] [PubMed] [Google Scholar]
- 29. Morrow A. N., Schmeisser H., Tsuno T., Zoon K. C. 2011. A novel role for IFN-stimulated gene factor 3II in IFN-gamma signaling and induction of antiviral activity in human cells. J. Immunol. 186:1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muller U., et al. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921 [DOI] [PubMed] [Google Scholar]
- 31. Paun A., Pitha P. M. 2007. The IRF family, revisited. Biochimie 89:744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pestka S., Krause C. D., Walter M. R. 2004. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202:8–32 [DOI] [PubMed] [Google Scholar]
- 33. Pfaffl M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pous J., et al. 2005. Structure of birnavirus-like particles determined by combined electron cryomicroscopy and X-ray crystallography. J. Gen. Virol. 86:2339–2346 [DOI] [PubMed] [Google Scholar]
- 35. Powers A. M., et al. 2001. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 75:10118–10131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qureshi S. A., Salditt-Georgieff M., Darnell J. E., Jr 1995. Tyrosine-phosphorylated Stat1 and Stat2 plus a 48-kDa protein all contact DNA in forming interferon-stimulated-gene factor 3. Proc. Natl. Acad. Sci. U. S. A. 92:3829–3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reed L., Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 38. Robertsen B. 2006. The interferon system of teleost fish. Fish Shellfish Immunol. 20:172–191 [DOI] [PubMed] [Google Scholar]
- 39. Robertsen B., Bergan V., Rokenes T., Larsen R., Albuquerque A. 2003. Atlantic salmon interferon genes: cloning, sequence analysis, expression, and biological activity. J. Interferon Cytokine Res. 23:601–612 [DOI] [PubMed] [Google Scholar]
- 40. Robertsen B., Trobridge G., Leong J. C. 1997. Molecular cloning of double-stranded RNA inducible Mx genes from Atlantic salmon (Salmo salar L.). Dev. Comp. Immunol. 21:397–412 [DOI] [PubMed] [Google Scholar]
- 41. Robertsen B., Zou J., Secombes C., Leong J. A. 2006. Molecular and expression analysis of an interferon-gamma-inducible guanylate-binding protein from rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 30:1023–1033 [DOI] [PubMed] [Google Scholar]
- 42. Rokenes T. P., Larsen R., Robertsen B. 2007. Atlantic salmon ISG15: expression and conjugation to cellular proteins in response to interferon, double-stranded RNA and virus infections. Mol. Immunol. 44:950–959 [DOI] [PubMed] [Google Scholar]
- 43. Samuel C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schoenborn J. R., Wilson C. B. 2007. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 96:41–101 [DOI] [PubMed] [Google Scholar]
- 45. Schroder K., Hertzog P. J., Ravasi T., Hume D. A. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163–189 [DOI] [PubMed] [Google Scholar]
- 46. Shivappa R. B., et al. 2004. Molecular characterization of Sp serotype strains of infectious pancreatic necrosis virus exhibiting differences in virulence. Dis. Aquat. Org. 61:23–32 [DOI] [PubMed] [Google Scholar]
- 47. Skjaeveland I., Iliev D. B., Strandskog G., Jorgensen J. B. 2009. Identification and characterization of TLR8 and MyD88 homologs in Atlantic salmon (Salmo salar). Dev. Comp. Immunol. 33:1011–1017 [DOI] [PubMed] [Google Scholar]
- 48. Skjaeveland I., Iliev D. B., Zou J., Jorgensen T., Jorgensen J. B. 2008. A TLR9 homolog that is up-regulated by IFN-gamma in Atlantic salmon (Salmo salar). Dev. Comp. Immunol. 32:603–607 [DOI] [PubMed] [Google Scholar]
- 49. Skjesol A., Aamo T., Hegseth M. N., Robertsen B., Jorgensen J. B. 2009. The interplay between infectious pancreatic necrosis virus (IPNV) and the IFN system: IFN signaling is inhibited by IPNV infection. Virus Res. 143:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Skjesol A., Hansen T., Shi C. Y., Thim H. L., Jorgensen J. B. 2010. Structural and functional studies of STAT1 from Atlantic salmon (Salmo salar). BMC Immunol. 11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stark G. R., Kerr I. M., Williams B. R., Silverman R. H., Schreiber R. D. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227–264 [DOI] [PubMed] [Google Scholar]
- 52. Stirnweiss A., et al. 2010. IFN regulatory factor-1 bypasses IFN-mediated antiviral effects through viperin gene induction. J. Immunol. 184:5179–5185 [DOI] [PubMed] [Google Scholar]
- 53. Stolte E. H., Savelkoul H. F., Wiegertjes G., Flik G., Lidy Verburg-van Kemenade B. M. 2008. Differential expression of two interferon-gamma genes in common carp (Cyprinus carpio L.). Dev. Comp. Immunol. 32:1467–1481 [DOI] [PubMed] [Google Scholar]
- 54. Sun B., Robertsen B., Wang Z., Liu B. 2009. Identification of an Atlantic salmon IFN multigene cluster encoding three IFN subtypes with very different expression properties. Dev. Comp. Immunol. 33:547–558 [DOI] [PubMed] [Google Scholar]
- 55. Takaoka A., et al. 2000. Cross talk between interferon-gamma and -alpha/beta signaling components in caveolar membrane domains. Science 288:2357–2360 [DOI] [PubMed] [Google Scholar]
- 56. Wergeland H. I., Aakre Johansen R. 2001. A salmonid cell line (TO) for production of infectious salmon anaemia virus (ISAV). Dis. Aquat. Org. 44:183–190 [DOI] [PubMed] [Google Scholar]
- 57. Xu C., et al. 2010. Alpha interferon and not gamma interferon inhibits salmonid alphavirus subtype 3 replication in vitro. J. Virol. 84:8903–8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang Y., Burke C. W., Ryman K. D., Klimstra W. B. 2007. Identification and characterization of interferon-induced proteins that inhibit alphavirus replication. J. Virol. 81:11246–11255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao J., et al. 2006. IRF-8/interferon (IFN) consensus sequence-binding protein is involved in Toll-like receptor (TLR) signaling and contributes to the cross-talk between TLR and IFN-gamma signaling pathways. J. Biol. Chem. 281:10073–10080 [DOI] [PubMed] [Google Scholar]
- 60. Zhu R., Zhang Y. B., Zhang Q. Y., Gui J. F. 2008. Functional domains and the antiviral effect of the double-stranded RNA-dependent protein kinase PKR from Paralichthys olivaceus. J. Virol. 82:6889–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zou J., et al. 2005. Identification and bioactivities of IFN-gamma in rainbow trout Oncorhynchus mykiss: the first Th1-type cytokine characterized functionally in fish. J. Immunol. 175:2484–2494 [DOI] [PubMed] [Google Scholar]
- 62. Zou J., et al. 2004. Identification of an interferon gamma homologue in Fugu, Takifugu rubripes. Fish Shellfish Immunol. 17:403–409 [DOI] [PubMed] [Google Scholar]